Abstract
Objective
Streptococcus suis (S. suis) is a causative agent for various syndromes in pigs. It can be transmitted to humans with typical symptoms of meningitis and death. Although human infections have been confirmed at Bali Referral Hospital, Indonesia, since 2014, the bacteria have not been isolated from pigs. Here, we provide confirmation of the presence of the bacteria in sick pigs in the province.
Results
Streptococcus suis was confirmed in 8 of 30 cases. The final confirmation was made using PCR and sequencing of the glutamate dehydrogenase (GDH) and recombination/repair protein (recN) gene fragments. Upon PCR serotyping, two were confirmed to be serotype 2 or 1/2. Prominent histopathological lesions of confirmed cases were meningitis, endocarditis, pericarditis, bronchopneumonia, enteritis and glomerulonephritis. The dominant inflammatory cells were neutrophils and macrophages. Further research is needed to understand the risk factors for human infection. Community awareness on the risk of contracting S. suis and vaccine development are needed to prevent human infections.
Keywords: Glutamate dehydrogenase (GDH) gene, Recombination/repair protein (recN) gene, Streptococcus suis, Sick pigs, Bali, Indonesia
Introduction
Streptococcus suis (S. suis) is an important agent of emerging zoonotic, community-acquired bacterial meningitis [1–3]. It is a global zoonotic agent originating in pigs [4]. Asia is a unique hotspot of S. suis infection in humans related to traditional pork consumption customs [5, 6]. The bacteria cause significant economic losses through mortality and decreased production [7–9] and indirect losses through decreased demand for pork if an outbreak occurs.
This emerging zoonotic bacterium is of particular concern in tourist destinations because it poses an infection risk to travelers and local people. Bali Province in Indonesia is one of world’s most prominent tourist destinations. More than six million travelers visited Bali in 2018 (https://bali.bps.go.id). Data from Bali Referral Hospital showed more than 40 confirmed human cases of S. suis meningitis since 2014 [10]. Cases of S. suis infection in pigs with encephalitis and arthritis signs have long been suspected in the province. Attempts to detect the bacteria have not yet been successful. This is likely because of identification problems with S. suis. Its growth characteristic of forming small colonies [11] means that this bacterium might be overlooked in a culture with mixed bacterial species. Misidentification of S. suis is also common [12–14]. The false negative result can be solved through a complete serotyping system [15], which is not available in Indonesia.
Main text
Methods
Source of animals
Sick pigs were owned by individual farmers, who contacted The Animal Biomedical and Molecular Biology Laboratory of Udayana University reporting sickness and mortality in their piggery. They agreed for their sick and dead animals to be included in the study. The farms were located across Bali Province (Table 1).
Table 1.
Clinical signs, animal age, number of animal in stall of confirmed S. suis infection in Bali, Indonesia, 2018
| No | Location (village and regency) | Number of animal in stall | Number of sick animals | Age (month) | Number of death | Clinical signs |
|---|---|---|---|---|---|---|
| 1 | Abang, Karangasem | 3 | 2 | 8 | 1 | In-appetence, depression, reddish skin discoloration, cough |
| 2 | Sesetan, Denpasar | 100 | 21 | 4 | 3 | Fever, anorexia, lethargic, limping, paralyzed, tremor, eye and nasal exudate, erythema ear, joint enlargement and bruising |
| 3 | Payangan, Gianyar | 4 | 1 | 8 | 5 | Weakness, anorectic, diarrhea, watery nose, eye exudate, reddish skin discoloration, limping |
| 4 | Kesiman Denpasar | 8 | 6 | 10 | 6 | Diarrhea, limping, anorexia, paralyzed, snot |
| 5 | Sidakarya, Denpasar | 150 | 14 | 3 | 3 | Yellowish diarrhea, dyspnea, nasal exudate, shivering, reddish skin discoloration |
| 6 | Kediri, Tabanan | 68 | 11 | 7 | 3 | Weakness, anorexia, diarrhea, nasal and eye exudate, limping, reddish skin discoloration |
| 7 | Perean Kangin, Tabanan | 12 | 7 | 6 | 3 | Lethargic, anorexia, reddish skin discoloration, shivering |
| 8 | Lod tunduh, Gianyar | 24 | 7 | 12 | 7 | Anorexia, lethargic, cough, reddish skin discoloration, swollen joint |
During January to July 2018, 30 suspected S. suis cases were recorded. The inclusion criteria were acute illness with at least one clinical sign of neurological disorder, reddish skin discoloration and arthritis. The animals were not treated with antibiotics.
Freshly dead animals were necropsied. Organs with clear pathological lesions were collected in Stuart Transport Medium (CM0111 Oxoid) and buffered formalin. Isolation and biochemical characterization were conducted according standard protocol [16]. The tissues from one animal were pooled and extracted. The suspension was plated on a 5% defibrinated sheep blood agar plate. The plate was incubated at 37 °C for 18–24 h. Some suspected colonies were Gram stained and grown in triple sugar iron agar and sulfide indole motility media. Other tests were catalase, oxidase, citrate, methyl red, Voges–Proskauer (VP), glucose and lactose tests. Three suspected colonies were injected to separate tryptic soy broth (Sigma Aldrich MFCD00132536) and incubated at 37 °C for 18–24 h. DNA was isolated using 10% chelex-100 (Biorad, CA) [17, 18]. The glutamate dehydrogenase (GDH) and recombination/repair protein (recN) gene fragments were amplified using polymerase chain reaction (PCR) using published specific primer sets for S. suis [19, 20]. The GDH and recN fragments were sequenced by Apical Scientific Sequencing (Malaysia) using an automatic chain termination method. Serotyping was conducted using PCR to detect the cps1I gene for serotype 1 and 14 and the cps2I gene for 2 and 1/2 as recommended [21].
Tissue was processed and stained with hematoxylin and eosin (H&E) staining based on a published protocol [22].
Results
Out of 30 cases, 8 were indicative for S. suis and showed small and non-hemolytic colonies that were Gram positive with coccus-chain forming appearance. Only positive cases are described further in this manuscript. The epidemiological and clinical data of the presumably positive cases are presented in Table 1. Listed from the most frequent, clinical signs were reddish skin discoloration, anorexia, nasal exudate, diarrhea, limping, eye exudate, lethargy, swollen joints, shivering, weakness, cough, lack of appetite, depression, dyspnea, tremor, fever and snot. Animal were aged 3–12 months. The cases were from Denpasar, Gianyar, Tabanan and Karangasem regencies.
The results of Gram staining and biochemical tests of suspected colonies from the eight suspected S. suis infections are presented in Table 2. All isolates were Gram (+), coccus form, short chained and positive in acid slant, catalase and lactose, but negative in VP test.
Table 2.
Gram staining and biochemical properties of bacterial colonies of confirmed S. suis infection in Bali, Indonesia
| No | Microscopic after Gram staining | TSIA | SIM | Catalase | VP | Lactose | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Acid slant | Acid butt | Gas | H2S | H2S | Indole | Motile | |||||
| 1 | Gram positive, coccus, short chain | + | − | − | − | − | − | − | + | − | + |
| 2 | Gram positive, coccus no chain | + | − | − | − | − | − | − | + | − | + |
| 3 | Gram positive, coccus, no chain | + | − | − | − | − | − | − | + | − | + |
| 4 | Gram positive, coccus, no chain | + | − | − | − | − | − | − | + | − | + |
| 5 | Gram positive, coccus, no chain | + | − | − | − | − | − | − | + | − | + |
| 6 | Gram positive, coccus, no chain | + | − | − | − | − | − | − | + | − | + |
| 7 | Gram positive, coccus, short chain | + | − | − | − | − | − | − | + | − | + |
| 8 | Gram positive, coccus, no chain | + | − | − | − | − | − | − | + | − | + |
The results of oxidase, citrate, MR, and glucose are not shown; all was negative
After electrophoresis of PCR products (not shown), positive samples showed a single band of around 700 bp for GDH and 350 bp for recN. The GDH sequences of five isolates of S. suis from our study were identical. The blast result showed a percentage identity (PID) of 99.09% to human isolates from Bali Indonesia as published previously [10]. The sequences of recN of two isolates show a PID of 100% to human isolates, while other two each have 98.5%. Upon PCR serotyping, two isolates were confirmed to be serotype 2 or 1/2. Two cps2I sequences of animal isolates in our study are completely identical to those of human isolates [10]. The GenBank Acc. No. of GDH, recN and cps2I for serotype 2 or 1/2 are MN334770–MN334774, MN520294–MN520297, and MN520292–MN520293, respectively.
A panel of histological pictures of confirmed cases is depicted in Fig. 1. The histopathologies of confirmed cases were similar with varying severities. The prominent pictures were congestion in the brain, meningitis, bronchopneumonia, endocarditis, myocarditis, pericarditis, erosion and enteritis along the gastrointestinal tract with obvious depletion of Payer patches, hemorrhagic hepatitis and glomerulonephritis, as well as lymphoid depletion, hemorrhage and accumulation of inflammatory cells in the spleen. Dominant inflammatory cell infiltration in those tissues was neutrophil and macrophage.
Fig. 1.
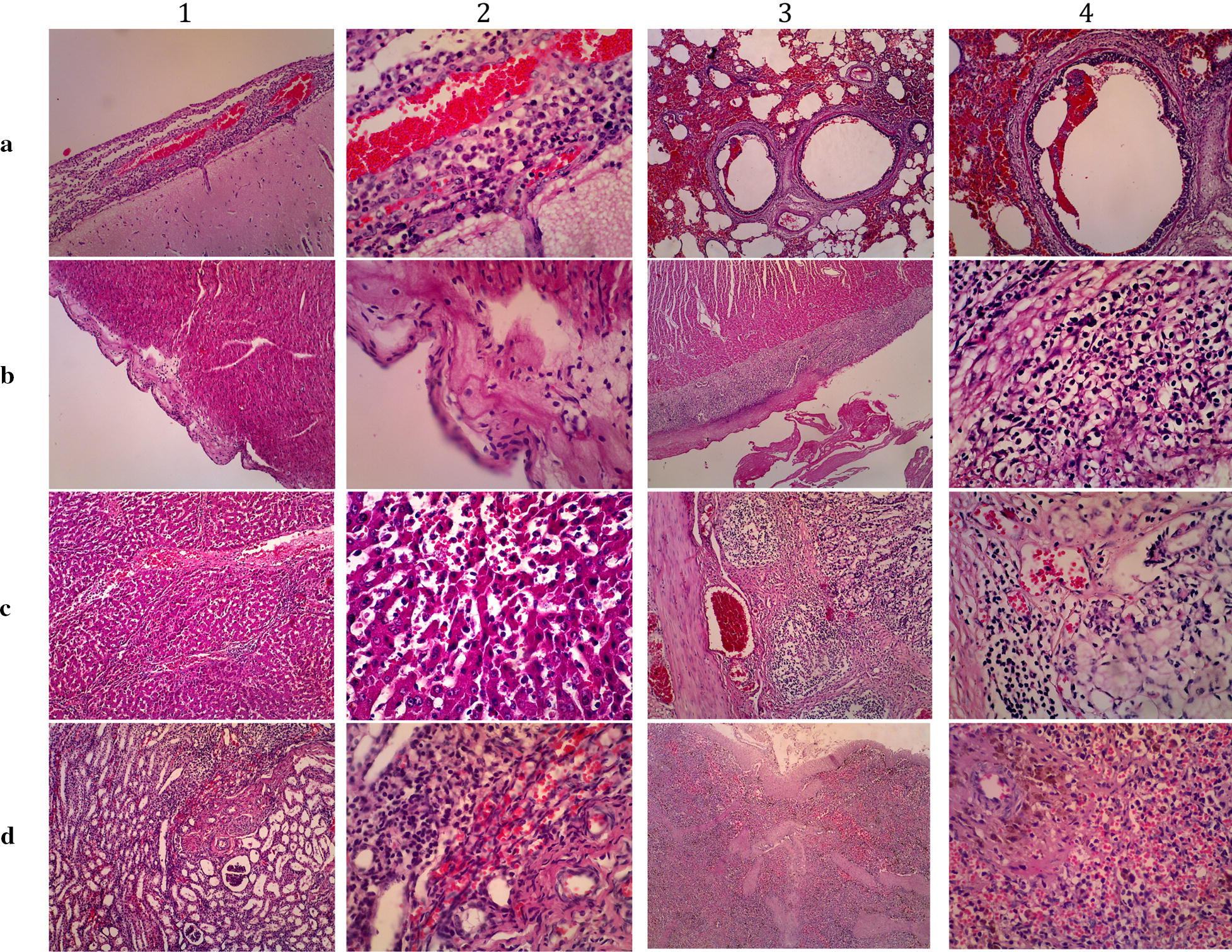
Histopathological pictures of various tissues of confirmed S. suis infection in pig cases in Bali, Indonesia, 2018. Panel a1–2 are brain and meninges showing meningitis; a3–4 are lung tissues showing bronchopneumonia; b1–2 are myocardium showing endocarditis; b3–4 are myocardium showing pericarditis; c1–2 are liver showing congestion and hepatitis; c3–4 are intestine showing hemorrhagic enteritis with depletion of Payer patches; d1–2 are kidney showing hemorrhagic glomerulonephritis; d3–4 are spleen showing perifollicular infiltration of inflammatory cells and hemorrhage; H&E stained. Magnifications in column 1 and 3 are ×100; Column 2 and 4 are ×400
Discussion
We confirmed that S. suis does present and cause illness in pigs in Bali. Human cases confirmed at Bali Referral Hospital [10] must have originated from pigs. Streptococcus suis meningitis is a global zoonotic community-acquired bacterial meningitis [1, 2, 4]. The bacterium is of extra importance in Asia as human outbreaks are related to traditional pork consumption practices [5, 6]. The practice of consuming a delicacy of raw pork with raw blood is also common in Bali.
In this study, we carefully selected suspected cases, especially those of acute cases with no history of antibiotic medication. The cases occurred in pigs under 1 year old. Although S. suis can be isolated from sick and healthy pigs at various ages [23], clinical manifestation seems more frequent in young animals [24]. A study in Canada [25] showed that S. suis was more frequently isolated from pigs aged between 5 and 10 weeks.
We recorded clinical signs in our cases involving many organs of central nervous, respiratory, urogenital and circulatory systems and gastrointestinal tract. Recorded clinical signs of S. suis infection in the literature can indeed be multi-organ. The signs can be pyrexia, lack of appetite, depression, nasal discharge, dyspnea, tremors, seizures, incoordination, unusual stances (such as sitting like a dog), inability to stand, paddling, opisthotonos, convulsions, nystagmus, skin disease, swollen limbs and death [26]. In some cases, the disease goes per-acute and ends with sudden death without obvious signs [26]. Although septicemia and meningitis are the most striking manifestations of the disease, endocarditis, pneumonia and arthritis have been reported [27]. Another review article also reported that the disease syndromes caused by S. suis in swine include arthritis, meningitis, pneumonia, septicemia, endocarditis, polyserositis, abortion and abscesses [28]. In a recent experimental infection, affected pigs presented clinical signs of anorexia, depression, fever, glazed eyes, reddened mucous membranes, severe nervous symptoms (incoordination, lateral prostration, paddling, opisthotonos, convulsions and lameness in the posterior limbs) [29].
Our suspected cases were from all regencies in Bali Province. However, the confirmed cases were from four regencies, namely Tabanan, Denpasar, Gianyar and Karangasem. Considering that Bali is a small island of 5.600 km2 with a high population density (http://www.baliprov.go.id/v1/geographi) and understanding the free movement of animals in the province, we assume that S. suis is distributed throughout the province. The morbidity, mortality and case fatality rates in our study were 18.7%, 8.4% and 44.9%, respectively. Morbidity, mortality and case fatality rates of S. suis in pigs vary [30]. Therefore, we assumed that our observation on the case epidemiology is plausible.
Our microbiological data confirmed S. suis. Gram staining, chain formation and biochemical characterization shows that identified isolates were Gram (+), coccus with grape-like or short chain. All were acid slant, catalase and lactose positive, while VP test was negative. S. suis is an encapsulated gram-positive bacterial coccus that occurs singly, frequently in pairs, or occasionally in short chains [3]. Most strains are alpha-hemolytic on bovine and sheep blood agar plates after 24 h of incubation at 37 °C [3]. Four tests are used for a presumptive identification of S. suis, that is, no growth in 6.5% NaCl agar, a negative VP test and production of acid in trehalose and salicin broths [25, 31]. The VP test is critical in differentiating S. suis from other Streptococcus species [15].
Final confirmation was made using PCR of GDH and recN. Both gene fragments are proposed as a system for reclassifying S. suis or as a specific PCR system for S. suis [19, 20]. We have established the system for S. suis detection from human and animal samples at Udayana University, Bali. The GDH blast result showed a PID of 99.09%, while four recN sequences showed PIDs of 98.5% and 100% to human isolates from Bal [10]. Upon PCR serotyping, two isolates were positive with primer sets for serotype 2 or 1/2 but not those of serotype 1 and 14. Only two primer sets were available in our lab. The readable sequences were identical to the S. suis serotype 2 and 1/2 Cps2I gene Ref. [21].
Although we carefully selected the cases with inclusion criteria of suspected cases that were acute and had at least one clinical signs of neurological disorder, reddish skin discoloration and arthritis, as well as no history of antibiotic treatment, only 8 of 30 suspected cases were confirmed as S. suis infection. The animals were not treated with antibiotics. The negative cases might have been caused by other infectious agents such as Haemophilus parasuis, pseudorabies or Escherichia coli [32].
Prominent histologic pictures were congestion in the brain and meningitis, bronchopneumonia, myocarditis, erosion and enteritis along the gastrointestinal tract, hemorrhagic liver and kidney. Dominant inflammatory cell infiltration in those tissues was neutrophilic. Gross and microscopic findings of S. suis include one or more of fibrinous polyserositis, fibrinous or hemorrhagic bronchopneumonia, purulent meningitis, myocardial necrosis, focal myocarditis and valvular endocarditis [30]. Moreover, meningoencephalitis, a striking lesion, has been found in China [29]. Meningitis histology in one case (Fig. 1, panel A1–2) resembles meningitis as described by that group.
This study was the result of passive surveillance in which the owners contacted our laboratory reporting sickness and mortality in their piggeries. The study did not investigate the link of animal cases to human infections. Active surveillance should be conducted to determine the overall risk factors and elucidate the link between animal isolates and human cases. That the GDH sequences of animal isolates are not identical to those of human isolates and the variation of recN and the confirmed serotype of 2 or 1/2 in two out of eight isolates expands our knowledge that the circulating bacteria might vary in the province. This should be mapped as it will benefit understanding of human transmission.
In conclusion, Streptococcus suis has been confirmed in sick pigs in Bali, Indonesia. Two isolates were confirmed to be serotype 2 or 1/2. Further research is needed to elucidate the risk factors for human infection and to map the distribution of S. suis in Indonesia. The fragment of GDH or recN might be used to map infections in Indonesia. Vaccines can be developed using inactivated strain [33] to reduce economic losses and the risk of human infection, including among domestic and international travelers.
Limitations
The distribution of S. suis in Bali and Indonesia is not yet available.
Acknowledgements
The authors thank to Mrs Krisna Dewi and Mrs Sutrisna Dewi for the laboratory works. The grammar and spelling of this manuscript have been edited professionally in Edanz Editing (https://www.edanzediting.com).
Abbreviations
- GDH
glutamate dehydrogenase
- MR
methyl red
- PCR
polymerase chain reaction
- recN
recombination/repair protein
- SIM
sulfide indole motility
- TSB
tryptic soy broth
- TSIA
triple sugar iron agar
- VP
Voges–Proskauer
Authors’ contributions
INKB and GNM designed the study and were major contributor in writing the manuscript. IGKS conducted the microbiology work. KKA, HS, and NKS collected field samples and data. IBOW conducted and judged the pathological examination. All authors read and approved the final manuscript.
Funding
The study was funded by Directorate General of Research and Development, The Ministry of Research, Technology, and Higher Education of Indonesia, with research contract no. 171.53/UN.14.4.A/LT/2018 dated 19 February 2018. The funding body had no role in the design of the study and collection, analysis, interpretation of data, and in writing the manuscript.
Availability of data and materials
The GenBank Acc. No. of GDH, recN and cps2I for serotype 2 or 1/2 are MN334770–MN334774, MN520294–MN520297, and MN520292–MN520293, respectively. The sequence data are in the process to be made available in dryad (https://datadryad.org).
Ethics approval and consent to participate
The study was not dealing with life animal. The ethical approval of this study was issued by The Ethical Committee on the use of animal in research of the Faculty of Veterinary Medicine Udayana University of Bali. The owners signed informed consent to use of the sick and dead animals in the study.
Consent to publish
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
I Nengah Kerta Besung, Email: kerta_besung@unud.ac.id.
Gusti Ngurah Mahardika, Email: gnmahardika@unud.ac.id.
References
- 1.Brouwer MC, van de Beek D. Epidemiology of community-acquired bacterial meningitis. Curr Opin Infect Dis. 2018;31(1):78–84. doi: 10.1097/QCO.0000000000000417. [DOI] [PubMed] [Google Scholar]
- 2.van de Beek D, Brouwer M, Hasbun R, Koedel U, Whitney CG, Wijdicks E. Community-acquired bacterial meningitis. Nat Rev Dis Primers. 2016;2:16074. doi: 10.1038/nrdp.2016.74. [DOI] [PubMed] [Google Scholar]
- 3.Goyette-Desjardins G, Auger JP, Xu J, Segura M, Gottschalk M. Streptococcus suis, an important pig pathogen and emerging zoonotic agent-an update on the worldwide distribution based on serotyping and sequence typing. Emerg Microbes Infect. 2014;3(6):e45. doi: 10.1038/emi.2014.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zou G, Zhou J, Xiao R, Zhang L, Cheng Y, Jin H, Li L, Zhang L, Wu B, Qian P, et al. Effects of environmental and management-associated factors on prevalence and diversity of Streptococcus suis in clinically healthy pig herds in China and the United Kingdom. Appl Environ Microbiol. 2018;84(8):e02590-17. doi: 10.1128/AEM.02590-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nghia HD, le Tu TP, Wolbers M, Thai CQ, Hoang NV, Nga TV, le Thao TP, Phu NH, Chau TT, Sinh DX, et al. Risk factors of Streptococcus suis infection in Vietnam. A case–control study. PLoS ONE. 2011;6(3):e17604. doi: 10.1371/journal.pone.0017604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Takeuchi D, Kerdsin A, Akeda Y, Chiranairadul P, Loetthong P, Tanburawong N, Areeratana P, Puangmali P, Khamisara K, Pinyo W, et al. Impact of a food safety campaign on Streptococcus suis infection in humans in Thailand. Am J Trop Med Hyg. 2017;96(6):1370–1377. doi: 10.4269/ajtmh.16-0456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ma F, Chang X, Wang G, Zhou H, Ma Z, Lin H, Fan H. Streptococcus suis serotype 2 stimulates neutrophil extracellular traps formation via activation of p38 MAPK and ERK1/2. Front Immunol. 2018;9:2854. doi: 10.3389/fimmu.2018.02854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ma Z, Zhu H, Su Y, Meng Y, Lin H, He K, Fan H. Screening of Streptococcus suis serotype 2 resistance genes with GWAS and transcriptomic microarray analysis. BMC Genomics. 2018;19(1):907. doi: 10.1186/s12864-018-5339-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou Y, Dong X, Li Z, Zou G, Lin L, Wang X, Chen H, Gasser RB, Li J. Predominance of Streptococcus suis ST1 and ST7 in human cases in China, and detection of a novel sequence type, ST658. Virulence. 2017;8(6):1031–1035. doi: 10.1080/21505594.2016.1243193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Susilawathi NM, Tarini NMA, Fatmawati NND, Mayura PIB, Suryapraba AAA, Subrata M, Sudewi AAR, Mahardika GN. Streptococcus suis-associated Meningitis, Bali, Indonesia, 2014–2017. Emerg Infect Dis. 2019;25(12):2235–2242. doi: 10.3201/eid2512.181709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Amass SF, Wu CC, Clark LK. Evaluation of antibiotics for the elimination of the tonsillar carrier state of Streptococcus suis in pigs. J Vet Diagn Invest. 1996;8(1):64–67. doi: 10.1177/104063879600800110. [DOI] [PubMed] [Google Scholar]
- 12.Huong VT, Ha N, Huy NT, Horby P, Nghia HD, Thiem VD, Zhu X, Hoa NT, Hien TT, Zamora J, et al. Epidemiology, clinical manifestations, and outcomes of Streptococcus suis infection in humans. Emerg Infect Dis. 2014;20(7):1105–1114. doi: 10.3201/eid2007.131594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fongcom A, Pruksakorn S, Netsirisawan P, Pongprasert R, Onsibud P. Streptococcus suis infection: a prospective study in northern Thailand. Southeast Asian J Trop Med Public Health. 2009;40(3):511–517. [PubMed] [Google Scholar]
- 14.Tsai HY, Liao CH, Liu CY, Huang YT, Teng LJ, Hsueh PR. Streptococcus suis infection in Taiwan, 2000–2011. Diagn Microbiol Infect Dis. 2012;74(1):75–77. doi: 10.1016/j.diagmicrobio.2012.05.013. [DOI] [PubMed] [Google Scholar]
- 15.Higgins R, Gottschalk M. An update on Streptococcus suis identification. J Vet Diagn Invest. 1990;2(3):249–252. doi: 10.1177/104063879000200324. [DOI] [PubMed] [Google Scholar]
- 16.Lehman DC, Mahon CR, Suvarna K. Streptococcus, enterococcus, and other catalase-negative, gram-positive cocci. In: Mahon CR, Lehman DC, Manuselis G, editors. Textbook of diagnostic microbiology. 5. Missouri: Saunders Elsivier; 2016. pp. 328–348. [Google Scholar]
- 17.Giraffa G, Rossetti L, Neviani E. An evaluation of chelex-based DNA purification protocols for the typing of lactic acid bacteria. J Microbiol Methods. 2000;42(2):175–184. doi: 10.1016/S0167-7012(00)00172-X. [DOI] [PubMed] [Google Scholar]
- 18.Reyes-Escogido L, Balam-Chi M, Rodriguez-Buenfil I, Valdes J, Kameyama L, Martinez-Perez F. Purification of bacterial genomic DNA in less than 20 min using chelex-100 microwave: examples from strains of lactic acid bacteria isolated from soil samples. Antonie Van Leeuwenhoek. 2010;98(4):465–474. doi: 10.1007/s10482-010-9462-0. [DOI] [PubMed] [Google Scholar]
- 19.Ishida S, le Tien HT, Osawa R, Tohya M, Nomoto R, Kawamura Y, Takahashi T, Kikuchi N, Kikuchi K, Sekizaki T. Development of an appropriate PCR system for the reclassification of Streptococcus suis. J Microbiol Methods. 2014;107:66–70. doi: 10.1016/j.mimet.2014.09.003. [DOI] [PubMed] [Google Scholar]
- 20.Okwumabua O, O’Connor M, Shull E. A polymerase chain reaction (PCR) assay specific for Streptococcus suis based on the gene encoding the glutamate dehydrogenase. FEMS Microbiol Lett. 2003;218(1):79–84. doi: 10.1111/j.1574-6968.2003.tb11501.x. [DOI] [PubMed] [Google Scholar]
- 21.Liu Z, Zheng H, Gottschalk M, Bai X, Lan R, Ji S, Liu H, Xu J. Development of multiplex PCR assays for the identification of the 33 serotypes of Streptococcus suis. PLoS ONE. 2013;8(8):e72070. doi: 10.1371/journal.pone.0072070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang Y, Si Y, Zhai L, Yang N, Yao S, Sang H, Zu D, Xu X, Qin S, Wang J. Celastrus orbiculatus Thunb. ameliorates high-fat diet-induced non-alcoholic fatty liver disease in guinea pigs. Pharmazie. 2013;68(10):850–854. [PubMed] [Google Scholar]
- 23.Luque I, Blume V, Borge C, Vela AI, Perea JA, Marquez JM, Fernandez-Garayzabal JF, Tarradas C. Genetic analysis of Streptococcus suis isolates recovered from diseased and healthy carrier pigs at different stages of production on a pig farm. Vet J. 2010;186(3):396–398. doi: 10.1016/j.tvjl.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 24.Cloutier G, D’Allaire S, Martinez G, Surprenant C, Lacouture S, Gottschalk M. Epidemiology of Streptococcus suis serotype 5 infection in a pig herd with and without clinical disease. Vet Microbiol. 2003;97(1–2):135–151. doi: 10.1016/j.vetmic.2003.09.018. [DOI] [PubMed] [Google Scholar]
- 25.Higgins R, Gottschalk M, Mittal KR, Beaudoin M. Streptococcus suis infection in swine. A sixteen month study. Can J Vet Res. 1990;54(1):170–173. [PMC free article] [PubMed] [Google Scholar]
- 26.Gottschalk M. Streptococcus suis infection. In: Allen DG, Constable PD, Dart A, Davies PR, Quesenberry KE, Reeves PT, Sharma JM, editors. Merck veterinary manual. New Jersey: Merck Sharp & Dohme Corp; 2016. [Google Scholar]
- 27.Sanford SE, Tilker ME. Streptococcus suis type II-associated diseases in swine: observations of a one-year study. J Am Vet Med Assoc. 1982;181(7):673–676. [PubMed] [Google Scholar]
- 28.Staats JJ, Feder I, Okwumabua O, Chengappa MM. Streptococcus suis: past and present. Vet Res Commun. 1997;21(6):381–407. doi: 10.1023/A:1005870317757. [DOI] [PubMed] [Google Scholar]
- 29.Zheng P, Zhao YX, Zhang AD, Kang C, Chen HC, Jin ML. Pathologic analysis of the brain from Streptococcus suis type 2 experimentally infected pigs. Vet Pathol. 2009;46(3):531–535. doi: 10.1354/vp.08-VP-0043-J-FL. [DOI] [PubMed] [Google Scholar]
- 30.John VS, Wilcock B, Kierstead M. Streptococcus suis type 2 infection in Swine in Ontario: a review of clinical and pathological presentations. Can Vet J. 1982;23(3):95–97. [PMC free article] [PubMed] [Google Scholar]
- 31.Tarradas C, Arenas A, Maldonado A, Luque I, Miranda A, Perea A. Identification of Streptococcus suis isolated from swine: proposal for biochemical parameters. J Clin Microbiol. 1994;32(2):578–580. doi: 10.1128/jcm.32.2.578-580.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fonseca_Junior AA, Nanoka CKV, Guedes EO, Lobato ZIP, Dias AS, Nascimento JAFB, Klein CS, Reis JKP, Heinemann MB. Detection of agents associated with respiratory diseases of swine by real time PCR. Rev Bras Saúde Prod Anim. 2015;16(2):300–307. doi: 10.1590/S1519-99402015000200005. [DOI] [Google Scholar]
- 33.Hsueh KJ, Cheng LT, Lee JW, Chung YC, Chung WB, Chu CY. Immunization with Streptococcus suis bacterin plus recombinant Sao protein in sows conveys passive immunity to their piglets. BMC Vet Res. 2017;13(1):15. doi: 10.1186/s12917-016-0937-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The GenBank Acc. No. of GDH, recN and cps2I for serotype 2 or 1/2 are MN334770–MN334774, MN520294–MN520297, and MN520292–MN520293, respectively. The sequence data are in the process to be made available in dryad (https://datadryad.org).


