Abstract
The epithelial-to-mesenchymal transition (EMT) has been reported to serve vital roles in regulating the progress of cancer metastasis. In addition, lipid rafts enriched in sphingolipids and cholesterol serve important roles in physiological and biochemical processes as a signaling platform. The present study explored the effects of hydroxypropyl-β-cyclodextrin (HP-β-CD), a cholesterol-depleting agent of lipid rafts, on the transforming growth factor (TGF)-β/Smad signaling pathway and endoplasmic reticulum (ER) stress in mediating EMT in MDA-MB-231 breast cancer cells. HP-β-CD treatment inhibited TGF-β1-induced EMT, based on increased expression of E-cadherin and decreased expression of vimentin. HP-β-CD reduced the expression of the TGF receptor TβRI and blocked the phosphorylation of Smad2. In addition, HP-β-CD increased the expression of ER stress-related proteins (binding immunoglobulin protein and activating transcription factor 6), but TGF-β1 could reverse these changes. Sodium 4-phenylbutyrate, an inhibitor of ER stress, suppressed these effects of HP-β-CD on EMT and TGF-β/Smad signaling pathway inhibition in breast cancer cells. Thus, HP-β-CD can block the TGF-β/Smad signaling pathway via diminishing the expression of TβRI which helps to activate ER stress and attenuate EMT in MDA-MB-231 cells, highlighting a potential target of lipid rafts for breast cancer treatment.
Keywords: lipid rafts, epithelial-mesenchymal transition, hydroxypropyl-β-cyclodextrin, endoplasmic reticulum stress, MDA-MB-231 cells
Introduction
Breast cancer (BC) has a high mortality rate, which is largely due to development of metastases (1). The epithelial-to-mesenchymal transition (EMT) serves a central role in metastasis formation, and is thus an important treatment target (2,3). Many signaling pathways are known to regulate the process of EMT (4). Among these pathways, the transforming growth factor (TGF)-β-dependent pathway has received relatively more research attention given its greater potency in inducing EMT (5). TGF-β1 binds to its receptors TβRI and TβRII, resulting in the phosphorylation of TβRII, which then activates TβRI to stimulate receptor-associated Smad 2/3 in the cytoplasm. Thereafter, phosphorylated Smad 2/3 (activated Smad 2/3) can form a stable complex with Smad4 and then regulate the transcription of target genes (6,7).
Lipid rafts are rich in sphingolipids and cholesterol, and serve numerous roles in physiological and biochemical processes as a signaling platform (8–10). The signal transduction of TGF-β receptors are depended on the lipid rafts (11,12) and we recently reported that increasing the content of sphingomyelin, a type of sphingolipid, in lipid rafts inhibited the process of EMT in breast cancer cells by suppressing the TGF-β/Smad signaling pathway (13). However, the effect of cholesterol, another important component of lipid rafts (14), on the EMT and its underlying mechanism remain to be elucidated. Therefore, the present study sought to explore the effects of the change in cholesterol in lipid rafts in the development of EMT regulated by the TGF-β/Smad signaling pathway. Toward accomplish this, hydroxypropyl-β-cyclodextrin (HP-β-CD) was used for in vitro treatment of MDA-MB-231 cells to deplete cholesterol in lipid rafts (15).
The endoplasmic reticulum (ER) is the main site of protein folding, calcium homeostasis, and thus also participates in regulating various intracellular signaling pathways (16). When the integrity of the ER is disturbed by adverse conditions, misfolded proteins will accumulate in the ER, giving rise to misfolded protein response, or ER stress, which is associated with many cellular biological functions, including EMT (17,18). In addition, a definite association has demonstrated that the TGF-β/Smad signaling pathway can regulate ER stress in lung cancer cells (19), podocytes (20) and even breast cancer cells (21). Therefore, it was hypothesized that HP-β-CD could regulate ER stress via TGF-β/Smad signaling pathway to influence EMT in MDA-MB-231 cells. To examine this hypothesis, the cells were treated with or without HP-β-CD and then stimulated with TGF-β1 or the ER stress inhibitor sodium 4-phenylbutyrate (4-PBA) to explore the effect of cholesterol in lipid rafts on the TGF-β/Smad pathway. These findings may provide novel insight into the mechanism of metastasis progression in breast cancer and in the meantime highlight new treatment targets.
Materials and methods
Cell culture and treatment
MDA-MB-231 cells (The Cell Bank of Type Culture Collection of the Chinese Academy of Sciences) were incubated in Dulbecco's modified Eagle's medium or RPMI-1640 medium (Beijing Solarbio Science & Technology Co., Ltd.) with penicillin (100 U/ml), 10% FBS and streptomycin 100 µg/ml, and cultured at 37°C in an atmosphere of 90% relative humidity and 5% CO2. HP-β-CD (BBI Life Sciences, Shanghai, China) was dissolved in phosphate-buffered saline (PBS) then filtered with a syringe-driven filter (Guangzhou Jet Bio-Filtration Co., Ltd.). The cells were treated with HP-β-CD diluted to the desired concentrations with complete medium. To choose the optimal concentration and treatment time of HP-β-CD, a previous study was referred to and cells treated with different concentrations (0, 2.5, 5, 10 mmol/l) for 48 h (22). Then the expression of EMT markers vimentin and E-cadherin was detected by western blotting. Cells were stimulated with 10 ng/ml TGF-β1 (13) (cat. no. 10804-HNAC; Sino Biological Inc.) dissolved in PBS for 48 h and the same amount of PBS was added to control group. For inhibition of ER stress, 5 mmol/l 4-PBA (23) (Shanghai Macklin Biochemical Co., Ltd.) was dissolved in DMSO and then diluted to the desired concentrations with complete medium; DMSO (<0.1%) was then added to the culture medium.
MTT assay
Untreated MDA-MB-231 cells were seeded on 96-well plates and incubated. When the cells reached 50% confluence, different concentrations (0, 2.5, 5, 10 mmol/l) of HP-β-CD were added to the medium. After 48 h, 20 µl MTT (5 mg/ml; cat. no. M8180; Beijing Solarbio Science & Technology Co., Ltd.) was added to each plate and after 4 h, the medium containing MTT was removed from 96-well plates and 200 µl DMSO was added to dissolve the formazan. Finally, the absorbance was measured at a wavelength of 490 nm; the experiment was performed in triplicate.
Wound healing assay
MDA-MB-231 cells were seeded on 12-well plates and incubated for 48 h at 37°C. When the cells reached 90% confluence, a straight line was drawn by a sterile 200 µl pipette tip perpendicular to a sterilized ruler in the middle of each well. The cells were then treated with 5 mmol/l HP-β-CD followed by 10 ng/ml TGF-β1 or 5 mmol/l 4-PBA in serum-free medium; untreated cells served as the control group. This time point was taken as 0 h, and then images of wound closure were acquired with a phase contrast inverted microscope after 48 h (Olympus IX71; Olympus Corporation; magnification, ×4).
Transwell invasion assay
The effects of HP-β-CD on the invasive ability of the breast cancer cells were evaluated with a Transwell assay (24 wells; cat. no. 353097; Corning Life Sciences). In brief, 2×105 cells cultured with 10% fetal calf serum-containing medium (cat. no. TBD11HT; Tianjin Haoyang Biological Products Technology Co., Ltd.) were plated in the top chamber of a Transwell plate pre-coated with Matrigel for 6 h at 37°C (cat. no. 356234; Corning Inc.) for 24 h. Then serum-free medium was filled in the upper chamber, along with HP-β-CD alone or in combination with TGF-β1 or 4-PBA. After 48 h, cells that did not emerge from the pores were removed by dabbing lightly with cotton swabs. Those cells that had migrated to the lower chamber were fixed in 4% paraformaldehyde for 30 min at room temperature and then stained with 1% crystal violet for 5 min at room temperature (Beijing Solarbio Science & Technology Co., Ltd.). The number of invading cells was counted under the same microscope as above (magnification, ×4).
Western blot analysis
Western blotting was used to assess the changes of biomarkers in the process of apoptosis, EMT, TGF-β/Smad signaling pathway and ER stress. Following treatment of the cells as described above, 2×106 cells were washed with pre-cooled PBS, and the total proteins were extracted with Protein Extraction Reagent (cat. no. WLA019b; Wanleibio Co., Ltd.). The 60 µg proteins were separated on 10–12% SDS-PAGE gels and then transferred on to a polyvinylidene difluoride membrane. After blocking with 10% skimmed milk for 2 h at room temperature, the membranes were incubated overnight at 4°C with constant agitation and the following antibodies: B-cell lymphoma 2 (Bcl-2; cat. no. BS-0032R; Polyclonal; 1:1,000; BIOSS), Bcl-2-associated X protein (Bax; cat. no. 60267-1-Ig; Monoclonal; 1:2,000; ProteinTech Group, Inc., Chicago, IL, USA), vimentin antibody (cat. no. 60330-1-Ig; Monoclonal; 1:4,000; ProteinTech Group, Inc.), E-cadherin antibody (cat. no. 20874-1-AP; Polyclonal; 1:4,000; ProteinTech Group, Inc.), phosphorylated-Smad2 antibody (cat. no. AF3450; 1:2,000; Polyclonal; Affinity Biosciences), Smad2 antibody (cat. no. WL02286; 1:2,000; Polyclonal; Wanleibio Co., Ltd.), TβRI antibody (cat. no. AF5347; 1:2,000; Polyclonal; Affinity Biosciences), binding immunoglobulin protein antibody (BIP/GRP78; cat. no. 66574-1-Ig; 1:4,500; Monoclonal; ProteinTech Group, Inc.), activating transcription factor 6 (ATF6) antibody (cat. no. WL02407; 1:2,000; Polyclonal; Wanleibio Co., Ltd.), and GAPDH antibody (cat. no. HRP-60004; 1:12,000; Monoclonal; ProteinTech Group, Inc.). Secondary anti-rabbit antibodies (cat. no. SA00001-2; 1:10,000; ProteinTech Group, Inc.) or anti-mouse antibodies (cat. no. SA00001-1; 1:12,000 ProteinTech Group, Inc.) were used to incubate the membranes for 1 h at room temperature with constant agitation. Proteins were visualized with an enhanced chemiluminescence reagent (Wanleibio Co., Ltd.) and a Chemiluminescence Detection System (Image Lab version 5.1; Bio-Rad Laboratories, Inc.); the GAPDH signal was used as a loading control. Each assay was repeated at least three times.
Immunofluorescence and confocal microscopy
A total of 1×104 cells were placed on coverslips in a 48-well plate and then treated with HP-β-CD alone or in combination with TGF-β1 for 48 h at 37°C. The cells were rinsed in PBS and then fixed in 4% paraformaldehyde for 30 min at room temperature, followed by another three washes in PBS. Then the cells were treated with 0.5% Triton X-100 in PBS to increase the permeability, and the coverslips were immersed in 5% bovine serum albumin (cat. no. A8020; Beijing Solarbio Science & Technology Co., Ltd.) at room temperature in PBS for 1 h. The cells were incubated with anti-TβRI antibodies (cat. no. AF5347; 1:100; Affinity Biosciences) overnight at 4°C, and then incubated with fluorescein isothiocyanate-conjugated goat anti-rabbit antibody (cat. no. SF134; 1:400; Beijing Solarbio Science & Technology Co., Ltd.) for 60 min at room temperature. DAPI (Wuhan Boster Biological Technology, Ltd.) was used to stain the nuclei for 10 min at room temperature, and the cells were rinsed with PBS. The cells were observed under a confocal microscope (Leica SP8; Leica Microsystems, Ltd.; magnification, ×40), and the fluorescence of TβRI was analyzed with ImageJ software (ver. 2.1; National Institutes of Health).
Statistical analysis
All data were analyzed using GraphPad Prism 6.0 software (GraphPad Software, Inc.). Results are expressed as the mean ± standard deviation. The differences between groups were analyzed using one-way analysis of variance followed by a Dunnett's post hoc test. All experiments were repeated ≥3 times. P<0.05 was considered to indicate a statistically significant difference.
Results
HP-β-CD suppresses EMT features in MDA-MB-231 cells
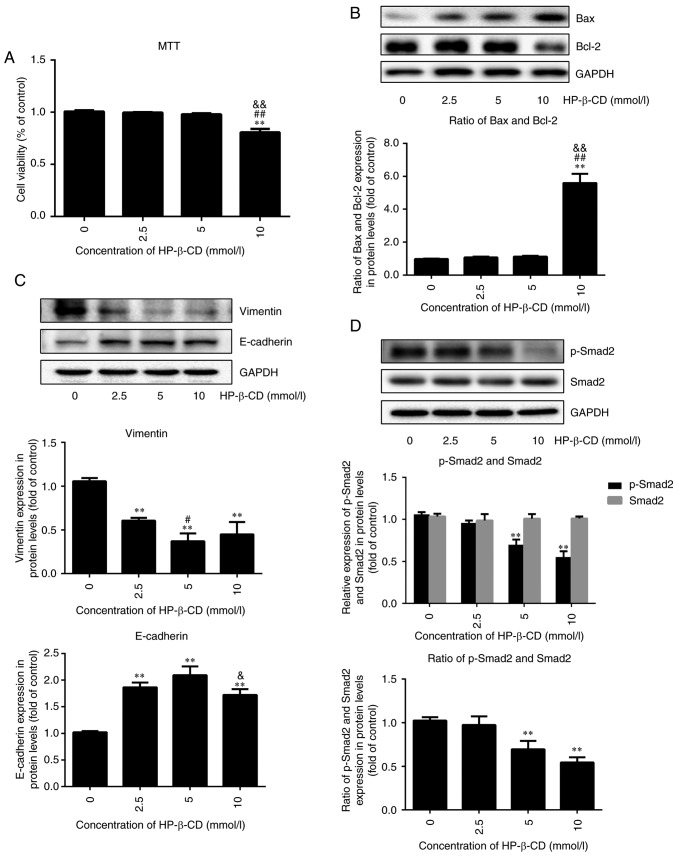
First, MTT and western blotting was used to test the cytotoxic ability of HP-β-CD. MTT demonstrated that HP-β-CD exhibited no significant cytotoxicity in the cells up to 5 mmol/l concentration (Fig. 1A). Western blotting demonstrated that the expression of Bax/Bcl-2 had no significant change up to 5 mmol/l concentration (Fig. 1B). However, HP-β-CD was found to significantly attenuate cellular growth at a concentration above 5 mmol/l in MTT (Fig. 1A) and western blotting demonstrated the same result (Fig. 1B).
Figure 1.
HP-β-CD suppresses EMT features and the TGF-β/Smad signaling pathway in MDA-MB-231 breast cancer cells. MDA-MB-231 cells were incubated in the presence of HP-β-CD at different concentrations (0, 2.5, 5 and 10 mmol/l). MTT was used to detect the cell viability after 48 h, and western blotting was used to measure the expression level of apoptosis, EMT and TGF-β/Smad pathway-related proteins. (A) Viability of MDA-MB-231 cells. (B) Expression of Bax and Bcl-2 in cells. (C) Expression of vimentin and E-cadherin in cells. (D) Expression of p-Smad2 and Smad2 in cells. Values are shown as the mean ± standard deviation (n=6 or n=3). **P<0.01 vs. the control group; #P<0.05, ##P<0.01 vs. 2.5 mmol/l HP-β-CD group; &P<0.05, &&P<0.01 vs. 5 mmol/l HP-β-CD group. HP-β-CD, hydroxypropyl-β-cyclodextrin; EMT, epithelial-to-mesenchymal transition; TGF, transforming growth factor; p-, phosphorylated.
HP-β-CD inhibited EMT in breast cancer cells in a dose-dependent pattern, with the greatest effect observed with treatment of 5 mmol/l HP-β-CD, resulting in a 64% decrease in the expression of vimentin and a 105% increase in E-cadherin expression (Fig. 1C; P<0.001; n=3). Therefore, 5 mmol/l HP-β-CD was chosen as the appropriate concentration to influence EMT for subsequent experiments. In addition, the expression of p-Smad2 was downregulated by HP-β-CD (Fig. 1D; P<0.001; n=3), but this had no effect on Smad2 expression. These results suggested that p-Smad2 might be involved in regulating the HP-β-CD-induced suppression of EMT in MDA-MB-231 cells.
HP-β-CD can inhibit the TGF-β/Smad signaling pathway
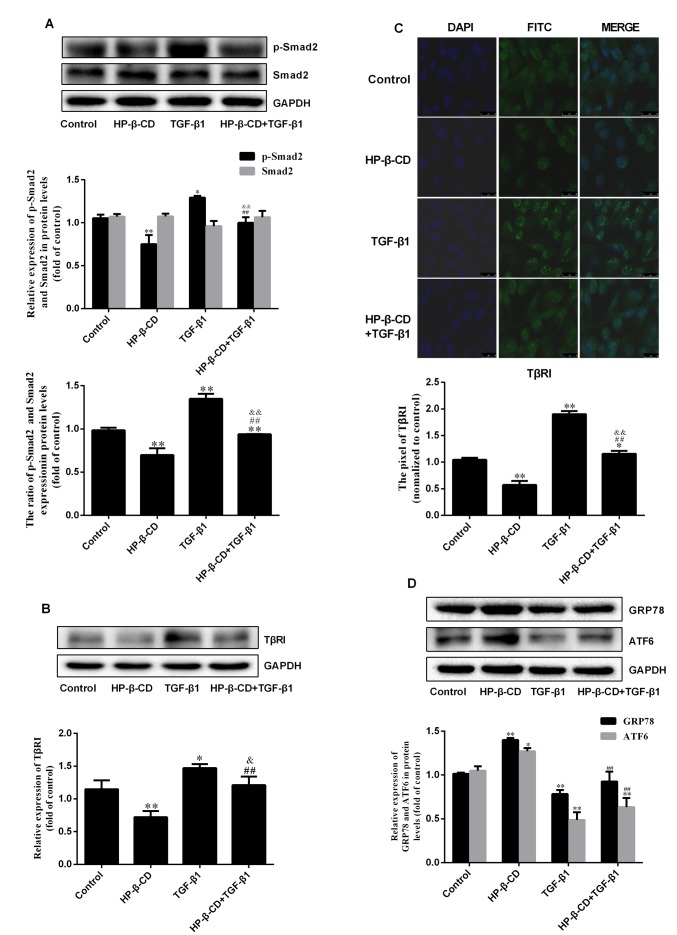
Consistent with the results above, treatment of 5 mmol/l HP-β-CD for 48 h significantly downregulated p-Smad2 (Fig. 2A; P<0.001; n=3) expression but demonstrated no significant effect on Smad2 expression. However, TGF-β1 treatment upregulated p-Smad2 expression by 70.7%, with no significant changes in Smad2 (Fig. 2A; P<0.001; n=3). Western blotting demonstrated that TGF-β1 treatment increased the expression of TβRI by 28.3%; however, the combination of HP-β-CD and TGF-β1 treatment diminished the expression of TβRI by 17.8% compared to TGF-β1 treatment alone (Fig. 2B; P<0.05; n=3), which was confirmed by immunofluorescence and confocal laser microscopy (Fig. 2C; P<0.001; n=3). Treatment of TGF-β1 alone downregulated the expression of the ER stress markers GRP78 and ATF6, and HP-β-CD treatment reversed these effects, increasing the expression of GRP78 and ATF6 by 18.8 and 37.6% respectively (Fig. 2D; P<0.05; n=3). These results indicated that HP-β-CD regulates ER stress.
Figure 2.
HP-β-CD can inhibit the TGF-β/Smad signaling pathway by decreasing the expression of TGF-β type I receptors while activating ER stress. MDA-MB-231 cells were treated with 5 mmol/l HP-β-CD then with or without 10 ng/ml TGF-β1 for 48 h. Western blotting was used to measure (A) the relative expression of TGF-β/Smad pathway-related proteins (p-Smad2 and Smad2) and (B) expression of TβRI. (C) Confocal microscope image for immunofluorescence staining of TβRI. Magnification, ×40 (D) ER stress-related proteins (GRP78 and ATF6) were also detected by western blotting. Values are shown as the mean ± standard deviation (n=3). *P<0.05, **P<0.01 vs. the control group; ##P<0.01 vs. HP-β-CD group; &P<0.05, &&P<0.01 vs. TGF-β1 group. HP-β-CD, hydroxypropyl-β-cyclodextrin; TGF, transforming growth factor; ER, endoplasmic reticulum; GRP78, binding immunoglobulin protein antibody; ATF6, activating transcription factor 6.
HP-β-CD inhibits the TGF-β1-induced migration and invasion of MDA-MB-231 cells
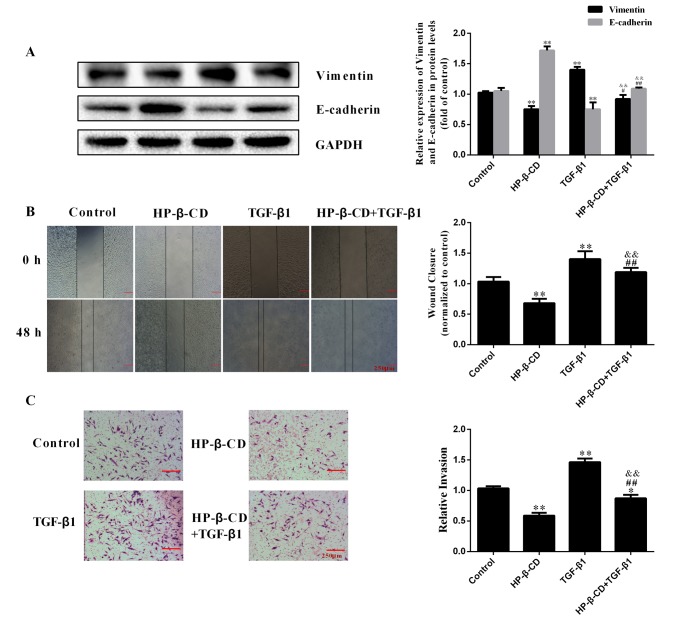
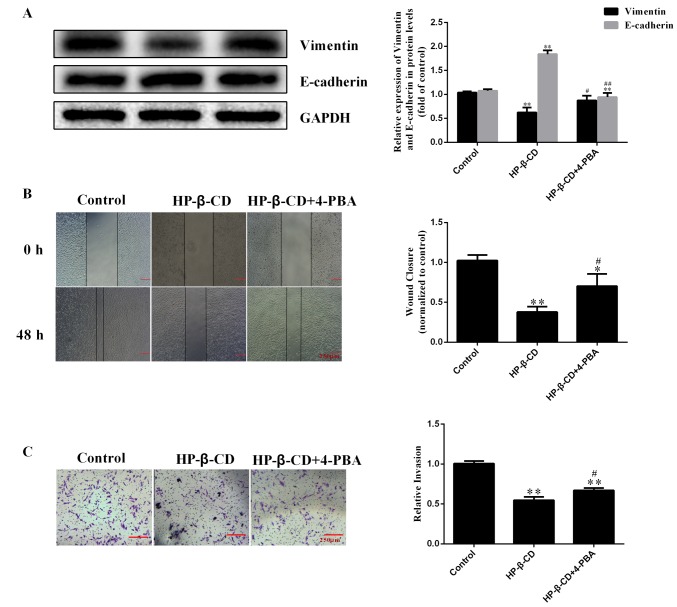
HP-β-CD reversed the effects of TGF-β1 on EMT, shifting MDA-MB-231 cells to a more epithelial phenotype, downregulating vimentin expression by 35.9% while upregulating E-cadherin expression by 28.5% (Fig. 3A; P<0.001; n=3). The wound healing assay demonstrated that TGF-β1 treatment significantly increased the migration ability of cells by 41.2%, which was significantly inhibited by HP-β-CD (Fig. 3B). The invasive ability of MDA-MB-231 cells was increased after 48 h of TGF-β1 treatment and this effect was significantly inhibited with HP-β-CD pre-treatment (Fig. 3C).
Figure 3.
HP-β-CD inhibits the migration and invasion of MDA-MB-231 cells induced by TGF-β1. MDA-MB-231 cells were treated with 5 mmol/l HP-β-CD then with or without 10 ng/ml TGF-β1 for 48 h. (A) Western blotting was used to measure the relative expression of EMT-related proteins (vimentin and E-cadherin) in cells. (B) Wound-healing assay and (C) Transwell invasion assay demonstrated the migratory and invasive ability of cells, respectively. Magnification, ×4. Values are shown as the mean ± standard deviation (n=3). *P<0.05, **P<0.01 vs. the control group; #P<0.05, ##P<0.01 vs. HP-β-CD group; &&P<0.01 vs. TGF-β1 group. HP-β-CD, hydroxypropyl-β-cyclodextrin; TGF, transforming growth factor; EMT, epithelial-to-mesenchymal transition.
HP-β-CD activates ER stress in MDA-MB-231 cells
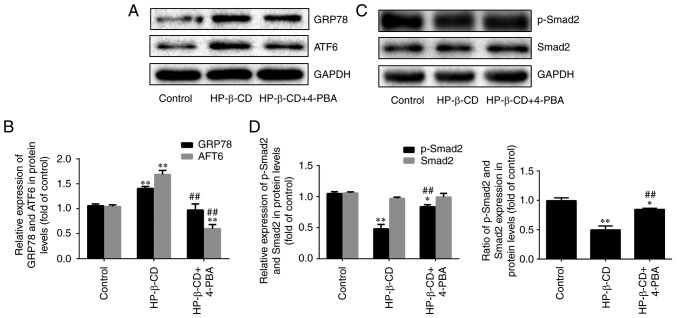
Treatment of cells with HP-β-CD alone upregulated the expression of ER stress-related proteins GRP78 and ATF6 by 37.7 and 61.8% respectively (Fig. 4A and B; P<0.001; n=3). However, treatment with the ER stress inhibitor 4-PBA blocked these effects, downregulating GRP78 and ATF6 expression by 30.7 and 64.2% respectively (Fig. 4A and B; P<0.001; n=3). In addition, p-Smad2 was downregulated by 54.3% after cells were treated with HP-β-CD alone. Whereas after the combination of HP-β-CD and 4-PBA treatment, it was upregulated by 77.1%. Consistent with the experiments described above, no significant changes in the expression of Smad2 were observed (Fig. 4C and D; P<0.001; n=3). These data confirmed that HP-β-CD can activate ER stress.
Figure 4.
4-PBA promotes HP-β-CD-inhibited EMT in MDA-MB-231 breast cancer cells. MDA-MB-231 cells were treated with HP-β-CD and then with or without 4-PBA for 48 h. Western blotting was used to detect (A and B) the relative expression of ER stress-related proteins (GRP78 and ATF6) and (C and D) relative expression of TGF-β/Smad pathway-related proteins (p-Smad2 and Smad2). Values are shown as the mean ± standard deviation (n=3). *P<0.05, **P<0.01 vs. the control group; ##P<0.01 vs. the HP-β-CD group. HP-β-CD, hydroxypropyl-β-cyclodextrin; EMT, epithelial-to-mesenchymal transition; 4-PBA, sodium 4-phenylbutyrate; ER, endoplasmic reticulum; GRP78, binding immunoglobulin protein antibody; ATF6, activating transcription factor 6; TGF, transforming growth factor.
HP-β-CD attenuates the EMT via activating ER stress in MDA-MB-231 cells
Inhibition of ER stress with 4-PBA also reversed the HP-β-CD-induced inhibition of EMT, resulting in upregulation of vimentin and downregulation of E-cadherin (Fig. 5A). Furthermore, the migratory ability of cells was increased after 4-PBA treatment compared with that detected under HP-β-CD treatment alone (Fig. 5B), and the inhibition of the invasive ability of MDA-MB-231 cells was also reversed by 4-PBA (Fig. 5C).
Figure 5.
4-PBA activates the migration and invasion inhibited by HP-β-CD. (A) Western blotting was used to measure the relative expression of EMT-related proteins (vimentin and E-cadherin) in MDA-MB-231 cells. (B) Wound healing analysis and (C) Transwell invasion assay demonstrated the migratory and invasive ability of cells, respectively. Magnification, ×4. Values are shown as the mean ± standard deviation (n=3). *P<0.05, **P<0.01 vs. the control group; #P<0.05, ##P<0.01 vs. the HP-β-CD group. 4-PBA, sodium 4-phenylbutyrate; HP-β-CD, hydroxypropyl-β-cyclodextrin; EMT, epithelial-to-mesenchymal transition.
Discussion
The present study demonstrated that HP-β-CD can suppress the EMT of breast cancer cells by activating ER stress. MTT analysis demonstrated that 5 mmol/l HP-β-CD has no significant effects on the viability of MDA-MB-231 cells, with no significant change in the relative expression of Bax/Bcl-2. Meanwhile, it was found that HP-β-CD suppressed EMT features in MDA-MB-231 cells by downregulating TβRI expression, interfering with TGF-β1-induced Smad2 phosphorylation, and thereby increasing the expression of E-cadherin and decreasing that of vimentin. In addition, HP-β-CD inhibited the migratory and invasive capacities of MDA-MB-231 cells. These effects were mediated through the induction of ER stress by HP-β-CD, since blocking ER stress with 4-PBA, an inhibitor of ER stress, promoted the migratory and invasive capability of cells treated with HP-β-CD. Notably, TGF-β1 decreased the expression levels of ER stress marker proteins, and 4-PBA increased the expression of p-Smad2. Overall, these results demonstrated that depletion of cholesterol in lipid rafts could inhibit TGF-β1-induced EMT in MDA-MB-231 cells, which could suppress metastasis.
Lipid raft is a signaling platform (8–10) and can regulate many signaling pathways, therefore it serves vital roles in many biological processes, including cytoskeleton rearrangement, proliferation, and the migration and apoptosis of various types of cancer cells (24–26). We previously identified that sphingomyelin synthase 1 (SMS1) overexpression could inhibit TGF-β1-induced EMT by increasing the content of sphingomyelin in lipid rafts (13). Thus, the present study focused on the effects of another important lipid raft component, cholesterol, in the context of breast cancer. HP-β-CD can deplete cholesterol in lipid rafts and this may affect the construction of lipid rafts and then cancer cell viability by regulating the transmembrane signaling (15,27). For example, Alawin et al (28) found that antiproliferative effects of γ-tocotrienol are associated with lipid raft disruption in HER2-positive human breast cancer cells and Badana et al (29). demonstrated that lipid rafts disruption induces apoptosis by attenuating expression of LRP6 and survivin in triple negative breast cancer. Although many reports have suggested that the transmembrane signaling of the TGF-β/Smad signaling pathway is dependent on lipid raft (30–33), to the best of the authors' knowledge, this is the first study to demonstrate its role in EMT regulated by TGF-β/Smad signaling pathway.
Multiple signaling pathways are known to mediate EMT, including TGF-β, Notch-, Hedgehog- and mitogen-activated protein kinase-dependent pathways (34,35). Among these pathways, the TGF-β-dependent pathway is considered to be a primary inducer of EMT (5). TGF-β receptors are internalized by clathrin-dependent endocytosis as a key regulatory event in signal transduction (30,36). Therefore, changing the main components of lipid rafts can influence the localization of TGF-β receptors in the lipid rafts/caveolae and the consequent signal transduction, as confirmed in our previous study (13). The present study further demonstrated that HP-β-CD negatively regulated TβRI expression, probably by influencing the distribution of TβRI in lipid rafts. These changes would then block the TGF-β1-induced activation of the TGF-β/Smad signaling pathway to suppress TGF-β1-induced EMT, as well as the corresponding migration and invasion abilities of the cancer cells. Lipid composition and membrane biophysical properties serve an important role in cell movement (37). Notably, our previous findings and those of the present study demonstrated that changing the composition of lipid rafts by using HP-β-CD to deplete cholesterol and overexpressing SMS1 to increase the content of sphingomyelin had the same effect on EMT, which means that cholesterol and sphingomyelin may serve opposite roles in the process of EMT. It also identified that cholesterol and sphingomyelin had the opposite roles in regulating the endothelial dysfunction (data not shown). Therefore, the detailed mechanism needs further investigations.
In the present study, TGF-β1 decreased the expression levels of ER stress markers, which could be reversed by HP-β-CD pre-treatment. In addition, 4-PBA activated the TGF-β/Smad signaling pathway and inhibited the effects of HP-β-CD on EMT. The role of ER stress in EMT is somewhat controversial. Huang et al (21) demonstrated that pterostilbene-induced ER stress could inhibit EMT in breast cancer, and recent studies have pointed to a relationship between the TGF-β/Smad signaling pathway and ER stress in a number of cell lines, including A549 cells and podocytes (19,38) suggesting that TGF-β1 can activate ER stress to promote the process of EMT. The present study demonstrated that HP-β-CD could block both the TGF-β/Smad signaling pathway and EMT, revealing a positive association between TGF-β/Smad signaling pathway and EMT, in line with the studies of Moon et al (38) and Yamashita et al (19). However, in contrast to these reports, the present study found that HP-β-CD could induce ER stress to attenuate the EMT, indicating a negative association, and this is in line with Huang et al (21) and Dasgupta et al (39) who found that pterostilbene and AECHL-1 suppressed the EMT by activating ER stress in breast cancer. These apparent contradictory findings highlight the cell type specificity of the effects of ER stress on EMT. In addition, other ER stress inducers, such as tunicamycin, can activate ER stress and then regulate the process of EMT; and inducing the ER stress can alter many other signaling pathways, including the AKT and MAPK signaling pathways (40) and the Wnt/β-catenin signaling pathway (41), therefore, further studies are required to compliment the present study. Although there are several studies that do not include a 4-PBA alone group (41–44), it would have been better if the present study had added a 4-PBA group to overcome the influence of 4-PBA on the experimental results. Collectively, the results of the present study suggested that HP-β-CD regulates the EMT through inhibition of the TGF-β/Smad signaling pathway, which then activates ER stress in MDA-MB-231 cells (Fig. 6). Since this can attenuate EMT in MDA-MB-231 cells, this mechanism holds promise as a treatment target to suppress the progression of breast cancer prior to metastasis formation.
Figure 6.
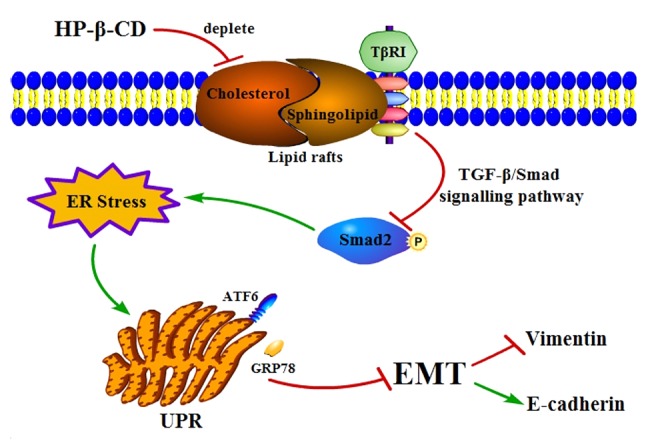
Proposed mechanism of how HP-β-CD attenuates EMT via ER stress in MDA-MB-231 breast cancer cells. HP-β-CD can deplete cholesterol in lipid rafts then regulates TβRI expression negatively to attenuate the TGF-β/Smad signaling pathway, which then activates ER stress, thereby leading to suppression of EMT. HP-β-CD, hydroxypropyl-β-cyclodextrin; EMT, epithelial-to-mesenchymal transition; ER, endoplasmic reticulum; TβRI, TGF receptor; TGF, transforming growth factor; GRP78, binding immunoglobulin protein antibody; ATF6, activating transcription factor 6; UPR, unfolded protein response.
Acknowledgements
Not applicable.
Funding
The present study was supported by grants from The National Natural Science Foundation of China (grant no. 81560151) and The Jiangxi Provincial Department of Science and Technology (grant no. 20181BAB205022).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
YW and YZ performed the experiments, analyzed data and were major contributors in writing the manuscript. LC, XH and ZH also analyzed and interpreted the data of the manuscript. TW and LW were responsible for the design and drafting of the manuscript. NY conceived the study and designed the experiments. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Wittkowski KM, Dadurian C, Seybold MP, Kim HS, Hoshino A, Lyden D. Complex polymorphisms in endocytosis genes suggest alpha-cyclodextrin as a treatment for breast cancer. PLoS One. 2018;13:e0199012. doi: 10.1371/journal.pone.0199012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li L, Li W. Epithelial-mesenchymal transition in human cancer: Comprehensive reprogramming of metabolism, epigenetics, and differentiation. Pharmacol Ther. 2015;150:33–46. doi: 10.1016/j.pharmthera.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 3.Ruben B, Gerhard C. The relevance of EMT in breast cancer metastasis: Correlation or causality? FEBS Lett. 2015;589:1577–1587. doi: 10.1016/j.febslet.2015.05.002. [DOI] [PubMed] [Google Scholar]
- 4.Das V, Bhattacharya S, Chikkaputtaiah C, Hazra S, Pal M. The basics of epithelial-mesenchymal transition (EMT): A study from a structure, dynamics, and functional perspective. J Cell Physiol. 2019 Feb 5; doi: 10.1002/jcp.28160. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 5.Heldin CH, Landström M, Moustakas A. Mechanism of TGF-beta signaling to growth arrest, apoptosis, and epithelial-mesenchymal transition. Curr Opin Cell Biol. 2009;21:166–176. doi: 10.1016/j.ceb.2009.01.021. [DOI] [PubMed] [Google Scholar]
- 6.Lamouille S, Xu J, Derynck R. Molecular mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell Biol. 2014;15:178–196. doi: 10.1038/nrm3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sánchez-Tilló E, Liu Y, de Barrios O, Siles L, Fanlo L, Cuatrecasas M, Darling DS, Dean DC, Castells A, Postigo A. EMT-activating transcription factors in cancer: Beyond EMT and tumor invasiveness. Cell Mol Life Sci. 2012;69:3429–3456. doi: 10.1007/s00018-012-1122-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beloribi-Djefaflia S, Vasseur S, Guillaumond F. Lipid metabolic reprogramming in cancer cells. Oncogenesis. 2016;5:e189. doi: 10.1038/oncsis.2015.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kraft ML. Plasma membrane organization and function: Moving past lipid rafts. Mol Biol Cell. 2013;24:2765–2768. doi: 10.1091/mbc.e13-03-0165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li YC, Park MJ, Ye SK, Kim CW, Kim YN. Elevated levels of cholesterol-rich lipid rafts in cancer cells are correlated with apoptosis sensitivity induced by cholesterol-depleting agents. Am J Pathol. 2006;168:1107–1118. doi: 10.2353/ajpath.2006.050959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Razani B, Zhang XL, Bitzer M, von Gersdorff G, Böttinger EP, Lisanti MP. Caveolin-1 regulates transforming growth factor (TGF)-beta/SMAD signaling through an interaction with the TGF-beta type I receptor. J Biol Chem. 2001;276:6727–6738. doi: 10.1074/jbc.M008340200. [DOI] [PubMed] [Google Scholar]
- 12.Le Roy C, Wrana JL. Clathrin- and non-clathrin-mediated endocytic regulation of cell signalling. Nat Rev Mol Cell Biol. 2005;6:112–126. doi: 10.1038/nrm1571. [DOI] [PubMed] [Google Scholar]
- 13.Liu S, Hou H, Zhang P, Wu Y, He Q, Li H, Yan N. Sphingomyelin synthase 1 regulates the epithelialtomesenchymal transition mediated by the TGF-β/Smad pathway in MDA-MB-231 cells. Mol Med Rep. 2019;19:1159–1167. doi: 10.3892/mmr.2018.9722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maxfield FR, Tabas I. Role of cholesterol and lipid organization in disease. Nature. 2005;438:612–621. doi: 10.1038/nature04399. [DOI] [PubMed] [Google Scholar]
- 15.Kline MA, O'Connor Butler ES, Hinzey A, Sliman S, Kotha SR, Marsh CB, Uppu RM, Parinandi NL. A simple method for effective and safe removal of membrane cholesterol from lipid rafts in vascular endothelial cells: Implications in oxidant-mediated lipid signaling. Methods Mol Biol. 2010;610:201–2011. doi: 10.1007/978-1-60327-029-8_12. [DOI] [PubMed] [Google Scholar]
- 16.Engin F, Hotamisligil GS. Restoring endoplasmic reticulum function by chemical chaperones: An emerging therapeutic approach for metabolic diseases. Diabetes Obes Metab. 2010;12:108–115. doi: 10.1111/j.1463-1326.2010.01282.x. [DOI] [PubMed] [Google Scholar]
- 17.Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 2007;8:519–529. doi: 10.1038/nrm2199. [DOI] [PubMed] [Google Scholar]
- 18.Wang S, Kaufman R. The impact of the unfolded protein response on human disease. J Cell Biol. 2012;197:857–867. doi: 10.1083/jcb.201110131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yamashita M, Ogasawara M, Kawasaki Y, Niisato M, Saito H, Kasai S, Maesawa C, Maemondo M, Yamauchi K. Deficiency of protein-L-isoaspartate (D-aspartate) O-methyl-transferase expression under endoplasmic reticulum stress promotes epithelial mesenchymal transition in lung adenocarcinoma. Oncotarget. 2018;9:13287–13300. doi: 10.18632/oncotarget.24324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen CA, Chang JM, Chang EE, Chen HC, Yang YL. Crosstalk between transforming growth factor-beta1 and endoplasmic reticulum stress regulates alpha-smooth muscle cell actin expression in podocytes. Life Sci. 2018;209:9–14. doi: 10.1016/j.lfs.2018.07.050. [DOI] [PubMed] [Google Scholar]
- 21.Huang Y, Du J, Mi Y, Li T, Gong Y, Ouyang H, Hou Y. Long non-coding RNAs contribute to the inhibition of proliferation and EMT by pterostilbene in human breast cancer. Fron Oncol. 2018;8:629. doi: 10.3389/fonc.2018.00629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Badana A, Chintala M, Varikuti G, Pudi N, Kumari S, Kappala RV, Malla RR. Lipid raft integrity is required for survival of triple negative breast cancer cells. J Breast Cancer. 2016;19:372–384. doi: 10.4048/jbc.2016.19.4.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee HM, Kang JH, Shin JM, Lee SA, Park HO. Chemical chaperone of endoplasmic reticulum stress inhibits epithelial-mesenchymal transition induced by TGF-β1 in airway epithelium via the c-src pathway. Mediators Inflamm. 2017;2017:1–9. doi: 10.1155/2017/4327237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Deans JP, Li H, Polyak MJ. CD20-mediated apoptosis: Signalling through lipid rafts. Immunology. 2010;107:176–182. doi: 10.1046/j.1365-2567.2002.01495.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gómez-Llobregat J, Buceta J, Reigada R. Interplay of cytoskeletal activity and lipid phase stability in dynamic protein recruitment and clustering. Sci Rep. 2013;3:2608. doi: 10.1038/srep02608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang R, Bi J, Ampah KK, Ba X, Liu W, Zeng X. Lipid rafts control human melanoma cell migration by regulating focal adhesion disassembly. Biochim Biophys Acta. 2013;1833:3195–3205. doi: 10.1016/j.bbamcr.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 27.Head BP, Patel HH, Insel PA. Interaction of membrane/lipid rafts with the cytoskeleton: Impact on signaling and function: Membrane/lipid rafts, mediators of cytoskeletal arrangement and cell signaling. Biochim Biophys Acta. 2014;1838:532–545. doi: 10.1016/j.bbamem.2013.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alawin OA, Ahmed RA, Ibrahim BA, Briski KP, Sylvester PW. Antiproliferative effects of γ-tocotrienol are associated with lipid raft disruption in HER2-positive human breast cancer cells. J Nutr Biochem. 2015;27:266–277. doi: 10.1016/j.jnutbio.2015.09.018. [DOI] [PubMed] [Google Scholar]
- 29.Badana AK, Chintala M, Gavara MM, Naika S, Kumari S, Kappala VR, Iska BR, Malla RR. Lipid rafts disruption induces apoptosis by attenuating expression of LRP6 and survivin in triple negative breast cancer. Biomed Pharmacother. 2017;97:359–369. doi: 10.1016/j.biopha.2017.10.045. [DOI] [PubMed] [Google Scholar]
- 30.Mitchell H, Choudhury A, Pagano RE, Leof EB. Ligand-dependent and -independent transforming growth factor-beta receptor recycling regulated by clathrin-mediated endocytosis and Rab11. Mol Biol Cell. 2004;15:4166–4178. doi: 10.1091/mbc.e04-03-0245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Le Roy C, Wrana JL. Clathrin- and non-clathrin-mediated endocytic regulation of cell signalling. Nat Rev Mol Cell Biol. 2005;6:112–126. doi: 10.1038/nrm1571. [DOI] [PubMed] [Google Scholar]
- 32.Midgley AC, Rogers M, Hallett MB, Clayton A, Bowen T, Phillips AO, Steadman R. Transforming growth factor-β1 (TGF-β1) stimulated fibroblast to myofibroblast differentiation is mediated by hyaluronan (HA)-facilitated epidermal growth factor receptor (EGFR) and CD44 co-localization in lipid rafts. J Biol Chem. 2013;288:14824–14838. doi: 10.1074/jbc.M113.451336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen YG. Endocytic regulation of TGF-beta signaling. Cell Res. 2009;19:58–70. doi: 10.1038/cr.2008.315. [DOI] [PubMed] [Google Scholar]
- 34.Derynck R, Muthusamy BP, Saeteurn KY. Signaling pathway cooperation in TGF-β-induced epithelial-mesenchymal transition. Curr Opin Cell Biol. 2014;31:56–66. doi: 10.1016/j.ceb.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Takebe N, Warren RQ, Ivy SP. Breast cancer growth and metastasis: Interplay between cancer stem cells, embryonic signaling pathways and epithelial-to-mesenchymal transition. Breast Cancer Res. 2011;13:211–211. doi: 10.1186/bcr2876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Conner SD, Schmid SL. Regulated portals of entry into the cell. Nature. 2003;422:37–44. doi: 10.1038/nature01451. [DOI] [PubMed] [Google Scholar]
- 37.Ewers H, Helenius A. Lipid-mediated endocytosis. Cold Spring Harb Perspect Biol. 2011;3:a004721. doi: 10.1101/cshperspect.a004721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moon SY, Kim HS, Nho KW, Jang YJ, Lee SK. Endoplasmic reticulum stress induces epithelial-mesenchymal transition through autophagy via activation of c-Src kinase. Nephron. Exp Nephrol. 2014;126:127–140. doi: 10.1159/000362457. [DOI] [PubMed] [Google Scholar]
- 39.Dasgupta A, Sawant MA, Kavishwar G, Lavhale M, Sitasawad S. AECHL-1 targets breast cancer progression via inhibition of metastasis, prevention of EMT and suppression of cancer stem cell characteristics. Sci Rep. 2016;6:38045. doi: 10.1038/srep38045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huang S, Wang D, Zhang S, Huang X, Wang D, Ljaz M, Shi Y. Tunicamycin potentiates paclitaxel-induced apoptosis through inhibition of PI3K/AKT and MAPK pathways in breast cancer. Cancer Chemother Pharmacol. 2017;80:685–696. doi: 10.1007/s00280-017-3393-7. [DOI] [PubMed] [Google Scholar]
- 41.He Z, He X, Liu M, Hua L, Wang T, Liu Q, Chen L, Yan N. Simvastatin attenuates H2O2-induced endothelial cell dysfunction by reducing endoplasmic reticulum stress. Molecules. 2019;24(pii):E1782. doi: 10.3390/molecules24091782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wu F, Xu K, Liu L, Zhang K, Xia L, Zhang M, Teng C, Tong H, He Y, Xue Y, et al. Vitamin B12 enhances nerve repair and improves functional recovery after traumatic brain injury by inhibiting ER stress-induced neuron injury. Front Pharmacol. 2019;10:406. doi: 10.3389/fphar.2019.00406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang EH, Yao SQ, Tao L, Xi JY. Sodium 4-phenylbutyrate attenuates high-fat diet-induced impaired spermatogenesis. Biomed Environ Sci. 2018;12:876–882. doi: 10.3967/bes2018.118. [DOI] [PubMed] [Google Scholar]
- 44.Guo Q, Hu H, Zhou Y, Yan Y, Wei X, Fan X, Yang D, He H, Oh Y, Chen K, et al. Glucosamine induces increased musclin gene expression through endoplasmic reticulum stress-induced unfolding protein response signaling pathways in mouse skeletal muscle cells. Food Chem Toxicol. 2018;125:95–105. doi: 10.1016/j.fct.2018.12.051. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.