Abstract
The major role of inner medullary collecting duct (IMCD) cells is to maintain water and sodium homeostasis. In addition to the major role, it also participates in the protection of renal and systemic inflammation. Although IMCD cells could take part in renal and systemic inflammation, investigations on renal inflammation in IMCD cells have rarely been reported. Although berberine (BBR) has been reported to show diverse pharmacological effects, its anti-inflammatory and protective effects on IMCD cells have not been studied. Therefore, in the present study, we examined the anti-inflammatory and protective effects of BBR in mouse IMCD-3 (mIMCD-3) cells against lipopolysaccharide (LPS). An MTT assay was carried out to investigate the toxicity of BBR on mIMCD-3 cells. Reverse transcription quantitative-PCR and western blotting were performed to analysis pro-inflammatory molecules and cytokines. Mechanisms of BBR were examined by western blotting and immunocytochemistry. According to previous studies, pro-inflammatory molecules, such as inducible nitric oxide synthase and cyclooxygenase-2, and pro-inflammatory cytokines, such as interleukin (IL)-1β, IL-6 and tumor necrosis factor-α are increased in LPS-exposed mIMCD-3 cells. However, the production of these pro-inflammatory molecules is significantly inhibited by treatment with BBR. In addition, BBR inhibited translocation of nuclear factor (NF)-κB p65 from the cytosol to the nucleus, and degradation of inhibitory κ-Bα in LPS-exposed mIMCD-3 cells. In conclusion, BBR could inhibit renal inflammatory responses via inhibition of NF-κB signaling and ultimately contribute to amelioration of renal injury during systemic inflammation.
Keywords: berberine, inner medullary collecting duct, lipopolysaccharide, nuclear factor-κB, renal inflammation
Introduction
Inner medullary collecting duct (IMCD) is the last part of the collecting duct system, which connects the nephrons to the ureter (1). The main function of IMCD is maintenance of body water homeostasis through regulation of electrolyte and fluid balance by managing the reabsorption and excretion of sodium ions (Na+) and water along with hormonal regulation (2–4). However surprisingly, IMCD also plays a pivotal role in inflammatory responses of the kidney, because it is a preferred site for Escherichia coli adherence (5). Previous studies have reported that pro-inflammatory mediators such as inducible nitric oxide synthase (iNOS), cyclooxygenase-2 (COX-2), and cytokines are produced by IMCD through various stimuli (6–8). However, regulation of inflammatory responses of IMCD cells during renal injury has not been studied in depth.
Berberine (BBR) is an isoquinoline alkaloid and the major compound of Coptidis rhizoma and Cortex Phellodendri. Both these plants have been used to treat gastroenteritis, secretory diarrhea, febrile illness, and hepatobiliary diseases in Chinese traditional medicine (9,10). BBR has been reported to have biochemical and pharmacological effects such as antidiabetic, anticancer, anti-fibrotic, antibacterial, antioxidant, and anti-inflammatory (11–15). Recent studies have reported that BBR ameliorates renal conditions such as podocyte injury, nephrotoxicity, acute hepato-renal toxicity, and type 2 diabetic nephropathy (9,16–18). Additionally, BBR inhibits LPS-induced inflammation in rat glomerular mesangial cells and LPS-induced oxidative stress in rat kidneys (19,20). However, the beneficial properties of BBR in LPS-induced inflammatory responses in mouse IMCD-3 cells have not been reported.
Therefore, we firstly examined the potential effects of BBR on LPS-treated mIMCD-3 cells. So, we investigated the production of inflammatory mediators such as iNOS, COX-2, IL-1β, IL-6, and TNF-α in LPS-exposed IMCD cells. Secondly, we examined the regulating mechanisms of BBR including NF-κB and MAPKs against LPS in mIMCD-3 cells.
Materials and methods
Chemicals and reagents
Dulbecco's Modified Eagle's Medium/Ham's Nutrient Mixture F-12 (DMEM/F-12) (cat. no. 11330-032), fetal bovine serum (FBS) (cat. no. 26140-079) and antibiotics (cat. no. 15140122) were obtained from Gibco; Thermo Fisher Scientific, Inc. N-acetylcysteine (NAC) (cat. no. A7250), Bay 11-7082 (cat. no. B5556), Parthenolide (cat. no. P0667) BBR (cat. no. B3412; Purity: 99%), LPS from Escherichia coli (cat. no. L2880; serotype 055:B5) and paraformaldehyde (cat. no. 158127) were purchased from Sigma-Aldrich; Merck KGaA. Tris-HCl (cat. no. #161-0798; #161-0799) and pre-stained sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) marker (cat. no. 1610395) were purchased from Bio-Rad Laboratories, Inc. Sodium dodecyl sulfate (cat. no. 18220) was purchased from Affymetrix; Thermo Fisher Scientific, Inc. Antibodies against iNOS (cat. no. sc-651), COX-2 (cat. No. sc-17454), Inhibitor kappa B alpha (Iκ-Bα) (cat. no. sc-371), NF-κB (cat. no. sc-372), β-actin (cat. no. sc-47778), and horseradish peroxidase-conjugated mouse anti-rabbit IgG (cat. no. sc-2357) and goat anti-mouse IgG (cat. no. sc-2005) were purchased from Santa Cruz Biotechnology. Antibodies against pERK (cat. no. #9101), ERK (cat. no. #9102), pJNK (cat. no. #9251), JNK (cat. no. #9252), pP38 (cat. no. #9211), P38 (cat. no. #9212), and Inhibitory kappa B alpha (Iκ-Bα) (cat. no. #2859) were purchased from Cell Signaling Technology, Inc. Easy-blue™ Total RNA extraction kit (cat. no. 17061) was purchased from iNtRON Biotechnology. Enzyme-linked immunosorbent assay (ELISA) kits for anti-mouse IL-1β (cat. no. MAB401), IL-6 (cat. no. MAB406) and TNF-α (cat. no. MAB425) antibodies, and mouse IL-1β (cat. no. BAF401), IL-6 (cat. no. BAF406) and TNF-α (cat. no. BAF425) biotinylated antibodies were purchased from R&D Systems. Nuclear extraction kit (cat. no. 2900) was purchased from EMD Millipore. Trans AM NF-κB activation assay kit (cat. no. 40096) was purchased from Active Motif.
Cell culture
Mouse inner medullary collecting duct (mIMCD-3) cells, the mouse renal epithelial cell line (cat. no. CRL2123), were purchased from the American Type Culture Collection (ATCC). mIMCD-3 cells were routinely cultured in DMEM/F-12 supplemented with 10% FBS and 1% penicillin/streptomycin, and were maintained in a humidified chamber containing 5% CO2 at 37°C and were used in passages 3–5.
Cell viability assay
3-(4,5-Dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) assay was performed to detect the cytotoxicity of BBR on mIMCD-3 cells. The cells were seeded in each well of 24-well plates (5×104 cells/well) and incubated with BBR at a concentration of 0.05, 0.1, 0.5, 1 or 10 µM for 24 h. Thereafter, the medium was changed, and cells were incubated with MTT solution (0.5 mg/ml) for 30 min at 37°C. The medium was removed and the purple colored precipitate of formazan was dissolved in 200 µl of dimethyl sulfoxide. Aliquots of the dissolved precipitate were taken in a 96-well plate, in duplicates, and estimated at 540 nm using a micro-ELISA plate reader.
Reverse transcription-quantitative PCR (RT-qPCR)
Total RNAs were isolated with Easy-Blue™, and the purity of the samples was confirmed by RNA calculator (Gene Quant Pro, Biochrom). The total RNA extraction kit was used according to the manufacturer's instructions and reverse transcription of RNA (10 µg) to cDNA was performed using the ABI cDNA synthesis kit (Applied Biosystems; Thermo Fisher Scientific, Inc.) (conditions: 37°C for 1 h, followed by 95°C for 5 min). TaqMan quantitative RT-PCR with an ABI StepOne Plus detection system was performed according to the manufacturer's instructions (Applied Biosystems; Thermo Fisher Scientific, Inc.). All qPCR data were normalized to the expression levels of the housekeeping gene hypoxanthine guanine phosphoribosyltransferase (HPRT). The thermocycling conditions used for qPCR were as follows: 50°C for 2 min and 95°C for 10 min, followed by 40 cycles of 95°C for 10 sec and 60°C for 30 sec. The commercial forward, reverse, and probe oligonucleotide primers for multiplex real-time TaqMan PCR were purchased from ABI (cat. no. 4304437; Applied Biosystems; Thermo Fisher Scientific, Inc.). The 2−ΔΔCq method was used to determine the relative mRNA expression level (21).
Flow cytometry analysis
mIMCD-3 cells treated with BBR (0.1, 0.5 or 1 µM) and LPS (5 µg/ml) for 24 h were harvested with cell scraper and washed with PBS by centrifugation for 5 min. Cells were then incubated with the blocking solution for 30 min at RT. Subsequently, cells were incubated with a PE-conjugated anti-mouse TLR4/MD-2 complex antibody (1:250; cat. no. 117605; BioLegend) for 30 min at 4°C. Data were acquired by BD FACS calibur cell analyser and analysed in CellQuest Pro software (BD Biosciences; Thermo Fisher Scientific, Inc.).
Western blotting
The cells were washed with PBS and lysed with lysis buffer (1% cocktail of protease inhibitor and 1% phosphatase inhibitor in 1X RIPA Buffer). Protein concentration was determined by bicinchoninic acid assay. Subsequently, Total cell proteins (20 µg) were then separated by SDS-PAGE on a 10% gel and transferred to a PVDF membrane (cat. no. 10600023; GE Healthcare Life Sciences). The membrane was blocked with 5% skim milk in phosphate-buffered saline (PBS) with Tween-20 (PBST) for 2 h at room temperature (RT) and washed PBST for 10 min, three times. Then, incubated with primary antibody (1:1,000) at 4°C overnight: iNOS, COX-2, Ik-Ba, b-actin, pERK, ERK, pJNK, JNK, pP38 and P38. After washing three times with PBST, each blot was incubated with horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG or donkey anti-goat IgG secondary antibody for 1 h at RT. The proteins were visualized using an enhanced chemiluminescence detection system (cat. no. RPN2232; Amersham; GE Healthcare). Captures protein bands and quantitative analysis were performed using Quantity One® software version 4.6.6 (Bio-Rad Laboratories, Inc.).
ELISA
ELISAs for IL-1β, IL-6, and TNF-α were carried out in duplicates in 96-well plates coated with anti-mouse IL-1β, IL-6, and TNF-α monoclonal antibodies in PBS (pH 7.4) at 4°C overnight. The plates were washed with PBST and blocked with PBS containing 10% FBS for 2 h at RT. After two times washes, standards and samples were added and incubated for 2 h at RT, and then the wells were washed and biotinylated anti-mouse IL-1β, IL-6, or TNF-α were added and incubated for 1 h at RT. The wells were washed, avidin-peroxidase was added, and plates were incubated for 30 min at RT. The wells washing again and followed by the addition of TMB substrate. Color development was measured at 450 nm using an automated microplate ELISA reader. Standard samples were run on each assay plate by using serial dilutions of recombinant IL-1β, IL-6, and TNF-α.
Immunocytochemistry
mIMCD-3 cells were plated in a chamber slide and incubated with LPS (5 µg/ml) for 30 min at 37°C. The cells were treated with BBR (1 µM) for 1 h before LPS treatment. The cells were fixed in 4% paraformaldehyde for 15 min at RT and washed 3 times with PBS. The cells were treated with 0.1% TritonX-100 for 15 min at RT. After washing, non-specific binding sites were blocked with serum (3% BSA) for 1 h at RT, and incubated with NF-κB antibody (1:250) at 4°C overnight. The cells were then washed and incubated with AlexaFluor®568 goat anti-rabbit IgG (1:2,000; cat. no. A-11011) for NF-κB antibody for 2 h at RT in a darkened room. For nuclear staining, the cells were incubated with DAPI (cat. no. H-1200; Vector Laboratories) at 5 µg/ml for 5 min at RT. The slide was finally washed and mounted for microscopic examination. Stained sections were visualized using a confocal laser microscope (Olympus).
Nuclear extraction
mIMCD-3 cells were plated in 100-mm dishes (1×107 cells/dish). Then, LPS (5 µg/ml) treated for 0, 15, 30 and 60 min with or without pretreatment of BBR (1 µM) for 1 h. Nuclear extraction assay performed according to the manufacturer's instructions. Briefly, cells were harvested with cell scraper and washed with PBS by centrifugation at 250 × g for 5 min. Add 1X Cytoplasmic Lysis Buffer (containing DTT and Protease inhibitor cocktail) to the pellet and incubation on ice for 15 min. Centrifuge at 250 × g for 5 min, discard the supernatant and add 1X Cytoplasmic Lysis Buffer. Five times drawing and ejecting by 27-gauge syringe, then centrifuge at 8,000 × g for 20 min. Discard supernatant, and add Nuclear Extraction Buffer (containing DTT and Protease inhibitor cocktail). Five times drawing and ejecting by 27-gauge syringe, then gently agitate on rotator at 4°C for 60 min. Centrifuge at 16,000 × g for 5 min, then supernatant was used for experiments.
NF-κB p65 activation assay
NF-κB activation was determined in the nuclear extracts of mIMCD-3 cells, following the manufacturer's instructions. Briefly, 15 µg nuclear extract was added to a biotinylated oligonucleotide containing the NF-κB consensus site attached to the streptavidin-coated 96-well plates with agitation on rocking platform at 100 rpm for 1 h. Plates were washed with wash buffer to remove all the unbound reagents. NF-κB p65 primary antibody (1:1,000) was added for 1 h at room temperature without agitation, followed by a goat anti-rabbit secondary antibody conjugated with horseradish peroxidase (1:1,000) at room temperature without agitation. Subsequent to an incubation for 1 h at room temperature, 100 µl developing solution was added to all wells for 3 min at room temperature protected from direct light. The blue color development in the sample wells was monitored until it turned medium to dark blue. Subsequently, 100 µl stop solution was added and the blue color turned yellow. Finally, the absorbance value was ascertained using a spectrophotometer at a wavelength of 450 nm. As a positive control for NF-κB p65 activation, nuclear extracts from Jurkat cells, provided with the kit, were used.
Statistical analysis
Results are expressed as the mean ± standard error of the mean (SEM). Statistical significance of intergroup differences was evaluated using two-way ANOVA, with time and dose as variables, followed by a Duncan's post-hoc test. All statistical analyses were performed using SPSS. P<0.05 was considered to indicate a statistically significant difference. All experiments were carried out in triplicates.
Results
Effects of BBR on production of iNOS and COX-2
Before studying the biological activity of BBR, we performed the MTT assay to evaluate the cytotoxicity of BBR in mIMCD-3 cells. As shown in Fig. 1A, up to a dose of 1 µM, BBR did not affect cell viability. Thus, we chose BBR concentrations of 0.1, 0.5 and 1 µM for further experiments.
Figure 1.
Cytotoxicity of BBR and inhibitory activity of BBR on iNOS and COX-2 production in mIMCD-3 cells. (A) mIMCD-3 cells were incubated with BBR (0.05, 0.1, 0.5, 1 or 10 µM) for 24 h. Subsequently, an MTT assay was performed to evaluate cytotoxicity of BBR. mIMCD-3 cells were incubated with BBR (0.1, 0.5 or 1 µM) for 1 h. Subsequently, cells were exposed to LPS (5 µg/ml) for 3 h (iNOS) and 1 h (COX-2). mRNA levels of (B) iNOS and (D) COX-2 were evaluated by reverse transcription-PCR. Protein levels of (C) iNOS and (E) COX-2 were measured by western blotting. Results are presented as the mean ± SEM of at least three independent experiments. *P<0.05 vs. control; †P<0.05 vs. LPS alone. BBR, berberine; iNOS, inducible nitric oxide synthase; COX-2, cyclooxygenase-2; mIMCD-3, mouse IMCD-3; LPS, lipopolysaccharide.
To determine the pro-inflammatory effects of BBR against LPS in mIMCD-3 cells, production of pro-inflammatory molecules such as iNOS and COX-2 after LPS stimulation in mIMCD-3 cells was investigated. In accordance with our previous report (2), production of iNOS and COX-2 was elevated in LPS-exposed mIMCD-3 cells. However, the elevation of iNOS and COX-2 was significantly inhibited by BBR in these cells (Fig. 1B-E).
Effects of BBR on production of pro-inflammatory cytokines
IL-1β, IL-6, and TNF-α are the representative pro-inflammatory cytokines known to be induced by LPS treatment in mIMCD-3 cells (2). Thus, to measure the effects of BBR on the production of these pro-inflammatory cytokines in LPS-exposed mIMCD-3 cells, we investigated the mRNA and protein expression of IL-1β, IL-6, and TNF-α after LPS stimulation. As reported earlier (2), LPS treatment increased the mRNA and protein levels of IL-1β, IL-6, and TNF-α in mIMCD-3 cells. However, BBR treatment inhibited the induction of IL-1β, IL-6, and TNF-α mRNA and protein levels in a dose-dependent manner (Fig. 2).
Figure 2.
Inhibitory effects of BBR on pro-inflammatory cytokine production in mIMCD-3 cells. mIMCD-3 cells were incubated with BBR (0.1, 0.5 or 1 µM) for 1 h. Subsequently, cells were exposed to LPS (5 µg/ml) for 1 h (IL-1β and TNF-α) and 24 h (IL-6). mRNA levels of (A) IL-1β, (B) IL-6 and (C) TNF-α were evaluated by reverse transcription-PCR. Protein levels of (D) IL-1β, (E) IL-6 and (F) TNF-α were evaluated by ELISA. Results are presented as the mean ± SEM of at least three independent experiments. *P<0.05 vs. control; †P<0.05 vs. LPS alone. BBR, berberine; mIMCD-3, mouse IMCD-3; LPS, lipopolysaccharide; IL, interleukin; TNF-α, tumor necrosis factor-α.
Effects of BBR on activation of NF-κB and MAPKs
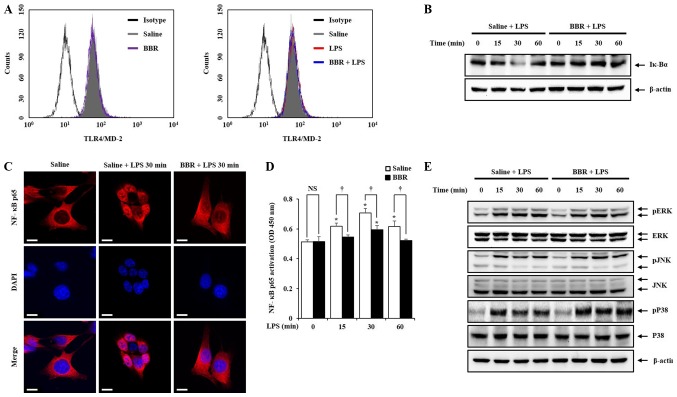
Since BBR was effective in inhibiting pro-inflammatory mediators in LPS-treated mIMCD-3 cells, we sought to investigate the mechanistic details of the beneficial activity of BBR. Firstly, to rule out the possibility that BBR directly regulates LPS or TLR4, we examined the effect of BBR on TLR4/MD2 complex expression. The methods of Limulus amebocyte lysate (LAL) assay and RT-qPCR are described in Data S1. As shown in Fig. 3A and Fig. S1, BBR did not directly affect LPS and TLR4 expression in mIMCD-3 cells, which means BBR regulates the down-streams upon TLR4-LPS interaction. Thus, we ought to examine the activation of NF-κB and MAPKs, because activation of both signaling pathways produce pro-einflammatory mediators (22). As shown in Fig. 3B-E, LPS stimulation triggered Iκ-Bα degradation, NF-κB p65 translocation from cytosol to nucleus, elevation of NF-κB p65 binding activity, and MAPK phosphorylation. However, treatment with BBR inhibited Iκ-Bα degradation, NF-κB p65 translocation and NF-κB p65 binding activity elevation but not MAPK phosphorylation.
Figure 3.
Inhibitory effects of BBR on the NF-κB and MAPK pathways in mIMCD-3 cells. (A) mIMCD-3 cells were pre-treated with BBR (1 µM) 1 h before LPS (5 µg/ml) treatment. The expression of TLR4/MD-2 complex was detected after 24 h LPS treatment by flow cytometry. mIMCD-3 cells were incubated with BBR (1 µM) for 1 h. Thereafter, mIMCD-3 cells were harvested after LPS (5 µg/ml) stimulation for 0, 15, 30 or 60 min. Western blotting was performed to demonstrate that BBR could inhibit (B) Iκ-Bα degradation and (E) MAPK phosphorylation in mIMCD-3 cells. (C) Immunofluorescence staining was performed to evaluate BBR could inhibit NF-κB translocation from cytosol to nucleus in mIMCD-3 cells. NF-κB p65 was stained with AlexaFluor 568 (red) and Nucleus was stained with DAPI (blue) in mIMCD-3 cells. (D) Binding activity of NF-κB p65 from mIMCD-3 cells was measured using the Trans AM NF-κB p65 assay kit. *P<0.05 vs. saline + LPS 0 min; †P<0.05. Figure shows a representative image from three independent experiments. Scale bar, 10 µm. BBR, berberine; mIMCD-3, mouse IMCD-3; LPS, lipopolysaccharide; Iκ-Bα, inhibitory κ-Bα; NF-κB, nuclear factor-κB; TLR4, toll-like receptor 4; NS, not significant.
Effects of NF-κB on production of inflammatory mediators
To evaluate whether NF-κB deactivation could contribute to amelioration of inflammatory responses in LPS-treated mIMCD-3 cells, we used three well known NF-κB inhibitors (NAC, Bay 11-7082, and Parthenolide). As is known, three inhibitors could block NF-κB p65 translocation from nucleus to cytosol against LPS treatment in mIMCD-3 cells (Fig. S2). Next, we hypothesized that NF-κB inhibitors might downregulate the production of pro-inflammatory mediators, similar to BBR. As expected, NF-κB inhibitors treatment reduced the mRNA levels of iNOS, COX-2, IL-1β, IL-6, and TNF-α after LPS treatment in mIMCD-3 cells to a similar degree as in the cells treated with BBR (Fig. 4).
Figure 4.
Reduction of pro-inflammatory molecules and cytokine expression by inhibition of the NF-κB pathway in mIMCD-3 cells. mIMCD-3 cells were incubated with 10 mM NAC, 5 5 µM Bay, and 10 µM Par, which is an NF-κB inhibitor/or BBR (1 µM) for 1 h. Thereafter, cells were exposed to LPS (5 µg/ml) for 3 h (iNOS), 1 h (COX-2, IL-1β and TNF-α), and 24 h (IL-6). mRNA levels of (A) iNOS, (B) COX-2, (C) IL-1β, (D) IL-6 and (E) TNF-α were evaluated by reverse transcription-PCR. Results are expressed as the mean ± SEM of at least three independent experiments. *P<0.05 vs. control; †P<0.05 vs. LPS alone. NF-κB, nuclear factor-κB; mIMCD-3, mouse IMCD-3; NAC, N-acetylcysteine; Bay, Bay11-7082; Par, Parthenolide; BBR, berberine; LPS, lipopolysaccharide; iNOS, inducible nitric oxide synthase; COX-2, cyclooxygenase-2; IL, interleukin; TNF-α, tumor necrosis factor-α.
Discussion
In this study, we revealed that BBR inhibited LPS-mediated inflammatory responses in mIMCD-3 cells. LPS stimulation induced the expression of pro-inflammatory mediators (iNOS and COX-2) and pro-inflammatory cytokines (IL-1β, IL-6, and TNF-α) in mIMCD-3 cells. However, BBR treatment could inhibit these inflammatory mediators and cytokines dramatically. In addition, the mechanism underlying the inhibitory effects of BBR upon LPS stimulation involved the inhibition of NF-κB activation. Our results show that BBR suppresses LPS-mediated inflammation via NF-κB deactivation in mIMCD-3 cells.
Since BBR is so popular with variety biochemical and pharmacological effects and cheap, there are already many ready-to-use drugs associated with BBR. Today, BBR has been developed to dietary supplement, and BBR was approved Unique ingredient identifier (UNII) by FDA (UNII: 0I8Y3P32UF). Based on this information, we can easily examine the beneficial potential of BBR in both non-clinical and clinical experiments. In non-clinical experiments, Pietra et al (13) reported that BBR reduce fibrosis and inflammatory cytokine in human dermal fibroblasts in vitro. It was also reported that BBR could protect ulcerative colitis and neuropathic pain in mice (23,24). In addition, many researches have focused on the beneficial effects of BBR in renal diseases. It has been reported that BBR could ameliorate cisplatin-induced nephrotoxicity in mice, that might be through the reduction of histopathological damage, renal functional markers, oxidative stress, cell death signals, and NF-κB activation (25). In addition, BBR have shown to protective and beneficial activities in various renal diseases such as diabetic nephropathy and renal dysfunction (26). Based on many researches about beneficial effects of BBR on renal injury, BBR has been applied to clinical trials in human renal diseases, ands has been reported to alleviate the renal injury in type 2 diabetes mellitus (27–29). In addition to previous reports, our results suggest that BBR could be applied in urinary tract infection (UTI), such as cystitis and pyelonephritis. The most common cause of UTI is Escherichia coli (30), and infection spreads from the bladder to the kidneys and collecting systems (31). Therefore, we suggest that BBR inhibits LPS-infected renal inflammation, especially in UTI.
Collecting duct system of the kidney is a renal tubular segment and plays a role in the reabsorption of filtered water (~15%). A key feature of the collecting duct is water reabsorption through hormonal regulation in an autocrine/paracrine manner (32–40). For instance, vasopressin, also called as antidiuretic hormone (ADH), affects osmotic water permeability and increases water reabsorption in IMCD cells (35). Hence, most reports of IMCD have been in the context of regulation of water homeostasis such as osmotic water, urea, and electrolyte permeability (35,41–44). However, because many renal diseases are accompanied by inflammatory mediator production as well as water imbalance (45), it is very important to identify the underlying patho-physiology of inflammatory responses in renal diseases. Thus, regulation of inflammatory mediators could be key in the treatment of renal diseases. Through this study, we propose BBR as a beneficial agent in managing renal inflammation.
Toll-like receptor 4 (TLR4), a transmembrane receptor, belongs to the super-family of pattern recognition receptors (PRRs). Generally, a well-known function of TLR4 is recognition of exogenous molecules from pathogens-associated molecular pattern molecules (PAMPs) and/or damage-associated molecular pattern molecules (DAMPs), which is released upon cellular or tissue damage. TLR4 existed in many cells such as endothelial cells, myocytes, thyroid cells, endometrial cells, mesangial cells, and adipocytes (46–51). We and other researchers identified the expression of TLR4 in IMCD cells (2,5,8). When TLR4 ligand such as LPS binds to TLR4, pro-inflammatory mediators such as iNOS, COX-2, IL-1β, IL-6, and TNF-α are up-regulated (5). As these inflammatory mediators released from collecting duct cells could trigger renal injury and inflammation, inhibition of inflammatory mediators in collecting duct cells might be important (5). In this study, BBR treatment inhibited TLR4-mediated elevation of inflammatory mediators such as iNOS, COX-2, IL-1β, IL-6, and TNF-α in LPS-treated mIMCD-3 cells, which suggests that BBR attenuated local inflammation in collecting duct cells (Figs. 1 and 2).
In this study, we examined whether activation of NF-κB could regulates the inflammatory mediators. NF-κB belongs to a family of dimeric transcription factors and participates in numerous biological procedures, including immune responses, inflammation, cellular differentiation, proliferation, and survival (52–55). NF-κB activation, followed by LPS treatment, results in induction of cytokines (IL-1, IL-6, and TNF-α) and inflammatory enzymes (iNOS and COX-2) (56–58). Thus, negative regulation of NF-κB might be responsible for inhibition of inflammatory mediators. Our findings showed that BBR treatment inhibited the degradation of Iκ-Bα and translocation of NF-κB into the nucleus after LPS treatment, suggesting that BBR inhibited the activation of NF-κB (Fig. 3). In addition, to examine whether NF-κB activation after LPS leads to production of inflammatory mediators in mIMCD-3 cells, we investigated the production of inflammatory mediators after NF-κB inhibition by 3 well known inhibitor such as NAC (59,60), Bay 11-7082 (61,62), and Parthenolide (62). As expected, the inhibitors showed the inhibitory effects on NF-κB activation in our mIMCD-3 models (Fig. S2). The inhibition of NF-κB by NAC, Bay 11-7082, and Parthenolide significantly inhibited the production of inflammatory mediators (Fig. 4), which suggest that BBR has anti-inflammatory effects mainly through NF-κB deactivation in LPS-exposed mIMCD-3 cells. Some previous researches have reported that BBR inhibits inflammatory mediators and NF-κB signaling in various different disease models (19,63–67). However, there is no report that BBR could have anti-inflammatory and protective effects in LPS-exposed inner medullary collecting duct (IMCD) cells. In this study, we firstly reported that BBR has protective effects upon LPS-induced inflammation by downregulation of NF-κB pathway in mouse IMCD-3 cells.
In summary, we firstly revealed that the anti-inflammatory effects of BBR via deactivation of NF-κB in LPS-stimulated mIMCD-3 cells. Our results suggest that BBR might be a potential and beneficial drug in renal injury. However, the current data are not sufficient to explain the beneficial effects of BBR in in vivo renal injury systems. Therefore, further studies in this area would be needed.
Supplementary Material
Acknowledgements
Not applicable.
Funding
The present study was supported in part by The National Research Foundation of Korea grant funded by the Korea government (grant no. NRF-2017R1A5A2015805).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
DGK, JWC and IJJ designed the study, conducted the data analysis and prepared the manuscript. MJK assisted with the experiments. HSL, HJS and SHH analyzed the data and prepared the manuscript. HJS revised the manuscript for intellectual and scientific content. GSB and SJP designed the study and prepared the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Tchapyjnikov D, Li Y, Pisitkun T, Hoffert JD, Yu MJ, Knepper MA. Proteomic profiling of nuclei from native renal inner medullary collecting duct cells using LC-MS/MS. Physiol Genomics. 2010;40:167–183. doi: 10.1152/physiolgenomics.00148.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim DG, Bae GS, Jo IJ, Choi SB, Kim MJ, Jeong JH, Kang DG, Lee HS, Song HJ, Park SJ. Guggulsterone attenuated lipopolysaccharide-induced inflammatory responses in mouse inner medullary collecting Duct-3 cells. Inflammation. 2016;39:87–95. doi: 10.1007/s10753-015-0226-x. [DOI] [PubMed] [Google Scholar]
- 3.Welch AK, Jeanette Lynch I, Gumz ML, Cain BD, Wingo CS. Aldosterone alters the chromatin structure of the murine endothelin-1 gene. Life Sci. 2016;159:121–126. doi: 10.1016/j.lfs.2016.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mohaupt MG, Schwöbel J, Elzie JL, Kannan GS, Kone BC. Cytokines activate inducible nitric oxide synthase gene transcription in inner medullary collecting duct cells. Am J Physiol. 1995;268:F770–F777. doi: 10.1152/ajprenal.1995.268.4.F770. [DOI] [PubMed] [Google Scholar]
- 5.Chassin C, Vimont S, Cluzeaud F, Bens M, Goujon JM, Fernandez B, Hertig A, Rondeau E, Arlet G, Hornef MW, Vandewalle A. TLR4 facilitates translocation of bacteria across renal collecting duct cells. J Am Soc Nephrol. 2008;19:2364–2374. doi: 10.1681/ASN.2007121273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chassin C, Goujon JM, Darche S, du Merle L, Bens M, Cluzeaud F, Werts C, Ogier-Denis E, Le Bouguénec C, Buzoni-Gatel D, Vandewalle A. Renal collecting duct epithelial cells react to pyelonephritis-associated escherichia coli by activating distinct TLR4-dependent and -independent inflammatory pathways. J Immunol. 2006;177:4773–4784. doi: 10.4049/jimmunol.177.7.4773. [DOI] [PubMed] [Google Scholar]
- 7.Choi JY, Nam SA, Jin DC, Kim J, Cha JH. Expression and cellular localization of inducible nitric oxide synthase in lipopolysaccharide-treated rat kidneys. J Histochem Cytochem. 2012;60:301–315. doi: 10.1369/0022155411436131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Küper C, Beck FX, Neuhofer W. Toll-like receptor 4 activates NF-κB and MAP kinase pathways to regulate expression of proinflammatory COX-2 in renal medullary collecting duct cells. Am J Physiol Renal Physiol. 2012;302:F38–F46. doi: 10.1152/ajprenal.00590.2010. [DOI] [PubMed] [Google Scholar]
- 9.Sun SF, Zhao TT, Zhang HJ, Huang XR, Zhang WK, Zhang L, Yan MH, Dong X, Wang H, Wen YM, et al. Renoprotective effect of berberine on type 2 diabetic nephropathy in rats. Clin Exp Pharmacol Physiol. 2015;42:662–670. doi: 10.1111/1440-1681.12402. [DOI] [PubMed] [Google Scholar]
- 10.Linn YC, Lu J, Lim LC, Sun H, Sun J, Zhou Y, Ng HS. Berberine-induced haemolysis revisited: Safety of Rhizoma coptidis and cortex phellodendri in chronic haematological diseases. Phytother Res. 2012;26:682–686. doi: 10.1002/ptr.3617. [DOI] [PubMed] [Google Scholar]
- 11.Dong Y, Chen YT, Yang YX, Zhou XJ, Dai SJ, Tong JF, Shou D, Li C. Metabolomics study of type 2 diabetes mellitus and the antidiabetic effect of berberine in zucker diabetic fatty rats using Uplc-ESI-Hdms. Phytother Res. 2016;30:823–828. doi: 10.1002/ptr.5587. [DOI] [PubMed] [Google Scholar]
- 12.Wang J, Peng Y, Liu Y, Yang J, Ding N, Tan W. Berberine, a natural compound, suppresses Hedgehog signaling pathway activity and cancer growth. BMC Cancer. 2015;15:595. doi: 10.1186/s12885-015-1596-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pietra D, Borghini A, Bianucci AM. In vitro studies of antifibrotic and cytoprotective effects elicited by proto-berberine alkaloids in human dermal fibroblasts. Pharmacol Rep. 2015;67:1081–1089. doi: 10.1016/j.pharep.2015.04.001. [DOI] [PubMed] [Google Scholar]
- 14.Peng L, Kang S, Yin Z, Jia R, Song X, Li L, Li Z, Zou Y, Liang X, Li L, et al. Antibacterial activity and mechanism of berberine against Streptococcus agalactiae. Int J Clin Exp Pathol. 2015;8:5217–5223. [PMC free article] [PubMed] [Google Scholar]
- 15.Kwon OJ, Kim MY, Shin SH, Lee AR, Lee JY, Seo BI, Shin MR, Choi HG, Kim JA, Min BS, et al. Antioxidant and anti-inflammatory effects of Rhei rhizoma and coptidis rhizoma mixture on reflux esophagitis in rats. Evid Based Complement Altern Med. 2016;2016:2052180. doi: 10.1155/2016/2052180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang B, Xu X, He X, Wang Z, Yang M. Berberine improved aldo-induced podocyte injury via inhibiting oxidative stress and endoplasmic reticulum stress pathways both in vivo and in vitro. Cell Physiol Biochem. 2016;39:217–228. doi: 10.1159/000445618. [DOI] [PubMed] [Google Scholar]
- 17.Adil M, Kandhare AD, Dalvi G, Ghosh P, Venkata S, Raygude KS, Bodhankar SL. Ameliorative effect of berberine against gentamicin-induced nephrotoxicity in rats via attenuation of oxidative stress, inflammation, apoptosis and mitochondrial dysfunction. Ren Fail. 2016;38:996–1006. doi: 10.3109/0886022X.2016.1165120. [DOI] [PubMed] [Google Scholar]
- 18.Chen X, Zhang Y, Zhu Z, Liu H, Guo H, Xiong C, Xie K, Zhang X, Su S. Protective effect of berberine on doxorubicin-induced acute hepatorenal toxicity in rats. Mol Med Rep. 2016;13:3953–3960. doi: 10.3892/mmr.2016.5017. [DOI] [PubMed] [Google Scholar]
- 19.Jiang Q, Liu P, Wu X, Liu W, Shen X, Lan T, Xu S, Peng J, Xie X, Huang H. Berberine attenuates lipopolysaccharide-induced extracelluar matrix accumulation and inflammation in rat mesangial cells: Involvement of NF-κB signaling pathway. Mol Cell Endocrinol. 2011;331:34–40. doi: 10.1016/j.mce.2010.07.023. [DOI] [PubMed] [Google Scholar]
- 20.Yokozawa T, Ishida A, Kashiwada Y, Cho EJ, Kim HY, Ikeshiro Y. Coptidis Rhizoma: Protective effects against peroxynitrite-induced oxidative damage and elucidation of its active components. J Pharm Pharmacol. 2004;56:547–556. doi: 10.1211/0022357023024. [DOI] [PubMed] [Google Scholar]
- 21.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 22.Pålsson-McDermott EM, O'Neill LA. Signal transduction by the lipopolysaccharide receptor, Toll-like receptor-4. Immunology. 2004;113:153–162. doi: 10.1111/j.1365-2567.2004.01976.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhu L, Gu P, Shen H. Protective effects of berberine hydrochloride on DSS-induced ulcerative colitis in rats. Int Immunopharmacol. 2019;68:242–251. doi: 10.1016/j.intimp.2018.12.036. [DOI] [PubMed] [Google Scholar]
- 24.Rezaee R, Monemi A, SadeghiBonjar MA, Hashemzaei M. Berberine alleviates paclitaxel-induced neuropathy. J Pharmacopuncture. 2019;22:90–94. doi: 10.3831/KPI.2019.22.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Domitrović R, Cvijanović O, Pernjak-Pugel E, Skoda M, Mikelić L, Crnčević-Orlić Ž. Berberine exerts nephroprotective effect against cisplatin-induced kidney damage through inhibition of oxidative/nitrosative stress, inflammation, autophagy and apoptosis. Food Chem Toxicol. 2013;62:397–406. doi: 10.1016/j.fct.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 26.Zhu L, Han J, Yuan R, Xue L, Pang W. Berberine ameliorates diabetic nephropathy by inhibiting TLR4/NF-κB pathway. Biol Res. 2018;51:9. doi: 10.1186/s40659-018-0157-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li ZY, Liu B, Zhuang XJ, Shen YD, Tian HR, Ji Y, Li LX, Liu F. Effects of berberine on the serum cystatin C levels and urine albumin/creatine ratio in patients with type 2 diabetes mellitus. Zhonghua Yi Xue Za Zhi. 2018;98:3756–3761. doi: 10.3760/cma.j.issn.0376-2491.2018.46.007. (In Chinese) [DOI] [PubMed] [Google Scholar]
- 28.Hu Y, Ehli EA, Kittelsrud J, Ronan PJ, Munger K, Downey T, Bohlen K, Callahan L, Munson V, Jahnke M, et al. Lipid-lowering effect of berberine in human subjects and rats. Phytomedicine. 2012;19:861–867. doi: 10.1016/j.phymed.2012.05.009. [DOI] [PubMed] [Google Scholar]
- 29.Yin J, Xing H, Ye J. Efficacy of berberine in patients with type 2 diabetes mellitus. Metabolism. 2008;57:712–717. doi: 10.1016/j.metabol.2008.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Flores-Mireles AL, Walker JN, Caparon M, Hultgren SJ. Urinary tract infections: Epidemiology, mechanisms of infection and treatment options. Nat Rev Microbiol. 2015;13:269–284. doi: 10.1038/nrmicro3432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stone SC, Mallon WK, Childs JM, Docherty SD. Emphysematous pyelonephritis: Clues to rapid diagnosis in the Emergency Department. J Emerg Med. 2005;28:315–319. doi: 10.1016/j.jemermed.2004.07.015. [DOI] [PubMed] [Google Scholar]
- 32.Kim JE, Jung HJ, Lee YJ, Kwon TH. Vasopressin-regulated miRNAs and AQP2-targeting miRNAs in kidney collecting duct cells. Am J Physiol Renal Physiol. 2015;308:F749–F764. doi: 10.1152/ajprenal.00334.2014. [DOI] [PubMed] [Google Scholar]
- 33.Gao M, Cao R, Du S, Jia X, Zheng S, Huang S, Han Q, Liu J, Zhang X, Miao Y, et al. Disruption of prostaglandin E2 receptor EP4 impairs urinary concentration via decreasing aquaporin 2 in renal collecting ducts. Proc Natl Acad Sci USA. 2015;112:8397–8402. doi: 10.1073/pnas.1509565112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kishore BK, Nelson RD, Miller RL, Carlson NG, Kohan DE. P2Y(2) receptors and water transport in the kidney. Purinergic Signal. 2009;5:491–499. doi: 10.1007/s11302-009-9151-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li YX, Huang Y, Liu S, Mao Y, Yuan CY, Yang X, Yao LJ. Glycogen synthase kinase-3 modulates hyperosmotic-induced urea transporter A1 relocation in the inner medullary collecting duct cells. Nephron. 2016;133:71–79. doi: 10.1159/000446158. [DOI] [PubMed] [Google Scholar]
- 36.Hyndman KA, Dugas C, Arguello AM, Goodchild TT, Buckley KM, Burch M, Yanagisawa M, Pollock JS. High salt induces autocrine actions of ET-1 on inner medullary collecting duct NO production via upregulated ETB receptor expression. Am J Physiol Regul Integr Comp Physiol. 2016;311:R263–R271. doi: 10.1152/ajpregu.00016.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pandit MM, Gao Y, van Hoek A, Kohan DE. Osmolar regulation of endothelin-1 production by the inner medullary collecting duct. Life Sci. 2016;159:135–139. doi: 10.1016/j.lfs.2015.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fuson AL, Komlosi P, Unlap TM, Bell PD, Peti-Peterdi J. Immunolocalization of a microsomal prostaglandin E synthase in rabbit kidney. Am J Physiol Renal Physiol. 2003;285:F558–F564. doi: 10.1152/ajprenal.00433.2002. [DOI] [PubMed] [Google Scholar]
- 39.Gueutin V, Vallet M, Jayat M, Peti-Peterdi J, Cornière N, Leviel F, Sohet F, Wagner CA, Eladari D, Chambrey R. Renal β-intercalated cells maintain body fluid and electrolyte balance. J Clin Invest. 2013;123:4219–4231. doi: 10.1172/JCI63492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huo TL, Grenader A, Blandina P, Healy DP. Prostaglandin E2 production in rat IMCD cells. II. Possible role for locally formed dopamine. Am J Physiol. 1991;261:F655–F662. doi: 10.1152/ajprenal.1991.261.4.F655. [DOI] [PubMed] [Google Scholar]
- 41.Chen M, Cai H, Klein JD, Laur O, Chen G. Dexamethasone increases aquaporin-2 protein expression in ex vivo inner medullary collecting duct suspensions. Front Physiol. 2015;6:310. doi: 10.3389/fphys.2015.00310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ranieri M, Tamma G, Di Mise A, Russo A, Centrone M, Svelto M, Calamita G, Valenti G. Negative feedback from CaSR signaling to aquaporin-2 sensitizes vasopressin to extracellular Ca2. J Cell Sci. 2015;128:2350–2360. doi: 10.1242/jcs.168096. [DOI] [PubMed] [Google Scholar]
- 43.Zheng P, Lin Y, Wang F, Luo R, Zhang T, Hu S, Feng P, Liang X, Li C, Wang W. 4-PBA improves lithium-induced nephrogenic diabetes insipidus by attenuating ER stress. Am J Physiol Renal Physiol. 2016;311:F763–F776. doi: 10.1152/ajprenal.00225.2016. [DOI] [PubMed] [Google Scholar]
- 44.Hyndman KA, Boesen EI, Elmarakby AA, Brands MW, Huang P, Kohan DE, Pollock DM, Pollock JS. Renal collecting duct NOS1 maintains fluid-electrolyte homeostasis and blood pressure. Hypertension. 2013;62:91–98. doi: 10.1161/HYPERTENSIONAHA.113.01291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Anders HJ, Muruve DA. The inflammasomes in kidney disease. J Am Soc Nephrol. 2011;22:1007–1018. doi: 10.1681/ASN.2010080798. [DOI] [PubMed] [Google Scholar]
- 46.Hijiya N, Miyake K, Akashi S, Matsuura K, Higuchi Y, Yamamoto S. Possible involvement of toll-like receptor 4 in endothelial cell activation of larger vessels in response to lipopolysaccharide. Pathobiology. 2002;70:18–25. doi: 10.1159/000066000. [DOI] [PubMed] [Google Scholar]
- 47.Tsukumo DM, Carvalho-Filho MA, Carvalheira JB, Prada PO, Hirabara SM, Schenka AA, Araújo EP, Vassalo J, Curi R, Velloso LA, Saad MJ. Loss-of-function mutation in toll-like receptor 4 prevents diet-induced obesity and insulin resistance. Diabetes. 2007;56:1986–1998. doi: 10.2337/db06-1595. [DOI] [PubMed] [Google Scholar]
- 48.Nicola JP, Vélez ML, Lucero AM, Fozzatti L, Pellizas CG, Masini-Repiso AM. Functional toll-like receptor 4 conferring lipopolysaccharide responsiveness is expressed in thyroid cells. Endocrinology. 2009;150:500–5008. doi: 10.1210/en.2008-0345. [DOI] [PubMed] [Google Scholar]
- 49.Hirata T, Osuga Y, Hirota Y, Koga K, Yoshino O, Harada M, Morimoto C, Yano T, Nishii O, Tsutsumi O, Taketani Y. Evidence for the presence of toll-like receptor 4 system in the human endometrium. J Clin Endocrinol Metab. 2005;90:548–556. doi: 10.1210/jc.2004-0241. [DOI] [PubMed] [Google Scholar]
- 50.Wolf G, Bohlender J, Bondeva T, Roger T, Thaiss F, Wenzel UO. Angiotensin II upregulates toll-like receptor 4 on mesangial cells. J Am Soc Nephrol. 2006;17:1585–1593. doi: 10.1681/ASN.2005070699. [DOI] [PubMed] [Google Scholar]
- 51.Vitseva OI, Tanriverdi K, Tchkonia TT, Kirkland JL, McDonnell ME, Apovian CM, Freedman J, Gokce N. Inducible toll-like receptor and NF-kappaB regulatory pathway expression in human adipose tissue. Obesity (Silver Spring) 2008;16:932–937. doi: 10.1038/oby.2008.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hayden MS, Ghosh S. NF-κB, the first quarter-century: Remarkable progress and outstanding questions. Genes Dev. 2012;26:203–234. doi: 10.1101/gad.183434.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vallabhapurapu S, Karin M. Regulation and function of NF-kappaB transcription factors in the immune system. Annu Rev Immunol. 2009;27:693–733. doi: 10.1146/annurev.immunol.021908.132641. [DOI] [PubMed] [Google Scholar]
- 54.Karin M, Greten FR. NF-kappaB: Linking inflammation and immunity to cancer development and progression. Nat Rev Immunol. 2005;5:749–759. doi: 10.1038/nri1703. [DOI] [PubMed] [Google Scholar]
- 55.Li Q, Verma IM. NF-kappaB regulation in the immune system. Nat Rev Immunol. 2002;2:725–734. doi: 10.1038/nri968. [DOI] [PubMed] [Google Scholar]
- 56.Ghosh S, May MJ, Kopp EB. NF-kappa B and Rel proteins: Evolutionarily conserved mediators of immune responses. Annu Rev Immunol. 1998;16:225–260. doi: 10.1146/annurev.immunol.16.1.225. [DOI] [PubMed] [Google Scholar]
- 57.Caamaño J, Hunter CA. NF-kappaB family of transcription factors: Central regulators of innate and adaptive immune functions. Clin Microbiol Rev. 2002;15:414–429. doi: 10.1128/CMR.15.3.414-429.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.May MJ, Ghosh S. Signal transduction through NF-kappa B. Immunol Today. 1998;19:80–88. doi: 10.1016/S0167-5699(97)01197-3. [DOI] [PubMed] [Google Scholar]
- 59.Oka S, Kamata H, Kamata K, Yagisawa H, Hirata H. N-acetylcysteine suppresses TNF-induced NF-kappaB activation through inhibition of IkappaB kinases. FEBS Lett. 2000;472:196–202. doi: 10.1016/S0014-5793(00)01464-2. [DOI] [PubMed] [Google Scholar]
- 60.Gupta SC, Sundaram C, Reuter S, Aggarwal BB. Inhibiting NF-κB activation by small molecules as a therapeutic strategy. Biochim Biophys Acta. 2010;1799:775–787. doi: 10.1016/j.bbagrm.2010.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Strickson S, Campbell DG, Emmerich CH, Knebel A, Plater L, Ritorto MS, Shpiro N, Cohen P. The anti-inflammatory drug BAY 11-7082 suppresses the MyD88-dependent signalling network by targeting the ubiquitin system. Biochem J. 2013;451:427–437. doi: 10.1042/BJ20121651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ghashghaeinia M, Toulany M, Saki M, Bobbala D, Fehrenbacher B, Rupec R, Rodemann HP, Ghoreschi K, Röcken M, Schaller M, et al. The NFĸB pathway inhibitors bay 11-7082 and parthenolide induce programmed cell death in anucleated erythrocytes. Cell Physiol Biochem. 2011;27:45–54. doi: 10.1159/000325204. [DOI] [PubMed] [Google Scholar]
- 63.Zhao C, Wang Y, Yuan X, Sun G, Shen B, Xu F, Fan G, Jin M, Li X, Liu G. Berberine inhibits lipopolysaccharide-induced expression of inflammatory cytokines by suppressing TLR4-mediated NF-ĸB and MAPK signaling pathways in rumen epithelial cells of Holstein calves. J Dairy Res. 2019;86:171–176. doi: 10.1017/S0022029919000323. [DOI] [PubMed] [Google Scholar]
- 64.Zhang H, Shan Y, Wu Y, Xu C, Yu X, Zhao J, Yan J, Shang W. Berberine suppresses LPS-induced inflammation through modulating Sirt1/NF-κB signaling pathway in RAW264.7 cells. Int Immunopharmacol. 2017;52:93–100. doi: 10.1016/j.intimp.2017.08.032. [DOI] [PubMed] [Google Scholar]
- 65.Gao MY, Chen L, Yang L, Yu X, Kou JP, Yu BY. Berberine inhibits LPS-induced TF procoagulant activity and expression through NF-κB/p65, Akt and MAPK pathway in THP-1 cells. Pharmacol Rep. 2014;66:480–484. doi: 10.1016/j.pharep.2013.12.004. [DOI] [PubMed] [Google Scholar]
- 66.Wang X, Feng S, Ding N, He Y, Li C, Li M, Ding X, Ding H, Li J, Wu J, Li Y. Anti-inflammatory effects of berberine hydrochloride in an LPS-induced murine model of mastitis. Evid Based Complement Alternat Med. 2018;2018:5164314. doi: 10.1155/2018/5164314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lee IA, Hyun YJ, Kim DH. Berberine ameliorates TNBS-induced colitis by inhibiting lipid peroxidation, enterobacterial growth and NF-κB activation. Eur J Pharmacol. 2010;648:162–170. doi: 10.1016/j.ejphar.2010.08.046. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.