Abstract
Context:
The clinical and prevention benefits of early initiation of antiretroviral therapy (ART) have led to the adoption of test and treat policy for HIV. Early diagnosis of HIV is crucial for maximal benefits from ART.
Aims:
This study aims to assess trends in CD4 cell counts at diagnosis and determinants of late presentation.
Settings and Design:
We analyzed 5-year data from a free HIV/sexually transmitted infection referral clinic immune.
Subjects and Methods:
Persons presenting for HIV testing from January 2011 to December 2015, for whom CD4 cell count results were available within 3 months of HIV diagnosis, were included in the analysis. Persons on ART were excluded from the study.
Statistical Analysis:
The predictors of CD4 cell count at presentation were assessed using univariate and multivariate linear regression.
Results:
Of 1001 persons diagnosed HIV-1 positive, 659 had received CD4 test within 3 months of diagnosis. The median CD4 count at presentation ranged from 212 to 352 cells/cmm in these 5 years and did not show any significant change with time. Nearly 40% had CD4 cell counts below 200 cells/cmm (AIDS); additionally, 23% presented below 350 cells/cmm. Older age (beta: -5.78; P = 0.001), education above matriculation (beta: -123.72; P = 0.014), having current opportunistic infections (beta: -173.58; P = 0.037), and being symptomatic (beta: -101.8; P = 0.002) were predictors of presenting at lower CD4 counts.
Conclusion:
Between 2011 and 2015, persons with HIV continued to present late in spite of changes in ART access program. Education focused on the benefits of early diagnosis and availability of free immediate treatment in the public sector, are crucial to the achievement of the India's 90-90-90 goals.
Keywords: CD4 cell count at presentation, HIV diagnosis, trends
INTRODUCTION
The sustained use of antiretroviral drugs has made HIV infection a chronic manageable condition.[1] India has been providing people living with HIV, free access to antiretroviral drugs since 2004. An estimated 2.1 million Indians were living with HIV in 2015, of whom 0.9 million were on antiretroviral therapy (ART).[2] Centers providing free ART access have increased exponentially in India, from 300 in March 2011 to 519 in March 2015 along with 1080 link ART centers. The number of ART centers in Pune too, increased from three in 2011 to seven in 2015[3] supported by numerous Integrated Counselling and Testing Centres for HIV testing.
The CD4 cell count, an immunological marker of HIV disease progression, has been the cornerstone for initiation of ART in India until very recently. It is also used by the National AIDS Control Organization as a marker for monitoring efficacy of ART.
The guidelines for starting ART for asymptomatic HIV-infected patients evolved over time from 200 cells/cmm (2004) to 250 cells/cmm in 2009 and 350 cells/cmm (2011) to finally 500 cells/cmm in June 2016. Recently in May 2017, the policy has changed to “test and treat.“[4] Here, every person confirmed HIV positive is immediately started on ART irrespective of CD4 cell count, thus delinking CD4 count status from eligibility for free ART initiation. This is an important and crucial step for achieving 90:90:90 goals by 2020, by India. These goals envisage that 90% of the HIV-infected patients should know their status, 90% of all people with diagnosed HIV infection will receive sustained ART, and 90% of these will achieve viral suppression.[5]
A low-CD4 cell count (<200 cells/cmm) is one of the ways to determine whether a person living with HIV has progressed to AIDS. Late diagnosis, i.e., when CD4 cell counts are low, is detrimental not only to the health of the infected person but the community as well.[6] For an effective control of HIV, it is important to identify infection in early stages and initiate treatment at the earliest. Various studies have shown the advantages of early initiation of ART.[7,8,9]
However, the CD4 cell count at HIV diagnosis in most parts of the world has not improved significantly over the years, whether in sub-Saharan African countries or developed countries such as the USA.[10,11] A Pune study in 2010 revealed that persons presenting with opportunistic infections (OIs) at the time of diagnosis was significantly high.[12] Ensuring that 90% of people living with human immunodeficiency virus infection PLHIV can access treatment and remain suppressed implies that we should be able to link almost all persons living with HIV to care at the earliest. As this strategy improves, it is anticipated that clinics should see persons with higher CD4 cell count at diagnosis. Moving forward, it will be important to understand the determinants of patients presenting late for HIV diagnosis to create effective strategies for early diagnosis.
We analyzed data from a free HIV/sexually transmitted infection (STI) referral clinic located in the industrial area of the Pimpri-Chinchwad, Pune, to assess trends in CD4 cell counts at presentation (diagnosis) from January 2011 to December 2015.
SUBJECTS AND METHODS
Persons presenting to this free HIV/STI referral clinic are first provided pretest counseling and after informed consent is seen by clinicians. Blood sample is collected for HIV testing, and appropriate symptomatic treatment is given if required. The demographic and clinical data are entered into electronic databases whereas the laboratory results are entered by concerned laboratories after testing into an electronic database using a unique identifying number delinked from personal identifiers. At posttest counseling, all HIV-infected patients are offered CD4 cell count testing, and they are linked to the ART center based on prevailing guidelines.
The current analysis is based on record review. All persons who were tested HIV-1 positive and had returned for their posttest and CD4 cell count testing within 3 months of HIV testing, from January 2011 to December 2015 were included in the study. Persons who were already on ART at the time of visit were excluded from the analysis.
Data were analyzed to look at the trends of CD4 counts at presentation using. IBM Corp. Released 2016. IBM SPSS Statistics for Windows, Version 24.0. Armonk, NY: IBM Corp.
The predictors of CD4 cell count at presentation/baseline were assessed using univariate and multivariate linear regression.
Ethics statement: All the patients were provided informed consent for HIV testing and data collection. The consent was reviewed annually by the Institutional Ethics Committee. No data with individual identifier is presented in this paper.
RESULTS
During the study (2011–2015), 4555 persons visited the clinic of whom 169 were on ART. In all, 3039 individuals received HIV test.
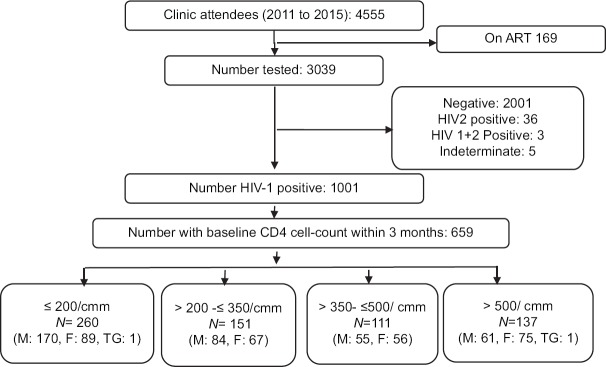
Of the 1001, who were diagnosed HIV-1 positive (n = 1001) in our clinic, 659 returned for posttest and CD4 cell count testing within 3 months of diagnosis and were analyzed further [Figure 1]. There was no significant difference between the characteristics of 659 PLHIV who underwent CD4 cell counts within 3 months of HIV test and those who did not (n = 342).
Figure 1.
Identification of eligible study participants for analyzing trends in CD4 cell counts at presentation to care. The figure shows schema for patients selection in this study. Patients who were on antiretroviral therapy or just coming for information or other reasons than HIV testing were not included in the analysis. TG = Transgender
Of these 659, 77.8% (513) had previously tested in private sector before approaching our clinic, for whom median CD 4 cell count was 230 (interquartile range [IQR]: 122, 421). The median duration between the test done in private and at our clinic was 8 days (IQR 4, 70 days). The proportion of persons presenting to our clinic for confirmation and further management more than 3 months after initial diagnosis in private sector, was 20.3% and they had a median CD4 cell count of 257 (IQR: 167.5, 456.5).
The median CD4 cell count at presentation (n = 659) overall ranged from 212 to 352 cells/cmm in these 5 years and did not show any significant change with time. Trends in CD4 cell count at presentation are shown in Figure 2. Nearly 40% (n = 260) overall had CD4 cell counts below 200 (AIDS), 23% (n = 151) between 200 and 350, and 20.8% (n = 137) had counts more than 500.
Figure 2.
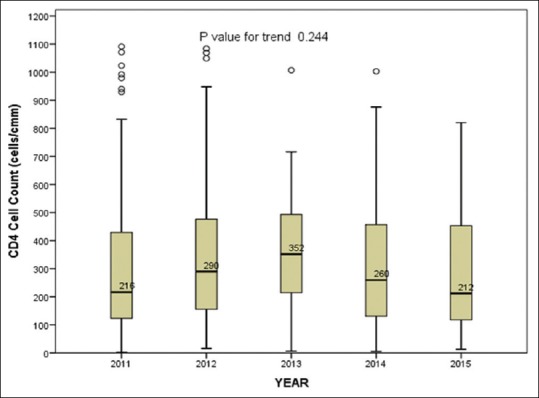
Trends in the median CD4 cell counts over time. The ends of the box are the upper and lower quartiles, and the median is marked by a horizontal line inside the box. The median CD4 count and number of HIV-1-positive cases per year are given in the table
Of the 287 (43.6%) women, 11 (3.8%) were pregnant at the time of HIV diagnosis and 86 widowed (30%). All pregnant women except one had CD4 cell count above 350 cells/cmm.
Univariate analysis revealed that those who were older, staying with families, completed matriculation, were employed, had past or current OIs and symptomatic were likely to have low CD4 cell count at presentation. Whereas females and unmarried persons were more likely to present at higher CD4 counts [Table 1].
Table 1.
Predictors of CD4 cell-count at presentation for HIV1-positive patients attending HIV/sexually transmitted infection referral clinic
| Variables | n | Univariate analysis | Multivariate model | ||
|---|---|---|---|---|---|
| β | P | β | P | ||
| Age | −7.5* | <0.001 | −5.78* | 0.001 | |
| Gender | |||||
| Male | 370 | Reference | |||
| Female | 287 | 68.64 | 0.003 | −19.23 | 0.699 |
| Origin | |||||
| Urban | 319 | Reference | |||
| Semi-urban | 38 | −0.46 | 0.993 | ||
| Rural | 88 | −45.23 | 0.187 | ||
| Family type | |||||
| Staying alone | 34 | Reference | 0.279 | Reference | |
| Nuclear family | 433 | −66.71 | 0.078 | −48.68 | 0.523 |
| Joint family | 154 | −88.07 | 0.038 | −94.31 | 0.232 |
| Education | |||||
| Illiterate | 102 | Reference | Reference | ||
| Upto matriculation | 410 | −82.07 | 0.003 | −72.66 | 0.079 |
| Above matriculation | 91 | −25.21 | 0.517 | −123.72 | 0.014 |
| Marital status | |||||
| Unmarried | 75 | 93.31 | 0.012 | 80.77 | 0.175 |
| Married living with spouse | 417 | Reference | |||
| Living away from spouse/widowed/divorced/separated | 131 | −24.05 | 0.413 | −9.03 | 0.835 |
| Occupation | |||||
| Housewife | 152 | Reference | |||
| Unemployed | 22 | −109.08 | 0.100 | −118.58 | 0.338 |
| Employed | 426 | −68.21 | 0.006 | −49.08 | 0.242 |
| Past history of STDs | 2 | −92.21 | 0.678 | ||
| Past history of OIs | 60 | −120.49 | 0.007 | −67.41 | 0.097 |
| Current OIs | 11 | −246.92 | 0.010 | −173.58 | 0.037 |
| Symptoms present | 273 | −134.67 | <0.001 | −101.80 | 0.002 |
| Any habit (alcohol/smoking/tobacco) | 613 | −83.33 | <0.001 | −30.47 | 0.468 |
*Negative beta coefficients shows decrease in CD4 count as compared to reference categories.[17] STDs=Sexually transmitted diseases; OIs=Opportunistic infections
| Year | 2011 | 2012 | 2013 | 2014 | 2015 |
|---|---|---|---|---|---|
| Median CD4 count (IQR) |
216 (122.5–429.25) |
290 (155.0–477.0) |
352 (206.8–497.5) |
259.5 (129.8–458.3) |
212 (114–458.3) |
| HIV-1 positives, n |
269 | 203 | 40 | 102 | 45 |
IQR=Interquartile range
However, multivariate analysis revealed that only older age (beta: -5.78; P = 0.001), education above matriculation (beta: -123.72; P = 0.014), having current OIs (beta: -173.58; P = 0.037), and being symptomatic (beta: -101.8; P = 0.002) were predictors of presenting at lower CD4 cell counts.
Those who had concurrent OIs were likely to have CD4 cell counts that were lower by about 173.53 cells (P = 0.037) and those presenting with any symptoms by 95 cells.
DISCUSSION
Our data highlight that in the 5-year period from 2011 to 2015, persons with HIV continued to present late in their disease, with nearly 40% presenting in AIDS stage and an additional 23% presenting below the 350 cell count cutoff. Even though the ART access cut off had increased over time, there was no change in CD4 trends at presentation. Thus, a significant proportion on PLHIV had already experienced immune damage before they could receive appropriate care.
Similar data have been reported from sub-Saharan Africa[11] during 2002–2013, south Africa,[13] and the US[10] while Nigeria[14] has reported even higher rates (51.9%) of late presentation. In the US study among HOPS participants,[10] older (above 35 years of age), heterosexual persons of nonwhite races presented late, i.e., with CD4 count <200 while 50.3% of the persons diagnosed developed AIDS within 1 year. Our patients presenting throughout the 5-year period showed no real change in characteristics as compared to data prior to 2010[12] and possibly reflect that community awareness of available treatment options may not have improved over time.
The current national guidelines advocate “Test and Treat” policy; however, our analysis shows that only 20% of the PLHIV had CD4 cell counts above 500 at diagnosis. Thus, a passive implementation of test and treat policy would result in only an additional 20% gain in number of persons linked to ART center.
We also noted that a majority of persons tested in our clinic had been tested earlier in private laboratories. Although the median duration for coming in for confirmation was 8 days, 20.7% presented 3 months after the first diagnosis, of whom at least a quarter had CD4 counts <200 (median: 257 [IQR: 167.5, 456.5]) and they had neither received treatment nor CD4 testing. The median CD4 cell count too was low (230) overall, among those screened positive prior to testing at our clinic, implying that identification even in private sector is rather later in the course of the disease. Ensuring timely identification of HIV infection would require sensitization of patient and provider community for self/provider-initiated the assessment of “risk” and early testing. It is important as well, to educate on benefits of immediate linkage to care for the individual PLHIV and for public health.
Women were diagnosed earlier in our study similar to African countries,[11,13,14] but it should be noted that their proportion was lower in our study. Pregnancy alone could not have been a reason for early diagnosis since only 3.8% were pregnant compared to nearly 6% in South Africa[13] which is probably indicative of their generalized high HIV prevalent epidemic. However, significant proportion of women was widowed, and their testing is most likely to have been instigated by the early demise of their spouses since in our local heterosexually driven epidemic, males were affected earlier.
HIV related symptoms and current OIs were significantly associated with HIV diagnosis and this is the same picture as in Thailand (55%)[15] in recent years. This is a sad testament to the fact that clinical conditions and not behaviors continue to drive test seeking and highlight the need for greater education in the community. The second and the third 90 of 90:90:90 depends largely on the early diagnosis of the HIV in community and linkage to care for its prevention gains. If we have to achieve the first 90 soon and ensure early diagnosis, the focus of our intervention should be, to increase the proportion of persons tested based on self-assessment of risk rather than on clinical suspicion by a physician.
Our study also highlights that education level and younger age[6] remains a significant factor that drives early testing as reported elsewhere.[16]
CONCLUSION
This is a retrospective record review of a relatively small sample, presenting to a single referral clinic and carries with it the attendant biases. In spite of this, it is evident that the years of programming and information, education, and communication (IEC) are yet to make a significant dent in the scenario of patients presenting late in the HIV infection. This challenge needs to be overcome if we want to reach the first 90% of HIV-infected patients in India. Future IEC campaigns and TI programs need to focus on the benefits of early diagnosis and availability of immediate treatment access to achieve India's 90-90-90 goals. The community-based approach of self-testing along with targeted education could be one method to ensure that people present early in their HIV disease, and access care in time under the new “Test and Treat” policy 17.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Deeks SG, Lewin SR, Havlir DV. The end of AIDS: HIV infection as a chronic disease. Lancet. 2013;382:1525–33. doi: 10.1016/S0140-6736(13)61809-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.National AIDS Control Organization. Department of AIDS Control, Ministry of Health and Family Welfare. Annual Report 2015-16. [Last accessed on 2016 Sep 09]. Available from: http://naco.gov.in/sites/default/files/Annual%20Report%202015-16_NACO.pdf .
- 3.Maharashtra State AIDS Control Society. [Last accessed on 2016 July 05]. Available from: http://mahasacs.org/images/PDFs/website_data1.pdf .
- 4.National AIDS Control Organization. [Last accessed on 2017 Oct 04]. Available from: http://naco.gov.in/test-and-treat-strategy .
- 5.90-90-90: An Ambitious Treatment Target to Help End the AIDS Epidemic. [Last accessed on 2017 Sep 29]. Available from: http://www.unaids.org/en/resources/documents/2017/90-90-90 .
- 6.Mugavero MJ, Castellano C, Edelman D, Hicks C. Late diagnosis of HIV infection: The role of age and sex. Am J Med. 2007;120:370–3. doi: 10.1016/j.amjmed.2006.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, Kumarasamy N, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365:493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.TEMPRANO ANRS 12136 Study Group. Danel C, Moh R, Gabillard D, Badje A, Le Carrou J, et al. Atrial of early antiretrovirals and isoniazid preventive therapy in Africa. N Engl J Med. 2015;373:808–22. doi: 10.1056/NEJMoa1507198. [DOI] [PubMed] [Google Scholar]
- 9.Grinsztejn B, Hosseinipour MC, Ribaudo HJ, Swindells S, Eron J, Chen YQ, et al. Effects of early versus delayed initiation of antiretroviral treatment on clinical outcomes of HIV-1 infection: Results from the phase 3 HPTN 052 randomised controlled trial. Lancet Infect Dis. 2014;14:281–90. doi: 10.1016/S1473-3099(13)70692-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buchacz K, Armon C, Palella FJ, Baker RK, Tedaldi E, Durham MD, et al. CD4 cell counts at HIV diagnosis among HIV outpatient study participants, 2000-2009. AIDS Res Treat. 2012;2012:869841. doi: 10.1155/2012/869841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Siedner MJ, Ng CK, Bassett IV, Katz IT, Bangsberg DR, Tsai AC. Trends in CD4 count at presentation to care and treatment initiation in Sub-Saharan Africa, 2002-2013: A meta-analysis. Clin Infect Dis. 2015;60:1120–7. doi: 10.1093/cid/ciu1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Antwal M, Gurjar R, Chidrawar S, Pawar J, Gaikwad S, Panchal N, et al. Clinical profile of HIV infected patients attending a HIV referral clinic in Pune, India. Indian J Med Res. 2014;140:271–7. [PMC free article] [PubMed] [Google Scholar]
- 13.Govender S, Otwombe K, Essien T, Panchia R, de Bruyn G, Mohapi L, et al. CD4 counts and viral loads of newly diagnosed HIV-infected individuals: Implications for treatment as prevention. PLoS One. 2014;9:e90754. doi: 10.1371/journal.pone.0090754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Akinbami A, Dosunmu A, Adediran A, Ajibola S, Oshinaike O, Wright K, et al. CD4 count pattern and demographic distribution of treatment-naïve HIV patients in Lagos, Nigeria. AIDS Res Treat. 2012;2012:352753. doi: 10.1155/2012/352753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thanawuth N, Chongsuvivatwong V. Late HIV diagnosis and delay in CD4 count measurement among HIV-infected patients in Southern Thailand. AIDS Care. 2008;20:43–50. doi: 10.1080/09540120701439303. [DOI] [PubMed] [Google Scholar]
- 16.Sharma M, Ying R, Tarr G, Barnabas R. Systematic review and meta-analysis of community and facility-based HIV testing to address linkage to care gaps in Sub-Saharan Africa. Nature. 2015;528:S77–85. doi: 10.1038/nature16044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Montgomery C, Peck EA, Geoffrey Vining G. Introduction to linear regression analysis. 5th ed. Wiley & Sons; 2006. [Google Scholar]