Abstract
Considering the increased resistance to antibiotics in the clinic and the ideal antibacterial properties of KR-12, the effects of KR-12-a6, an important analogue of KR-12, on the osteogenic differentiation of human bone marrow mesenchymal stem cells (hBMSCs) were investigated. Osteogenic differentiation-associated experiments were conducted in hBMSCs, and KR-12-a6 was used as an additional stimulating factor during osteogenic induction. Quantitative analysis of alkaline phosphatase (ALP) and alizarin red staining, and reverse transcription-quantitative PCR analysis of the expression of osteogenesis-associated genes were performed to determine the effects of KR-12-a6 on the osteogenic differentiation of hBMSCs. LDN-212854 was selected to selectively suppress BMP/SMAD signaling. Western blotting was performed to investigate the underlying mechanisms. The intensity of ALP and alizarin red staining gradually increased with increasing KR-12-a6 concentrations. KR-12-a6 induced the strongest staining at 40 µg/ml, whereas 60 µg/ml and 80 µg/ml concentrations did not further increase the intensity of staining. The mRNA expression levels of RUNX2 and ALP increased in a dose-dependent manner as early as 3 days post-KR-12-a6 treatment. The mRNA expression of COL1A1, BSP and BMP2 exhibited significant upregulation from day 7 post-KR-12-a6 treatment. In contrast, the mRNA levels of OSX, OCN and OPN were enhanced dramatically at day 14 following KR-12-a6 stimulation. Additionally, KR-12-a6 significantly promoted the phosphorylation of Smad1/5. Furthermore, LDN-212854 suppressed the activation of Smad1/5 and inhibited the upregulation of several osteogenic differentiation-associated genes in KR-12-a6-treated hBMSCs. KR-12-a6 promoted the osteogenic differentiation of hBMSCs via BMP/SMAD signaling.
Keywords: KR-12-a6, antimicrobial peptide, osteogenic differentiation, human bone marrow mesenchymal stem cells, bone morphogenic protein/SMAD signaling
Introduction
As an important part of the immune system, antimicrobial peptides (AMPs) can physically destroy microbial membranes and induce their cleavage, indicating their potential as substitutes for traditional antibiotics (1). Following the identification of LL-37, the only natural human cathelicidin (an AMP subfamily), in 1995 (2,3), its presence has been detected in various types of cells and epithelia, including intestinal epithelial cells (4), mast cells (5), monocytes (6) and lymphocytes (7). Natural human AMPs are the first line of defense against local infection, serving an important role in the invasion of systemic pathogens into local areas or local wound infections (8,9). As the peptide chain of LL-37 is too long and difficult to synthesize, it is not a routine therapeutic drug for bacterial infections or other inflammatory diseases in the clinic; however, the cost of producing short-chain AMPs is relatively low. Furthermore, the removal of hydrophobic amino acids from the N-terminus of natural LL-37 can reduce its toxicity to eukaryotic cells, as well as reduce its interactions with human plasma proteins (10,11). Thus, short-chain AMPs have attracted the attention of researchers. Compared with natural LL-37, KR-20 and KS-30, two derivatives of LL-37, exhibit improved antimicrobial properties (12,13). KR-12 is the shortest derivative of LL-37 with antimicrobial activity (10,11). Compared with LL-37 and its other derivatives, the cost of KR-12 synthesis is lower, and its cytotoxicity is reduced; for example, LL-37, but not KR-12, induces hemolytic effectsin human erythrocytes (14). As an analogue of KR-12 (amino acid sequence: KRIVQRIKDFLR-NH2), KR-12-a6 (amino acid sequence: LRIVKLILKWLR-NH2) has similar antibacterial properties and biocompatibility (15); also, as a highest hydrophobic analogue of KR-12, KR-12-a6 displays greater inhibition of lipopolysaccharide (LPS)-stimulated tumor necrosis factor-α production and higher LPS-binding activity than KR-12 (15). Therefore, KR-12-a6 may be a potential therapeutic drug for the treatment of clinical infectious diseases, including osteomyelitis.
Osteomyelitis is a common infectious disease that can cause serious symptoms locally or systemically, such as severe pain, high fever, swelling and tenderness around the infection site, posing a great challenge to clinical treatment (16). In addition to the surgical removal of infected bone tissues, local or systemic application of antibiotics is the most common treatment (17); however, with the increasing use of antibiotics to treat bone infections in the clinic, bacterial resistance to antibiotics has gradually developed (18). In addition, osteolysis caused by infection cannot be treated with traditional antibiotics. Gentamicin (19) and vancomycin (20), two commonly used antibiotics in the treatment of bone-related infections, have been shown to inhibit the viability of osteoblasts and reduce their numbers. Recently, certain small polypeptides have been reported to promote bone integration while controlling infection (21). Therefore, drugs with favorable antibacterial properties and pro-osseointegration abilities are of great importance for the treatment of clinical bone infections.
Human bone marrow mesenchymal stem cells (hBMSCs) play a key role in the process of bone regeneration (22–24). hBMSCs can differentiate into osteoblasts during bone formation, and are the main source of bone progenitor cells (25). When local infections are controlled, hBMSCs are activated and differentiate into osteoblasts to repair the local osteolysis (26). However, when hBMSCs fail to completely repair the local bone defect caused by infection, local osteoporosis and even pathological fracture can result (27). At present, drugs that inhibit osteoclast activity and bone resorption, such as vitamin D analogues, calcitonin and estrogen, are commonly used in the treatment of osteoporosis (28). However, these drugs do not promote osteogenic differentiation or exhibit antibacterial properties. Considering the increased resistance to antibiotics in the clinic and the ideal antibacterial properties of AMPs, it was proposed that AMPs may exhibit positive therapeutic effects for the treatment of osteomyelitis (29). Therefore, the present study examined whether its analogue KR-12-a6 could also promote the osteogenic differentiation of hBMSCs and investigated the underlying mechanisms.
Materials and methods
Isolation and culture of hBMSCs
Bone marrow was obtained from the iliac crest marrow aspirates of 3 healthy donors (one 34-year old male, two females at 19 and 38-year's old) undergoing iliac crest bone transplantation at Jingzhou Central Hospital, Tongji Medical College of Huazhong University of Science and Technology (Jingzhou, China) between January and July 2018. The procedure was performed following approval from the Ethics Committee of Huazhong University of Science and Technology and after obtaining donors' informed consent. The collected bone marrow was treated with heparin anticoagulant and then diluted in α-minimal essential medium (α-MEM; Gibco; Thermo Fisher Scientific, Inc.) (30). hBMSCs were then isolated by density gradient centrifugation (400 × g for 20 min at room temperature) with Ficoll-Paque (GE Healthcare) and plastic adherence, purified by discarding suspended cells through exchanging medium, and then grown in α-MEM supplemented with 20% fetal bovine serum (Atlanta Biologicals; Bio-Techne Corporation), 100 U/ml penicillin (Invitrogen; Thermo Fisher Scientific, Inc.), 100 µg/ml streptomycin (Invitrogen; Thermo Fisher Scientific, Inc.), and 2 mM L-glutamine (Invitrogen; Thermo Fisher Scientific, Inc.). Cells were then cultured at 37°C with 5% humidified CO2. The hBMSCs were passaged and maintained at low density. Adherent cells reaching 80% confluence were harvested by using 0.25% trypsin at 37°C for 3 min. Then, 1×105 cells were replated, and the remainder was used for the analysis of gene expression.
Osteoblast differentiation
hBMSCs were plated in 6-well plates in standard growth medium. At 80% confluence, the medium was replaced with osteogenic medium consisting of α-MEM + 10% fetal calf serum (Gibco; Thermo Fisher Scientific, Inc.) and 1% penicillin/streptomycin supplemented with 10 mM β-glycerophosphate (Calbiochem; Merck KGaA), 50 µg/ml L-ascorbic acid (Wako Chemicals GmbH), 10 nM dexamethasone (Sigma-Aldrich; Merck KGaA), and 10 nM calcitriol (Sigma-Aldrich; Merck KGaA). The medium was replaced every 3 days. The control cells were grown in standard growth medium. Cell pellets were harvested for RNA isolation at different time points following induction.
Alkaline phosphatase (ALP) staining and quantitative analysis
hBMSCs were cultured in 24-well plates at 2×104 cells/cm2. At 80% confluence, cells were induced to differentiate into osteoblasts using StemPro™ Osteogenesis Differentiation kit (Gibco; Thermo Fisher Scientific, Inc.) supplemented with KR-12-a6 (synthesized and purified by GL Biochem (Shanghai) Ltd.) at 0, 20, 30, 40, 60, or 80 µg/ml at 37°C for 7 days. For ALP staining, hBMSCs in each group were washed twice with PBS at the end of the 7-day KR-12-a6-treatment period, fixed with 4% paraformaldehyde for 30 s, and then stained using the ALP staining kit (Maokang Biotechnology, Shanghai) according to the manufacturer's instructions. Images were acquired by an inverted phase contrast microscope (CKX41, Olympus) under ×100 magnification. At least five fields per sample were randomly selected and observed. To quantitatively analyze ALP activity, hBMSCs after the 7-day KR-12-a6-treatment period were washed twice with PBS and lysed with Triton X-100 (1%) for 15 min. Then, the activity was measured by Alkaline Phosphatase Assay kit (Beyotime Institute of Biotechnology) according to the manufacturer's instructions. The final estimation was based on the absorbance at 405 nm measured by a spectrophotometer.
Alizarin red staining and quantitative analysis
Assessment of ex vivo mineralization was performed by employing alizarin red staining. hBMSCs at the density of 5×104 cells/well in 6-well plates underwent osteoblast differentiation in medium supplemented with KR-12-a6 at 0, 20, 30, 40, 60, or 80 µg/ml at 37°C for 21 days. Then cells were washed in PBS, fixed in 70% ethanol at −20°C for 1 h, and rinsed in dH2O. The cultures were stained with 40 mM alizarin red (Sigma-Aldrich; Merck KGaA) at pH 4.2 for 10 min at room temperature with rotation. Cells were then rinsed twice with dH2O, followed by washing three times with PBS to reduce nonspecific staining. Images were acquired by an inverted phase contrast microscope (CKX41, Olympus Corporation) under ×100 magnification. At least five fields per sample were randomly selected and observed. For further quantitative analysis, a 10% chlorinated 16 alkyl pyridine solution of sodium phosphate (pH 7.0) was added into each sample to dissolve the dye, and the absorbance was measured at 620 nm by a spectrophotometer.
RNA isolation and reverse transcription-quantitative PCR (RT-qPCR)
hBMSCs at the density of 5×104 cells/well in 12-well plates were treated with KR-12-a6 at 0, 20, 30, and 40 µg/ml and then cultured at 37°C for 3, 7, and 14 days. On day 3, 7, and 14 post-KR-12-a6 treatment, hBMSCs were harvested in Buffer RLT using an RNeasy Mini kit (Qiagen, Inc.). Total RNA was prepared according to standard protocols. Complementary DNAs (cDNAs) were synthesized from 0.5 µg total RNA using a QuantiTect Reverse Transcription kit (Qiagen, Inc.) according to the manufacturer's protocols. qPCR was conducted using a QuantiTect SYBR Green PCR kit (Qiagen, Inc.) and an ABI PRISM 7900HT SDS instrument (Applied Biosystems; Thermo Fisher Scientific, Inc.). qPCR was performed as follows: 2 min activation at 95°C, then 40 cycles of 15 sec denaturation at 95°C and 15 sec annealing at 62°C, and a final 20-sec extension at 68°C. All reactions were in a final volume of 20 µl consisting of 2X master mix (10 µl), forward and reverse primers (2 µl), cDNA, and RNase H2O, and each sample was run in triplicate. The specificity of PCR products was confirmed at the end of each run via melting curve analysis. All signals were normalized to β-actin, and the quantification cycle was determined. RT-qPCR was quantified by 2−∆∆Cq method (31). Oligonucleotide primers used for RT-qPCR are presented in Table I. β-actin was used as the housekeeping gene to normalize gene expression levels.
Table I.
Primer sequences for reverse transcription-quantitative PCR.
| Gene | Forward primer | Reverse primer |
|---|---|---|
| RUNX2 | 5′-GCCACCACTCACTACCACACCTA-3′ | 5′-TCCTGACGAAGTGCCATAGTAGAGATAT-3′ |
| ALP | 5′-GGACCATTCCCACGTCTTCAC-3′ | 5′-CCTTGTAGCCAGGCCCATTG-3′ |
| COL1A1 | 5′-TGGGAGGAAGCAAAAGACTC-3′ | 5′-GGGTCATTTCCACATGCTTT-3′ |
| BSP | 5′-TGCCTTGAGCCTGCTTCC-3′ | 5′-GCAAAATTAAAGCAGTCTTCATTTTG-3′ |
| BMP2 | 5′-AACACTGTGCGCAGCTTCC-3′ | 5′-CTCCGGGTTGTTTTCCCAC-3′ |
| OSX | 5′-CCCCACCTCTTGCAACCA-3′ | 5′-CCCCACCTATTGCAACCA-3′ |
| OPN | 5′-GCCGACCAAGGAAAACTCACT-3′ | 5′-GGCACAGGTGATGCCTAGGA-3′ |
| OCN | 5′-CCCAGGCGCTACCTGTATCAA-3′ | 5′-GGTCAGCCAACTCGTCACAGTC-3′ |
| β-actin | 5′-TGGCACCCAGCACAATGAA-3′ | 5′-CTAAGTCATAGTCCGCCTAGAAGCA-3′ |
ALP, alkaline phosphatase; BMP, bone morphogenic protein; BSP, bone sialoprotein; COL1A1, type 1 collagen α1 chain; OCN, osteocalcin; OPN, osteopontin; OSX, osterix; RUNX2, runt-related transcription factor 2.
Western blot analysis
hBMSCs at the density of 5×104 cells/well in 12-well plates were treated with KR-12-a6 at 0, 20, 30, and 40 µg/ml and then cultured at 37°C for 7 days. On day 7 post-KR-12-a6 treatment, hBMSCs were harvested and lysed in immunoprecipitation lysis buffer (10 mM Tris, 0.15 M NaCl, 1% NP-40, and 10% glycerol, pH 7.4, at 22°C) containing protease and phosphatase inhibitor cocktails (Sigma-Aldrich; Merck KGaA). Cell lysates were then centrifuged at 15,000 × g for 45 min at 4°C. The supernatants were collected, and the protein concentration was determined by BCA assay with a Bio-Rad Model 680 Plate Reader. Then, 40 µg/sample of protein was separated via 7.5% SDS-PAGE and transferred onto nitrocellulose membranes. The membranes were blocked with 5% non-fat milk for 1 h at 4°C, and then incubated with primary antibodies targeting phosphorylated (p)-Smad1/5 (cat. no. 9516S, Cell Signaling Technology, 1:500 dilution), Smad1/5 (cat. no. sc-6201, Santa Cruz Biotechnology, 1:200 dilution), or β-actin (cat. no. sc-1616, Santa Cruz Biotechnology, 1:200 dilution) overnight at 4°C. The following day, membranes were washed and incubated with bovine anti-rabbit IgG-HRP secondary antibody (cat. no. sc-2370, Santa Cruz Biotechnology, 1:1,000 dilution) or bovine anti-goat IgG-HRP secondary antibody (cat. no. sc-2350, Santa Cruz Biotechnology, 1:1,000 dilution) for 1 h at room temperature, and immunoreactive bands were detected and visualized by ECL reagent (EMD Millipore) and a FluorChem FC Imaging system (Alpha Innotech). The protein expression was then quantified by AlphaEase FC StandAlone software (version 6.0.0.14, Alpha Innotech).
LDN-212854 treatment of hBMSCs
hBMSCs at the density of 5×104 cells/well in 12-well plates were pre-treated with 10 µM LDN-212854 (Selleck Chemicals, Shanghai) or DMSO (vehicle control) at 37°C for 14 h, then washed 3 times with plain culture medium. hBMSCs continued to be cultured in medium containing 1 µM LDN-212854 alone or 1 µM LDN-212854 in combination with 40 µg/ml KR-12-a6 at 37°C for 7 days. After washing for times with PBS, hBMSCs were harvested and subjected to western blot analysis.
Statistical analysis
Each experiment was repeated at least three times, data are expressed as the mean ± standard deviation. One-way analysis of variance (ANOVA) followed by Tukey's post hoc test was used to determine statistical significance. All statistical analyses were performed using SPSS 19.0 (IBM Corp.). P<0.05 was considered to indicate a statistically significant difference.
Results
Intensity of ALP and alizarin red staining increases with elevated concentrations of KR-12-a6 in hBMSCs
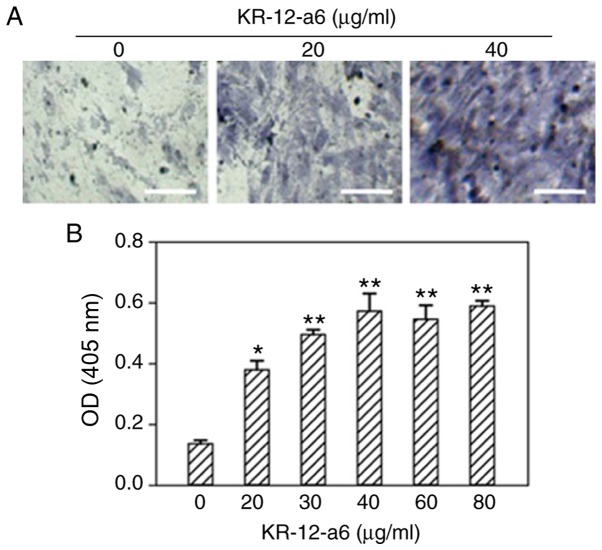
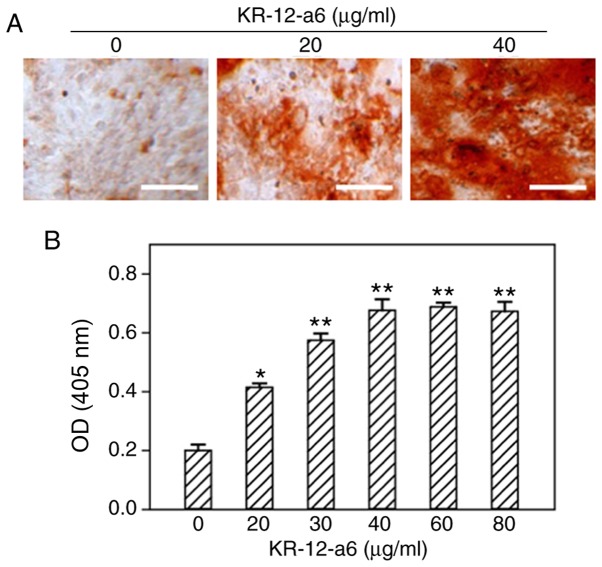
To determine the effects of KR-12-a6 on the osteogenic differentiation of hBMSCs, ALP staining and alizarin red staining were performed. With increasing concentrations of KR-12-a6, the intensity of ALP (Fig. 1) and alizarin red staining (Fig. 2) also increased. KR-12-a6 at 40 µg/ml exhibited the strongest effects on both ALP (Fig. 1) and alizarin red staining (Fig. 2), whereas KR-12-a6 at 60 and 80 µg/ml did not notably increase the staining intensity compared with KR-12-a6 at 40 µg/ml.
Figure 1.
Osteogenic ALP staining of hBMSCs and quantitative analysis following KR-12-a6 treatment. ALP staining was performed following KR-12-a6 stimulation at different concentrations (0, 20, 30, 40, 60, and 80 µg/ml) in hBMSCs. (A) Representative images following stimulation with 0, 20, and 40 µg/ml KR-12-a6. (B) Optical density of staining at 405 nm. Data are presented asthe mean ± SD (n=4). *P<0.05, **P<0.01 vs. KR-12-a6 at 0 µg/ml. Scale bar=100 µm. ALP, alkaline phosphate; OD, optical density; hBMSC, human bone marrow mesenchymal stem cell.
Figure 2.
Osteogenic alizarin red staining of hBMSCs and quantitative analysis following KR-12-a6 treatment. Alizarin red staining was performed following KR-12-a6 stimulation at different concentrations (0, 20, 30, 40, 60, and 80 µg/ml) in hBMSCs. (A) Representative images following stimulation with 0, 20, and 40 µg/ml KR-12-a6. (B) Optical density of staining at 620 nm. Data are presented as the mean ± SD (n=4). *P<0.05, **P<0.01 vs. KR-12-a6 at 0 µg/ml. Scale bar=100 µm. OD, optical density; hBMSC, human bone marrow mesenchymal stem cell.
mRNA expression of osteoblastic differentiation-associated genes increases after KR-12-a6 stimulation at different time pointsin hBMSCs
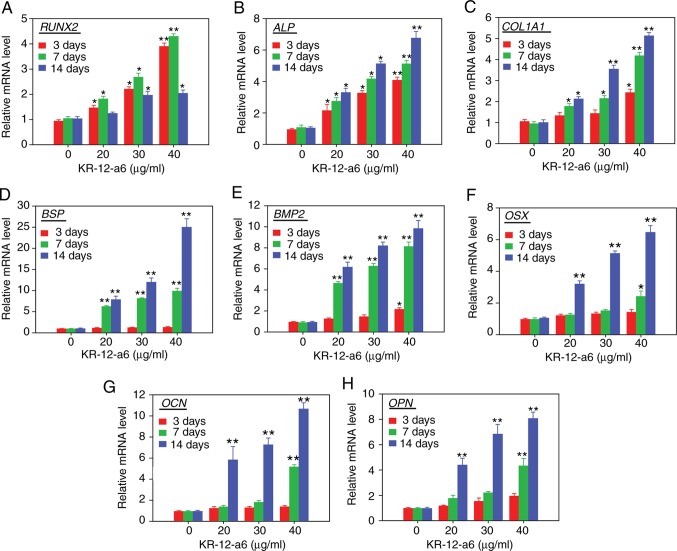
The mRNA expression levels of osteoblastic differentiation-associated genes, including RUNX2 (encoding runt-related transcription factor 2; Fig. 3A), ALP (Fig. 3B), COL1A1 (encoding type 1 collagen alpha 1 chain; Fig. 3C), BSP (encoding bone sialoprotein; Fig. 3D), BMP2 (encoding bone morphogenic protein 2; Fig. 3E), OSX (encoding osterix; Fig. 3F), OCN (encoding osteocalcin; Fig. 3G) and OPN (encoding osteopontin; Fig. 3H), were determined via RT-qPCR analysis following treatment of hBMSCs with KR-12-a6 for 3, 7 or 14 days. The mRNA levels of RUNX2 and ALP increased in a dose-dependent manner as early as 3 days post-KR-12-a6 treatment. The mRNA expression of COL1A1, BSP and BMP2 was significantly upregulated from day 7 post-KR-12-a6 treatment compared with the control. In contrast, the mRNA levels of OSX, OCN and OPN were only significantly upregulated at day 14 following KR-12-a6 stimulation.
Figure 3.
Effects of KR-12-a6 on the mRNA expression of osteogenic differentiation markers. Human bone marrow mesenchymal stem cells were treated with KR-12-a6 at concentrations of 0, 20, 30 or 40 µg/ml, and the mRNA levels of (A) RUNX2, (B) ALP, (C) COL1A1, (D) BSP, (E) BMP2, (F) OSX, (G) OCN and (H) OPN were determined via reverse transcription-quantitative PCR on days 3, 7 and 14 post-KR-12-a6 treatment. Data are presented as the mean ± SD (n=4). *P<0.05, **P<0.01 vs. KR-12-a6 at 0 µg/ml. ALP, alkaline phosphatase; BMP, bone morphogenic protein; BSP, bone sialoprotein; COL1A1, type 1 collagen α1 chain; OCN, osteocalcin; OPN, osteopontin; OSX, osterix; RUNX2, runt-related transcription factor 2.
BMP/SMAD signaling is activated during KR-12-a6-induced hBMSC osteogenic differentiation
As a significant elevation of BMP2 mRNA was observed in Fig. 3E, it was next investigated as to whether BMP/SMAD signaling was involved in KR-12-a6-induced hBMSC osteogenic differentiation. The activation of SMAD signaling was examined via western blotting following KR-12-a6-induced hBMSC osteogenesis. The results showed that KR-12-a6 promoted the phosphorylation of Smad1/5 in a dose-dependent manner following 7 days of KR-12-a6 treatment (Fig. 4A and B) and exhibited the maximum activation at 40 µg/ml. These results suggested that KR-12-a6 activated BMP/SMAD signaling in a dose-dependent manner.
Figure 4.
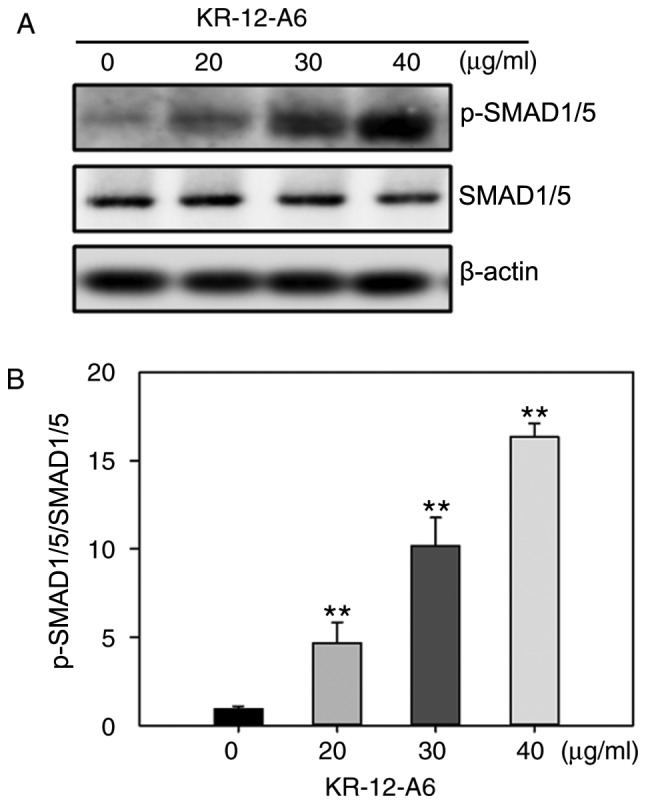
Effects of KR-12-a6 on the activation of BMP/SMAD signaling during the osteogenic differentiation of human bone marrow mesenchymal stem cells. (A) Western blotting was performed to determine the protein expression of p-Smad1/5 and Smad1/5 after 7 days of KR-12-a6 treatment at different concentrations (0, 20, 30 and 40 µg/ml). β-actin served as the loading control. (B) Quantitative analysis of Smad1/5 phosphorylation. Data are presented as the mean ± SD (n=4). **P<0.01 vs. KR-12-a6 at 0 µg/ml. p, phosphorylated.
Inhibition of BMP/SMAD signaling suppresses KR-12-a6-induced osteogenic differentiation of hBMSCs
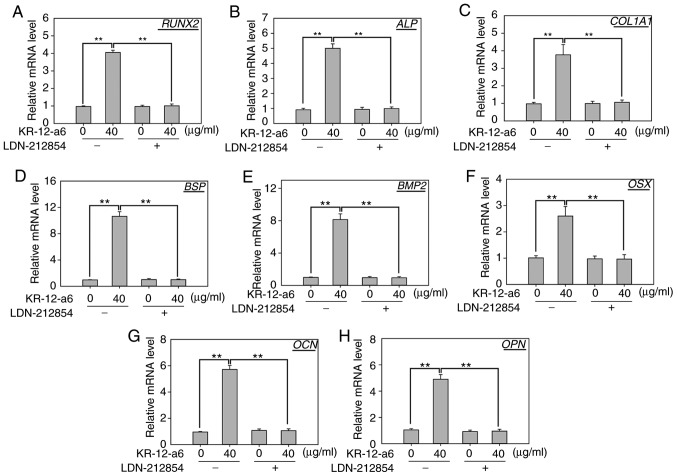
To further elucidate the role of BMP/SMAD signaling in osteoblast differentiation, LDN-212854, a novel BMP inhibitor that exhibits greater selectivity for BMP compared with the TGF-β type I receptors, was used to suppress BMP/SMAD signaling. Western blotting was performed to observe the changes of several Smad proteins after 7 days of KR-12-a6 treatment with or without LDN-212854 (Fig. 5). The results showed that KR-12-a6 at 40 µg/ml significantly promoted the phosphorylation of Smad1/5 in hBMSCs, which was consistent with the results in Fig. 4. However, the use of LDN-212854 significantly reduced Smad1/5 phosphorylation in KR-12-a6-treated hBMSCs (Fig. 5). RT-qPCR analysis was then performed to further examine the effects of LDN-212854 (Fig. 6). The results showed that LDN-212854, which inhibited BMP/SMAD signaling, significantly suppressed the mRNA expression of several osteoblastic differentiation-associated genes, including RUNX2 (Fig. 6A), ALP (Fig. 6B), COL1A1 (Fig. 6C), BSP (Fig. 6D), BMP2 (Fig. 6E), OSX (Fig. 6F), OCN (Fig. 6G), and OPN (Fig. 6H) in hBMSCs at day 7 post-KR-12-a6 treatment. Collectively, these results indicated that BMP/SMAD signaling exerts a positive role in KR-12-a6-induced hBMSC osteogenesis.
Figure 5.
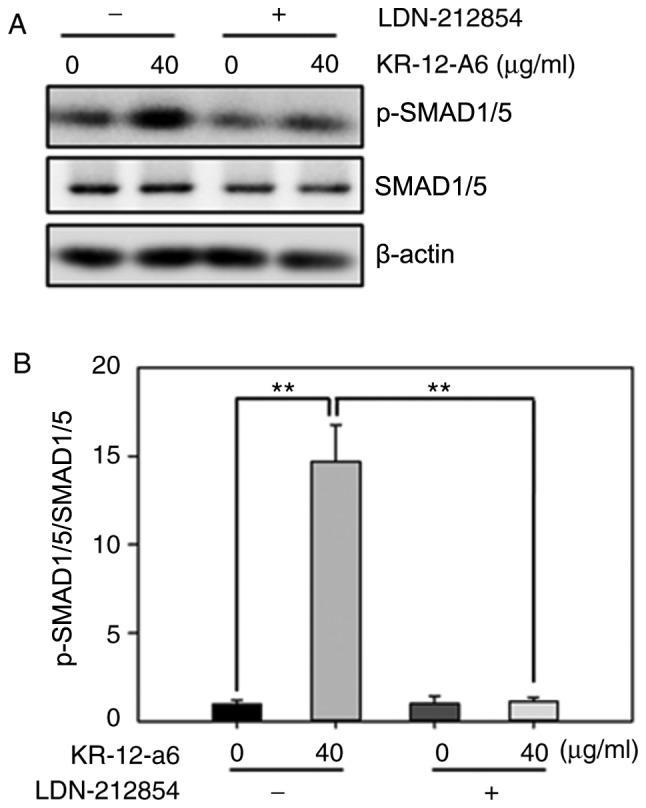
Effects of LDN-212854 on the activation of BMP/SMAD signaling in KR-12-a6-treated hBMSCs. hBMSCs were divided into four treatment groups as follows: Control, KR-12-a6, LDN-212854, and KR-12-a6+LDN-212854. (A) Western blot analysis of the protein expression of p-Smad1/5 and Smad1/5 in the different treatment groups. β-actin served as the loading control. (B) Quantitative analysis of Smad1/5 phosphorylation. Data are presented as the mean ± SD (n=4). **P<0.01 vs. KR-12-a6 at 40 µg/ml without LDN-212854. hBMSC, human bone marrow mesenchymal stem cell; p, phosphorylated.
Figure 6.
Effects of LDN-212854 on the mRNA expression of osteogenic differentiation-associated genes in KR-12-a6-treated hBMSCs. Reverse transcription-quantitative PCR was performed to examine them RNA expression of (A) RUNX2, (B) ALP, (C) COL1A1, (D) BSP, (E) BMP2, (F) OSX, (G) OCN and (H) OPN in hBMSCs on day 7 following the various treatments: Control, KR-12-a6, LDN-212854, and KR-12-a6+LDN-212854. Data are presented as the mean ± SD (n=4). **P<0.01 vs. KR-12-a6 at 40 µg/ml without LDN-212854. ALP, alkaline phosphatase; BMP, bone morphogenic protein; BSP, bone sialoprotein; COL1A1, type 1 collagen alpha 1 chain; hBMSC, human bone marrow mesenchymal stem cell; OCN, osteocalcin; OPN, osteopontin; OSX, osterix; RUNX2, runt-related transcription factor 2.
Discussion
Bone infection and osteolysis are common clinical symptoms of osteomyelitis, which requires local or systemic treatment with antibiotics (32). With the rise of drug-resistant bacteria in clinical bone infection, traditional antibiotics have become less effective (18–20). Due to their low drug resistance and excellent antimicrobial properties, AMPs have received increasing attention. AMPs are an important part of the innate immune system, and are regarded as potential substitutes for traditional antibiotics (33). Antimicrobial peptide LL-37 is an important antimicrobial substance that is naturally synthesized in the human body (7,13). It is the first line of defense against local infection and systemic pathogen invasion; more importantly, it does not lead to bacterial resistance (9). However, because of its long amino acid sequence, LL-37 is difficult to develop as a clinical drug for infectious diseases (10,11). As the shortest active fragment of LL-37, KR-12 has the advantages of low cost for synthesis and low cytotoxicity; it is thus predicted to serve an important role in the treatment of infections caused by drug-resistant bacteria (10,11). As an analogue of KR-12, KR-12-a6 has the same antibacterial properties, and is also a potential drug for the clinical treatment of osteomyelitis (15).
Antibiotics that are commonly used to control infection do not promote the formation of new bones; rather, they inhibit the local osteogenic microenvironment and show no effect on the clinical treatment of infection-associated osteolysis (19,20). Studies have shown that antibiotics, including gentamicin and vancomycin, inhibit osteoblast proliferation and differentiation (19,20). In contrast, certain small peptides can control infection while promoting new bone formation (21). Various small AMPs and their analogues may have the ability to promote bone differentiation while also possessing antibacterial properties. Therefore, treating osteomyelitis with AMPs may promote bone repair following infection-induced osteolysis while also controlling the infection. Due to the ability to differentiate into osteoblasts, hBMSCs have been widely used in the study of osteogenic differentiation (24). To verify whether KR-12-a6, a KR-12 analogue, can also affect the osteogenic differentiation of hBMSCs, primary hBMSCs were selected as experimental subjects in this study.
The results showed that KR-12-a6 enhanced the osteogenic differentiation and mineralization of hBMSCs in a dose-dependent manner. KR-12-a6 at 40 µg/ml significantly promoted osteogenic differentiation, indicating that KR-12-a6can effectively promote osteogenic differentiation at high concentrations. To accurately evaluate the expression levels of osteoblast-associated genes after KR-12-a6 stimulation, several osteoblast-associated genes were detected at different stages of osteogenic differentiation. During the first 7 days of osteogenesis, the mRNA levels of RUNX2, ALP, COL1A1, BSP and BMP2 increased significantly. In contrast, the late-stage markers of osteogenesis, including OSX, OCN and OPN, were upregulated during the second week of osteogenic differentiation. RT-qPCR analysis at different stages revealed that KR-12-a6 enhanced the expression of osteoblast-associated genes in hBMSCs; these effects were more notable at high concentrations of KR-12-a6. These findings suggested that KR-12-a6 may promote the osteogenic differentiation of hBMSCs in vitro. Further mechanistic studies revealed that KR-12-a6 significantly promoted the phosphorylation of Smad1/5. When LDN-212854, a selective BMP inhibitor, was used to block BMP/SMAD signaling, the activation of p-Smad1/5 was suppressed. Furthermore, the mRNA expression of several osteogenic differentiation-associated genes was also inhibited in KR-12-a6-treated hBMSCs, suggesting the involvement of BMP/SMAD signaling in the KR-12-a6-induced osteogenic differentiation of hBMSCs. However, the current study showed certain limitations. First, results obtained with the use of primary hBMSCs could not fully represent the biophysiological events that happen in vivo and the translational relevance of the study should be strictly confirmed in animal studies in vivo. Second, the activation of BMP/SMAD signaling might not be the only mechanism through which KR-12-a6 enhanced osteogenesis, other potential signaling pathways involved in osteogenesis should also be investigated.
In conclusion, KR-12-a6 promoted the osteogenic differentiation of hBMSCs in a dose-dependent manner, and BMP/SMAD signaling was involved in the process. In addition, considering the potential for the use of AMPs in the treatment of osteomyelitis and other infections, discovering more peptides with antibacterial properties and bone formation regulation is of clear importance for clinical treatment. KR-12-a6 may be an effective drug for the prevention and treatment of local osteomyelitis and infectious osteolysis.
Acknowledgements
The authors would like to thank Dr Yan C. Cheng (senior scientist, Center for Biomedical Research, the Rockefeller University, United States) for his valuable opinions in the discussion of the present study.
Funding
This work was supported by the Shaanxi Province Key Research & Development Projects (grant no. 2017kw-043) and the Talent Support Program of Air Force Military Medical University ‘Project Ling Yun’ (grant no. cyjhsll).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
LS and LF designed the research and wrote the manuscript; LF and PJ performed experiments and analyzed data; YH and HL collected and analyzed data; LS and LF revised the manuscript and approved the final submission. All authors discussed the results and reviewed the manuscript.
Ethics approval and consent to participate
The present study was approved by the Ethics Committee of Huazhong University of Science and Technology. Informed consents were obtained from all donors.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Bechinger B, Gorr SU. Antimicrobial peptides: Mechanisms of action and resistance. J Dent Res. 2017;96:254–260. doi: 10.1177/0022034516679973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Larrick JW, Hirata M, Balint RF, Lee J, Zhong J, Wright SC. Human CAP18: A novel antimicrobial lipopolysaccharide-binding protein. Infect Immun. 1995;63:1291–1297. doi: 10.1128/iai.63.4.1291-1297.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cowland JB, Johnsen AH, Borregaard N. hCAP-18, a cathelin/pro-bactenecin-like protein of human neutrophil specific granules. FEBS Lett. 1995;368:173–176. doi: 10.1016/0014-5793(95)00634-L. [DOI] [PubMed] [Google Scholar]
- 4.Chakraborty K, Ghosh S, Koley H, Mukhopadhyay AK, Ramamurthy T, Saha DR, Mukhopadhyay D, Roychowdhury S, Hamabata T, Takeda Y, Das S. Bacterial exotoxins downregulate cathelicidin (hCAP-18/LL-37) and human beta-defensin 1 (HBD-1) expression in the intestinal epithelial cells. Cell Microbiol. 2008;10:2520–2537. doi: 10.1111/j.1462-5822.2008.01227.x. [DOI] [PubMed] [Google Scholar]
- 5.Agier J, Brzezińska-Błaszczyk E, Żelechowska P, Wiktorska M, Pietrzak J, Różalska S. Cathelicidin LL-37 affects surface and intracellular toll-like receptor expression in tissue mast cells. J Immunol Res. 2018;2018:7357162. doi: 10.1155/2018/7357162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chamilos G, Gregorio J, Meller S, Lande R, Kontoyiannis DP, Modlin RL, Gilliet M. Cytosolic sensing of extracellular self-DNA transported into monocytes by the antimicrobial peptide LL37. Blood. 2012;120:3699–2707. doi: 10.1182/blood-2012-01-401364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Agerberth B, Charo J, Werr J, Olsson B, Idali F, Lindbom L, Kiessling R, Jörnvall H, Wigzell H, Gudmundsson GH. The human antimicrobial and chemotactic peptides LL-37 and alpha-defensins are expressed by specific lymphocyte and monocyte populations. Blood. 2000;96:3086–3093. doi: 10.1182/blood.V96.9.3086. [DOI] [PubMed] [Google Scholar]
- 8.Dorschner RA, Pestonjamasp VK, Tamakuwala S, Ohtake T, Rudisill J, Nizet V, Agerberth B, Gudmundsson GH, Gallo RL. Cutaneous injury induces the release of cathelicidin anti-microbial peptides active against group A Streptococcus. J Invest Dermatol. 2001;117:91–97. doi: 10.1046/j.1523-1747.2001.01340.x. [DOI] [PubMed] [Google Scholar]
- 9.Duplantier AJ, van Hoek ML. The human cathelicidin antimicrobial peptide LL-37 as a potential treatment for polymicrobial infected wounds. Front Immunol. 2013;4:143. doi: 10.3389/fimmu.2013.00143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang G. Structures of human host defense cathelicidin LL-37 and its smallest antimicrobial peptide KR-12 in lipid micelles. J Biol Chem. 2008;283:32637–32643. doi: 10.1074/jbc.M805533200. [DOI] [PubMed] [Google Scholar]
- 11.Mishra B, Epand RF, Epand RM, Wang G. Structural location determines functional roles of the basic amino acids of KR-12, the smallest antimicrobial peptide from human cathelicidin LL-37. RSC Adv. 2013 Nov 14; doi: 10.1039/c3ra42599a. (Epub ahead of print). doi: 10.1039/C3RA42599A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rico-Mata R, De Leon-Rodriguez LM, Avila EE. Effect of antimicrobial peptides derived from human cathelicidin LL-37 on Entamoeba histolytica trophozoites. Exp Parasitol. 2013;133:300–306. doi: 10.1016/j.exppara.2012.12.009. [DOI] [PubMed] [Google Scholar]
- 13.Feng X, Sambanthamoorthy K, Palys T, Paranavitana C. The human antimicrobial peptide LL-37 and its fragments possess both antimicrobial and antibiofilm activities against multidrug-resistant Acinetobacter baumannii. Peptides. 2013;49:131–137. doi: 10.1016/j.peptides.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 14.Luo Y, McLean DT, Linden GJ, McAuley DF, McMullan R, Lundy FT. The naturally occurring host defense peptide, LL-37, and its truncated mimetics KE-18 and KR-12 have selected biocidal and antibiofilm activities against Candida albicans, Staphylococcus aureus, and Escherichia coli in vitro. Front Microbiol. 2017;8:544. doi: 10.3389/fmicb.2017.00544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jacob B, Park IS, Bang JK, Shin SY. Short KR-12 analogs designed from human cathelicidin LL-37 possessing both antimicrobial and antiendotoxic activities without mammalian cell toxicity. J Pept Sci. 2013;19:700–707. doi: 10.1002/psc.2552. [DOI] [PubMed] [Google Scholar]
- 16.Geurts J, Hohnen A, Vranken T, Moh P. Treatment strategies for chronic osteomyelitis in low- and middle-income countries: Systematic review. Trop Med Int Health. 2017;22:1054–1062. doi: 10.1111/tmi.12921. [DOI] [PubMed] [Google Scholar]
- 17.Mortazavi MM, Khan MA, Quadri SA, Suriya SS, Fahimdanesh KM, Fard SA, Hassanzadeh T, Taqi MA, Grossman H, Tubbs RS. Cranial osteomyelitis: A comprehensive review of modern therapies. World Neurosurg. 2018;111:142–153. doi: 10.1016/j.wneu.2017.12.066. [DOI] [PubMed] [Google Scholar]
- 18.Fily F, Ronat JB, Malou N, Kanapathipillai R, Seguin C, Hussein N, Fakhri RM, Langendorf C. Post-traumatic osteomyelitis in Middle East war-wounded civilians: Resistance to first-line antibiotics in selected bacteria over the decade 2006–2016. BMC Infect Dis. 2019;19:103. doi: 10.1186/s12879-019-3741-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ince A, Schütze N, Karl N, Löhr JF, Eulert J. Gentamicin negatively influenced osteogenic function in vitro. Int Orthop. 2007;31:223–228. doi: 10.1007/s00264-006-0144-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mantripragada VP, Jayasuriya AC. Effect of dual delivery of antibiotics (vancomycin and cefazolin) and BMP-7 from chitosan microparticles on Staphylococcus epidermidis and pre-osteoblasts in vitro. Mater Sci Eng C Mater Biol Appl. 2016;67:409–417. doi: 10.1016/j.msec.2016.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Choe H, Narayanan AS, Gandhi DA, Weinberg A, Marcus RE, Lee Z, Bonomo RA, Greenfield EM. Immunomodulatory peptide IDR-1018 decreases implant infection and preserves osseointegration. Clin Orthop Relat Res. 2015;473:2898–2907. doi: 10.1007/s11999-015-4301-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zou W, Greenblatt MB, Brady N, Lotinun S, Zhai B, de Rivera H, Singh A, Sun J, Gygi SP, Baron R, et al. The microtubule-associated protein DCAMKL1 regulates osteoblast function via repression of Runx2. J Exp Med. 2013;210:1793–1806. doi: 10.1084/jem.20111790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mansour A, Abou-Ezzi G, Sitnicka E, Jacobsen SE, Wakkach A, Blin-Wakkach C. Osteoclasts promote the formation of hematopoietic stem cell niches in the bone marrow. J Exp Med. 2012;209:537–549. doi: 10.1084/jem.20110994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Crane JL, Cao X. Bone marrow mesenchymal stem cells and TGF-β signaling in bone remodeling. J Clin Invest. 2014;124:466–472. doi: 10.1172/JCI70050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li B. MicroRNA regulation in osteogenic and adipogenic differentiation of bone mesenchymal stem cells and its application in bone regeneration. Curr Stem Cell Res Ther. 2018;13:26–30. doi: 10.2174/1574888X12666170605112727. [DOI] [PubMed] [Google Scholar]
- 26.Bidwell JP, Alvarez MB, Hood M, Jr, Childress P. Functional impairment of bone formation in the pathogenesis of osteoporosis: The bone marrow regenerative competence. Curr Osteoporos Rep. 2013;11:117–125. doi: 10.1007/s11914-013-0139-2. [DOI] [PubMed] [Google Scholar]
- 27.Shen GS, Zhou HB, Zhang H, Chen B, Liu ZP, Yuan Y, Zhou XZ, Xu YJ. The GDF11-FTO-PPARγ axis controls the shift of osteoporotic MSC fate to adipocyte and inhibits bone formation during osteoporosis. Biochim Biophys Acta Mol Basis Dis. 2018;1864:3644–3654. doi: 10.1016/j.bbadis.2018.09.015. [DOI] [PubMed] [Google Scholar]
- 28.Pavone V, Testa G, Giardina SMC, Vescio A, Restivo DA, Sessa G. Pharmacological therapy of osteoporosis: A systematic current review of literature. Front Pharmacol. 2017;8:803. doi: 10.3389/fphar.2017.00803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Faber C, Stallmann HP, Lyaruu DM, Joosten U, von Eiff C, van NieuwAmerongen A, Wuisman PI. Comparable efficacies of the antimicrobial peptide human lactoferrin 1–11 and gentamicin in a chronic methicillin-resistant Staphylococcus aureus osteomyelitis model. Antimicrob Agents Chemother. 2005;49:2438–2444. doi: 10.1128/AAC.49.6.2438-2444.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 31.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 32.Xing K, Huang G, Hua S, Xu G, Li M. Systematic review of randomized controlled trials on antibiotic treatment for osteomyelitis in diabetes. Diabet Med. 2019;36:546–556. doi: 10.1111/dme.13935. [DOI] [PubMed] [Google Scholar]
- 33.Sierra JM, Fusté E, Rabanal F, Vinuesa T, Viñas M. An overview of antimicrobial peptides and the latest advances in their development. Expert Opin Biol Ther. 2017;17:663–676. doi: 10.1080/14712598.2017.1315402. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.