Abstract
Iridoid glycosides of Radix Scrophulariae (IGRS) are a group of the major bioactive components from Radix Scrophulariae with extensive pharmacological activities. The present study investigated the effects of IGRS on cerebral ischemia-reperfusion injury (CIRI) and explored its potential mechanisms of action. A CIRI model in rats was established by occlusion of the right middle cerebral artery for 90 min, followed by 24 h of reperfusion. Prior to surgery, 30, 60 or 120 mg/kg IGRS was administered to the rats once a day for 7 days. Then, the neurological scores, brain edema and volume of the cerebral infarction were measured. The apoptosis index was determined by terminal deoxynucleotidyl transferase mediated dUTP nick end labeling. The effects of IGRS on the histopathology of the cortex in brain tissues and the endoplasmic reticulum ultrastructure in the hippocampus were analyzed. Finally, the expression of endoplasmic reticulum stress (ERS)-regulating mediators, endoplasmic reticulum chaperone BiP (GRP78), DNA damage-inducible transcript 3 protein (CHOP) and caspase-12, were detected by reverse transcription quantitative polymerase chain reaction (RT-qPCR) and western blot analysis. The volume of cerebral infarction and brain water content in the IGRS-treated groups treated at doses of 60 and 120 mg/kg were decreased significantly compared with the Model group. The neurological scores were also significantly decreased in the IGRS-treated groups. IGRS treatment effectively decreased neuronal apoptosis resulting from CIRI-induced neuron injury. In addition, the histopathological damage and the endoplasmic reticulum ultrastructure injury were partially improved in CIRI rats following IGRS treatment. RT-qPCR and western blot analysis data indicated that IGRS significantly decreased the expression levels of GRP78, CHOP and caspase-12 at both mRNA and protein levels. The results of the present study demonstrated that IGRS exerted a protective effect against CIRI in brain tissue via the inhibition of apoptosis and ERS.
Keywords: iridoid glycosides from Radix Scrophulariae, cerebral ischemia-reperfusion injury, endoplasmic reticulum stress, apoptosis
Introduction
Stroke is one of the most prevalent diseases in the world, with high mortality and morbidity rates, and ~80% of all stroke events are cerebral arterial thrombosis- or embolism-induced ischemia (1–3). The most effective and basic treatment is the restoration of the blood supply by recanalization of the occluded arteries (4). However, this thrombolytic therapy is often accompanied by additional injury, which is referred to as cerebral ischemia-reperfusion injury (CIRI). Under such injury, brain damage has been demonstrated to be aggravated, with intracellular calcium overload, energy metabolism dysfunction and apoptosis functioning as the primary processes involved (5).
The endoplasmic reticulum (ER) is an important organelle, which serves a major role in maintaining the balance of cellular Ca2+ and modifying proteins following translation (6,7). Cerebral ischemia-reperfusion has been demonstrated to cause ER stress (ERS), which leads to the false folding and accumulation of proteins in the ER, triggering the unfolded protein response (UPR). Endoplasmic reticulum chaperone BiP (GRP78) is a central regulator for ERS as it is able to control the activation of transmembrane ERS sensors, serine/threonine-protein kinase/endoribonuclease IRE1, eukaryotic translation initiation factor 2-alpha kinase 3 and cyclic AMP-dependent transcription factor ATF-6 alpha, through a binding-release mechanism. If the stress persists or becomes more severe, cell apoptosis will be triggered by the UPR via the activation of caspase-12 and DNA damage-inducible transcript 3 protein (CHOP) (8,9). According to previous studies, ERS is one of the essential signaling mechanisms in the process of neuronal injury caused by cerebral ischemia (10,11). ERS inhibition may protect against neuronal injury (12).
Radix Scrophulariae, known as ‘Xuanshen’, obtained from the dried root of Scrophularia ningpoensis Hemsl, is widely used for treating ischemic cerebrovascular and cardiovascular diseases (13,14). Pharmacological research and clinical practice have demonstrated that Radix Scrophulariae may delay the blood clotting process (15), ameliorate cerebral ischemia injury (16) and that it exhibits anti-neurotoxic activities (17). Iridoid glycosides of Radix Scrophulariae (IGRS) are a group of the major bioactive components of Radix Scrophulariae, including harpagoside and harpagide. A previous study presented evidence that acute cerebral ischemia may be prevented by harpagide, which is known as one of the bioactive components of IGRS as it exhibits anti-apoptotic effects (18).
However, the total range of pharmacological effects of IGRS remain unknown. It is unclear whether IGRS protects against CIRI, and to the best of our knowledge, the therapeutic effect of IGRS on in vivo CIRI has not been investigated yet. Therefore, the present study aimed to evaluate the effects of IGRS on CIRI and to investigate the underlying mechanisms caused by ERS.
Materials and methods
Experimental drugs
The IGRS components were provided by Chinese Medicinal Resources Laboratory of Zhejiang Chinese Medical University. A total of 53.19% of the IGRS was iridoid glycosides. Edaravone was purchased from Jiangsu Simcere Pharmaceutical Co. Ltd.
Laboratory animals
A total of 96 healthy male Sprague Dawley rats (6–8 weeks old, 200±20 g) were provided by the Experimental Animal Center of Zhejiang Chinese Medical University [lot no. SCXK (Shanghai) 2013–0016]. The animals were housed in the room under a controlled temperature (20-24°C) for 7 days prior to use, with a 12 h light/dark cycle. All experiments were performed according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals and approved by the Animal Care Committee of Zhejiang Chinese Medical University. The procedures were implemented in accordance with the National Centre for the Replacement, Refinement and Reduction of Animals in Research ARRIVE guidelines (www.nc3rs.org.uk/arrive-guidelines) (19) and the AVMA euthanasia guidelines 2013 (20). All efforts were made to minimize the number of animals used and the suffering.
Preparation of CIRI rat model
The CIRI rat model was prepared according to an intraluminal suture method, as previously described (21). Briefly, the rats were anesthetized by an intraperitoneal (I.P.) injection of 350 mg/kg 10% chloral hydrate. No signs of peritonitis were observed following the administration of the 10% chloral hydrate. Following a midline neck incision, the right common carotid artery and external carotid arteries were isolated. A 0.28-mm nylon filament (Beijing Cinontech Co., Ltd.) was inserted through the external carotid arteries into the right internal carotid artery to block the right middle cerebral artery with an insertion length of 18–20 mm (22). Reperfusion was initiated 90 min after the onset of ischemia by gently removing the filament. Sham-operated rats underwent the same surgery, with the exception that the filament was inserted and withdrawn immediately. The rats were kept at 37±0.5°C with a heating lamp during the procedure. Following recovery from the anesthesia, the rats were placed back into their cages with ad libitum access to food and water.
Experimental design
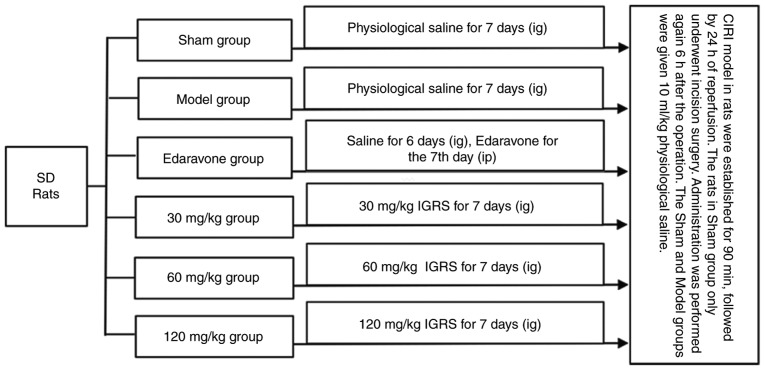
All animals were randomly divided into six groups: Sham-operation with saline treatment (Sham); CIRI with saline treatment (Model); model establishment and 3 mg/kg edaravone administration (edaravone-treated); model establishment and 30, 60 or 120 mg/kg IGRS administration. Prior to surgery, each group was given the corresponding drugs once a day for 7 days, with the exception of the edaravone-treated group, which was given physiological saline (10 ml/kg). A total of 1 h prior to the procedure, all the rats in the Sham, Model and the different IGRS groups received gastric perfusions of their respective drugs, while the rats in the edaravone-treated group were given an I.P. injection of edaravone. The administration was performed again 6 h after the operation. A total of 10 ml/kg physiological saline was administered to the Sham and Model groups (Fig. 1).
Figure 1.
Experimental design. A CIRI model in rats was established by occluding the right middle cerebral artery for 90 min and followed by 24 h of reperfusion. Prior to surgery, each group was given the corresponding drugs once a day for 7 days, with the exception of the edaravone-treated group, which was given physiological saline (10 ml/kg). A total of 1 h prior to surgery, rats in the Sham, Model and the IGRS groups had their drugs administered IG, while the rats in the edaravone group were given edaravone IP. The drugs were re-administration again 6 h after the surgical procedure. CIRI, cerebral ischemia-reperfusion injury; IGRS, iridoid glycosides from Radix Scrophulariae; IG, intragastric; IP, intraperitoneal; SD, Sprague Dawley.
The duration of the experiment was 8 days, including 7 days of IGRS pretreatment, 90 min of cerebral ischemia and 24 h of reperfusion. When cerebral ischemia reperfusion lasted for 24 h, CIRI rats exhibited hemiplegic symptoms to different degrees on one side of limbs, which affected their normal eating. The weight of CIRI rats dropped by 20% on average, accompanied by symptoms of rapid breathing. Therefore, it was determined that the rats should be euthanized after 24 h of cerebral ischemia reperfusion. All the animals used were anesthetized with 10% chloral hydrate (350 mg/kg body weight; I.P.) and decapitated rapidly after 24 h CIRI. No treatment-associated mortalities were observed. A combination of criteria were used to confirm death, including: Lack of pulse, breathing, corneal reflex and response to firm toe pinch; inability to hear respiratory sounds and heartbeat by use of a stethoscope; graying of the mucous membranes; and rigor mortis. Following confirmation of death, the brain tissues were removed immediately for subsequent study.
Neurological scores
The rats underwent a neurological severity score test as previously described (23) at 24 h of reperfusion, including a set of exercise, sensation, reflection and balance tests. Neurological function was graded on a scale of 0–18 (Table I).
Table I.
Modified neurological severity score grading system.
| Points | Degree of injury |
|---|---|
| 0 | Normal |
| 1–6 | Mild injury |
| 7–12 | Moderate injury |
| 13–18 | Serious damage |
| 18 | Most severe neurological deficits |
Measurement of ischemic infarction volume
At 24 h post-reperfusion, rats were anesthetized with 10% chloral hydrate (350 mg/kg body weight; I.P.) prior to sacrifice. Their brains were removed, divided into 6 parts of 2 mm coronal slices, and dyed with 2% 2,3,5-triphenyltetrazolium chloride (TTC) in PBS at 37°C for 15 min, which was then replaced with 4% paraformaldehyde for 10 min (2). The white areas of the brains were labeled as infarct tissue and the red areas indicated normal tissue. Images of the TTC-stained sections were captured and analyzed with Image-Pro Plus 6.0 (Media Cybernetics, Inc.). The infarct volume was calculated as a percentage as described previously to avoid inaccurate secondary measurements of edema (24,25).
Measurement of brain water content
Rats were sacrificed and the brain tissues were immediately weighed to obtain the wet weight (WW). The tissues were then dried at 60°C for 24 h and weighed again to obtain the dry weight (DW). The water content was calculated according to the following formula: (WW-DW)/WW ×100 as described previously (26,27).
Histology analysis
Brain tissues were harvested and fixed in 4% paraformaldehyde for 24 h at 4°C. An ethanol gradient was used to dehydrate the samples according to the following concentration: 50% ethanol (2 h), 60% ethanol (2 h), 75% ethanol (2 h), 85% ethanol (3 h), 95% ethanol (2 h) and 100% ethanol (2 h). Then the tissues were cleared, paraffin-embedded, sectioned and stuck to glass slides at 4°C. Following conventional de-waxing and washing, the sections were stained with hematoxylin and eosin Staining kit (Phygene Life Sciences Co., Ltd). Briefly, the sections were incubated with hematoxylin (~0.5%) for 5 min and with eosin (~0.5%) for 2 min at room temperature. Subsequently, the histological outcomes were evaluated under a light microscope at magnification, ×400. Denatured cells exhibited a shrinking nucleus, while live cells retained normal cellular morphology. The numbers of denatured cells in the cortex were obtained from observing 4 non-overlapping microscopic fields of view. The degree of injury was indicated according to the scores of denatured cell index (DCI) by comparing the number of denatured cells to the number of total cells as described previously (28).
Detection of apoptotic cell death using a terminal deoxynucleotidyl transferase mediated dUTP nick end labeling (TUNEL) assay
The degree of apoptosis in the brain cortex of paraffin-embedded coronal brain sections of animals from all the groups was analyzed by TUNEL assay using an In situ Cell Death Detection kit (Roche Diagnostics). Briefly, the tissue sections were fixed with 4% paraformaldehyde for 24 h at 4°C. Following conventional washing, the slides were incubated with Protein K (20 µg/ml) for 20 min at 37°C. Then the slides were rinsed twice with 0.1 mol/l phosphate buffered solution (PBS; pH 7.4). The TUNEL reaction mixture (50 µl; 45 µl Label Solution with 5 µl Enzyme Solution) was added to the sample. The section was incubated for 60 min at 37°C in a humidified chamber in the dark. After that, the slide was rinsed 4 times with PBS. Sections were mounted with DAPI (0.5–10 µg/ml) and placed under a fluorescence microscope (magnification, ×400). A total of 4 sections were used as specimens and ten fields were randomly selected per section, and statistically analyzed using Image-Pro Plus 6.0 software(Media Cybernetics, Inc.). The level of apoptosis was expressed as a ratio of TUNEL-positive neurons to DAPI-labeled entire neurons as described previously (29,30).
Transmission electron microscopy (TEM)
Brain tissues were harvested and the cerebral hippocampus was dissected and cut into 1×1×1 mm-sized sections and immediately placed in 2.5% glutaraldehyde in 0.1 mol/l PBS (pH 7.4) at 4°C overnight. The sections were rinsed three times with 0.1 mol/l PBS and immersed in 1% osmium tetroxide in 0.1 mol/l PBS for 2 h at 4°C. The tissue block was dehydrated in graded ethanol solutions and embedded in epoxy resin. Polymerization was performed at 70°C overnight and the samples were sectioned into a thickness of 70 nm. Following staining with 50% ethanol saturated solution of uranyl acetate for 1 h and lead citrate solution for 15 min at room temperature, the sections were observed under a TEM (H-7650 TEM; Hitachi, Ltd.) (31). The observations of cell structures in sections were made visually.
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
Rats were sacrificed at 24 h post-reperfusion. To analyze the expression levels of GRP78, CHOP and caspase-12, the hippocampus tissue was separated from the injured side of the brain. The total RNA was extracted using TRIzol® reagent (Thermo Fisher Scientific, Inc.), cDNA was produced using a PrimeScript™ RT reagent kit with gDNA Eraser (Takara Biotechnology Co., Ltd.). RT-qPCR was conducted on an Applied Biosystems 7500 and 7500 FAST Real-Time PCR detection system (Applied Biosystems; Thermo Fisher Scientific, Inc.) using SYBR Green (Beijing ComWin Biotech, Co., Ltd.) for fluorescent quantification. All reactions were repeated 3 times. Data normalization was completed using GAPDH as an endogenous control, and the normalized values were assessed using the 2−ΔΔCq formula to compute the fold difference between the control and experimental groups (32). The sequences of the primers used in this experiment are presented in Table II.
Table II.
Primer sequences used in the quantitative polymerase chain reaction assay.
| Gene | Forward | Reverse |
|---|---|---|
| GRP78 | 5′-TGTCTTCTCAGCATCAAGCAAGG-3′ | 5′-CCAACACTTCCTGGACAGGCTT-3′ |
| CHOP | 5′-GGAGGTCCTGTCCTCAGATGAA-3′ | 5′-GCTCCTCTGTCAGCCAAGCTAG-3′ |
| Caspase-12 | 5′-CAGATGAGGAACGTGTGTTGAGC-3′ | 5′-GGAACCAGTCTTGCCTACCTTC-3′ |
| GAPDH | 5′-ACAGCAACAGGGTGGTGAC-3′ | 5′-TTTGAGGGTGCAGCGAACTT-3′ |
GRP78, endoplasmic reticulum chaperone BiP; CHOP, DNA damage-inducible transcript 3 protein.
Western blot analysis
The hippocampus tissue was separated from the injured side of the brain and frozen quickly in liquid nitrogen, then transferred to a −80°C freezer for storage. The tissue were homogenized in 300 µl RIPA lysis buffer containing PMSF (cat. no. ST506; Beyotime institute of Biotechnology) and then incubated at 0°C for 30 min, followed by centrifugation at 12,000 × g at 4°C for 5 min. The supernatant was collected and denatured by boiling for 5 min. Protein concentration was quantified by the micro-bicinchoninic acid (BCA) kit (cat. no. CW0014; Beijing ComWin Biotech, Co., Ltd.). The lysates were loaded onto 10% SDS-PAGE for the separation of protein GRP78 and caspase-12, 15% SDS-PAGE for the separation of protein CHOP (30 µg of protein was loaded per lane). The separated protein bands were transferred onto polyvinylidene fluoride membranes (EMD Millipore) at 300 mA for 1.5 h. The membrane was blocked with blocking buffer containing 5% fat-free milk for 2 h at room temperature and incubated with the following primary antibodies at 4°C overnight: Rabbit anti-GRP78 (1:1,000, cat. no. ab108613), rabbit anti-CHOP (1:1,000, cat. no. ab11419), rabbit anti-caspase-12 (1:1,000, cat. no. ab62484) and mouse anti-β-actin (1:1,000, cat. no. ab8226; all from Abcam). The membranes were washed three times with Tris-buffered saline (TBS) containing 0.1% Tween-20 (TBST; pH 7.4) and then incubated in horseradish peroxidase-conjugated secondary antibody (goat anti-rabbit, 1:2,000, cat. no. C50113, LI-COR Biosciences; goat anti-mouse, 1:15,000, cat. no. C50331, LI-COR Biosciences) for 2 h at room temperature in the dark and then washed three times with TBST. The membranes were developed using the Odyssey Fluorescence Scanning Imaging System (LI-BOR Biosciences). To minimize experimental variation, each protein expression experiment was processed in parallel (33). The protein results were analyzed with Image-Pro Plus 6.0 analysis software (Media Cybernetics, Inc. USA). The ratio of the gray value of the target protein to that of the internal reference protein was taken as the relative gray value (34).
Statistical analysis
The experimental data were analyzed by SPSS v.17.0 (SPSS, Inc.) and GraphPad Prism v.5.0 software (GraphPad Software, Inc.). The results were expressed as mean ± standard deviation and analyzed by one-way analysis of variance followed by a Dunnett's post-hoc test. P<0.05 was considered to indicate a statistically significant difference.
Results
IGRS confers a protective effect against CIRI
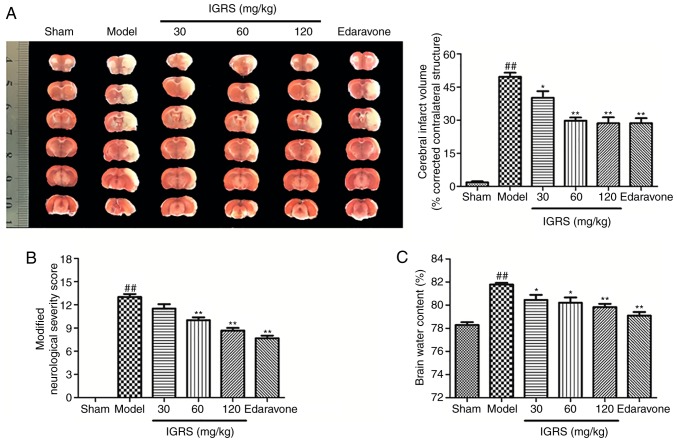
The present study first evaluated whether pretreatment with IGRS conferred a protective effect in CIRI. After 24 h of reperfusion, infarct volumes were evaluated by using TTC staining. The 30, 60 and 120 mg/kg IGRS-treated groups exhibited significantly decreased infarct volumes compared with the Model group (Fig. 2A). The protective effect of IGRS was confirmed by comparing the volumes of cerebral infarction. The cerebral infarct volumes of the 30, 60 and 120 mg/kg IGRS-treated groups were 40.2, 29.75 and 28.61%, respectively, while that the Model group was 49.69%. Neurological scores were examined at 24 h after reperfusion and scored on an 18-point scale (Fig. 2B). The neurological scores decreased from 13 points in the Model group to 8.67 in the 120 mg/kg IGRS-treated group, indicating that the degree of injury was significantly decreased.
Figure 2.
Effect of IGRS on cerebral ischemia-reperfusion injury. At 24 h post-reperfusion, rats were anesthetized by intraperitoneal injection with 10% chloral hydrate (350 mg/kg body weight) prior to sacrifice. Neurological scores were performed, and ischemic infarction volumes and brain water content were measured. (A) Representative images of TTC staining and a cerebral infarction volume histogram in coronal brain sections (n=6 per group). (B) Histogram of neurologic scores (n=6 per group). (C) Histogram of brain water content (n=6 per group). Values are presented as mean ± standard deviation of each group. ##P<0.01 vs. Sham group. *P<0.05 and **P<0.01 vs. Model group. TTC, 2,3,5-triphenyltetrazolium chloride; IGRS, iridoid glycosides from Radix Scrophulariae.
Brain water content was subsequently evaluated using the wet-dry method after reperfusion of 24 h compared with the Model group, the brain water content in the 30, 60 and 120 mg/kg IGRS-treated groups were decreased significantly (Fig. 2C). Altogether, the results demonstrated that IGRS decreased CIRI-induced neurological deficits and attenuated CIRI infarct volumes and brain edema, suggesting its therapeutic potential for ischemic brain injury.
IGRS ameliorates CIRI-induced neuronal damage
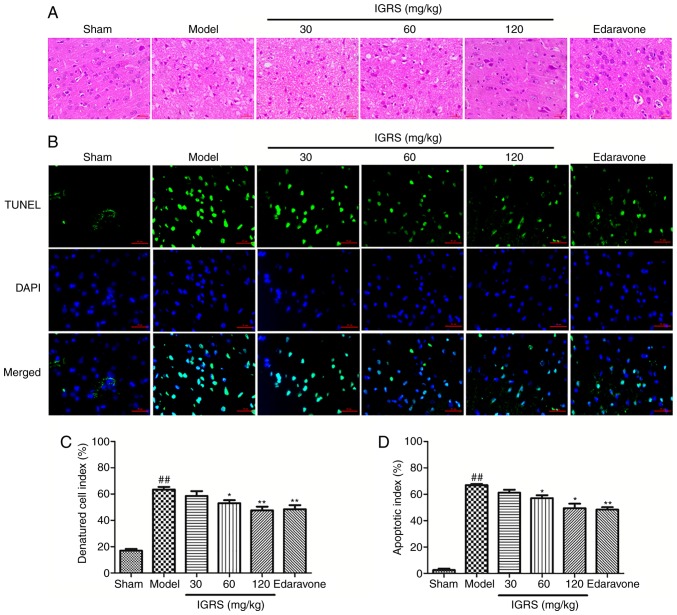
In order to investigate the neuroprotective effect of IGRS on CIRI-induced neuronal damage, the cortical tissues of the rats were stained, and the morphological changes were evaluated under a microscope (Fig. 3A and C). In the Sham group, the cerebral cortex was normal in morphology and structure; it had a large number of nerve cells with abundant cytoplasm, and large and round nuclei. Compared with the normal neurons in the Sham group, severe cellular edema, condensed nuclei, nuclear loss and a significant DCI rises were observed in the Model group (P<0.01). A protective effect of IGRS was observed in the 30, 60 and 120 mg/kg IGRS-treated groups; the tissues were less edematous and the neurons possessed clearer nuclei (Fig. 3A and C). Furthermore, IGRS treatment normalized the glial cells in CIRI rats and markedly decreased the DCI scores.
Figure 3.
Effect of IGRS on histopathology and neuronal apoptosis in CIRI rats. Following removal of the brain tissue from CIRI rats, the sections were embedded in paraffin, and HE staining and TUNEL staining were performed. The slides of brain tissue were observed under a microscope. The number of cortical nerves was analyzed and the apoptosis rate was calculated. Representative images of (A) HE-stained and (B) TUNEL-stained cerebral cortex sections from CIRI brain tissues at 24 h after reperfusion (magnification, ×400). Histograms of the (C) denatured cell index and (D) apoptosis rate in the cortex (n=4 per group) are presented. Values are presented as mean ± standard deviation of each group. ##P<0.01 vs. Sham group. *P<0.05 and **P<0.01 vs. Model group. CIRI, cerebral ischemia-reperfusion injury; HE, hematoxylin and eosin; TUNEL, terminal deoxynucleotidyl transferase mediated dUTP nick end labeling; IGRS, iridoid glycosides from Radix Scrophulariae.
TUNEL staining was used to detect nerve cell apoptosis in the cerebral cortex at 24 h after reperfusion (Fig. 3B and D). Apoptotic cells exhibited a green fluorescence signal, which was regarded as positive TUNEL staining. Fewer TUNEL-positive cells were observed in the Sham group. Conversely, the number of apoptotic cells in the Model group increased significantly within the cortical ischemic region (P<0.01). The apoptosis rate of cortical nerve cells was 2.57% in Sham group and 66.89% in Model group, while in the 30, 60 and 120 mg/kg IGRS-treated groups the apoptosis rates were 61.25, 57.16 and 49.48%, respectively. Therefore, in contrast to the Model group, the apoptosis rate of nerve cells in the IGRS-treated groups, especially at 120 mg/kg, was decreased significantly (P<0.05).
IGRS improves ER morphological changes
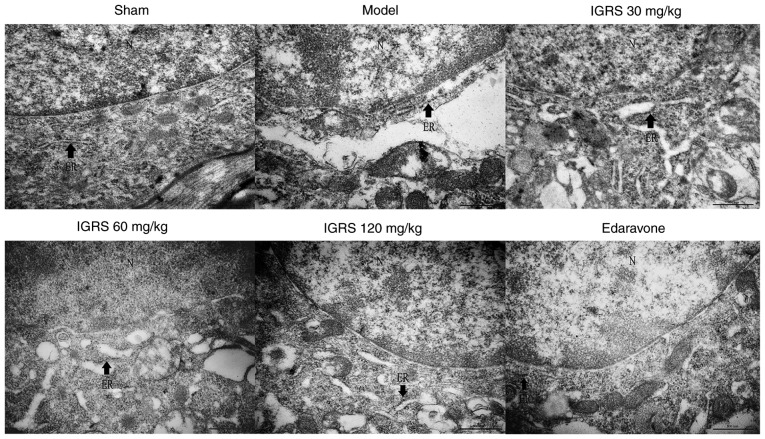
To provide further insight into the protective effect of IGRS on ER morphological changes in neurons induced by CIRI, the hippocampus tissue was examined for ultrastructural changes by TEM. The neurons in the Sham group exhibited integrated structures, there were large numbers of rough (R)ER with normal morphology and the mitochondria around the nuclei were normal. Compared with the Sham group, the neurons in the Model group were significantly swollen, the RER was dilated and swollen, and the ribosomes had disassociated from the RER. These observations are characteristic changes of ER morphology in states of ERS. In contrast to the Model group, the ER morphology in the neurons of the 30, 60 and 120 mg/kg IGRS-treated groups and the edaravone-treated group exhibited different degrees of recovery. The degree of swelling and the loss of ribosomes were notably alleviated, and the number of ribosomes in the cytoplasmic matrix was notably decreased (Fig. 4).
Figure 4.
Effect of IGRS on the ultrastructural changes of the ER in hippocampal neurons of cerebral ischemia-reperfusion injured rats. The cerebral hippocampus was dissected and fixed. Following dehydration and embedding, the samples were sliced into 70-nm thick slices. The sections were stained with a 50% ethanol saturated solution of uranyl acetate and lead citrate, and then observed under a transmission electron microscope (magnification, ×50,000). Arrows indicate ER. Scale bar, 500 nm. N, nucleus; ER, endoplasmic reticulum; IGRS, iridoid glycosides from Radix Scrophulariae.
IGRS ameliorates the expression of apoptosis factors mediated by ERS
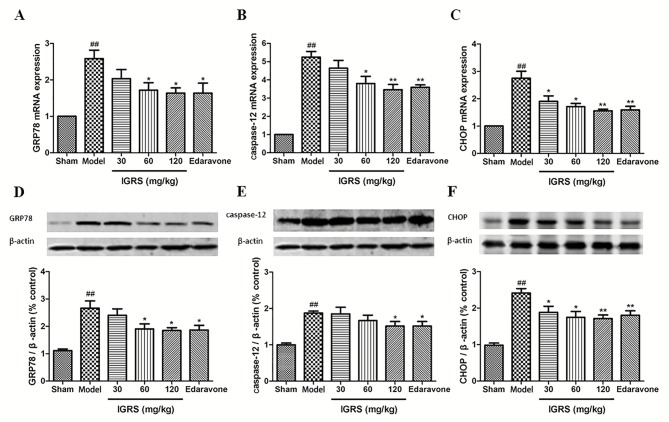
The aforementioned results indicated that IGRS protected against CIRI by suppressing ERS. To additionally confirm the regulation mechanisms of IGRS, the expression levels of ERS indicators, GRP78, CHOP and caspase-12, were determined in the Model and IGRS-treated groups using RT-qPCR and western blot analyses. The RT-qPCR data indicated that the mRNA levels of GRP78, CHOP and caspase-12 were markedly upregulated in the Model group compared with the Sham group, whereas IGRS treatment significantly downregulated the mRNA levels of GRP78, CHOP and caspase-12 (P<0.05; Fig. 5A-C). The relative expression levels of GRP78 mRNA in hippocampus of rats in the 120 mg/kg IGRS group was 1.64, which was decreased by ~36.4% compared with the Model group (P<0.05). The relative expression of CHOP mRNA in the 120 mg/kg IGRS group was 1.56, which was decreased by ~43.3% compared with the Model group (P<0.01). The relative expression of all mRNA transcripts in the 30 mg/kg IGRS group was slightly increased compared with that in the 120 mg/kg IGRS group, but much lower compared with that in the Model group. The relative expression levels of caspase-12 mRNA in the 60 and 120 mg/kg groups were similar to each other.
Figure 5.
Effect of IGRS on the expression of GRP78, caspase-12 and CHOP mRNA and protein in cerebral ischemia-reperfusion injured rats. The brain tissues of the experimental rats were removed, and the hippocampus tissues were separated from the injured side of the brain at 24 h post-reperfusion. The total RNA was extracted, cDNA was produced, and RT-qPCR was conducted. The expression of GAPDH was used as a loading control. The expression of β-actin was used as a loading control for western blot analysis. (A) GRP78, (B) caspase-12 and (C) CHOP mRNA levels were determined by RT-qPCR (n=5 per group). The expression levels of (D) GRP78, (E) caspase-12 and (F) CHOP protein were determined by western blot analysis (n=5 per group). Values are presented as mean ± standard deviation of each group. ##P<0.01 vs. Sham group. *P<0.05 and **P<0.01 vs. Model group. RT-qPCR, reverse transcription-quantitative polymerase chain reaction; IGRS, iridoid glycosides from Radix Scrophulariae; GRP78, endoplasmic reticulum chaperone BiP; CHOP, DNA damage-inducible transcript 3 protein.
The western blot analysis data demonstrated similar protein expression results for GRP78, CHOP and caspase-12, which were significantly increased in the Model group when compared with that of the Sham group. The ERS-induced protein expression levels was significantly downregulated following IGRS treatment (P<0.05) and had similar trends to the mRNA results (Fig. 5D-F).
Discussion
Acute ischemic stroke remains a leading cause of mortality and long-term disability in the word. Ischemic stroke is the result of a transient or permanent decrease of cerebral blood flow caused by the blocking of a cerebral artery; in animal models, this is achieved by simulating a local thrombus with an embolus. During the prompt recovery of blood flow to the ischemic tissue, reperfusion injury occurs and aggravates the initial injury, known as secondary neuronal damage (35,36). In cases of strokes in humans, cerebral vessel occlusion is seldom permanent; the majority of cases of human ischemic stroke result in spontaneous or thrombolytic therapy-induced reperfusion. Currently, the thread embolism method is commonly used in surgical procedures to establish a CIRI model without the need for a craniotomy. Using this method, different states of human transient and permanent focal cerebral ischemia may be simulated, and ischemia and reperfusion times may be accurately controlled (37–39). Intravenous tissue plasminogen activator is an approved medication demonstrated to improve functional outcomes when stroke occurs (40). The aim of the present study was to explore the effect of IGRS on the injury caused by cerebral ischemia-reperfusion. Various mechanisms are involved in the pathological process of CIRI; there is considerable evidence indicating that apoptosis serves an essential role in its progression (41–42). In the present study, a CIRI model was successfully established in rats, leading to brain damage, which was consistent with previous results (43–45). Previous studies had used TUNEL staining to evaluate the apoptosis of neurons in a CIRI model (46). Therefore, TUNEL staining was used to detect neuronal apoptosis in CIRI rats in the present study.
Radix Scrophulariae is widely used in Traditional Chinese Medicine for a broad range of diseases as it has a high concentration of iridoids. The iridoids possess a wide range of pharmacological properties, including anti-angiogenesis, neuroprotection and cardiovascular protection (47). The iridoids from Radix Scrophulariae have been identified as the active class of compounds with the neuroprotective effect (48). There are a number of previous studies demonstrating that early administration of extracts from natural plants may improve cerebral ischemia reperfusion injury and neurological function (49–51). Therefore, prior to surgery, the IGRS-treated groups were given the corresponding drugs once a day for 7 days. In addition, edaravone, a potent free radical scavenger, was used to verify the protective effect of IGRS in the cerebral ischemia-reperfusion injury model.
It was observed that neurological deficits were effectively improved, the cerebral infarct volume was decreased, brain edema was alleviated and neuronal cells were protected subsequent to treatment with IGRS following CIRI. The results indicated dose-dependent neuroprotective effects, suggesting the neuroprotective roles of IGRS. Consistent with the previous studies (52,53), the apoptosis rate was high in the Model group according to the results of the TUNEL assay. The decrease in the apoptosis rate in the IGRS-treated groups suggested that IGRS has the potential to inhibit neuronal apoptosis and apoptosis pathways.
ERS is the primary intracellular signal transduction pathway of cell apoptosis, serving a critical role in ischemic neuronal cell apoptosis, as described previously (9). ER chaperones including GRP78, CHOP and caspase-12 are highly sensitive to CIRI and are representative of ERS (54). Previous data has indicated that GRP78 and caspase-12 serve major roles in cerebral ischemia, and that cell apoptosis was induced by the overexpression of CHOP via the inhibition of Bcl-2; therefore, it was suggested that CIRI could be alleviated by modulating CHOP (55,56). In the present study, it was identified that the marked increase in the expression levels of GRP78, CHOP and caspase-12 was caused by CIRI, but was markedly downregulated following IGRS treatment. Therefore, IGRS may be a potential neuroprotective medicine by inhibiting the expression of GRP78, CHOP and caspase-12 induced by ERS.
Due to the severe clinical consequences of stroke, prevention and acute management are of paramount importance in clinical treatment. In addition, secondary stroke prevention is concerned with averting recurrent strokes following an initial stroke or transient ischemic attack (40). The majority of patients survive a first-time ischemic stroke event but are at high risk for recurrent stroke and concomitant cardio- and peripheral vascular diseases. Therefore, preventive measures are also an indispensable part in the treatment of patients who have suffered a stroke, especially for the prevention of recurrent stroke in individuals with a history of ischemic stroke. Therefore, in the present study, a week of pre-administration was performed prior to construction of the CIRI model, to relieve tissue damage caused by stroke. Permanent necrosis of nerve cells can occur easily following cerebral ischemia reperfusion; therefore, an effective rescue time of 6 h has been accepted to improve the penumbra in the ischemic area. Consolidating treatment on the basis of prevention can decrease injury to a great extent, which is consistent with the goal of the clinical prevention and treatment of stroke. In drug-based therapy, there are usually ≥2 types of drugs used in combination, to function synergistically. In the present study, IGRS were administered early in the establishment of the model and post-surgery. The results of the present study also provided evidence that IGRS attenuated CIRI and the protective effects were related to the inhibition of ERS, demonstrating the feasibility of this drug delivery method. In summary, the present study provided a theoretical basis for the development and application of IGRS in the prevention and treatment of stroke.
Acknowledgements
Not applicable.
Glossary
Abbreviations
- IGRS
iridoid glycosides from Radix Scrophulariae
- CIRI
cerebral ischemia-reperfusion injury
- ERS
endoplasmic reticulum stress
Funding
The present study was supported by the National Natural Science Foundation of China (grant no. 81573643).
Availability of data and materials
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.
Authors' contributions
ZH and XZ conceived, designed and carried out the experiments. YC and LZ performed the experiments and were major contributors in writing the manuscript. XG and HG assisted in parts of the experiment and participated in the data analysis. RC participated in the interpretation of data statistics and revised the manuscript. FQ participated in the establishment of cerebral ischemia-reperfusion model in rats and the acquisition of data during the experiments. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The rats were provided by the Experimental Animal Center of Zhejiang Chinese Medical University. All experiments were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals, and approved by the Animal Care Committee of Zhejiang Chinese Medical University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Sun K, Fan J, Han J. Ameliorating effects of traditional Chinese medicine preparation, Chinese materia medica and active compounds on ischemia/reperfusion-induced cerebral microcirculatory disturbances and neuron damage. Acta Pharm Sin B. 2015;5:8–24. doi: 10.1016/j.apsb.2014.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen HS, Qi SH, Shen JG. One-compound-multi-target: Combination prospect of natural compounds with thrombolytic therapy in acute ischemic stroke. Curr Neuropharmacol. 2017;15:134–156. doi: 10.2174/1570159X14666160620102055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cheng CY, Lee YC. Anti-inflammatory effects of traditional chinese medicines against ischemic injury in in vivo models of cerebral ischemia. Evid Based Complement Alternat Med. 2016;2016:5739434. doi: 10.1155/2016/5739434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen XM, Chen HS, Xu MJ, Shen JG. Targeting reactive nitrogen species: A promising therapeutic strategy for cerebral ischemia-reperfusion injury. Acta Pharmacol Sin. 2013;34:67–77. doi: 10.1038/aps.2012.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hu Y, Deng H, Xu S, Zhang J. MicroRNAs regulate mitochondrial function in cerebral ischemia-reperfusion injury. Int J Mol Sci. 2015;16:24895–24917. doi: 10.3390/ijms161024895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang HY, Wang ZG, Lu XH, Kong XX, Xiao J. Endoplasmic reticulum stress: Relevance and therapeutics in central nervous system diseases. Mol Neurobiol. 2015;51:1343–1352. doi: 10.1007/s12035-014-8813-7. [DOI] [PubMed] [Google Scholar]
- 7.Li JQ, Yu JT, Jiang T, Tan L. Endoplasmic reticulum dysfunction in Alzheimer's disease. Mol Neurobiol. 2015;51:383–395. doi: 10.1007/s12035-014-8695-8. [DOI] [PubMed] [Google Scholar]
- 8.Lee AS. The ER chaperone and signaling regulator GRP78/BIP as a monitor of endoplasmic reticulum stress. Methods. 2005;35:373–381. doi: 10.1016/j.ymeth.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 9.Gong L, Tang Y, An R, Lin M, Chen L, Du J. RTN1-C mediates cerebral ischemia/reperfusion injury via ER stress and mitochondria-associated apoptosis pathways. Cell Death Dis. 2017;8:e3080. doi: 10.1038/cddis.2017.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cao G, Zhou H, Jiang N, Han Y, Hu Y, Zhang Y, Qi J, Kou J, Yu B. Yi Qi Fu Mai powder injection ameliorates cerebral ischemia by inhibiting endoplasmic reticulum stress-mediated neuronal apoptosis. Oxid Med Cell Longev. 2016;2016:5493279. doi: 10.1155/2016/5493279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lin YW, Chen TY, Hung C, Tai SH, Huang SY, Chang CC, Hung HY, Lee EJ. Melatonin protects brain against ischemia/reperfusion injury by attenuating endoplasmic reticulum stress. Int J Mol Med. 2018;42:182–192. doi: 10.3892/ijmm.2018.3607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chaudhari N, Talwar P, Parimisetty A, Lefebvre d'Hellencourt C, Ravanan P. A molecular web: Endoplasmic reticulum stress, inflammation, and oxidative stress. Front Cell Neurosci. 2014;8:213. doi: 10.3389/fncel.2014.00213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li X, Fang D, Cong X, Cao G, Cai H, Cai B. Application of Fourier transform near-infrared spectroscopy combined with high-performance liquid chromatography in rapid and simultaneous determination of essential components in crude Radix Scrophulariae. AAPS PharmSciTech. 2012;13:1428–1435. doi: 10.1208/s12249-012-9867-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang CC, Gu WL, Wu XM, Li YM, Chen CX, Huang XY. Active components from Radix Scrophulariae inhibits the ventricular remodeling induced by hypertension in rats. Springerplus. 2016;5:358. doi: 10.1186/s40064-016-1985-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li YM, Zeng HW, He X, Jiang YY, Jiang SH, Zhu DY. Iridoid and phenylpropanoid glycosides of Scrophularia ningpoensis inhibit the formation of LTB (4) and platelet aggregation Ti Erh Chun i Ta Hsueh Pao. Acad J Sec Mil Med Univ. 1999;2:301–303. [Google Scholar]
- 16.Huang Q, Gong QY, Yao MH, Yu R, Shi NC. Protective effect of Scrophularia ningpoensis extracts on cerebral ischemia injury in rats. Chin J New Drugs Clini Remedies. 2004;23:323–327. [Google Scholar]
- 17.Kim A, Im M, Jin YM. SRVF, a novel herbal formula including scrophulariae radix and fructus viticis, disrupts focal adhesion and causes detachment-induced apoptosis in malignant cancer cells. Sci Rep. 2017;7:12756. doi: 10.1038/s41598-017-12934-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ying Xl, Zhong XM, Xu MD, Chen MJ, Xiao WX, Li GZ, Wang H, Huang Z. Neuro-protective effect of harpagide on acute cerebral ischemic injury in mice and its mechanism involving mitochondria. Chin Pharm J. 2015;50:1026–1031. [Google Scholar]
- 19.Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG. Improving bioscience research reporting: The ARRIVE guidelines for reporting animal research. PLoS Biol. 2010;8:e1000412. doi: 10.1371/journal.pbio.1000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leary S, Underwood W, Anthony R, Cartner S, Corey D, Grandin T, Greenacre C, Gwaltney-Brant S, McCrackin MA, Meyer R, et al. AVMA guidelines for the euthanasia of animals: 2013 edition. Am Vet Med Assoc. 2013 [Google Scholar]
- 21.Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989;20:84–91. doi: 10.1161/01.STR.20.1.84. [DOI] [PubMed] [Google Scholar]
- 22.Lv MR, Li B, Wang MG, Meng FG, Yu JJ, Guo F, Li Y. Activation of the PI3K-Akt pathway promotes neuroprotection of the δ-opioid receptor agonist against cerebral ischemia-reperfusion injury in rat models. Biomed Pharmacother. 2017;93:230–237. doi: 10.1016/j.biopha.2017.05.121. [DOI] [PubMed] [Google Scholar]
- 23.Zhai ZY, Feng J. Left-right asymmetry influenced the infarct volume and neurological dysfunction following focal middle cerebral artery occlusion in rats. Brain Behav. 2018;8:e01166. doi: 10.1002/brb3.1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu F, Schafer DP, McCullough LD. TTC, fluoro-Jade B and NeuN staining confirm evolving phases of infarction induced by middle cerebral artery occlusion. J Neurosci Methods. 2009;179:1–8. doi: 10.1016/j.jneumeth.2008.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Swanson RA, Morton MT, Tsao-Wu G, Savalos RA, Davidson C, Sharp FR. A semiautomated method for measuring brain infarct volume. J Cereb Blood Flow Metab. 1990;10:290–293. doi: 10.1038/jcbfm.1990.47. [DOI] [PubMed] [Google Scholar]
- 26.Ya BL, Li HF, Wang HY, Wu F, Xin Q, Cheng HJ, Li WJ, Lin N, Ba ZH, Zhang RJ, et al. 5-HMF attenuates striatum oxidative damage via Nrf2/ARE signaling pathway following transient global cerebral ischemia. Cell Stress Chaperones. 2017;22:55–65. doi: 10.1007/s12192-016-0742-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tyagi N, Qipshidze N, Munjal C, Vacek JC, Metreveli N, Givvimani S, Tyagi SC. Tetrahydrocurcumin ameliorates homocysteinylated cytochrome-c mediated autophagy in hyperhomocysteinemia mice after cerebral ischemia. J Mol Neurosci. 2012;47:128–138. doi: 10.1007/s12031-011-9695-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu P, Yang X, Hei C, Meli Y, Niu J, Sun T, Li PA. Rapamycin reduced ischemic brain damage in diabetic animals is Associated with suppressions of mTOR and ERK1/2 signaling. Int J Biol Sci. 2016;12:1032–1040. doi: 10.7150/ijbs.15624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li W, Yang Y, Hu Z, Ling S, Fang M. Neuroprotective effects of DAHP and Triptolide in focal cerebral ischemia via apoptosis inhibition and PI3K/Akt/mTOR pathway activation. Front Neuroanat. 2015;9:48. doi: 10.3389/fnana.2015.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jiang CJ, Wang ZJ, Zhao YJ, Zhang ZY, Tao JJ, Ma JY. Erythropoietin reduces apoptosis of brain tissue cells in rats after cerebral ischemia/reperfusion injury: A characteristic analysis using magnetic resonance imaging. Neural Regen Res. 2016;11:1450–1455. doi: 10.4103/1673-5374.191219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Janyou A, Wicha P, Jittiwat J, Suksamrarn A, Tocharus C, Tocharus J. Dihydrocapsaicin attenuates blood brain barrier and cerebral damage in focal cerebral ischemia/reperfusion via oxidative stress and inflammatory. Sci Rep. 2017;7:10556. doi: 10.1038/s41598-017-11181-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2 (Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 33.Villamil-Ortiz JG, Cardona-Gomez GP. Comparative analysis of autophagy and tauopathy related markers in cerebral ischemia and Alzheimer's disease animal models. Fron Aging Neurosci. 2015;7:84. doi: 10.3389/fnagi.2015.00084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li XB, Ding MX, Ding CL, Li LL, Feng JZ, Yu XJ. Toll-Like receptor 4 promotes the phosphorylation of CRMP2 via the activation of Rho-kinase in MCAO rats. Mol Med Rep. 2018;18:342–348. doi: 10.3892/mmr.2018.8968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Caltagirone C, Cisari C, Schievano C, Di Paola R, Cordaro M, Bruschetta G, Esposito E, Cuzzocrea S, Stroke Study Group Co-ultramicronized palmitoylethanolamide/luteolin in the treatment of cerebral ischemia: From rodent to man. Transl Stroke Res. 2016;7:54–69. doi: 10.1007/s12975-015-0440-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ferlito M, Wang Q, Fulton WB, Colombani PM, Marchionni L, Fox-Talbot K, Paolocci N, Steenbergen C. Correction: Hydrogen sulfide increases survival during sepsis: Protective effect of CHOP inhibition. J Immunol. 2014;192:1806–1814. doi: 10.4049/jimmunol.1490009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miao M, Yan X, Guo L, Shao S. Effects of the Rabdosia rubescens total flavonoids on focal cerebral ischemia reperfusion model in rats. Saudi Pharm J. 2017;25:607–614. doi: 10.1016/j.jsps.2017.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jung SY, Kim KM, Cho S, Lim S, Lim C, Kim YK. Effects of pretreatment with methanol extract of Peucedani Radix on transient ischemic brain injury in mice. Chin Med. 2017;12:30–40. doi: 10.1186/s13020-017-0151-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang C, Liu M, Pan Y, Bai B, Chen J. Global gene expression profile of cerebral ischemia-reperfusion injury in rat MCAO model. Oncotarget. 2017;8:74607–74622. doi: 10.18632/oncotarget.22995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Caprio FZ, Sorond FA. Cerebrovascular disease: Primary and secondary stroke prevention. Med Clin North Am. 2019;103:295–308. doi: 10.1016/j.mcna.2018.10.001. [DOI] [PubMed] [Google Scholar]
- 41.Xiao B, Chai Y, Lv S, Ye M, Wu M, Xie L, Fan Y, Zhu X. Endothelial cell-derived exosomes protect SH-SY5Y nerve cells against ischemia/reperfusion injury. Int J Mol Med. 2017;40:1201–1209. doi: 10.3892/ijmm.2017.3106. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 42.Zhao Q, Wang X, Chen A, Cheng X, Zhang G, Sun J, Zhao Y, Huang Y, Zhu Y. Rhein protects against cerebral ischemic-/reperfusion-induced oxidative stress and apoptosis in rats. Int J Mol Med. 2018;41:2802–2812. doi: 10.3892/ijmm.2018.3488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.He F, Zhang N, Lv Y, Sun W, Chen H. Low-dose lipopolysaccharide inhibits neuronal apoptosis induced by cerebral ischemia/reperfusion injury via the PI3K/Akt/FoxO1 signaling pathway in rat. Mol Med Rep. 2019;19:1443–1452. doi: 10.3892/mmr.2019.9827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen S, Sun M, Zhao X, Yang Z, Liu W, Cao J, Qiao Y, Luo X, Wen A. Neuroprotection of hydroxysafflor yellow A in experimental cerebral ischemia/reperfusion injury via metabolic inhibition of phenylalanine and mitochondrial biogenesis. Mol Med Rep. 2019;19:3009–3020. doi: 10.3892/mmr.2019.9959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hou Y, Wang Y, He Q, Li L, Xie H, Zhao Y, Zhao J. Nrf2 inhibits NLRP3 inflammasome activation through regulating Trx1/TXNIP complex in cerebral ischemia reperfusion injury. Behav Brain Res. 2018;336:32–39. doi: 10.1016/j.bbr.2017.06.027. [DOI] [PubMed] [Google Scholar]
- 46.Su D, Ma J, Zhang Z, Tian Y, Shen B. Protective effects of UCF-101 on cerebral ischemia-reperfusion (CIR) is depended on the MAPK/p38/ERK signaling pathway. Cell Mol Neurobiol. 2016;36:907–914. doi: 10.1007/s10571-015-0275-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shen X, Eichhorn T, Greten HJ, Efferth T. Effects of Scrophularia ningpoensis hemsl. On inhibition of proliferation, apoptosis induction and NF-κB signaling of immortalized and cancer cell lines. Pharmaceuticals (Basel) 2012;5:189–208. doi: 10.3390/ph5020189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liang W, Lam WP, Tang HC, Leung PC, Yew DT. Current evidence of Chinese herbal constituents with effects on NMDA receptor blockade. Pharmaceuticals (Basel) 2013;6:1039–1054. doi: 10.3390/ph6081039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang Q, An RD, Tian XC, Yang M, Li MH, Lou J, Xu L, Dong Z. β-Caryophyllene pretreatment alleviates focal cerebral ischemia-reperfusion injury by activating PI3K/Akt signaling pathway. Neurochem Res. 2017;42:1459–1469. doi: 10.1007/s11064-017-2202-3. [DOI] [PubMed] [Google Scholar]
- 50.Gong G, Xiang L, Yuan L, Hu L, Wu W, Cai L, Yin L, Dong H. Protective effect of glycyrrhizin, a direct HMGB1 inhibitor, on focal cerebral ischemia/reperfusion-induced inflammation, oxidative stress, and apoptosis in rats. PLoS One. 2014;9:e89450. doi: 10.1371/journal.pone.0089450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang Y, Ren QY, Zhang X, Lu HL, Chen J. Neuroprotective mechanisms of calycosin against focal cerebral ischemia and reperfusion injury in rats. Cell Physiol Biochem. 2018;45:537–546. doi: 10.1159/000487031. [DOI] [PubMed] [Google Scholar]
- 52.Liu B, Li F, Shi J, Yang D, Deng Y, Gong Q. Gastrodin ameliorates subacute phase cerebral ischemia-reperfusion injury by inhibiting inflammation and apoptosis in rats. Mol Med Rep. 2016;14:4144–4152. doi: 10.3892/mmr.2016.5785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li X, Guo H, Zhao L, Wang B, Liu H, Yue L, Bai H, Jiang H, Gao L, Feng D, Qu Y. Adiponectin attenuates NADPH oxidase-mediated oxidative stress and neuronal damage induced by cerebral ischemia-reperfusion injury. Biochim Biophys Acta Mol Basis Dis. 2017;1863:3265–3276. doi: 10.1016/j.bbadis.2017.08.010. [DOI] [PubMed] [Google Scholar]
- 54.Song W, Guo F, Zhong H, Liu L, Yang R, Wang Q, Xiong L. Therapeutic window of globular adiponectin against cerebral ischemia in diabetic mice: The role of dynamic alteration of adiponectin/adiponectin receptor expression. Sci Rep. 2015;5:17310. doi: 10.1038/srep17310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhao Y, Fang Y, Zhao H, Li J, Luo Y. Chrysophanol inhibits endoplasmic reticulum stress in cerebral ischemia and reperfusion mice. Eur J Pharmacol. 2018;818:1–9. doi: 10.1016/j.ejphar.2018.01.046. [DOI] [PubMed] [Google Scholar]
- 56.Liu X, Zhao S, Liu F, Kang J, Xiao A, Li F, Zhang C, Yan F, Zhao H, Luo M, et al. Remote ischemic postconditioning alleviates cerebral ischemic injury by attenuating endoplasmic reticulum stress-mediated apoptosis. Transl Stroke Res. 2014;5:692–700. doi: 10.1007/s12975-014-0359-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.