Abstract
ERCP is the mainstay of therapy for pancreatobiliary diseases in patients with native upper gastrointestinal (UGI) anatomy. However, when UGI anatomy is surgically altered, standard ERCP becomes technically challenging or not possible. In such instances, EUS-guided biliary drainage (EUS-BD) has been increasingly employed by advanced endoscopists as a safe and effective method of access to the biliary tree. In this study, we review the technical aspects and outcomes of EUS-BD in patients with surgical UGI anatomy.
Keywords: Advanced endoscopy, advanced endoscopy, bariatric surgery, biliary access, biliary stones, cholangiography, ERCP, EUS, new technologies, Roux-en-Y gastric bypass, surgically altered anatomy
INTRODUCTION
ERCP is the standard procedure for accessing the biliary tree in patients with native upper gastrointestinal (UGI) anatomy. However, when UGI anatomy is surgically altered, standard ERCP becomes technically challenging or not possible at times.[1] The most common types of surgically altered anatomy (SAA) include Billroth II, post-Whipple (pancreaticoduodenectomy), Roux-en-Y gastric bypass (RYGB), and Roux-en-Y hepaticojejunostomy (RYHJ); these surgeries are performed for malignant indications, such as gastric and pancreaticobiliary malignancies, with either curative or palliative intent, or for benign indications, such as obesity, chronic pancreatitis, and complications of peptic ulcer disease.
In particular, considering that obesity has a prevalence of nearly 30% in the US, bariatric surgery (most commonly RYGB) has become increasingly prevalent in recent years.[2] Up to one-third of RYGBs may be complicated by gallstone disease because of rapid weight loss.[3] In addition, UGI surgeries can be complicated by bilioenteric anastomotic leaks and/or strictures. Because of an increase in the trends for bariatric surgery[4] and cancer incidence,[5] it is predicted that the need for biliary access in patients with SAA will increase in the future. Standard ERCP is difficult or even impossible in many of these cases.[1] Thus, identification of minimally invasive alternative options for this growing patient population is of paramount importance.
Traditional alternatives in patients with SAA include surgical exploration of the bile duct or surgery-assisted ERCP and percutaneous transhepatic biliary drainage (PTBD). Direct surgical exploration of the bile duct has been largely abandoned because of the availability of less invasive alternatives.[6] Laparoscopy-assisted ERCP has high technical success rates, but is very expensive, is limited to patients with RYGB anatomy and by the need to coordinate different specialty teams, and carries risks of surgical procedures.[7,8,9,10] PTBD also has a high technical success rate, but it is relatively contraindicated in patients with ascites or hepatic metastases; has a high rate of reinterventions due to occlusion or dislocation of the external drains; and reduces the overall quality of life of patients due to skin irritation/infection, cholangitis, bile leakage, and pain.[11,12,13] More recent alternatives to biliary access in patients with SAA include enteroscopy-assisted ERCP (e-ERCP) and EUS-guided biliary drainage (EUS-BD). e-ERCP is a labor-intensive procedure with suboptimal technical success rates.[14,15] EUS-BD has been increasingly utilized in this patient population as it allows a relatively safe and effective access to the biliary tree in the setting of SAA.[16,17]
POSTSURGICAL ANATOMY
In some types of SAA, such as sleeve gastrectomy and Billroth I distal gastrectomy, standard ERCP can be performed because the remnant stomach is in continuity with the duodenum, and thus the papilla is accessible with the traditional approach. However, other more common types of SAA render standard ERCP very challenging or impossible, due to the lack of continuity between the stomach and the duodenum and the presence of anastomotic angulations and adhesions. The most common types of SAA encountered in clinical practice are partial gastrectomy with Billroth II reconstruction or gastrojejunostomy (GJ) without gastric resection, Whipple anatomy, RYGB, and RYHJ.[1]
Gastrojejunostomy (including Billroth II)
Partial gastrectomy with Billroth II reconstruction is indicated for distal gastric cancer and more frequently in the past, for complications of severe peptic ulcer disease. The distal stomach is resected, and an end-to-side GJ is created. The afferent limb of the anastomosis leads to the duodenum and portion of the proximal jejunum, whereas the efferent limb leads to the distal small bowel. In cases of gastric outlet obstruction, GJ without gastric resection can be performed as palliative treatment. Depending on the length of the afferent limb and the angulation of the anastomosis, standard ERCP may be feasible, but challenging.
Roux-en-Y gastric bypass
The stomach is typically divided into a small proximal pouch and a larger distal gastric remnant. The small bowel is usually divided into two limbs: the biliopancreatic limb, which consists of the duodenum and proximal jejunum, is connected to the excluded stomach and contains the biliopancreatic orifices, and the Roux limb, which consists of the small bowel distal to the division. The Roux limb is “brought up” and anastomosed to the gastric pouch through a GJ, whereas the biliopancreatic limb is anastomosed about 75–150 cm distally through a jejunojejunostomy. In this case, standard ERCP is practically impossible because the papilla cannot be reached, while e-ERCP may be performed, but has a low success rate.[14,15]
Whipple procedure
Pancreaticoduodenectomy (Whipple procedure) is typically performed for treating biliary, pancreatic head, or ampullary cancers. The classical surgery consists of removal of the pancreatic head, distal stomach, duodenum, proximal jejunum, distal common bile duct (CBD), and gallbladder. Reconstruction is done by creating a pancreaticojejunostomy (PJ), choledochojejunostomy (CJ), and GJ similar to that described with the Roux-en-Y. The biliopancreatic limb in this case consists of the jejunum that contains the PJ and CJ, whereas the Roux limb consists of the distal small bowel anastomosed to the remnant stomach.
EUS-BILIARY DRAINAGE TECHNIQUES
There are three basic approaches to EUS-BD: EUS-rendezvous (EUS-RV), transluminal, and EUS-guided antegrade approaches.[18] These procedures should be performed with CO2 insufflation under profound sedation or general anesthesia, and intravenous antibiotics should be administered to minimize potential infectious adverse events.[17]
EUS-rendezvous
The EUS-RV technique involves EUS-guided access to the dilated bile tree using fine-needle aspiration (FNA) needles. Biliary access can be intrahepatic (through the cardia or lesser curvature) or extrahepatic (through the distal antrum or, more commonly, the duodenum). Once access to the biliary tree is ascertained through bile aspiration and contrast medium injection, a guidewire is inserted through the needle and pushed distally across the stricture and the papilla or surgical anastomosis into the small bowel. Then, the echoendoscope is removed while leaving the wire in place. A standard duodenoscope is used to retrieve the guidewire with snare or biopsy forceps and use it for retrograde cannulation and/or over-the-wire stent placement.[19,20]
Transluminal EUS-biliary drainage
The direct transluminal technique involves the creation of a new fistula, between the extrahepatic CBD and the duodenum (EUS-choledochoduodenostomy [EUS-CDS]) or between the left intrahepatic bile duct and the stomach (EUS-hepatogastrostomy [EUS-HGS]). More rarely, a fistula can be created between the biliary tree and the Roux limb (EUS-hepaticojejunostomy [EUS-HJS]). Biliary access and guidewire insertion is achieved as described in the EUS-RV technique above. Once the guidewire is inserted, the tract can be dilated[21,22] followed by stent placement [Figures 1 and 2].[19]
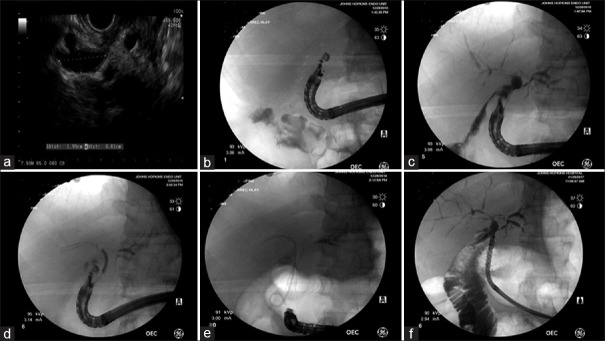
Figure 1.
EUS-guided choledochoduodenostomy in a 53-year-old male for recurrent cholangitis status postorthotopic liver transplantation with Roux-en-Y hepaticojejunostomy and hepaticojejunostomy stricture. (a and b) Linear echoendoscope was advanced to D1 and the dilated bile duct was identified. (c) Under EUS guidance, a 19-gauge needle was advanced into the bile duct, and a cholangiogram was performed by injecting contrast. (d) A 0.025-inch guidewire was advanced through the needle into the intrahepatic ducts. A 10 mm × 40 mm fully covered self-expanding metal stent was advanced over the wire and deployed successfully creating the choledochoduodenostomy. (e) A 7 Fr × 5 cm double-pig tail was deployed through the fully covered self-expanding metal stent to avoid stent migration. (f) On a different session, the two stents were removed, and pediatric gastroscope was advanced through the created choledochoduodenostomy. Cholangiogram revealed diffuse intrahepatic strictures with beaded appearance
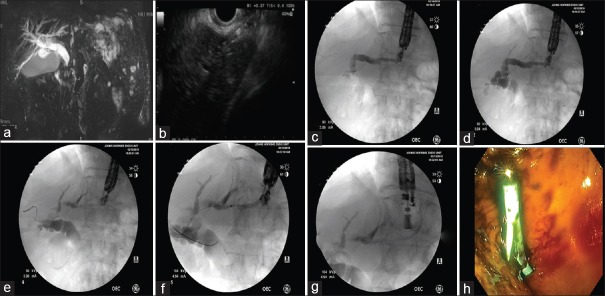
Figure 2.
EUS-hepatogastrostomy for malignant biliary obstruction in a 75-year-old male status postpartial gastrectomy and Roux-en-Y reconstruction for gastric cancer. (a) Significant dilatation of the common bile duct can be appreciated in the magnetic resonance imaging. (b) A linear echoendoscope was advanced to the stomach. Following the identification of the left intrahepatic duct, a 19-gauge needle was advanced transgastrically into the left intrahepatic duct. (c and d) Contrast was injected and anterograde cholangiography was performed, confirming correct positioning within the biliary tree. Dilatation of the intrahepatic and extrahepatic bile ducts can be appreciated. (e and f) A guidewire was passed into the left hepatic duct across the hilum into the common hepatic duct. (g) A 7 Fr × 10 cm plastic stent with internal and external flaps was placed over the wire into the left intrahepatic duct. (h) Endoscopic view of the deployed plastic stent
Antegrade EUS-biliary drainage
The antegrade approach involves EUS-guided biliary access, guidewire insertion, and dilation as described above. In addition, a stent is placed through the echoendoscope over the guidewire and passed across the stricture and through the papilla (transpapillary) or anastomosis/stricture (transanastomotic) for “downstream” drainage into the duodenum/jejunum.[23,24]
In cases of biliary stones, balloon sphincteroplasty can be performed similar to standard ERCP, but in an antegrade fashion, and then the same balloon or a biliary stone retrieval balloon may be used to push the stones distally across the papilla or the stenosis. Mechanical lithotripters can also be employed in an antegrade fashion to crush the biliary stones.[25,26,27,28,29,30,31,32] A nasobiliary drainage may be placed at the operator's discretion to prevent a possible bile leak from the puncture site and to maintain an access route in case of needed repeat procedures.[25,26,27,28,29,30,31,32]
Application of traditional EUS-biliary drainage techniques in patients with surgically altered anatomy
All three of the above-described techniques can be used in cases of native GI anatomy and failed ERCP or in the presence of ampullary distortions/diverticulum or duodenal stents.[18] However, in some patients with SAA (RY anatomy), the EUS-RV and/or EUS-CDS approaches are usually not feasible/applicable due to prior resection of the extrahepatic biliary system (e.g., RY-HJ) or inability to reach the ampulla/bilioenteric anastomosis (e.g., RYGB and RY-HJ). In these cases, the intrahepatic antegrade approaches or EUS-HGS/EUS-HJS are preferred. In some types of SAA (e.g., Billroth II), EUS-RV may be performed with standard duodenoscope or with the use of an enteroscope/colonoscope (e-ERCP).
The most common EUS-guided access to the biliary tree in patients with SAA is through the left intrahepatic ducts in the 2nd/3rd hepatic segment from the distal esophagus, proximal stomach, or transjejunal in patients with esophagojejunostomy.[16] A rare exception to this rule may include cases in which the proximal CBD is sufficiently dilated that it can also be visualized from the mid/proximal stomach.[33] Even rarer is the access of the biliary tree through dilated right intrahepatic ducts in patients with SAA and no left intrahepatic duct dilation.[34]
More than one technique can be utilized in the same patient as well. Antegrade EUS-BD and EUS-HGS (or EUS-HJS) were performed during the same session in a subgroup of patients with malignant[35,36] or benign[32] indications. Other authors have proposed a two-stage procedure for benign indications. The first stage consists of the creation of EUS-HGS or EUS-HJS. The second stage, executed 1–4 weeks after to allow tract maturation, was performed by antegrade biliary access through the mature fistula with standard duodenoscopic devices or with cholangioscopes.[29,30,31]
EUS-directed transgastric ERCP
In recent years, a novel approach, coined EUS-directed transgastric ERCP (EDGE), was developed for patients who have undergone bariatric RYGB. The original EDGE was a two-stage procedure: the first stage of identifying the gastric remnant under EUS guidance and placing a percutaneous endoscopic gastrostomy (PEG) in it and after few days the second stage of replacing the PEG with a self-expanding metal stent and then performing antegrade ERCP through the gastrocutaneous stent.[37,38]
More recently, a single-stage fully endoscopic procedure has been developed. The excluded stomach is identified under EUS guidance from the gastric pouch or the Roux limb. Once identified, the excluded stomach is accessed with a EUS-FNA needle (usually 19-gauge). Contrast medium and water is injected to confirm the location of the excluded stomach and distend it. The excluded stomach is then accessed with a cautery-enhanced lumen-apposing metal stent (LAMS). The distal flange of the LAMS is deployed under EUS/fluoroscopic guidance in the excluded stomach, and the proximal flange is deployed under sonographic and endoscopic guidance in the gastric pouch. Once the LAMS is placed, the lumen of the stent is dilated with a dilating balloon to the diameter of the stent (15–20 mm). The newly created gastrogastric fistula allows the antegrade passage of a standard duodenoscope from the remnant pouch to the excluded stomach and to the papilla, where conventional ERCP can be performed. Once ampullary access is no longer needed, the LAMS can be removed and the fistula is left to close by secondary intention. This is confirmed by obtaining a contrast study 8 weeks after LAMS removal or via repeat endoscopy. Endoscopic suturing or use of an over-the-scope clip can be utilized for closure of fistulae that remain patent after 8 weeks.[39,40]
Some endoscopists have advocated for a two-stage procedure due to the risk of LAMS dislodgement if ERCP is performed during the index procedure: the first stage of LAMS placement, followed by the second stage 1–4 weeks after antegrade ERCP after fistula maturation.[41] This approach, however, is costly, time-consuming, and not an option in cases when urgent ERCP is needed (e.g., cholangitis and bile leak). Our approach is to place a 20-mm LAMS, secure it with endoscopic suturing, and then perform ERCP during the same session.[40] This approach minimizes the number of procedures, allows for ERCP to be performed promptly, while minimizing the risk of the stent dislodgement and perforation. The 20-mm LAMS provides 78% increase in the surface area compared to the 15-mm LAMS. Placement of sutures allows robust stent fixation to the gastric/jejunal wall. In combination, this technique seems to be effective and safe.
OUTCOMES OF EUS-BILIARY DRAINAGE IN PATIENTS WITH SURGICALLY ALTERED ANATOMY
EUS-BD has shown promising results in managing biliary diseases where ERCP fails or is not feasible. However, the evidence on the outcomes of these procedures in patients with SAA is relatively weak, consisting mostly of small and/or retrospective studies.
Antegrade EUS-biliary drainage
Most reported antegrade EUS-BD procedures in patients with SAA have been for biliary stones. These small retrospective studies show an overall success rate between 60% and 100%, and adverse rates between 15% and 20%, mostly mild to moderate in severity.[25,26,27,28,29,30,31,32] In a multicenter study that included 29 patients with SAA and bile duct stones, antegrade EUS-BD was successful in 72% of cases. Five (17%) patients had adverse events including mild abdominal pain, mild biliary peritonitis, moderate cholecystitis, and mild elevation of C-reactive protein, all managed conservatively.[26] In another recent study that included twenty patients with SAA and biliary stones, combined EUS-HJS/HGS and antegrade EUS were technically and clinically successful in 90% of cases, with mild adverse events in 15% of patients.[32]
Dilation of bilioenteric anastomosis and inflammatory strictures and closure of bile leaks are other benign indications for antegrade EUS-BD in patients with SAA.[28,30,32,42,43] A recent study included 37 patients with SAA and benign biliary diseases (16 cases of biliary stones and 21 cases of anastomotic strictures), who failed e-ERCP.[30] In this study, eight cases were treated with single-stage antegrade EUS-BD, whereas the remaining required two-stage combined EUS-HGS/EUS-HJS and antegrade EUS-BD. Peroral cholangioscopy-assisted antegrade interventions (guidewire manipulation or lithotripsy) were performed in 19 of these patients. The overall clinical success was 92%, with 3 (8%) cases of moderate biliary peritonitis.[30]
Antegrade EUS-BD has also been reported for malignant biliary obstruction in twenty patients with SAA. Technical and clinical success was observed in 95% of cases, with mild pancreatitis and fever in 5 (20%) patients.[44] In another recent study that included 79 patients with SAA and malignant biliary obstruction, combined EUS-HGS and antegrade EUS-BD procedure showed a lower technical success rate than EUS-HGS alone (83.8 vs. 97.6%, P = 0.03), but had similar clinical success rate (90.2 vs. 90.3%) and less adverse events (10.8 vs. 26.1%, P = 0.03).[35]
EUS-biliary drainage versus enteroscopy-assisted ERCP
An international multicentric study, which compared the outcomes of 98 patients with SAA and benign or malignant biliary obstruction who underwent either e-ERCP or EUS-BD, found that EUS-BD can be performed with a higher degree of clinical efficacy (98% vs. 65.3%, P = 0.001) and shorter procedure time (55 min vs. 95 min, P < 0.0001) than e-ERCP. However, more adverse events occurred in the EUS-BD group (20% vs. 4%, P = 0.01), even though most were mild to moderate, and hospital stay was significantly longer in the EUS-BD group (6.6 vs. 2.4 days, P < 0.0001). Based on these results, the authors concluded that whether e-ERCP or EUS-BD should be first-line treatment in SAA depends on the indications for the procedure, patient's anatomy, and local practice and expertise.[45]
EUS-directed transgastric ERCP
When the EDGE procedure was first proposed, some authors expressed reservations concerning the potential long-term adverse events following the creation of gastrogastric fistula, such as abdominal pain, marginal ulcers, and weight regain,[46,47] especially because nonfistula-creating alternatives were available.[14] However, a recent retrospective study that compared 29 EDGE procedures with 43 laparoscopic-assisted ERCP (LA-ERCP) found that EDGE had similar technical success to LA-ERCP (96.5% and 97.7%, respectively), but shorter procedural time (73 vs. 184 min, respectively) and shorter hospital stay (0.8 vs. 2.65 days, respectively). In addition, no weight gain was observed after an average 28-week follow-up.[41] Similarly, a recent multicenter study compared the outcomes of 30 EDGE and 30 e-ERCP procedures. EDGE had a significantly higher success rate (100% vs. 60.0%, P < 0.001), shorter procedural time (49.8 min vs. 90.7 min, P < 0.001), shorter hospital stay (1 vs. 10.5 days, P = 0.02), and a similar safety profile to e-ERCP. No weight gain or marginal ulcers were observed after a median follow-up of 209 days.[48]
Right intrahepatic EUS-biliary drainage
As mentioned previously, the left intrahepatic route is the preferred route of EUS-guided biliary cannulation. However, one study reported the outcomes of EUS-BD in six consecutive patients with isolated right hepatic duct obstruction and failed or unfeasible ERCP, three of whom had SAA. Cholangiography was achieved in all patients, and biliary decompression was achieved in 5/6 cases, without any adverse events.[34]
Adverse events
The rate of adverse events varies widely across studies, with most reports falling between 10% and 20%. In a recent meta-analysis, common adverse events related to EUS-BD included bleeding (4.03%), bile leakage (4.03%), pneumoperitoneum (3.02%), stent migration (2.68%), cholangitis (2.43%), abdominal pain (1.51%), and peritonitis (1.26%).[49] Roughly similar numbers were reported in other meta-analyses.[13,50] While the majority of these adverse events are mild to moderate, some studies have reported also severe or even fatal outcomes, such as massive bleeding, perforation, or stent migration into the peritoneal cavity. The rate of adverse events of EUS-BD in patients with SAA seems to be roughly similar to that reported in patients with native GI anatomy.[51]
Some of the factors associated with adverse outcomes include the use of noncoaxial electrocautery dilation, such as dilation with a needle-knife,[21,22] overly aggressive tract dilation,[21,22] and plastic stenting when compared to metal stenting.[22,49,52,53] Another significant factor associated with adverse events is operator experience, with most of the adverse events occurring during the first 33–50 cases.[54,55,56]
FUTURE DIRECTIONS
EUS-BD is a safe and effective minimally invasive method of biliary cannulation in cases when ERCP has failed or is not feasible, such as in patients with SAA. However, several shortcomings still need to be addressed.
First, adequate training is of paramount importance.[16,57] Advanced endoscopists, besides being proficient in EUS/ERCP, should strive to master the entire range of EUS-BD techniques. While access to training facilities is inherently limited to only few participants, hands-on training with ex vivo animal models seems beneficial.[58,59] However, because EUS-BD procedures are relatively rare and very complex, and considering that there is a significant learning curve associated with adverse outcomes,[54,55,56] we also recognize the need to have specifically accredited centers with adequate volume and expertise operating at a hospital equipped with interventional radiology and/or pancreaticobiliary surgery backup.[16,57]
Second, accessories tailored specifically for EUS-BD are sorely needed.[18,52] A recent development is the new one-step LAMS preloaded onto a hot delivery system (Axios, Boston Scientific, Natick, Mass) that allows to perform transmural EUS-BD quickly and efficiently by overcoming several cumbersome phases such as multiple guidewire exchanges. Three recent studies, including a total of 122 participants, have shown that LAMSs are 93%–98% technically and clinically effective for creating single-stage EUS-CDS in patients with inoperable malignant distal biliary obstruction and failed ERCP.[60,61,62] Adverse event rates were 7%–37%, and the majority were mild to moderate.[60,61,62] However, the utility of LAMS in patients with SAA is unclear. Recently, we developed a new technique, named EUS-directed transenteric ERCP (EDEE), in which a LAMS is used to create a gastroenteric or enteroenteric fistula and allows access to the biliary tree in altered non-RYGB anatomy [Figure 3]. We described this procedure in a multicenter study that included 18 patients who underwent EDEE.[63] We successfully created a fistula in all patients and achieved a 94% technical and clinical success. In 4/18 (22%) patients, we successfully performed standard ERCP in the same session as the creation of the fistula. Adverse events were observed only in 2/18 patients: mild infection in one and moderate postprocedural pain in the other, both successfully managed with medical treatment.[63] Another potentially significant innovation is EUS with forward-viewing echoendoscopes (FV-EUS).[64] One of its main advantages is the ability to advance more easily in the GI tract and to allow the exit of accessories parallel to the longitudinal axis of the echoendoscope. Even though these features can be very useful in some types of SAA, such as Billroth II, FV-EUS seems to be much less useful in RY anatomy.[65]
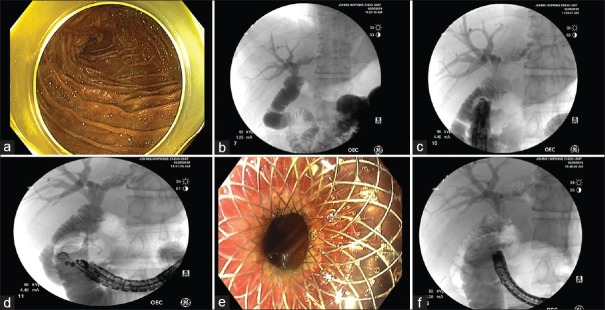
Figure 3.
EUS-directed transenteric ERCP for choledocholithiasis in a 50-year-old male with a history of Roux-en-Y hepaticojejunostomy due to bile duct injury postcholecystectomy. (a and b) The endoscope was advanced to the jejunum, and a mixture of contrast and saline was injected into the afferent limb, confirming correct position. (c) The endoscope was withdrawn, and a linear EUS was advanced to the duodenum and a location suitable for the duodenojejunal anastomosis was established under fluoroscopy. (d) The small bowel was punctured with a 19-G FNA needle. The small bowel adjacent to the hepaticojejunostomy anastomosis was dilated using 330 cc of saline mixed with contrast. Under EUS guidance, a cautery-assisted lumen-apposing metal stent, 15 mm × 10 mm, was then deployed creating the duodenojejunostomy. (e) Endoscopic view of the proximal flange of lumen-apposing metal stent post-deployment. (f) Under fluoroscopic guidance, using a therapeutic gastroscope, a guidewire was advanced across the lumen-apposing metal stent and hepaticojejunostomy was successfully cannulated
Finally, high-quality studies on EUS-BD in patients with SAA are needed. Currently, most studies are small, single center, and/or retrospective and include only short-term outcomes. Considering that the population with SAA is projected to grow in the future, such knowledge is crucial.
OUR APPROACH TO EUS-BILIARY DRAINAGE IN PATIENTS WITH SURGICALLY ALTERED ANATOMY
Because EUS-BD is a relatively rare procedure[66] performed only at a few tertiary referral centers and largely depends on personal experience and locally available accessories, no specific algorithm has been universally accepted for patients with SAA. Based on our experience,[19] and that of others,[67,68] we suggest the following approach for patients with SAA [Figure 4]. If the patient has RYGB anatomy, then we perform EDGE for pancreaticobiliary access and interventions. In patients with other forms of SAA and the papilla/anastomosis is accessible, we perform intrahepatic EUS-RV (if the extrahepatic route is available, extrahepatic EUS-RV can also be performed). If EUS-RV is not feasible/successful or if the papilla/anastomosis is not accessible, then consider EUS-HGS/EUS-HJS. We prefer this approach to antegrade EUS-BD because it maintains access to the biliary tree. If transmural biliary access is not feasible/successful, the novel EDEE procedure can be attempted. If the endoscopic approach is not feasible/successful, we refer the patient for PTBD.
Figure 4.
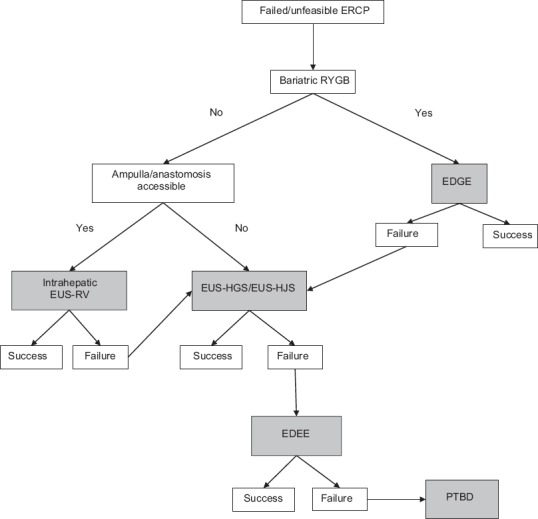
Our approach to managing complex pancreatobiliary pathology. RYGB: Roux-en-Y Gastric Bypass, EDGE: EUS-directed transgastric ERCP, EUS-RV: EUS-rendezvous, EUS-HGS: EUS-guided hepatogastrostomy, EUS-HJS: EUS-guided hepaticojejunostomy, EUS-BD: EUS-guided biliary drainage, EDEE: EUS-directed transenteric ERCP, PTBD: Percutaneous transhepatic biliary drainage
CONCLUSIONS
EUS-BD is an effective and safe minimally invasive method of biliary cannulation in cases when ERCP has failed or is not feasible, such as in patients with SAA. It is expected that the need for EUS-BD in patients with SAA will increase rapidly, and therefore, this technique deserves widespread adaptation. Future efforts should focus on creating adequate training programs, developing novel devices specifically for EUS-BD, and designing large multicenter studies to obtain data on indications, most appropriate methods/devices, and long-term outcomes of EUS-BD in patients with SAA.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient has given his consent for his images and other clinical information to be reported in the journal. The patient understands that his name and initials will not be published and due efforts will be made to conceal identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Lee A, Shah JN. Endoscopic approach to the bile duct in the patient with surgically altered anatomy. Gastrointest Endosc Clin N Am. 2013;23:483–504. doi: 10.1016/j.giec.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 2.Wang BC, Furnback W. Modelling the long-term outcomes of bariatric surgery: A review of cost-effectiveness studies. Best Pract Res Clin Gastroenterol. 2013;27:987–95. doi: 10.1016/j.bpg.2013.08.022. [DOI] [PubMed] [Google Scholar]
- 3.Uy MC, Talingdan-Te MC, Espinosa WZ, et al. Ursodeoxycholic acid in the prevention of gallstone formation after bariatric surgery: A meta-analysis. Obes Surg. 2008;18:1532–8. doi: 10.1007/s11695-008-9587-7. [DOI] [PubMed] [Google Scholar]
- 4.Nguyen NT, Masoomi H, Magno CP, et al. Trends in use of bariatric surgery, 2003-2008. J Am Coll Surg. 2011;213:261–6. doi: 10.1016/j.jamcollsurg.2011.04.030. [DOI] [PubMed] [Google Scholar]
- 5.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 6.Artifon EL, Loureiro JF, Baron TH, et al. Surgery or EUS-guided choledochoduodenostomy for malignant distal biliary obstruction after ERCP failure. Endosc Ultrasound. 2015;4:235–43. doi: 10.4103/2303-9027.163010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schreiner MA, Chang L, Gluck M, et al. Laparoscopy-assisted versus balloon enteroscopy-assisted ERCP in bariatric post-Roux-en-Y gastric bypass patients. Gastrointest Endosc. 2012;75:748–56. doi: 10.1016/j.gie.2011.11.019. [DOI] [PubMed] [Google Scholar]
- 8.Gutierrez JM, Lederer H, Krook JC, et al. Surgical gastrostomy for pancreatobiliary and duodenal access following Roux en Y gastric bypass. J Gastrointest Surg. 2009;13:2170–5. doi: 10.1007/s11605-009-0991-7. [DOI] [PubMed] [Google Scholar]
- 9.Frederiksen NA, Tveskov L, Helgstrand F, et al. Treatment of common bile duct stones in gastric bypass patients with laparoscopic transgastric endoscopic retrograde cholangiopancreatography. Obes Surg. 2017;27:1409–13. doi: 10.1007/s11695-016-2524-2. [DOI] [PubMed] [Google Scholar]
- 10.Abbas AM, Strong AT, Diehl DL, et al. Multicenter evaluation of the clinical utility of laparoscopy-assisted ERCP in patients with Roux-en-Y gastric bypass. Gastrointest Endosc. 2018;87:1031–9. doi: 10.1016/j.gie.2017.10.044. [DOI] [PubMed] [Google Scholar]
- 11.Nennstiel S, Weber A, Frick G, et al. Drainage-related complications in percutaneous transhepatic biliary drainage: An analysis over 10 years. J Clin Gastroenterol. 2015;49:764–70. doi: 10.1097/MCG.0000000000000275. [DOI] [PubMed] [Google Scholar]
- 12.Khashab MA, Valeshabad AK, Afghani E, et al. A comparative evaluation of EUS-guided biliary drainage and percutaneous drainage in patients with distal malignant biliary obstruction and failed ERCP. Dig Dis Sci. 2015;60:557–65. doi: 10.1007/s10620-014-3300-6. [DOI] [PubMed] [Google Scholar]
- 13.Sharaiha RZ, Khan MA, Kamal F, et al. Efficacy and safety of EUS-guided biliary drainage in comparison with percutaneous biliary drainage when ERCP fails: A systematic review and meta-analysis. Gastrointest Endosc. 2017;85:904–14. doi: 10.1016/j.gie.2016.12.023. [DOI] [PubMed] [Google Scholar]
- 14.Skinner M, Popa D, Neumann H, et al. ERCP with the overtube-assisted enteroscopy technique: A systematic review. Endoscopy. 2014;46:560–72. doi: 10.1055/s-0034-1365698. [DOI] [PubMed] [Google Scholar]
- 15.Shah RJ, Smolkin M, Yen R, et al. A multicenter, U.S. experience of single-balloon, double-balloon, and rotational overtube-assisted enteroscopy ERCP in patients with surgically altered pancreaticobiliary anatomy (with video) Gastrointest Endosc. 2013;77:593–600. doi: 10.1016/j.gie.2012.10.015. [DOI] [PubMed] [Google Scholar]
- 16.Kahaleh M, Artifon EL, Perez-Miranda M, et al. Endoscopic ultrasonography guided drainage: Summary of consortium meeting, May 21, 2012, San Diego, California. World J Gastroenterol. 2015;21:726–41. doi: 10.3748/wjg.v21.i3.726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jirapinyo P, Lee LS. Endoscopic ultrasound-guided pancreatobiliary endoscopy in surgically altered anatomy. Clin Endosc. 2016;49:515–29. doi: 10.5946/ce.2016.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khashab MA, Levy MJ, Itoi T, et al. EUS-guided biliary drainage. Gastrointest Endosc. 2015;82:993–1001. doi: 10.1016/j.gie.2015.06.043. [DOI] [PubMed] [Google Scholar]
- 19.Khashab MA, Valeshabad AK, Modayil R, et al. EUS-guided biliary drainage by using a standardized approach for malignant biliary obstruction: Rendezvous versus direct transluminal techniques (with videos) Gastrointest Endosc. 2013;78:734–41. doi: 10.1016/j.gie.2013.05.013. [DOI] [PubMed] [Google Scholar]
- 20.Saxena P, Aguila G, Kumbhari V, et al. Untying the knot: Technique of unraveling a guidewire knot created during EUS-guided biliary drainage. Endoscopy. 2014;46(Suppl 1):E140–1. doi: 10.1055/s-0033-1359240. [DOI] [PubMed] [Google Scholar]
- 21.Park DH, Jang JW, Lee SS, et al. EUS-guided biliary drainage with transluminal stenting after failed ERCP: Predictors of adverse events and long-term results. Gastrointest Endosc. 2011;74:1276–84. doi: 10.1016/j.gie.2011.07.054. [DOI] [PubMed] [Google Scholar]
- 22.Khashab MA, Messallam AA, Penas I, et al. International multicenter comparative trial of transluminal EUS-guided biliary drainage via hepatogastrostomy vs. choledochoduodenostomy approaches. Endosc Int Open. 2016;4:E175–81. doi: 10.1055/s-0041-109083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kumbhari V, Tieu AH, Khashab MA. EUS-guided biliary drainage made safer by a combination of hepaticogastrostomy and antegrade transpapillary stenting. Gastrointest Endosc. 2015;81:1015–6. doi: 10.1016/j.gie.2014.10.019. [DOI] [PubMed] [Google Scholar]
- 24.Saxena P, Kumbhari V, El Zein M, et al. EUS-guided biliary drainage with antegrade transpapillary placement of a metal biliary stent. Gastrointest Endosc. 2015;81:1010–1. doi: 10.1016/j.gie.2014.06.038. [DOI] [PubMed] [Google Scholar]
- 25.Weilert F, Binmoeller KF, Marson F, et al. Endoscopic ultrasound-guided anterograde treatment of biliary stones following gastric bypass. Endoscopy. 2011;43:1105–8. doi: 10.1055/s-0030-1256961. [DOI] [PubMed] [Google Scholar]
- 26.Iwashita T, Nakai Y, Hara K, et al. Endoscopic ultrasound-guided antegrade treatment of bile duct stone in patients with surgically altered anatomy: A multicenter retrospective cohort study. J Hepatobiliary Pancreat Sci. 2016;23:227–33. doi: 10.1002/jhbp.329. [DOI] [PubMed] [Google Scholar]
- 27.Itoi T, Sofuni A, Tsuchiya T, et al. Endoscopic ultrasonography-guided transhepatic antegrade stone removal in patients with surgically altered anatomy: Case series and technical review (with videos) J Hepatobiliary Pancreat Sci. 2014;21:E86–93. doi: 10.1002/jhbp.165. [DOI] [PubMed] [Google Scholar]
- 28.Iwashita T, Yasuda I, Doi S, et al. Endoscopic ultrasound-guided antegrade treatments for biliary disorders in patients with surgically altered anatomy. Dig Dis Sci. 2013;58:2417–22. doi: 10.1007/s10620-013-2645-6. [DOI] [PubMed] [Google Scholar]
- 29.Hosmer A, Abdelfatah MM, Law R, et al. Endoscopic ultrasound-guided hepaticogastrostomy and antegrade clearance of biliary lithiasis in patients with surgically-altered anatomy. Endosc Int Open. 2018;6:E127–30. doi: 10.1055/s-0043-123188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mukai S, Itoi T, Sofuni A, et al. EUS-guided antegrade intervention for benign biliary diseases in patients with surgically altered anatomy (with videos) Gastrointest Endosc. 2019;89:399–407. doi: 10.1016/j.gie.2018.07.030. [DOI] [PubMed] [Google Scholar]
- 31.Nakai Y, Isayama H, Koike K. Two-step endoscopic ultrasonography-guided antegrade treatment of a difficult bile duct stone in a surgically altered anatomy patient. Dig Endosc. 2018;30:125–7. doi: 10.1111/den.12965. [DOI] [PubMed] [Google Scholar]
- 32.James TW, Fan YC, Baron TH. EUS-guided hepaticoenterostomy as a portal to allow definitive antegrade treatment of benign biliary diseases in patients with surgically altered anatomy. Gastrointest Endosc. 2018;88:547–54. doi: 10.1016/j.gie.2018.04.2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kahaleh M, Yoshida C, Kane L, et al. Interventional EUS cholangiography: A report of five cases. Gastrointest Endosc. 2004;60:138–42. doi: 10.1016/s0016-5107(04)01528-7. [DOI] [PubMed] [Google Scholar]
- 34.Park SJ, Choi JH, Park DH, et al. Expanding indication: EUS-guided hepaticoduodenostomy for isolated right intrahepatic duct obstruction (with video) Gastrointest Endosc. 2013;78:374–80. doi: 10.1016/j.gie.2013.04.183. [DOI] [PubMed] [Google Scholar]
- 35.Imai H, Takenaka M, Omoto S, et al. Utility of endoscopic ultrasound-guided hepaticogastrostomy with antegrade stenting for malignant biliary obstruction after failed endoscopic retrograde cholangiopancreatography. Oncology. 2017;93(Suppl 1):69–75. doi: 10.1159/000481233. [DOI] [PubMed] [Google Scholar]
- 36.Ogura T, Masuda D, Imoto A, et al. EUS-guided hepaticogastrostomy combined with fine-gauge antegrade stenting: A pilot study. Endoscopy. 2014;46:416–21. doi: 10.1055/s-0034-1365020. [DOI] [PubMed] [Google Scholar]
- 37.Kedia P, Kumta NA, Sharaiha R, et al. Bypassing the bypass: EUS-directed transgastric ERCP for Roux-en-Y anatomy. Gastrointest Endosc. 2015;81:223–4. doi: 10.1016/j.gie.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 38.Kedia P, Kumta NA, Widmer J, et al. Endoscopic ultrasound-directed transgastric ERCP (EDGE) for Roux-en-Y anatomy: A novel technique. Endoscopy. 2015;47:159–63. doi: 10.1055/s-0034-1390771. [DOI] [PubMed] [Google Scholar]
- 39.Kedia P, Sharaiha RZ, Kumta NA, et al. Internal EUS-directed transgastric ERCP (EDGE): Game over. Gastroenterology. 2014;147:566–8. doi: 10.1053/j.gastro.2014.05.045. [DOI] [PubMed] [Google Scholar]
- 40.Irani S, Yang J, Khashab MA. Mitigating lumen-apposing metal stent dislodgment and allowing safe, single-stage EUS-directed transgastric ERCP. VideoGIE. 2018;3:322–4. doi: 10.1016/j.vgie.2018.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kedia P, Tarnasky PR, Nieto J, et al. EUS-directed transgastric ERCP (EDGE) versus laparoscopy-assisted ERCP (LA-ERCP) for roux-en-Y gastric bypass (RYGB) anatomy: A multicenter early comparative experience of clinical outcomes. J Clin Gastroenterol. 2019;53:304–8. doi: 10.1097/MCG.0000000000001037. [DOI] [PubMed] [Google Scholar]
- 42.Park DH, Jang JW, Lee SS, et al. EUS-guided transhepatic antegrade balloon dilation for benign bilioenteric anastomotic strictures in a patient with hepaticojejunostomy. Gastrointest Endosc. 2012;75:692–3. doi: 10.1016/j.gie.2011.04.013. [DOI] [PubMed] [Google Scholar]
- 43.Miranda-García P, Gonzalez JM, Tellechea JI, et al. EUS hepaticogastrostomy for bilioenteric anastomotic strictures: A permanent access for repeated ambulatory dilations? Results from a pilot study. Endosc Int Open. 2016;4:E461–5. doi: 10.1055/s-0042-103241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Iwashita T, Yasuda I, Mukai T, et al. Endoscopic ultrasound-guided antegrade biliary stenting for unresectable malignant biliary obstruction in patients with surgically altered anatomy: Single-center prospective pilot study. Dig Endosc. 2017;29:362–8. doi: 10.1111/den.12800. [DOI] [PubMed] [Google Scholar]
- 45.Khashab MA, El Zein MH, Sharzehi K, et al. EUS-guided biliary drainage or enteroscopy-assisted ERCP in patients with surgical anatomy and biliary obstruction: An international comparative study. Endosc Int Open. 2016;4:E1322–7. doi: 10.1055/s-0042-110790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Diehl DL, Gabrielsen JD, Strodel WE. The challenges of endoscopic retrograde cholangiopancreatography in gastric bypass patients: The game is not yet over. Gastroenterology. 2015;148:857–8. doi: 10.1053/j.gastro.2014.11.052. [DOI] [PubMed] [Google Scholar]
- 47.Abu Dayyeh BK, Thompson CC, Gostout C. Endoscopic retrograde cholangiopancreatography after Roux-en-Y gastric bypass: Challenges and cautions. Gastroenterology. 2015;148:858–9. doi: 10.1053/j.gastro.2014.11.051. [DOI] [PubMed] [Google Scholar]
- 48.Bukhari M, Kowalski T, Nieto J, et al. An international, multicenter, comparative trial of EUS-guided gastrogastrostomy-assisted ERCP versus enteroscopy-assisted ERCP in patients with Roux-en-Y gastric bypass anatomy. Gastrointest Endosc. 2018;88:486–94. doi: 10.1016/j.gie.2018.04.2356. [DOI] [PubMed] [Google Scholar]
- 49.Wang K, Zhu J, Xing L, et al. Assessment of efficacy and safety of EUS-guided biliary drainage: A systematic review. Gastrointest Endosc. 2016;83:1218–27. doi: 10.1016/j.gie.2015.10.033. [DOI] [PubMed] [Google Scholar]
- 50.Khan MA, Akbar A, Baron TH, et al. Endoscopic ultrasound-guided biliary drainage: A systematic review and meta-analysis. Dig Dis Sci. 2016;61:684–703. doi: 10.1007/s10620-015-3933-0. [DOI] [PubMed] [Google Scholar]
- 51.Mandai K, Uno K, Yasuda K. Relationship between the intraperitoneal stent length in endoscopic ultrasound-guided hepaticogastrostomy and surgically altered upper gastrointestinal anatomy in patients with malignant biliary obstruction. Gastroenterology Res. 2018;11:305–8. doi: 10.14740/gr1059w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gupta K, Perez-Miranda M, Kahaleh M, et al. Endoscopic ultrasound-assisted bile duct access and drainage: Multicenter, long-term analysis of approach, outcomes, and complications of a technique in evolution. J Clin Gastroenterol. 2014;48:80–7. doi: 10.1097/MCG.0b013e31828c6822. [DOI] [PubMed] [Google Scholar]
- 53.Khashab MA, Van der Merwe S, Kunda R, et al. Prospective international multicenter study on endoscopic ultrasound-guided biliary drainage for patients with malignant distal biliary obstruction after failed endoscopic retrograde cholangiopancreatography. Endosc Int Open. 2016;4:E487–96. doi: 10.1055/s-0042-102648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Poincloux L, Rouquette O, Buc E, et al. Endoscopic ultrasound-guided biliary drainage after failed ERCP: Cumulative experience of 101 procedures at a single center. Endoscop y. 2015;47:794–801. doi: 10.1055/s-0034-1391988. [DOI] [PubMed] [Google Scholar]
- 55.Vila JJ, Pérez-Miranda M, Vazquez-Sequeiros E, et al. Initial experience with EUS-guided cholangiopancreatography for biliary and pancreatic duct drainage: A Spanish national survey. Gastrointest Endosc. 2012;76:1133–41. doi: 10.1016/j.gie.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 56.Oh D, Park DH, Song TJ, et al. Optimal biliary access point and learning curve for endoscopic ultrasound-guided hepaticogastrostomy with transmural stenting. Therap Adv Gastroenterol. 2017;10:42–53. doi: 10.1177/1756283X16671671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kahaleh M. Training the next generation of advanced endoscopists in EUS-guided biliary and pancreatic drainage: Learning from master endoscopists. Gastrointest Endosc. 2013;78:638–41. doi: 10.1016/j.gie.2013.05.034. [DOI] [PubMed] [Google Scholar]
- 58.Dhir V, Itoi T, Fockens P, et al. Novel ex vivo model for hands-on teaching of and training in EUS-guided biliary drainage: Creation of “Mumbai EUS” stereolithography/3D printing bile duct prototype (with videos) Gastrointest Endosc. 2015;81:440–6. doi: 10.1016/j.gie.2014.09.011. [DOI] [PubMed] [Google Scholar]
- 59.Bhutani MS, Aveyard M, Stills HF., Jr Improved model for teaching interventional EUS. Gastrointest Endosc. 2000;52:400–3. doi: 10.1067/mge.2000.108408. [DOI] [PubMed] [Google Scholar]
- 60.Anderloni A, Fugazza A, Troncone E, et al. Single-stage EUS-guided choledochoduodenostomy using a lumen-apposing metal stent for malignant distal biliary obstruction. Gastrointest Endosc. 2019;89:69–76. doi: 10.1016/j.gie.2018.08.047. [DOI] [PubMed] [Google Scholar]
- 61.Kunda R, Pérez-Miranda M, Will U, et al. EUS-guided choledochoduodenostomy for malignant distal biliary obstruction using a lumen-apposing fully covered metal stent after failed ERCP. Surg Endosc. 2016;30:5002–8. doi: 10.1007/s00464-016-4845-6. [DOI] [PubMed] [Google Scholar]
- 62.Tsuchiya T, Teoh AY, Itoi T, et al. Long-term outcomes of EUS-guided choledochoduodenostomy using a lumen-apposing metal stent for malignant distal biliary obstruction: A prospective multicenter study. Gastrointest Endosc. 2018;87:1138–46. doi: 10.1016/j.gie.2017.08.017. [DOI] [PubMed] [Google Scholar]
- 63.Yang J, James T, Baron T, et al. EUS-guided creation of entero-enterostomy using lumen apposing metal stents for pancreaticobiliary access in non-RYGB surgical anatomy patients. DDW 2019 abstracts. Gastrointest Endosc. 2019;89:AB642–AB643. [Google Scholar]
- 64.Fusaroli P, Ceroni L, Caletti G. Forward-view endoscopic ultrasound: A systematic review of diagnostic and therapeutic applications. Endosc Ultrasound. 2013;2:64–70. doi: 10.4103/2303-9027.117689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fusaroli P, Serrani M, Lisotti A, et al. Performance of the forward-view echoendoscope for pancreaticobiliary examination in patients with status post-upper gastrointestinal surgery. Endosc Ultrasound. 2015;4:336–41. doi: 10.4103/2303-9027.170427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Holt BA, Hawes R, Hasan M, et al. Biliary drainage: Role of EUS guidance. Gastrointest Endosc. 2016;83:160–5. doi: 10.1016/j.gie.2015.06.019. [DOI] [PubMed] [Google Scholar]
- 67.Tyberg A, Desai AP, Kumta NA, et al. EUS-guided biliary drainage after failed ERCP: A novel algorithm individualized based on patient anatomy. Gastrointest Endosc. 2016;84:941–6. doi: 10.1016/j.gie.2016.05.035. [DOI] [PubMed] [Google Scholar]
- 68.Park DH, Jeong SU, Lee BU, et al. Prospective evaluation of a treatment algorithm with enhanced guidewire manipulation protocol for EUS-guided biliary drainage after failed ERCP (with video) Gastrointest Endosc. 2013;78:91–101. doi: 10.1016/j.gie.2013.01.042. [DOI] [PubMed] [Google Scholar]