Abstract
EUS-guided gallbladder drainage (EUS-GBD) is utilized for the treatment of acute cholecystitis and symptomatic cholelithiasis in patients who are poor operative candidates. Over the last several years, improved techniques and accessories have facilitated GBD. Recent literature demonstrated effectiveness and safety of EUS-guided GBD. Available data suggest at least similar results when compared to percutaneous cholecystostomy. EUS-guided GBD can be performed as a primary intervention in patients with cholecystitis who are unfit for urgent surgical intervention and as a secondary intervention to internalize biliary drainage in patients with indwelling percutaneous cholecystostomy catheters. Various stents can be used for -EUS-guided GBD. The optimal device and technique have yet to be determined, although at the present time, the use of luminal apposing stents is preferred. The purpose of this review is to provide the highlights of the most recent literature on EUS-guided GBD.
Keywords: Cholecystitis, endoscopic retrograde cholangiography, ERCP, EUS, gallbladder drainage
INTRODUCTION
In the majority of patients with acute cholecystitis, the etiology is gallstones, with a small subsegment of <10% of cases found in patients with acalculous cholecystitis.[1] Acute cholecystitis may develop in 6%–11% of patients with symptomatic gallstones over a period of 7–11 years.[1] Conversely, chronic cholecystitis is a histopathologic term that describes chronic inflammatory cell infiltration of the gallbladder and is also associated with the presence of gallstones. Chronic cholecystitis is felt to be a sequela of long-term inflammation from either the presence of gallstones or repeated episodes of acute cholecystitis.
Acute calculus cholecystitis occurs when the cystic duct is obstructed by an impacted stone and may become further inflamed by lecithin, a constituent of bile.[2] Infection of bile within the biliary tree very likely contributes to the development of cholecystitis, although this is not present in all patients with cholecystitis.[3]
Patients with acute cholecystitis classically develop abdominal pain in the right upper quadrant or epigastrium. This pain is steady and severe lasting >4 h. The pain may radiate to the right shoulder or to the back and is often associated with fever, nausea, vomiting, and anorexia. Physical examination of patients with acute cholecystitis often demonstrates in ill-appearing, febrile, tachycardic patient with signs of parietal peritoneal inflammation such as worsening pain with movement and desire to lie perfectly still. A Murphy's sign may be present, which is positive when increased discomfort is present with palpation of the right upper quadrant on deep inspiration. The sensitivity and specificity of a positive Murphy's sign, when compared to cholescintigraphy, is 97% and 48%, respectively.[4] On laboratory evaluation of the patient with acute cholecystitis, leukocytosis with a prominent left shift is typically present. In addition, serum total bilirubin and alkaline phosphatase may be elevated but is not always present if the obstruction is limited to the gallbladder. Mirizzi syndrome may be present if an impacted cystic duct stone causes extramural compression of the common bile duct; in this case, an elevation in the total bilirubin maybe seen.
The most commonly obtained diagnostic imaging for evaluation of acute cholecystitis is a transabdominal ultrasound, which will demonstrate thickening of the gallbladder wall (>5 mm) or pericholecystic edema. Further, a sonographic Murphy's sign may be seen when the ultrasound transducer palpates near the gallbladder.
Laparoscopic cholecystectomy is now considered the standard approach for surgical treatment of acute calculus cholecystitis. In cases of severe inflammation, adhesive disease, bleeding in the surgical field, or suspected bile duct injury, an open cholecystectomy may be preferred to ensure safe dissection and gallbladder resection. Laparoscopic cholecystectomy has a very low complication rate and is one of the most commonly performed surgeries in the worldwide. Several relative and absolute contraindications to surgical resection of the gallbladder in acute cholecystitis exist, including patients with American Society of Anesthesiologists (ASA) scores of three, four, or five, patients with thrombocytopenia, patients with advanced malignancy, or patients with impaired coagulability, including from cirrhosis of the liver.
Left untreated, cholecystitis may lead to significant complications including gangrenous cholecystitis, perforation, and cholecystoenteric fistula formation. Gangrenous cholecystitis occurs in up to 20% of untreated cases and often leads to sepsis.[5] Gallbladder perforation occurs in approximately 10% of cases of untreated cholecystitis, the perforation typically localized to the fundus of the gallbladder following gangrene development. This perforation may be contained as a pericholecystic abscess, but in cases of free perforation into the peritoneum, generalized peritonitis results with a high associated mortality.[6] Cholecystoenteric fistula may result from long-standing pressure necrosis from stones in the gallbladder, independent from acute cholecystitis; however, when occurring as a consequence of untreated acute cholecystitis, the fistula may occur from perforation of the gallbladder directly into the gastrointestinal lumen.
In patients, not fit to undergo surgery, nonoperative management is typically advised with antibiotic therapy and gallbladder drainage (GBD). Percutaneous transhepatic GBD (PT-GBD) has served as a temporizing method for GBD for many years. Its role in management is ideally to function as a bridge to definitive surgical treatment following improvement of surgical candidacy. However, many patients who undergo PT-GBD never undergo surgical resection of the gallbladder due to advanced age and/or comorbid medical illnesses. In this population, a permanent percutaneous drain may result. This drain has several notable disadvantages including risk of bleeding, bile leakage, pain at the insertion site, and cosmetic dissatisfaction.[7,8] Further, in patients who have received PT-GBD as a stand-alone treatment, cholecystitis recurs in 22%–47% of cases.[9] As a result, percutaneous drainage is associated with a decrease in patient quality of life, and patients overwhelmingly prefer internal biliary drainage when possible.[10] Internal drainage consists of either ERCP with transpapillary cystic duct stent placement or EUS-guided GBD.[11] ERCP with cystic duct stent placement is technically challenging with a high rate of both primary technical failure and need for reintervention due to stent occlusion.[12,13]
EUS-GBD was first described in 2007 using transmural placement of double pigtail biliary stents into the gallbladder of a patient with unresectable hilar cholangiocarcinoma and acute cholecystitis who was felt to be a poor operative candidate.[14] The procedure has been refined considerably over the following years and is utilized more readily in the management of gallbladder disease in patients unable to undergo operative management. In this review, we will discuss the different approaches to EUS-GBD, indications, elements to consider in EUS-GBD, postprocedural care of the EUS-GBD patient, and the outcomes in EUS-GBD. Finally, we will briefly discuss limitations of the current technologies and aspects of the procedure that require further research and development.
PREPROCEDURAL CONSIDERATIONS
As with all complex endoscopic procedures, a preprocedural interdisciplinary planning session among the endoscopist, surgeon, and interventional radiologist can help to create a plan in the event of procedural adverse events and to affirm the sequence of techniques.
EUS-GBD involves placement of a covered self-expandable metal stent (SEMS) or lumen-apposing metal stent (LAMS) into a portion of the gallbladder to create an anastomosis with the duodenum (cholecystoduodenostomy) or stomach (cholecystogastrostomy) [Figure 1]. In cases of surgically altered anatomy, such as Roux-en-Y gastric bypass, the anastomotic tract may be between the gallbladder and jejunum (cholecystojejunostomy). EUS-GBD allows for decompression of the gallbladder regardless of the etiology or degree of obstruction, as it directly bypasses any obstruction. The decision to create a cholecystoduodenostomy, cholecystogastrostomy, or cholecystojejunostomy is based on operator preference, patient-specific anatomy, and proximity of the gallbladder to the lumen. Preprocedural imaging, including computed tomography, is essential in determining if EUS-GBD is feasible and for selecting the site of puncture.
Figure 1.
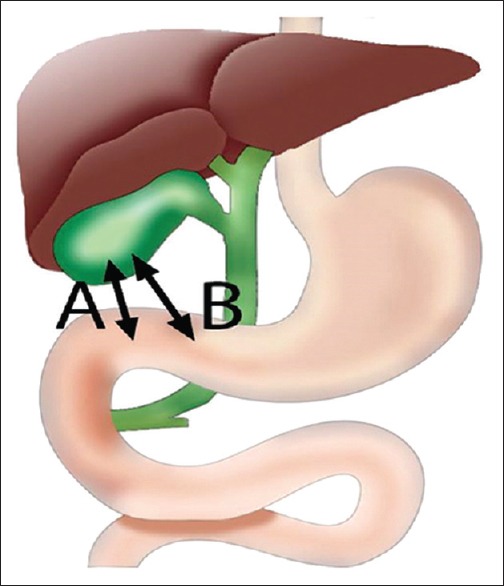
Initial puncture site in EUS-guided gallbladder drainage: (A) Duodenal bulb (B) Gastric antrum
The health and viability of the gastrointestinal mucosa should be considered when selecting an initial puncture site, as interposed cholangiocarcinoma, gastric, or pancreatic cancer may increase the difficulty of the procedure and lead to technical failure and poor clinical outcomes. Malignant tissue is often hypervascular, which can increase the risk of procedure-related bleeding. In addition, invasive cancer in the fourth portion of the duodenum or jejunum can greatly affect the safety and feasibility of the procedure. Ascites, owing to portal hypertension or peritoneal disease, may impair stent anchoring into the targeted bowel; this is less of a concern if the stomach or duodenum is the site of anastomosis as it has a relatively fixed position. Because of the technical difficulty of the procedure, EUS-GBD should be ideally performed using endotracheal intubation.
Pre- and post-procedural administration of intravenous antibiotics may decrease the risk of infection; due to the systemic infection that results from acute cholecystitis, patients are often already on sufficient antibiotic therapy. Since the procedure is both an endoscopic and fluoroscopic procedure, high-resolution X-ray equipment is desirable to increase procedural success.
LUMEN-APPOSING METAL STENT PLACEMENT IN EUS-GUIDED GALLBLADDER DRAINAGE
The LAMS available in the United States used for EUS-GBD is the AXIOS™ stent (Boston Scientific, Marlborough, MA, USA) which comes in diameters of 10, 15, and 20 mm, with a length of 10 mm. Binmoeller and Shah first described LAMS use in a porcine model for the creation of a gastric and enteric anastomosis in 2012.[15] The first use of LAMS for EUS-GBD occurred around 2015.[16] Through tissue apposition, LAMS creates a tract between 2 hollow organs and promotes tissue adhesion while maintaining a patent lumen within the tract, similar to a surgically created anastomosis.
The development of the AXIOS-EC™, an electrocautery-enabled access catheter-enhanced AXIOS™ stent (commonly referred to as “Hot Axios”), allows the option of avoiding initial needle puncture and guidewire placement, and as a result may avoid targeting errors and stent misdeployment as the gallbladder or bowel shifts. The AXIOS-EC™ consists of a fully covered metal stent with bilateral anchoring flanges [Figure 2]. Following deployment, the stent expands until the anchoring flange diameter is 24 mm and the inner portion is 15 mm. These flanges decrease stent migration by evenly distributing pressure on the luminal and gallbladder walls to provide secure anchoring. The stent additionally approximates the duodenal or gastric wall against the gallbladder wall to maintain attachment of the 2 structures.
Figure 2.
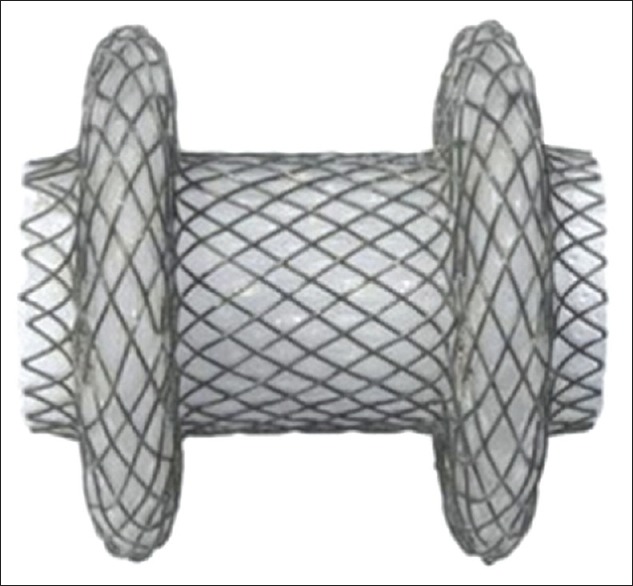
AXIOS™ stent (Boston Scientific, Marlborough, MA, USA)
FULLY COVERED SELF-EXPANDABLE METAL BILIARY STENTS IN EUS-GUIDED GALLBLADDER DRAINAGE
Before the advent of LAMS, fully covered self-expandable metal biliary stents (fcSEMS) were utilized for EUS-GBD. The most commonly used fcSEMS for this purpose is the VIABIL® Biliary Endoprosthesis (W.L. Gore and Associates, Flagstaff, AZ, USA) and is food and drug administration-approved for the palliation of malignant biliary strictures [Figure 3]. Although fcSEMS have largely been replaced by LAMS at most institutions, in instances where the distance between gallbladder and gastrointestinal lumen is beyond the distance safely spanned by a LAMS (10 mm), fcSEMS may be employed. Important characteristics of this stent include nonforeshortening during and after deployment, lack of movement of the stent during deployment as the constraining material is unraveled and prevents the need for counter maneuvers, and the presence of antimigration fins.
Figure 3.

VIABIL® Biliary Endoprosthesis (W.L. Gore and Associates, Flagstaff, AZ, USA)
PROCEDURE DESCRIPTION
The procedure is most commonly performed under endoscopic, endosonographic, and fluoroscopic guidance. A linear array echoendoscope is advanced into the prepyloric antrum or duodenal bulb, depending on proximity to the gallbladder. Color Doppler imaging is utilized to evaluate for interposing vessels and reduce the risk of procedure-related bleeding. Two approaches are used depending on available stents and operator preference. The first approach involves an initial puncture with a 19-gauge FNA needle, advancing through the gastrointestinal lumen and into the gallbladder body or neck while avoiding any intervening vessels.
Bile is then aspirated to initially confirm needle placement and is often sent for bacterial culture and Gram stain to guide antibiotic therapy. Contrast media is then injected into the gallbladder under fluoroscopic guidance to definitively confirm access. A 0.035-inch, 450 cm-long guidewire (Jagwire; Boston Scientific, Natick, Mass) or 0.025-inch guidewire (Visiglide; Olympus, Tokyo, Japan) is then passed through the needle and allowed to be coiled in the gallbladder. The needle is then removed, and if an electrocautery-enhanced delivery system is not used, balloon dilation or use of a needle-knife catheter or cystotome using electrocautery may be used to widen the luminal opening followed by additional tract dilation, if necessary to allow for stent placement. The dilation can be performed using either bougie or balloon dilation, typically up to a diameter of 4–5 mm. If an electrocautery-enhanced stent delivery system is used, needle-knife and dilation of the tract is not required.
Next, the stent is advanced over the guidewire, with the distal portion deployed into the gallbladder and proximal into the gastrointestinal lumen. The distal portion is typically deployed under EUS guidance followed by changing to the endoscopic view for proximal stent release. Correct position is confirmed on endoscopic view by flow of bile through the stent and/or visualization of gallstones. After deployment, the stent may be dilated to its full diameter with a through-the-scope balloon if the clinician plans to evaluate the interior of the gallbladder using an adult upper endoscope. This may be done to facilitate stone removal or lithotripsy at the time of the initial procedure. However, if there is no plan for further intervention after stent placement, the LAMS will self-expand over the next several hours and does not require balloon dilation to function correctly.
The second approach, originally described by Teoh et al., uses a single-step EUS-GBD utilizing the Axios-EC and has become the dominant method when the gallbladder is abutted closely with the duodenum or stomach.[17] In this method, the gallbladder is directly punctured with the cautery-enhanced tip of the Axios-EC delivery system, without prior needle or guidewire insertion. Immediately after entry into the gallbladder, the LAMS is deployed under EUS and endoscopic guidance. This approach is ideal when the gallbladder is distended.
Stent occlusion by food particles can lead to recurrent cholecystitis and has been noted when the drainage site is the stomach rather than the duodenum. In addition, there is a theoretical risk of mucosal bleeding from gallbladder tissue abrading against the stent. For these reasons, we recommend plastic double-pigtail stent placement within the metal stent [Figure 4]. This is typically a 7 French by 3 or 4 cm stent when a 10 mm LAMS or fcSEMS is placed and 10 Fr when larger diameters are chosen. The distance between gallbladder and lumen will need to be considered when selecting the appropriate plastic stent length.
Figure 4.
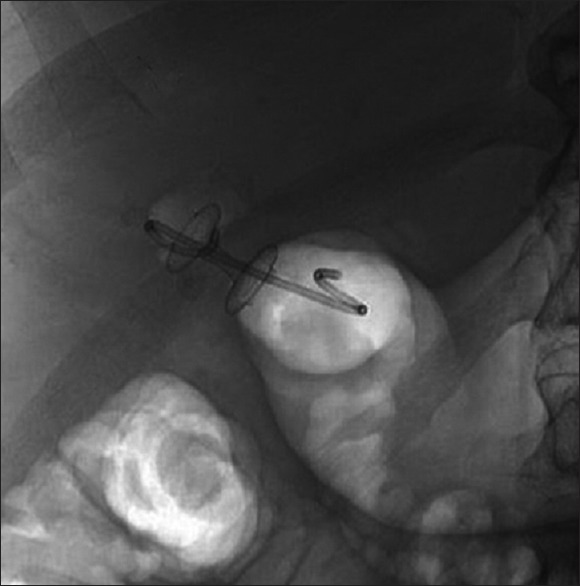
EUS-guided cholecystoduodenostomy using a 10 mm × 15 mm lumen-apposing metal stent with a coaxially placed 7 French by 4 cm double-pigtail plastic stent
MANAGING STENT DISPLACEMENT
EUS-GBD requires perforation of the bowel to create an anastomotic tract with the gallbladder. As long as a stent is deployed correctly and remains in correct position, the gallbladder will be approximated to the gastric wall and negates any clinical consequences of perforation. If the stent is entirely maldeployed such that the stent is fully within the stomach or duodenum, closure of both the gastric/small bowel perforation is desirable, especially if the tract has been balloon dilated. The gastric wall is extremely forgiving and closure often occurs with nasogastric suction. The small bowel puncture site may be closed using through-the-scope or over-the-scope clip placement. A critical principle for technical success is to ensure the guidewire remains in place until it is absolutely certain that the stent is in proper position; this will allow for rescue maneuvers if needed. The main rescue maneuver is placement of an additional LAMS or a through-the-scope SEMS. When a 10-mm LAMS is used, a standard 10-mm biliary fcSEMS is used. In the event when a larger diameter (15 or 20 mm) LAMS is misdeployed, larger stents are needed. In the US and elsewhere, a fully covered 18 or 20 mm mid-body diameter esophageal stent (Niti-S, TaeWoong Medical, Seoul, South Korea) with various lengths is available. We prefer using the 18 mm or 20 mm × 60 mm version. The use of this stent as a rescue maneuver is most useful when the LAMS has been properly deployed on either the luminal or gallbladder side and the other end is deployed in the space between the two organs. Again, as long as wire placement has not been lost, deployment of the stent is relatively easy.
POSTPROCEDURAL CARE
There is no standardized protocol for the care of patients following EUS-GBD; outpatients may not require hospitalization in uncomplicated procedures as long as they meet discharge criteria. Inpatients remain hospitalized for a minimum of 24 h as they are usually quite ill and may require systemic antibiotics. A liquid diet is initially administered and advanced as tolerated after the 1st 24–48 h to a regular diet. There is insufficient evidence to support the use of a soft or low residue diet, often referred to as a “stent diet,” to prevent occlusion of the cholecystoenteric stent by food particles. The patient is ready for discharge from the hospital when they demonstrate resolution of systemic inflammatory response syndrome or biliary pain is improved in the case of a nonseptic patient. While patients may have some postprocedural discomfort, narcotic medications should be avoided when possible to minimize effects on gallbladder motility.
STENT EXCHANGE
There is currently no agreed on duration to leave an expandable metal stent in place following EUS-GBD. However, there are concerns for delayed bleeding and for degradation to the stent covering, which can prevent removal if bleeding or perforation by the stent occurs and may cause obstruction due to tissue hyperplasia. Many centers choose to replace the stent after tract maturation with plastic pigtail stents to allow for continuous drainage without concern for recurrent cholecystitis.
COMPARATIVE OUTCOMES IN EUS-GUIDED GALLBLADDER DRAINAGE
Studies comparing the efficacy of EUS-GBD to PT-GBD have found similar clinical success in the 2 groups; however, adverse events, postprocedure pain scores, length of stay and need for repeat interventions were fewer in the endoscopic group.[18] In a study by Irani et al., the number of reinterventions per patient was 2.5 ± 2.8 in the PT-GBD group, as compared to 0.2 ± 0.4 in the EUS-GBD group.[19] In addition, the median pain scores and postprocedure length of hospital stay were also lower in the EUS-GBD group.
The largest study comparing ERCP with cystic duct stent placement to EUS-GBD in patients unfit for cholecystectomy was performed in Japan by Oh et al.[20] This study retrospectively assessed 172 patients with 76 in the ERCP group and 96 in the EUS-GBD group. The procedural success rate was 83.3% for ERCP and 100% in EUS-GBD. In the 16 failed ERCPs, 12 were due to inability to selectively cannulate the cystic duct and 4 were due to nonvisualization of the cystic duct on fluoroscopy as a result of obstruction. These patients went on to PT-GBD (n = 3), medical treatment (n = 6), and 7 crossed over to EUS-GBD with 100% technical success. The rate of procedural adverse events was similar between ERCP and EUS-GBD at 9.4% and 7.2%, respectively. Of note, recurrent cholecystitis or cholangitis occurred at a higher rate in the ERCP group (17.4%) as compared to the EUS-GBD group (3.9%).
FUTURE DIRECTIONS OF EUS-GUIDED GALLBLADDER DRAINAGE
Despite significant advances in stent technology, EUS-GBD remains a challenging technical feat, often owing to an unstable scope position in the duodenal bulb, decompressed gallbladder, or gallbladder distance from the gastrointestinal lumen. A major obstacle to widespread use of EUS-GBD is the challenge in targeting the gallbladder if it is not in the immediate vicinity of the gastrointestinal lumen or if it is insufficiently distended. To address the issues related to the proximity of the gallbladder, Zhang et al. have investigated the use of a retrievable puncture anchor traction method in a porcine model.[21] These investigators utilized a specialized T-tag to approximate and immobilize the gallbladder to facilitate EUS-GBD followed by removal of the T-tag at the end of the procedure. Use of this method significantly improved the technical success rate of EUS-GBD without issues of gallbladder collapse leading to stent maldeployment. Ultimately, this method may become the standard approach for EUS-GBD, but time will tell.
CONCLUSIONS
EUS-GBD is a promising development in the management of cholecystitis, both acute and chronic, in patients unable to undergo cholecystectomy. Larger comparative studies between percutaneous drain placement and EUS-GBD are required to determine the optimal strategy based on patient characteristics. In addition, long-term care of patients who have undergone EUS-GBD as destination therapy is not well known and additional work is needed to determine the optimal stent exchange interval. Many important technical challenges remain in the safe and successful application of EUS-GBD and require active research and development. As with all patients with complex medical considerations, care should be coordinated through multidisciplinary discussion between therapeutic endoscopists, surgeons, and interventional radiologists.
Financial support and sponsorship
Dr. James receives research and training support in part by a grant from the NIH (T32DK007634).
Conflicts of interest
Dr. Baron is a consultant and speaker for Medtronic, Boston Scientific, W.L. Gore, Cook Endoscopy and Olympus America.
REFERENCES
- 1.Friedman GD. Natural history of asymptomatic and symptomatic gallstones. Am J Surg. 1993;165:399–404. doi: 10.1016/s0002-9610(05)80930-4. [DOI] [PubMed] [Google Scholar]
- 2.Roslyn JJ, DenBesten L, Thompson JE, Jr, et al. Roles of lithogenic bile and cystic duct occlusion in the pathogenesis of acute cholecystitis. Am J Surg. 1980;140:126–30. doi: 10.1016/0002-9610(80)90428-6. [DOI] [PubMed] [Google Scholar]
- 3.Csendes A, Burdiles P, Maluenda F, et al. Simultaneous bacteriologic assessment of bile from gallbladder and common bile duct in control subjects and patients with gallstones and common duct stones. Arch Surg. 1996;131:389–94. doi: 10.1001/archsurg.1996.01430160047008. [DOI] [PubMed] [Google Scholar]
- 4.Singer AJ, McCracken G, Henry MC, et al. Correlation among clinical, laboratory, and hepatobiliary scanning findings in patients with suspected acute cholecystitis. Ann Emerg Med. 1996;28:267–72. doi: 10.1016/s0196-0644(96)70024-0. [DOI] [PubMed] [Google Scholar]
- 5.Reiss R, Nudelman I, Gutman C, et al. Changing trends in surgery for acute cholecystitis. World J Surg. 1990;14:567–70. doi: 10.1007/BF01658790. [DOI] [PubMed] [Google Scholar]
- 6.Derici H, Kara C, Bozdag AD, et al. Diagnosis and treatment of gallbladder perforation. World J Gastroenterol. 2006;12:7832–6. doi: 10.3748/wjg.v12.i48.7832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Winick AB, Waybill PN, Venbrux AC. Complications of percutaneous transhepatic biliary interventions. Tech Vasc Interv Radiol. 2001;4:200–6. doi: 10.1016/s1089-2516(01)90026-5. [DOI] [PubMed] [Google Scholar]
- 8.Carrasco CH, Zornoza J, Bechtel WJ. Malignant biliary obstruction: Complications of percutaneous biliary drainage. Radiology. 1984;152:343–6. doi: 10.1148/radiology.152.2.6739796. [DOI] [PubMed] [Google Scholar]
- 9.Choi JH, Lee SS, Choi JH, et al. Long-term outcomes after endoscopic ultrasonography-guided gallbladder drainage for acute cholecystitis. Endoscopy. 2014;46:656–61. doi: 10.1055/s-0034-1365720. [DOI] [PubMed] [Google Scholar]
- 10.Nam K, Kim DU, Lee TH, et al. Patient perception and preference of EUS-guided drainage over percutaneous drainage when endoscopic transpapillary biliary drainage fails: An international multicenter survey. Endosc Ultrasound. 2018;7:48–55. doi: 10.4103/eus.eus_100_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kjaer DW, Kruse A, Funch-Jensen P. Endoscopic gallbladder drainage of patients with acute cholecystitis. Endoscopy. 2007;39:304–8. doi: 10.1055/s-2007-966335. [DOI] [PubMed] [Google Scholar]
- 12.Itoi T, Sofuni A, Itokawa F, et al. Endoscopic transpapillary gallbladder drainage in patients with acute cholecystitis in whom percutaneous transhepatic approach is contraindicated or anatomically impossible (with video) Gastrointest Endosc. 2008;68:455–60. doi: 10.1016/j.gie.2008.02.052. [DOI] [PubMed] [Google Scholar]
- 13.Mutignani M, Iacopini F, Perri V, et al. Endoscopic gallbladder drainage for acute cholecystitis: Technical and clinical results. Endoscopy. 2009;41:539–46. doi: 10.1055/s-0029-1214727. [DOI] [PubMed] [Google Scholar]
- 14.Baron TH, Topazian MD. Endoscopic transduodenal drainage of the gallbladder: Implications for endoluminal treatment of gallbladder disease. Gastrointest Endosc. 2007;65:735–7. doi: 10.1016/j.gie.2006.07.041. [DOI] [PubMed] [Google Scholar]
- 15.Binmoeller KF, Shah JN. Endoscopic ultrasound-guided gastroenterostomy using novel tools designed for transluminal therapy: A porcine study. Endoscopy. 2012;44:499–503. doi: 10.1055/s-0032-1309382. [DOI] [PubMed] [Google Scholar]
- 16.Irani S, Baron TH, Grimm IS, et al. EUS-guided gallbladder drainage with a lumen-apposing metal stent (with video) Gastrointest Endosc. 2015;82:1110–5. doi: 10.1016/j.gie.2015.05.045. [DOI] [PubMed] [Google Scholar]
- 17.Teoh AY, Binmoeller KF, Lau JY. Single-step EUS-guided puncture and delivery of a lumen-apposing stent for gallbladder drainage using a novel cautery-tipped stent delivery system. Gastrointest Endosc. 2014;80:1171. doi: 10.1016/j.gie.2014.03.038. [DOI] [PubMed] [Google Scholar]
- 18.Kedia P, Sharaiha RZ, Kumta NA, et al. Endoscopic gallbladder drainage compared with percutaneous drainage. Gastrointest Endosc. 2015;82:1031–6. doi: 10.1016/j.gie.2015.03.1912. [DOI] [PubMed] [Google Scholar]
- 19.Irani S, Ngamruengphong S, Teoh A, et al. Similar efficacies of endoscopic ultrasound gallbladder drainage with a lumen-apposing metal stent versus percutaneous transhepatic gallbladder drainage for acute cholecystitis. Clin Gastroenterol Hepatol. 2017;15:738–45. doi: 10.1016/j.cgh.2016.12.021. [DOI] [PubMed] [Google Scholar]
- 20.Oh D, Song TJ, Cho DH, et al. EUS-guided cholecystostomy versus endoscopic transpapillary cholecystostomy for acute cholecystitis in high-risk surgical patients. Gastrointest Endosc. 2019;89:289–98. doi: 10.1016/j.gie.2018.08.052. [DOI] [PubMed] [Google Scholar]
- 21.Zhang K, Sun S, Guo J, et al. Retrievable puncture anchor traction method for EUS-guided gallbladder drainage: A porcine study. Gastrointest Endosc. 2018;88:957–63. doi: 10.1016/j.gie.2018.07.019. [DOI] [PubMed] [Google Scholar]


