Abstract
The EUS-guided biliary drainage (EUS-BD) has gained broad acceptance as the preferred approach after failed ERCP for malignant biliary obstruction. Despite the drainage route, namely, transhepatic or transduodenal, the technical and clinical success rates are high. Because of such good outcomes with tolerable adverse events (AEs) rate, the EUS-BD might soon even replace the ERCP for primary biliary decompression in patients at high risk of failed biliary cannulation. Among the EUS-BD techniques, the choledochoduodenostomy seems to carry the lower risk of AEs and should be considered the first-line EUS approach for biliary decompression.
Keywords: Cholangiopancreatography, EUS-guided biliary drainage, limitations, outcomes
INTRODUCTION
The ERCP is the gold-standard technique to perform the biliary drainage (BD) in either benign or malignant obstruction context. The success rate for biliary cannulation is over 90% in patients with naive gastrointestinal anatomy.[1,2] However, a failed biliary cannulation renders advanced biliary access techniques necessary which carry higher rates of adverse events (AEs) compared to the standard retrograde transpapillary access.[3] Even after employing advanced cannulation techniques or a redo ERCP, the BD cannot be achieved in a small portion of cases.
Until the 2000s, the alternatives to an ERCP failure included surgical or percutaneous transhepatic biliary drainage (PTHD). However, since the Giovannini et al. first reported the EUS-guided BD, it has gained broad acceptance as another plausible alternative.[4,5] In fact, recent guidelines even recommend the EUS-BD to be the preferred approach after a failed ERCP.[6]
The EUS-guided choledochoduodenostomy (CDS) is a type of EUS-guided transluminal technique that creates a fistula communicating the duodenal bulb with the common bile duct (CBD). A therapeutic curved linear array echoendoscope with a large working channel is introduced into the duodenal bulb to perform the biliary access and the stent placement as a one-step procedure. Then, the CBD should be interrogated to assess the severity of dilation and the location of the obstruction. Besides its proximity to the duodenum, the retroperitoneal position renders the CBD an attractive puncture site even in patients with ascites.
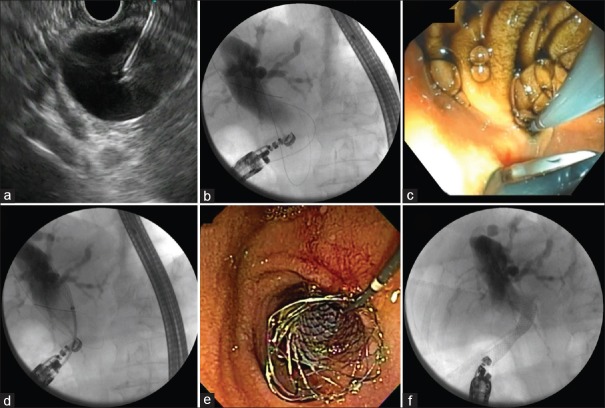
After puncturing the CBD with a 19G needle (directed toward the hilum), the assistant should aspirate bile and inject contrast to obtain a cholangiogram, thus confirming the biliary access. Then, a hydrophilic 0.035-inch guidewire is used to negotiate the CBD above the hepatic confluence. The needle is withdrawn with the wire left in place, and the tract should be dilated using a cystotome or a needle-knife, followed by Soehendra catheter or balloon dilation. Finally, the stent is inserted under endoscopic and fluoroscopic guidance with the proximal end inside the common bile duct (CDB) and the distal in the bulb. The most commonly employed stent is the fully covered biliary stent which can be associated with a double-pigtail plastic stent [Figure 1]. However, partially covered, double pigtails alone [Figure 2], and novel-specific stents – the lumen apposing metallic stents (LAMS) – have also been reported.[6] More recently, the LAMS delivery system has been electrocautery enhanced, which obviated the need for guidewire placement and tract dilation thus shortening the duration of the procedure. Figure 3 illustrates the whole CDS procedure.
Figure 1.
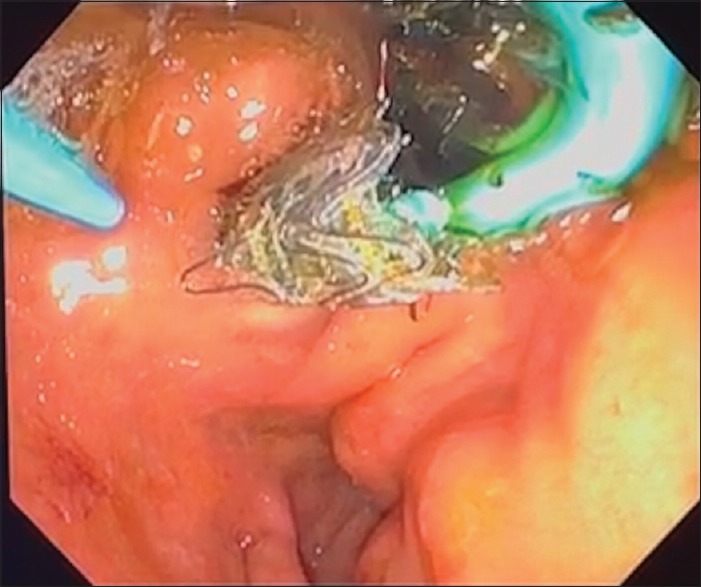
The final aspect of an EUS-guided choledochoduodenostomy using a combination of a self-expandable metallic stent and a double-pigtail plastic stent
Figure 2.
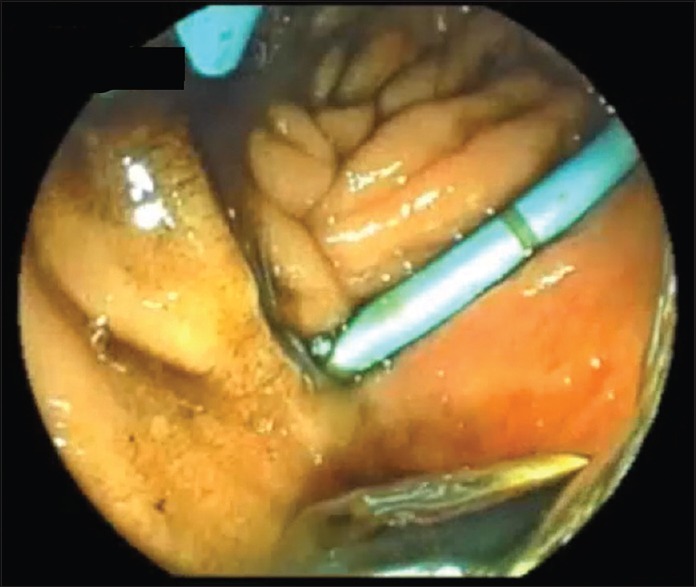
The final aspect of an EUS-guided choledochoduodenostomy using a double-pigtail plastic stent alone
Figure 3.
The step-by-step of an EUS-guided choledochoduodenostomy procedure: (a) Puncture of the dilated common bile duct with a 19G needle; (b) Cholangiogram confirming the position of the needle and the guidewire directed to the hilum; (c) Dilation of the puncture tract using a Soehendra catheter; (d) Fluoroscopic control of the deployment of a metallic stent; (e) Endoscopic final aspect after the deployment of the stent; (f) Fluoroscopic final aspect after the deployment of the stent
Most studies report extremely high technical success rates for the CDS, but clinical success tends to be slightly lower. Its main drawback is the incidence of AEs: Overall rates up to 14%.[7,8] This review article focuses on summarizing the current data concerning outcomes and limitations of the CDS at treating obstructive biliary disorders.
OUTCOMES
Overall
Several recent well-conducted systematic reviews have assessed the efficacy and safety of the CDS, most reporting technical success rates around 90%–95% and clinical success rates around 85%–90%. Hedjoudje et al. included nine articles with a total of 283 patients undergoing CDS. The pooled technical and clinical success rates were 94.6% and 86.9%, respectively, while the overall AEs rate was 20%.[9] Ikeuchi and Itoi included 348 cases from 41 studies and reported a pooled technical success rate of 91.8%. The reasons for technical failure were stent dysfunction, failure to dilate the tract, and guidewire dislodgement. Only 34 articles have also described clinical outcomes: among the 236 CDSs procedures, 223 presented clinical success (94.5%).[10]
Finally, Mohan et al. included 13 articles in a meta-analysis focused exclusively on CDS. A total of 572 patients underwent BD and the pooled overall technical and clinical success rates were 91.9% and 91.9%. The pooled AEs rate was 14.5% among which cholangitis, bleeding, bile leak, and perforation were the most common ones.[11]
COMPARISONS OF OUTCOMES
Surgery versus choledochoduodenostomy
The surgical biliary bypass was the first palliative treatment to address obstructive jaundice and was the main modality to alleviate symptoms of biliary obstruction after failed ERCP for several decades. Although the related mortality has decreased from around 24% to 6.5%, morbidity is still high: rates up to 35%.[12,13,14] In a few centers, especially in underdeveloped countries, the surgical approach is still the first-line palliative therapy after failed ERCP, mainly because of the lack of expertise and availability of alternative techniques such as the percutaneous transhepatic drainage (PTHD) or the EUS-BD [Table 1].
Table 1.
Comparison of outcomes
| EUS-CDS | Surgical HJT | PTHD | EUS-HGT | ERCP (primary) |
|---|---|---|---|---|
| Technical success rate | No difference | No difference | No difference | No difference |
| Clinical success rate | No difference | Favors EUS-BD | No difference | No difference |
| Adverse events | No difference | Favors EUS-BD | Favors EUS-CDS[24] | Favors EUS-CDS[26] |
| Stent patency | - | Favors EUS-BD | No difference | Favors EUS-CDS[26] |
| Level of evidence* | 1B[15] | 1A[16] | 1B[11,23,24] | 1B[4,26,27] |
*According to the Oxford classification.[28] EUS-BD: EUS-guided biliary drainage, EUS-CDS: EUS-guided choledochoduodenostomy, HJT: Hepaticojejunostomy, PTHD: Percutaneous transhepatic biliary drainage, EUS-HGT: EUS-guided hepaticogastrostomy
Artifon et al.[15] compared specifically the CDS with a conventional surgical technique, namely the hepaticojejunostomy (HJT). This prospective randomized study enrolled 32 patients with malignant biliary obstruction (MBO) in whom the ERCP failed to achieve BD. One HJT and 2 CDS could not be performed; thus, the technical success rate was 93.7% in the HJT group and 87.5% in the CDS group. The clinical success rate (93% in the HJT group vs. 71% in the Choledochoduodenostomy (CDT) group) and the complications rate (13.33% in the HJT group vs. 21.42% in the CDT group) were statistically similar for both surgical and endoscopic groups. However, the procedure time was significantly shorter in the CDS group (45.3 min) than in the HJT group (107 min) (P = 0.027).
PTHD versus choledochoduodenostomy
Over the last few years, the EUS-BD techniques have replaced the PTHD. Besides avoiding the loss of electrolytes, the internal drainage also exempts the unpleasant need for an external device.[16]
Téllez-Ávila et al.[17] retrospectively compared 62 patients undergoing either PTHD or EUS-BD. Among the EUS-BD patients, 48.5% were CDSs. The EUS-BD was superior to PTHD in terms of technical success (90% vs. 78%; P = 0.03), clinical success (96% vs. 63%; P = 0.04), AEs (6.6% vs. 28%; P = 0.04), length of stay (6.5 days [0–11] vs. 12.5 days [6–25] P = 0.009), and costs (1440.15 ± 240.94 vs. 2165.87 ± 241.10 American Dollars (USD); P = 0.03).
Sharaiha et al.[16] reviewed nine studies with a total of 482 patients comparing PTHD to the EUS-BD. The EUS-BD was associated with significantly higher clinical success rates, a lower rate of postprocedural AEs, and fewer reinterventions. There was no difference regarding technical success between the two techniques.
As to controlled data, only two prospective randomized trials to date compared directly PTHD to the CDS. Artifon et al.[18] published the first randomized controlled trial (RCT) in 2012 including 25 patients (EUS-CDS = 13 vs. PTHD = 12). The baseline characteristics were similar for both groups. All procedures were technically and clinically successful. At 1-week follow-up, the total bilirubin had reduced significantly in both groups (EUS-CDS: 16.4–3.3; P = 0.002 and PTHD: 17.2–3.8; P = 0.01). Accordingly, there was no difference concerning the final total bilirubin levels (3.3 vs. 3.8, P = 0.2). The AEs rates were 15.3% (2/13) and 25% (3/12) for EUS-CDS and PTBD, respectively (P = 0.44). In addition, overall costs were similar for both groups ($ 5673 EUS-CDS vs. $ 7570 PTHD; P = 0.39).
In 2015, Lee et al. reported the second RCT enrolling 66 patients randomly allocated to the EUS-BD or the PTHD group. There was no loss to follow-up; therefore, 34 and 32 cases were analyzed in the endoscopic and percutaneous groups, respectively. Among the EUS-BD patients, 8 underwent CDS with a clinical success rate of 87.5% (7/8). Overall EUS-BD technical and functional success rates were 94.1% (32/34) and 87.5% (28/32), respectively, versus 96.9% (31/32) and 87.1% (27/31) for the PTHD group. For a noninferiority margin of 15%, the difference found in technical success rates achieved statistical significance (P = 0.008). As to procedure-related AEs (8.8% vs. 31.2%, P = 0.022), re-intervention rate (25% vs. 54.8%, P = 0.015), and length of hospital stay (6 days vs. 12 days), the endoscopic group was superior to the standard PTHD.[19]
Despite limited controlled data, the quality of evidence is adequate to support the current employment of EUS-CDS as a safe alternative to PTHD. In fact, recent guidelines even recommend the EUS-guided interventions to be preferred over the PTHD if adequate endoscopic expertise and logistics are available.[6,20]
Hepaticogastrostomy versus cholodochoduodenostomy
In patients with a distal biliary obstruction, both hepaticogastrostomy (HGT) through a left-sided intrahepatic bile duct and CDS are feasible options.[8] Currently, there is no consensus on the preferred route, but most data show similar functional success with a slightly higher complication rate for the transhepatic approach.[21]
In an international multicenter retrospective cohort published in 2016, Khashab et al.[22] evaluated 121 patients with MBO undergoing CDS (n = 60) or HGT (n = 61). There was no difference regarding technical success rate (CDS 93.3% vs. HGT 91.8%, P = 0.75) and clinical success rate (CDS 85.5% vs. HGT 82.1%, P = 0.64). AEs occurred more frequently in the HGT group (19.67% vs. 13.3%), albeit no statistical difference was found (P = 0.37). Length of the hospital stay was significantly shorter in the CDS group (5.6 ± 6 days vs. 12.7 ± 11.5 days, P < 0.001). Interestingly, the use of noncoaxial electrocautery (odds ratio [OR] 3.95, 95% confidence interval [CI] 1.16–13.40, P = 0.03) and plastic stenting (OR 4.95, 95% CI 1.41–17.38, P = 0.01) were independently associated with the occurrence of AEs in the multivariable analysis.
In a randomized trial published in 2015, Artifon et al.[8] enrolled 49 patients with MBO who either failed ERCP and Rendezvous (n = 9) or had inaccessible papilla (n = 40). The individuals were randomly allocated to CDS (n = 24) or HGT (n = 25). The technical success rate was 96% (24/25) for HGT and 91% (22/24) for CDS (P = 0.60) while clinical success rate was 91% (22/24) for HGT and 77% (17/22) for CDS (P = 0.23). The mean procedure time and the immediate AEs rate were also similar for both groups. Nine (37.5%) and twelve (54.5%) patients died in the HPT and CDS groups, respectively, but no death was related to the procedure or cholangitis. Accordingly, there was no difference concerning the survival time between groups (P = 0.60) while the quality of life (QOL) improved significantly despite the draining route.
Some recent systematic reviews have compared the efficacy and safety profiles of the CDS versus the HGT. Uemura et al.[23] included 10 studies with a total of 434 patients comparing those techniques (CDS = 226, HGT = 208). In highly homogeneous analyses, the authors found similar technical and clinical success rates: 94.1% and 88.5% for CDS, 93.7% and 84.5% for HGT. In addition, there was no difference regarding AEs (OR = 0.97 95% CI 0.60–1.56, I = 37%). Conversely, in a previous meta-analysis including only seven studies, Khan et al.[24] demonstrated fewer AEs for the CDS with a pooled OR = 0.40 (0.18, 0.87).
Finally, Mohan et al.[11] recently published a meta-analysis including 14 independent cohort studies with a total of 596 patients – the largest meta-analysis to date. The primary aim was to assess the AEs rate. The pooled rate of overall AEs was 14.5% in the CDS compared with 20.9% in the HGT group, although the difference did not reach statistical significance (P = 0.10). Interestingly, a subgroup analysis showed fewer complications when LAMS (10.1% vs. 15.9% other stents) and metallic stents (13.4% vs. 19.2% for metallic/plastic) were employed.
ERCP versus choledochoduodenostomy (primary approach)
With the improvement of technique and devices over time, the EUS-BD procedure has become more effective and safer. In this sense, Wang et al. showed higher technical success rates in studies published after 2013 compared to previous publications.[25] This improvement allowed some authors to investigate the outcomes of EUS-BD as a primary approach to address MBO.
To date, three randomized studies compared ERCP to the EUS-BD in this context. Paik et al.[26] included 125 patients (EUS = 64 vs. ERCP = 61) with unresectable malignant distal biliary obstruction from four tertiary centers in South Korea. From the EUS group, 32 patients underwent CDS and the remaining underwent HGT. The overall technical and clinical success rates were 93.8% (60/64) and 90.0% (54/60) for the EUS-BD, and 90.2% (55/61) and 94.5% (52/55) for the ERCP. There was no difference in clinical success (P = 0.49). Interestingly, the EUS-BD group presented lower rates of overall AEs (6.3% vs. 19.7%, P = 0.03). The incidence of postprocedure pancreatitis (0% vs. 14.8%), the need for reintervention (15.6% vs. 42.6%), and the rate of stent patency (85.1% vs. 48.9%) also favored the EUS approach. Finally, the EUS-guided stenting was associated with a greater QOL than the transpapillary approach at the 12-week follow-up. In the EUS group, the technical success rate, clinical success rate, AEs, reintervention, stent patency, and overall survival were similar between CDS and HGT.
Park et al. enrolled 30 patients with distal MBO to undergo either EUS-BD or ERCP. All cases from the EUS-BD were CDSs. Technical success, clinical success, and stent dysfunction rates were similar for both methods. However, the etiologies for stent dysfunction differed significantly: Four cases of tumor in growth in the ERCP group and two cases of food impaction plus two cases of stent migration in the EUS group.[27]
The final RCT was recently published by Bang et al. This trial included 67 patients who were randomly allocated for EUS-BD (n = 33) or ERCP (n = 34). Again, all patients from the EUS group underwent the CDSs. The primary outcome was the rate of AEs: 21.2% versus 14.7% for EUS and ERCP, respectively (P = 0.46). In addition, the procedures had similar rates of technical success (90.9% vs. 94.1%, P = 0.67), clinical success (97% vs. 91.2%, P = 0.61), and need for reinterventions (3.0% vs. 2.9%, P = 0.99).[4]
Nonetheless, there is no comparative meta-analysis to date. Once all RCTs showed equivalent technical and clinical success for ERCP and EUS-BD, pooling data are unlikely to change these observations, but it might favor EUS in terms of AEs. Conclusively, the role of EUS-BD might change in the near future from a rescue to a primary procedure to address the MBO.
ADVERSE EVENTS
The most common AEs of the CDS are duodenal bleeding, biliary fistula formation, and stent migration. The most serious one is the early stent migration or a stent misplacement leading to the need for surgical repair. Besides them, the vast majority of AEs can be managed endoscopically or through interventional radiology, therefore, exempting the need for exploration.[15]
Hedjoudje et al. showed a 20% overall AEs rate in recently published meta-analysis. The most common ones were: stent dysfunction due to clogging/shrinkage/occlusion (3.95%); peritonitis (2.77%); pneumoperitoneum (2.37%); cholangitis (1.98%); and bleeding (1.98%).[9] The AEs rate following the HGT was significantly higher than the CDS (OR = 2.0 1.09–2.17 P < 0.001). Similarly, Khan et al. demonstrated lower complication rates for the CDS (OR = 0.40 0.18–0.87 P < 0.05).[24]
In another recent meta-analysis, Mohan et al. included a total of 572 CDSs and showed a pooled AEs rate of 13.6%. As in the other aforementioned studies, cholangitis (4.2%), bleeding (4.1%), bile leak (3.7%), and perforation (2.9%) were the most frequent complications. Interestingly, a subgroup analysis demonstrated that the AE rate associated with the LAMSs was only 9.3%.[11]
LIMITATIONS OF CHOLEDOCHODUODENOSTOMY
The most common limitation of the CDS is a hilar block which typically only dilates intrahepatic ducts. Because the CBD is close to the portal vein at the duodenal bulb and second portion, the puncture may be risky, particularly in patients with mild extrahepatic dilatation. Moreover, the stent delivery system requires some space to adequately allow deployment.[29] Consequently, most guidelines suggest the transhepatic route in cases of hilar tumors.[6,20] On the other hand, the presence of ascites makes the transhepatic access (whether percutaneous or EUS-guided) more difficult and hazardous.[30] In this context, the CDS becomes the first EUS-guided option over the HGT.
Another very common limitation is the lack of expertise. In fact, the aforementioned guidelines recommend the EUS-BD to be the first option after failed ERCP only if expertise is available. The MBO requiring biliary decompression is a rare condition, and the EUS-BD learning curve is particularly extended: Some studies suggest that expertise is achieved after fifty cases.[31] Consequently, the access to EUS-BD is limited, and procedures should be concentrated in highly specialized centers.
Furthermore, the absence of a written informed consent may hamper the EUS-BD since the safety profile and possible complications differ expressively from those related to the ERCP. In this sense, Khashab et al.[32] have suggested the endoscopist to obtain the informed consent for EUS-BD from all patients at the time of the ERCP, especially from those at high risk of failed biliary cannulation (e.g., surgical anatomy, previous failed ERCP, periampullary cancer with duodenal invasion on imaging, and duodenal stent covering the ampulla).
Finally, the indication to treat both biliary and duodenal obstruction does not restrain the CDS. It can be performed, and a duodenal stent can be placed during the same procedure.[33,34]
CONCLUSION
The EUS-BD is a safe option for BD after failed ERCP, mainly in a context of MBO. Despite the drainage route, the technical and clinical success rates of the EUS-BD are high. Because of such good outcomes with tolerable AEs rate, the EUS-BD might soon replace the ERCP for primary biliary decompression in patients at high risk for failed biliary cannulation. Among the EUS-BD techniques, the CDS seems to carry the lower risk of AEs and should be considered the first-line EUS approach for biliary decompression.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Varadarajulu S, Kilgore ML, Wilcox CM, et al. Relationship among hospital ERCP volume, length of stay, and technical outcomes. Gastrointest Endosc. 2006;64:338–47. doi: 10.1016/j.gie.2005.05.016. [DOI] [PubMed] [Google Scholar]
- 2.Kumar S, Sherman S, Hawes RH, et al. Success and yield of second attempt ERCP. Gastrointest Endosc. 1995;41:445–7. doi: 10.1016/s0016-5107(05)80001-x. [DOI] [PubMed] [Google Scholar]
- 3.Glomsaker T, Hoff G, Kvaløy JT, et al. Patterns and predictive factors of complications after endoscopic retrograde cholangiopancreatography. Br J Surg. 2013;100:373–80. doi: 10.1002/bjs.8992. [DOI] [PubMed] [Google Scholar]
- 4.Bang JY, Navaneethan U, Hasan M, et al. Stent placement by EUS or ERCP for primary biliary decompression in pancreatic cancer: A randomized trial (with videos) Gastrointest Endosc. 2018;88:9–17. doi: 10.1016/j.gie.2018.03.012. [DOI] [PubMed] [Google Scholar]
- 5.Giovannini M, Moutardier V, Pesenti C, et al. Endoscopic ultrasound-guided bilioduodenal anastomosis: A new technique for biliary drainage. Endoscopy. 2001;33:898–900. doi: 10.1055/s-2001-17324. [DOI] [PubMed] [Google Scholar]
- 6.Teoh AY, Dhir V, Kida M, et al. Consensus guidelines on the optimal management in interventional EUS procedures: Results from the Asian EUS group RAND/UCLA expert panel. Gut. 2018;67:1209–28. doi: 10.1136/gutjnl-2017-314341. [DOI] [PubMed] [Google Scholar]
- 7.Kawakubo K, Isayama H, Kato H, et al. Multicenter retrospective study of endoscopic ultrasound-guided biliary drainage for malignant biliary obstruction in Japan. J Hepatobiliary Pancreat Sci. 2014;21:328–34. doi: 10.1002/jhbp.27. [DOI] [PubMed] [Google Scholar]
- 8.Artifon EL, Marson FP, Gaidhane M, et al. Hepaticogastrostomy or choledochoduodenostomy for distal malignant biliary obstruction after failed ERCP: Is there any difference? Gastrointest Endosc. 2015;81:950–9. doi: 10.1016/j.gie.2014.09.047. [DOI] [PubMed] [Google Scholar]
- 9.Hedjoudje A, Sportes A, Grabar S, et al. Outcomes of endoscopic ultrasound-guided biliary drainage: A systematic review and meta-analysis. United European Gastroenterol J. 2019;7:60–8. doi: 10.1177/2050640618808147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ikeuchi N, Itoi T. Endoscopic ultrasonography-guided biliary drainage: An alternative to percutaneous transhepatic puncture. [Last accessed on 2019 Mar 23];Gastrointest Interv. 2015 4:31–9. Available from: http://www.sciencedirect.com/science/article/pii/S2213179515000139 . [Google Scholar]
- 11.Mohan BP, Shakhatreh M, Garg R, et al. Efficacy and safety of endoscopic ultrasound-guided choledochoduodenostomy: A systematic review and meta-analysis. J Clin Gastroenterol. 2019;53:243–50. doi: 10.1097/MCG.0000000000001167. [DOI] [PubMed] [Google Scholar]
- 12.Trede M. Treatment of pancreatic carcinoma: The surgeon's dilemma. Br J Surg. 1987;74:79–80. doi: 10.1002/bjs.1800740202. [DOI] [PubMed] [Google Scholar]
- 13.Tol JA, Eshuis WJ, Besselink MG, et al. Non-radical resection versus bypass procedure for pancreatic cancer – A consecutive series and systematic review. Eur J Surg Oncol. 2015;41:220–7. doi: 10.1016/j.ejso.2014.11.041. [DOI] [PubMed] [Google Scholar]
- 14.Kelz RR, Roses RE, Bartlett EK, et al. Surgical palliation for pancreatic malignancy: Practice patterns and predictors of morbidity and mortality. J Gastrointest Surg. 2014;18:1292–8. doi: 10.1007/s11605-014-2502-8. [DOI] [PubMed] [Google Scholar]
- 15.Artifon EL, Loureiro JF, Baron TH, et al. Surgery or EUS-guided choledochoduodenostomy for malignant distal biliary obstruction after ERCP failure. Endosc Ultrasound. 2015;4:235–43. doi: 10.4103/2303-9027.163010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sharaiha RZ, Khan MA, Kamal F, et al. Efficacy and safety of EUS-guided biliary drainage in comparison with percutaneous biliary drainage when ERCP fails: A systematic review and meta-analysis. Gastrointest Endosc. 2017;85:904–14. doi: 10.1016/j.gie.2016.12.023. [DOI] [PubMed] [Google Scholar]
- 17.Téllez-Ávila FI, Herrera-Mora D, Duarte-Medrano G, et al. Biliary drainage in patients with failed ERCP: Percutaneous versus EUS-guided drainage. Surg Laparosc Endosc Percutan Tech. 2018;28:183–7. doi: 10.1097/SLE.0000000000000528. [DOI] [PubMed] [Google Scholar]
- 18.Artifon EL, Aparicio D, Paione JB, et al. Biliary drainage in patients with unresectable, malignant obstruction where ERCP fails: Endoscopic ultrasonography-guided choledochoduodenostomy versus percutaneous drainage. J Clin Gastroenterol. 2012;46:768–74. doi: 10.1097/MCG.0b013e31825f264c. [DOI] [PubMed] [Google Scholar]
- 19.Lee TH, Choi JH, Park do H, et al. Similar efficacies of endoscopic ultrasound-guided transmural and percutaneous drainage for malignant distal biliary obstruction. Clin Gastroenterol Hepatol. 2016;14:1011–9.e3. doi: 10.1016/j.cgh.2015.12.032. [DOI] [PubMed] [Google Scholar]
- 20.Guo J, Giovannini M, Sahai AV, et al. A multi-institution consensus on how to perform EUS-guided biliary drainage for malignant biliary obstruction. Endosc Ultrasound. 2018;7:356–65. doi: 10.4103/eus.eus_53_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dhir V, Artifon EL, Gupta K, et al. Multicenter study on endoscopic ultrasound-guided expandable biliary metal stent placement: Choice of access route, direction of stent insertion, and drainage route. Dig Endosc. 2014;26:430–5. doi: 10.1111/den.12153. [DOI] [PubMed] [Google Scholar]
- 22.Khashab MA, Messallam AA, Penas I, et al. International multicenter comparative trial of transluminal EUS-guided biliary drainage via hepatogastrostomy vs. choledochoduodenostomy approaches. Endosc Int Open. 2016;4:E175–81. doi: 10.1055/s-0041-109083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Uemura RS, Khan MA, Otoch JP, Kahaleh M, Montero EF, Artifon ELA. EUS-guided Choledochoduodenostomy Versus Hepaticogastrostomy: A Systematic Review and Meta-analysis? J Clin Gastroenterol. 2018;52:123–130. doi: 10.1097/MCG.0000000000000948. doi: 10.1097/MCG.0000000000000948. [DOI] [PubMed] [Google Scholar]
- 24.Khan MA, Akbar A, Baron TH, et al. Endoscopic ultrasound-guided biliary drainage: A systematic review and meta-analysis. Dig Dis Sci. 2016;61:684–703. doi: 10.1007/s10620-015-3933-0. [DOI] [PubMed] [Google Scholar]
- 25.Wang K, Zhu J, Xing L, et al. Assessment of efficacy and safety of EUS-guided biliary drainage: A systematic review. Gastrointest Endosc. 2016;83:1218–27. doi: 10.1016/j.gie.2015.10.033. [DOI] [PubMed] [Google Scholar]
- 26.Paik WH, Lee TH, Park DH, et al. EUS-guided biliary drainage versus ERCP for the primary palliation of malignant biliary obstruction: A multicenter randomized clinical trial. Am J Gastroenterol. 2018;113:987–97. doi: 10.1038/s41395-018-0122-8. [DOI] [PubMed] [Google Scholar]
- 27.Park JK, Woo YS, Noh DH, et al. Efficacy of EUS-guided and ERCP-guided biliary drainage for malignant biliary obstruction: Prospective randomized controlled study. Gastrointest Endosc. 2018;88:277–82. doi: 10.1016/j.gie.2018.03.015. [DOI] [PubMed] [Google Scholar]
- 28.Howick J, Chalmers I, Glasziou P, et al. The Oxford Levels of Evidence. Oxford Centre for Evidence-Based Medicine. [Last accessed on 2015 Mar 02]. Available from: http://www.cebm.net/index.aspx?o=5653 .
- 29.Itoi T, Yamao K EUS 2008 Working Group. EUS 2008 working group document: Evaluation of EUS-guided choledochoduodenostomy (with video) Gastrointest Endosc. 2009;69:S8–12. doi: 10.1016/j.gie.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 30.Artifon EL, Ferreira FC, Sakai P. Endoscopic ultrasound-guided biliary drainage. Korean J Radiol. 2012;13(Suppl 1):S74–82. doi: 10.3348/kjr.2012.13.S1.S74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Poincloux L, Rouquette O, Buc E, et al. Endoscopic ultrasound-guided biliary drainage after failed ERCP: Cumulative experience of 101 procedures at a single center. Endoscopy. 2015;47:794–801. doi: 10.1055/s-0034-1391988. [DOI] [PubMed] [Google Scholar]
- 32.Khashab MA, Levy MJ, Itoi T, et al. EUS-guided biliary drainage. Gastrointest Endosc. 2015;82:993–1001. doi: 10.1016/j.gie.2015.06.043. [DOI] [PubMed] [Google Scholar]
- 33.Artifon EL, Frazão MS, Wodak S, et al. Endoscopic ultrasound-guided choledochoduodenostomy and duodenal stenting in patients with unresectable periampullary cancer: One-step procedure by using linear echoendoscope. Scand J Gastroenterol. 2013;48:374–9. doi: 10.3109/00365521.2012.763176. [DOI] [PubMed] [Google Scholar]
- 34.Rebello C, Bordini A, Yoshida A, et al. A one-step procedure by using linear echoendoscope to perform EUS-guided choledochoduodenostomy and duodenal stenting in patients with irresectable periampullary cancer. Endosc Ultrasound. 2012;1:156–61. doi: 10.7178/eus.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]