INTRODUCTION
Endoscopic transpapillary procedure, so-called ERCP-related procedure, should be considered as the first-line treatment method for biliary disorders such as acute cholangitis, obstructive jaundice, or choledocholithiasis because of its less invasiveness and lower risk of adverse events than other techniques despite the risk of post-ERCP pancreatitis.[1,2,3,4,5] However, ERCP-related procedure is not always successfully accomplished, even when performed by skilled endoscopists, in cases of inability of selective biliary cannulation because of the presence of intradiverticular papillae, a long narrow distal segment of the distal bile duct, or inaccessible papilla because of gastric outlet obstruction or surgically altered anatomy (SAA) even using balloon enteroscope.[6,7] Recently, in such cases, EUS-guided biliary drainage (EUS-BD) approach has been developed and reported as a novel useful alternative technique when standard ERCP-related procedure has failed.[8,9,10,11,12,13,14] As regards EUS-BD for obstructive jaundice due to malignant biliary stricture, EUS-BD is divided into several techniques depending on the approach route.[15] At present, there is no universal consensus on the optimal strategy for which the EUS-BD technique should be selected. Thus far, the selection of the EUS-BD approach depends on the patient's condition, which may involve the presence of gastric outlet obstruction, the site of biliary obstruction, the anastomosis after surgical intervention, or the preference of the endoscopist.[16] In several EUS-BD techniques, EUS-guided choledochoduodenostomy (EUS-CDS) and EUS-guided hepaticogastrostomy (EUS-HGS) are common mostly in patients with malignant diseases at the end stage of disease because these techniques are simple. However, bile flow in EUS-CDS or EUS-HGS is not physiological, resulting in short stent patency rather than retrograde biliary metal stent placement across the stricture site by ERCP. Furthermore, as there are as yet no commercially available dedicated metal stents for EUS-CDS or EUS-HGS around the world, a braided-type covered metal stent is conventionally used on EUS-CDS or EUS-HGS, which has some concern about stent misplacement or migration due to its high shortening rate.[17] Therefore, EUS-guided antegrade stenting (EUS-AGS) has been developed and reported as a useful option of EUS-BD because of the theoretical physiological bile flow in patients with inaccessible ampulla.[18] Moreover, EUS-antegrade intervention (EUS-AI) for benign biliary diseases in patients with surgically altered anatomy that is an antegrade treatment via the approach route created by EUS-HGS has been developed by application of EUS-AGS.[19] Herein, we describe technical details regarding such EUS-guided antegrade procedure for malignant and benign biliary diseases.
EUS-GUIDED ANTEGRADE STENTING FOR MALIGNANT BILIARY OBSTRUCTION
EUS-AGS is a technique to place the drainage stent across the obstruction via the fistula of EUS-BD. The first case report of EUS-AGS was reported by Nguyen-Tang et al. in 2010.[18] EUS-guided antegrade metal stenting was achieved in 5 patients with no procedure-related adverse event. Standard procedures for performing EUS-AGS consist of bile duct puncture, guidewire (GW) manipulation, tract dilation, and stenting as same as EUS-CDS or EUS-HGS. As our way, the left intrahepatic bile duct (IHBD) is punctured with a 19-gauge fine needle under EUS visualization after confirming the absence of intervening blood vessels using color Doppler imaging to prevent bleeding. After the contrast medium is injected into the bile duct for cholangiography, an 0.025-inch GW is inserted into the biliary tract through the needle. In a case with nondilated bile duct, a 0.018-inch GW is used after puncture with a 22-gauge fine needle. Needle tract dilation is performed using a ERCP catheter or a novel ultratapered mechanical dilator, the tip of which is extremely tapered to 2.5-Fr designed for EUS-guided transluminal drainage.[20] Then, the GW is manipulated and advanced through the stricture and the papilla of Vater to the duodenum. Additional cholangiography through the ERCP catheter is carried out to evaluate the length of the obstruction. Thereafter, a stent delivery system of metal stent is inserted, and the metal stent is placed at the stricture site across or above the papilla. Since stent migration does not occur with this technique, it seems to be safer than EUS-HGS.
Iwashita et al.[21] showed in their review that the overall success and adverse event rates of EUS-AGS were 77% and 5%, respectively. The success rate of EUS-AGS is inferior to that of EUS-HGS or EUS-CDS owing to the difficulty of GW passage and stent delivery system insertion across the strictures. To achieve technical success, the use of the ERCP catheter for the GW manipulation approach is effective, which helps achieve better pushability and torque characteristics of the GW within the biliary system, as well as the capability of immediate additional cholangiography through the ERCP catheter, if needed. If it is difficult to pass through the stricture, a hydrophilic GW is useful for manipulation. As regards the selection of the puncture route, compared to accessing the B3 branch, accessing the B2 branch yields a relatively straight course toward the papilla and can improve transmission of the pushing force to the devices to overcome the malignant biliary obstruction. It was reported that, with such technical expertise, high technical and clinical success rates of EUS-AGS (both 95%) could be achieved.[22]
EUS-GUIDED ANTEGRADE STENTING COMBINED WITH EUS-GUIDED HEPATICOGASTROSTOMY
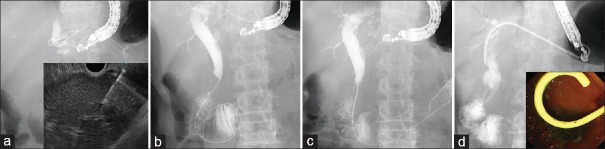
EUS-AGS has some disadvantages. Even if a stent is successfully placed across the stricture, bile leakage from the hepatic puncture site is concerned in cases of stent dysfunction. Furthermore, reintervention owing to stent occlusion is not possible, and EUS-HGS or additional EUS-AGS is required. However, the IHBD may not always be sufficiently dilated to allow puncturing. As one of the options to overcome this disadvantage of EUS-AGS, combination stenting, namely, simultaneous EUS-AGS combined with EUS-HGS, has been developed [Figure 1].[23,24,25] Because of double-stent placement, bile leakage from the fistula may be decreased and longer stent patency may be obtained compared with EUS-HGS or EUS-AGS alone. In fact, the procedure-related adverse event rates in EUS-AGS combined with EUS-HGS were approximately 10% in each institution, which was better than in EUS-AGS alone (20.0%)[21] or EUS-guided hepaticoenterostomy (EUS-HES) alone (26.1%)[23] even in the same institution. In a multicenter prospective study including 49 patients, sufficient stent patency (median 114 days) was achieved, which was similar to median overall survival in the study.[24] Stent dysfunction was seen in only 7 patients (17.5%) until those patients died, and the mean time to dysfunction was 320 days. However, it is necessary to consider the cost-benefit of EUS-AGS combined with EUS-HGS because two stents are placed. As EUS-BD is usually indicated for patients with advanced malignant cancer, the survival time may be short despite chemotherapy. Yamamoto et al.[25] reported the clinical result of EUS-AGS using a metal stent combined with EUS-HGS using a dedicated plastic stent. In this study, stent dysfunction was seen in 13.0% (3/23) of patients in 267, 263, and 135 days after the procedure. The time to stent dysfunction of this study was similar to the previously reported results of EUS-AGS combined with EUS-HGS using two metal stents and the cost performance of EUS-AGS using a metal stent combined with EUS-HGS using a dedicated plastic stent is better. Nevertheless, EUS-AGS with EUS-HGS is still technically challenging. Although the clinical outcome of EUS-AGS with EUS-HGS seems to be better than EUS-HGS alone, conventional EUS-HGS alone is sufficiently acceptable in cases with the difficulty of GW passage across the stricture and papilla, in particular by nonexperts of EUS-BD. The long procedure time leads to the concerns about the peritonitis due to bile leakage. Then, in such cases, it would be better to finish EUS-HGS alone without trying too much, and at a later date, additional AGS would be attempted through the matured HGS tract if necessary.
Figure 1.
EUS-guided antegrade stenting combined with EUS-guided hepaticogastrostomy for malignant biliary obstruction. (a) The left intrahepatic bile duct was punctured with a 19-gauge needle under EUS visualization. (b) The guidewire was advanced to the duodenum through the stricture. (c) An uncovered metal stent was antegradely placed at the stricture site above the papilla. (d) An 8-Fr dedicated plastic stent was placed across the hepaticogastrostomy route
AN OPTIMAL STENT FOR EUS-ANTEGRADE STENTING
Which is the suitable stent placed across the obstruction site for EUS-AGS? First, as there is no stent that can be removed via the fistula antegradely, an uncovered metal stent was chosen in the many institutions to prevent pancreatitis due to blocking the flow of pancreatic juice or cholecystitis due to blocking the cystic duct. However, in the prospective pilot study of EUS-AGS using an uncovered self-expandable metallic stent (uncovered-SEMS), pancreatitis occurred in 15% (3/20) of patients although the pancreatitis was a mild degree and was successfully managed conservatively in all patients.[22] In the multicenter study of EUS-AGS using an uncovered-SEMS, hyperamylasemia was observed in 8.1% (4/49) of patients in spite of hyperamylasemia without any symptoms suggesting acute pancreatitis.[24] Although majority of patients with the indication of EUS-AGS have pancreatic atrophy due to the malignant pancreatic duct obstruction, pancreatitis should be concerned. If possible, the antegrade placement of metal stent above the papilla may be ideal, especially in patients with normal pancreas. Second, the thin delivery system of metal stent is required for EUS-AGS to be inserted via the thin fistula created by EUS-HGS. Moreover, the delivery system should have the good pushability and flexibility in order to advance across the bent IHBD and the severe malignant biliary obstruction. At this point, an uncovered metal stent with 6-Fr delivery systems recently developed to facilitate a single-step simultaneous side-by-side placement for the management of unresectable malignant hilar biliary obstruction would be suitable for EUS-AGS.[26] Third, stent deployment is performed under only fluoroscopic guidance without endoscopic guidance. To prevent stent dislocation or misplacement, a metal stent with low shortening would be suitable for EUS-AGS. Therefore, a laser-cut-type metal stent is well chosen in many institutions. Recently, Yamada et al. reported the technical feasibility and safety of EUS-AGS combined with EUS-HGS using a novel covered metal stent to prolong stent patency more than an uncovered metal stent because patient survival has improved with advances in chemotherapy.[27] This covered metal stent consists of laser-cut metal membranes, which minimizes shortening of the stent, and the size of the stent delivery system is only 7.5-Fr and it has a fine gauge tip to facilitate the insertion into the obstruction site through the fistula. They reported that the novel covered metal stent was placed successfully in 17 patients without pancreatitis, and time to stent dysfunction of the novel covered metal stent group was significantly longer than that of the uncovered metal stent group (not available vs. 150 days, P = 0.02). Further evaluation would be required in terms of the optimal stent for EUS-AGS.
EUS-GUIDED ANTEGRADE INTERVENTION
The efficacy and safety of balloon enteroscopy-assisted ERCP (BE-ERCP) for the management of benign biliary diseases such as anastomotic stricture and/or bile duct stones in patients with SAA has been reported.[6,28,29] However, BE-ERCP is challenging due to the technical difficulty and time constraints, even when performed by skilled pancreaticobiliary endoscopists. Percutaneous transhepatic intervention or surgical intervention has been traditionally performed as an alternative procedure, but these procedures may lead to patient burden owing to the long hospitalization, as well as cosmetic issues, resulting in a decrease in the quality of life.
Recently, antegrade treatment via the approach route created by EUS-BD, so-called EUS-AI, has been developed for the management of benign biliary diseases in patients with SAA.[19,30,31] EUS-BD techniques have been not only BD procedure but also treatment procedure like a transpapillary ERCP-related procedure. The first step of EUS-AI is the creation of the hepatoenteric tract by EUS-HES, including EUS-HGS and EUS-guided hepaticojejunostomy (EUS-HJS). Standard procedures for performing EUS-HES consists of bile duct puncture, guide-wire manipulation, tract dilation, and stenting as same as EUS-HGS procedure for the malignant biliary obstruction. Furthermore, EUS-AI is mainly divided into two procedures: one-stage EUS-AI by EUS and two-stage EUS-AI by ERCP. In one-stage EUS-AI, the antegrade procedure is performed at the same session following the creation of the hepatoenteric tract by the EUS-HES. On the other hand, in two-stage EUS-AI, the antegrade procedure is performed electively 1 or 2 months after the creation of the hepatoenteric tract by the EUS-HES. Two-stage EUS-AI after hepatoenteric tract maturation may be safer than one-stage EUS-AI in terms of the prevention of bile leakage.[32,33]
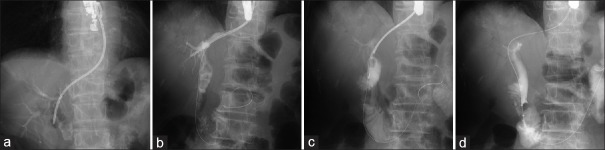
EUS-AI mainly includes antegrade stone removal (ASR) for common bile duct stones or IHBD stones,[34] so-called EUS-guided ASR, and the GW manipulation across the anastomotic stricture after HJS following the antegrade balloon dilation and the AGS for the stricture.[35] In EUS-ASR, after the dilation of the papilla or stricture of the anastomosis was performed using a standard or large balloon, bile duct stones were pushed out of the bile duct in the duodenum or jejunum across the major papilla or the anastomosis and/or were extracted across the fistula by using a basket catheter over the wire [Figure 2]. Two-stage EUS-AI may be recommended for EUS-ASR because the matured access route to the bile duct allows the safe use of mechanical lithotripsy to crush the stones over the wire if necessary. However, if the creation of a hepatoenteric tract by the EUS-HES is not difficult in cases of bile duct stones that are not large (< 15 mm), sequential one-stage EUS-AI would be acceptable, resulting in a decrease of the number of interventions. In case of anastomotic stricture that is not severe (passage of the GW across the anastomotic stricture in <3 min), sequential one-stage EUS-AI would be also acceptable. In fact, we reported that all antegrade treatment under fluoroscopic guidance was achieved with no adverse event related to bile leakage in cases of one-stage EUS-AI even though a number of cases were small (n = 8).[19] Further evaluation is necessary to clarify that the characteristics of stones and/or anatomy are maximally suitable for one-stage EUS-AI.
Figure 2.
EUS-guided antegrade stone removal in patients with Roux-en-Y reconstruction. (a) Creation of the hepaticoenteric tract by EUS-guided hepaticogastrostomy with an 8-Fr dedicated plastic stent. (b) One month later cholangiography was evaluated via the matured hepaticogastrostomy. (c) Mechanical lithotripsy was antegradely performed. (d) The crushed bile duct stones were pushed to the duodenum using a balloon catheter
In the previously reported clinical results about EUS-AI for benign biliary diseases in patients with SAA, the technical success rate ranged from 57% to 100% for the entire cohort.[19,22,30,31,34,35,36,37,38] Adverse events occurred in about 20% of cases and included abdominal pain due to bile leakage, mild pancreatitis, postprocedural cholangitis, and hepatic subcapsular hematoma. These data indicated that EUS-AI could be a useful alternative procedure for benign biliary diseases in patients with SAA although the adverse events' rate is a little high. EUS-AI appears to be a useful procedure, but many cases are particularly demanding technical difficulties. Because the dilated IHBD in cases of benign biliary diseases is often thinner than in cases of obstructive jaundice due to malignant biliary stricture, the puncture of the bile duct may be difficult. Actually, in a multicenter retrospective study from Japan, the treatment success by EUS-AI for bile duct stones in patients with SAA was achieved in 72% (21/29), and reasons for failed EUS-AI were unsuccessful bile duct puncture in 6 patients.[31] On the other hand, the better technical and clinical success rates of EUS-guided AGS for malignant biliary obstruction in patients with SAA (95%, 19/20) have been reported.[22] Furthermore, the fistula dilation and stent placement are difficult in cases in which repeated cholangitis results in a rigid bile duct wall or cases of bile ducts containing many IHBD stones.
AN OPTIMAL STENT FOR EUS-ANTEGRADE INTERVENTION
In the case of two-stage EUS-AI, the drainage stent is placed at the HES site by EUS-HES. A plastic stent or a self-expandable metal stent is available as a drainage stent in EUS-HES. At present, a covered metal stent has become more widely used for malignant biliary obstruction than a plastic stent because of the better drainage made possible by a large bore and the prevention of bile leakage and bile peritonitis. However, the significance of EUS-HES for benign biliary diseases lies more in the creation of an access route than in drainage of bile juice. Basically, the drainage stent is removed for following AI about 1 or 2 months later or after antegrade treatment would be completed. Therefore, long-term patency is not highly important. James et al.[38] reported the clinical experience of EUS-HES for benign biliary diseases in 20 patients with SAA. In this study, they performed EUS-HES using a fully covered metal stent, and in almost all cases, the indwelling metal stents were removed after a mean of 91 days, which is as same as the patency of a plastic stent. When considered from the viewpoint of medical cost, the plastic stent is better than the MS. Furthermore, a fully covered metal stent has the risk of stent misplacement or migration, leading to the possibility of an emergency operation or a fatal adverse event.[17] Other adverse events such as liver abscess, biloma, and focal cholangitis are also concerned by blocking the branch of bile duct.[39,40,41] Previously, Umeda et al.[42] reported the technical feasibility and safety of a newly designed 8F plastic stent dedicated for EUS-HES. They showed that in 23 cases of EUS-HES using their dedicated plastic stent, the technical and clinical success rate was 100% with no stent migration and no bile leakage. This dedicated plastic stent appears to be further suitable for EUS-HES in cases of benign biliary diseases. The advantages of this plastic stent for EUS-HES in benign biliary diseases are as follows: (1) the tapered and straight distal tip can be advanced easily in the bile duct even if many IHBD stones disturb the insertion of the stent, (2) reintervention or stent exchange is easy to perform after stent removal by using a forceps via the HES tract, and (3) there is no concern about proximal and distal stent migration.
PERORAL CHOLANGIOSCOPY-ASSISTED ANTEGRADE INTERVENTION
One of the advantages of EUS-AI over BE-ERCP is that in the complicated bile duct, stone cases or severe anastomotic stricture cases with failure of antegrade GW manipulation under fluoroscopic guidance peroral cholangioscopy are available via the matured hepaticoenteric tract, so-called peroral cholangioscopy-assisted AI (POCS-AI), like a transpapillary procedure.[19] The digital cholangioscope cannot be inserted into the bile duct through the available enteroscope at present.[43,44] To selectively advance the cholangioscope through a curved and tortuous IHBD and directly view the target stones and pinhole anastomosis site, it is particularly effective to use the novel digital cholangioscope that can be operated with 4-way steering.[45] Moreover, specially designed working channels for passing accessories such as electrohydraulic lithotripsy and dedicated irrigation and aspiration channels enable efficient treatment for stones, with good direct visualization. The digital cholangioscope can be advanced directly via the lumen of the metal stent or the matured fistula created by EUS-HE using a plastic stent after tract dilation by a 10-Fr dilation catheter. After the insertion of the cholangioscope, cholangioscopy-guided lithotripsy using electrohydraulic lithotripsy for the stones or GW manipulation across the anastomotic stricture can be performed under direct visualization. POCS-AI is usually conducted by 2 experienced endoscopists, so-called mother–baby method. Actually, it was reported that the technical success rate of EUS-AI could be risen using POCS-AI (91.9%).[19] According to the proposed algorithm, EUS-AI (POCS-AI) may be selected as first-line therapy for cases in which the digital cholangioscope is likely to be used.
BALLOON ENTEROSCOPY-ASSISTED ERCP VERSUS EUS-ANTEGRADE INTERVENTION
In the systematic review including 23 relevant reports of BE-ERCP overall BE-ERCP success for all attempts was approximately 74% with an adverse event rate of 3.4%.[29] In a multicenter U.S. experience of BE-ERCP and rotational overtube-assisted enteroscopy ERCP, successful ERCP was achieved in 63% of patients with an adverse event rate of 12.4%.[28] As BE-ERCP is technically challenging even if it is performed by an experienced endoscopist, the clinical success of EUS-AI appears to be higher than that of BE-ERCP. However, at that time, BE-ERCP appears to be a less invasive and safer procedure than EUS-AI because a systematic review of EUS-BD showed an adverse event rate of 23.3%.[46] Therefore, it is considered that at this time, BE-ERCP is basically first-line therapy, especially for benign biliary diseases, because of a minimally invasive procedure, and EUS-AI should be performed as an alternative procedure. In the international multicenter comparative study between BE-ERCP and EUS-BD for patients with SAA, adverse events occurred more commonly in the EUS-BD group (20% vs. 4%; P = 0.01) although EUS-BD procedures were significantly higher technical success rate (98% vs. 65%, P = 0.001) and less time-consuming with an average of 40 min saved per procedure.[14] A sequential antegrade procedure such as ASR technique is not established, and the devices that can be used are limited compared with BE-ERCP. For example, although some reports showed the efficacy of EUS-ASR in patients with SAA, the risk of bleeding during or after balloon dilation of the papilla or the anastomosis should be a matter of concern. If the bleeding adverse event occurs, endoscopic antegrade hemostasis without an endoscopic view may be difficult. However, in the future, EUS-BD might be considered as a first-line therapy, especially in patients with malignant biliary obstruction or expected long surgical limbs such as Roux-en-Y gastric bypass in which technical failure rate of BE-ERCP is high if expert of EUS-BD procedure is available. Further evaluation will be required about the comparison between BE-ERCP and EUS-antegrade procedure.
CONCLUSION
We described the overview of recently developed EUS-guided antegrade procedures including EUS-AGS for malignant biliary obstruction and EUS-AI for benign biliary diseases. These EUS-guided antegrade procedures were feasible with short procedure time, although the procedure method has not been established and high endoscopic technical skill is required. Further development of dedicated devices for EUS-guided antegrade procedures can expand the indications for the procedure in the future.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
The authors are grateful to Professor Emeritus J. Patrick Barron of Tokyo Medical University for his editorial guidance.
REFERENCES
- 1.Mukai S, Itoi T, Baron TH, et al. Indications and techniques of biliary drainage for acute cholangitis in updated Tokyo guidelines 2018. J Hepatobiliary Pancreat Sci. 2017;24:537–49. doi: 10.1002/jhbp.496. [DOI] [PubMed] [Google Scholar]
- 2.Lai EC, Mok FP, Tan ES, et al. Endoscopic biliary drainage for severe acute cholangitis. N Engl J Med. 1992;326:1582–6. doi: 10.1056/NEJM199206113262401. [DOI] [PubMed] [Google Scholar]
- 3.Leung JW, Chung SC, Sung JJ, et al. Urgent endoscopic drainage for acute suppurative cholangitis. Lancet. 1989;1:1307–9. doi: 10.1016/s0140-6736(89)92696-2. [DOI] [PubMed] [Google Scholar]
- 4.Boender J, Nix GA, de Ridder MA, et al. Endoscopic sphincterotomy and biliary drainage in patients with cholangitis due to common bile duct stones. Am J Gastroenterol. 1995;90:233–8. [PubMed] [Google Scholar]
- 5.Lau JY, Chung SC, Leung JW, et al. Endoscopic drainage aborts endotoxaemia in acute cholangitis. Br J Surg. 1996;83:181–4. [PubMed] [Google Scholar]
- 6.Ishii K, Itoi T, Tonozuka R, et al. Balloon enteroscopy-assisted ERCP in patients with roux-en-Y gastrectomy and intact papillae (with videos) Gastrointest Endosc. 2016;83:377–86.e6. doi: 10.1016/j.gie.2015.06.020. [DOI] [PubMed] [Google Scholar]
- 7.Tsuchiya T, Sofuni A, Tsuji S, et al. Endoscopic management of acute cholangitis according to the TG13. Dig Endosc. 2017;29(Suppl 2):94–9. doi: 10.1111/den.12799. [DOI] [PubMed] [Google Scholar]
- 8.Giovannini M, Moutardier V, Pesenti C, et al. Endoscopic ultrasound-guided bilioduodenal anastomosis: A new technique for biliary drainage. Endoscopy. 2001;33:898–900. doi: 10.1055/s-2001-17324. [DOI] [PubMed] [Google Scholar]
- 9.Kahaleh M, Hernandez AJ, Tokar J, et al. Interventional EUS-guided cholangiography: Evaluation of a technique in evolution. Gastrointest Endosc. 2006;64:52–9. doi: 10.1016/j.gie.2006.01.063. [DOI] [PubMed] [Google Scholar]
- 10.Park DH, Koo JE, Oh J, et al. EUS-guided biliary drainage with one-step placement of a fully covered metal stent for malignant biliary obstruction: A prospective feasibility study. Am J Gastroenterol. 2009;104:2168–74. doi: 10.1038/ajg.2009.254. [DOI] [PubMed] [Google Scholar]
- 11.Artifon EL, Safatle-Ribeiro AV, Ferreira FC, et al. EUS-guided antegrade transhepatic placement of a self-expandable metal stent in hepatico-jejunal anastomosis. JOP. 2011;12:610–3. [PubMed] [Google Scholar]
- 12.Khashab MA, Valeshabad AK, Afghani E, et al. A comparative evaluation of EUS-guided biliary drainage and percutaneous drainage in patients with distal malignant biliary obstruction and failed ERCP. Dig Dis Sci. 2015;60:557–65. doi: 10.1007/s10620-014-3300-6. [DOI] [PubMed] [Google Scholar]
- 13.Itoi T, Dhir V, Moon JH. EUS-guided biliary drainage: Moving into a new era of biliary drainage. Gastrointest Endosc. 2017;85:915–7. doi: 10.1016/j.gie.2017.01.026. [DOI] [PubMed] [Google Scholar]
- 14.Khashab MA, Bukhari M, Baron TH, et al. International multicenter comparative trial of endoscopic ultrasonography-guided gastroenterostomy versus surgical gastrojejunostomy for the treatment of malignant gastric outlet obstruction. Endosc Int Open. 2017;5:E275–81. doi: 10.1055/s-0043-101695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mukai S, Itoi T. How should we use endoscopic ultrasonography-guided biliary drainage techniques separately? Endosc Ultrasound. 2016;5:65–8. doi: 10.4103/2303-9027.180468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Artifon EL, Loureiro JF, Baron TH, et al. Surgery or EUS-guided choledochoduodenostomy for malignant distal biliary obstruction after ERCP failure. Endosc Ultrasound. 2015;4:235–43. doi: 10.4103/2303-9027.163010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martins FP, Rossini LG, Ferrari AP. Migration of a covered metallic stent following endoscopic ultrasound-guided hepaticogastrostomy: Fatal complication. Endoscopy. 2010;42(Suppl 2):E126–7. doi: 10.1055/s-0029-1243911. [DOI] [PubMed] [Google Scholar]
- 18.Nguyen-Tang T, Binmoeller KF, Sanchez-Yague A, et al. Endoscopic ultrasound (EUS)-guided transhepatic anterograde self-expandable metal stent (SEMS) placement across malignant biliary obstruction. Endoscopy. 2010;42:232–6. doi: 10.1055/s-0029-1243858. [DOI] [PubMed] [Google Scholar]
- 19.Mukai S, Itoi T, Sofuni A, et al. EUS-guided antegrade intervention for benign biliary diseases in patients with surgically altered anatomy (with videos) Gastrointest Endosc. 2019;89:399–407. doi: 10.1016/j.gie.2018.07.030. [DOI] [PubMed] [Google Scholar]
- 20.Honjo M, Itoi T, Tsuchiya T, et al. Safety and efficacy of ultra-tapered mechanical dilator for EUS-guided hepaticogastrostomy and pancreatic duct drainage compared with electrocautery dilator (with video) Endosc Ultrasound. 2018;7:376–82. doi: 10.4103/eus.eus_2_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iwashita T, Doi S, Yasuda I. Endoscopic ultrasound-guided biliary drainage: A review. Clin J Gastroenterol. 2014;7:94–102. doi: 10.1007/s12328-014-0467-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Iwashita T, Yasuda I, Mukai T, et al. Endoscopic ultrasound-guided antegrade biliary stenting for unresectable malignant biliary obstruction in patients with surgically altered anatomy: Single-center prospective pilot study. Dig Endosc. 2017;29:362–8. doi: 10.1111/den.12800. [DOI] [PubMed] [Google Scholar]
- 23.Imai H, Takenaka M, Omoto S, et al. Utility of endoscopic ultrasound-guided hepaticogastrostomy with antegrade stenting for malignant biliary obstruction after failed endoscopic retrograde cholangiopancreatography. Oncology. 2017;93(Suppl 1):69–75. doi: 10.1159/000481233. [DOI] [PubMed] [Google Scholar]
- 24.Ogura T, Kitano M, Takenaka M, et al. Multicenter prospective evaluation study of endoscopic ultrasound-guided hepaticogastrostomy combined with antegrade stenting (with video) Dig Endosc. 2018;30:252–9. doi: 10.1111/den.12976. [DOI] [PubMed] [Google Scholar]
- 25.Yamamoto K, Itoi T, Tsuchiya T, et al. EUS-guided antegrade metal stenting with hepaticoenterostomy using a dedicated plastic stent with a review of the literature (with video) Endosc Ultrasound. 2018;7:404–12. doi: 10.4103/eus.eus_51_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kawakubo K, Kawakami H, Kuwatani M, et al. Single-step simultaneous side-by-side placement of a self-expandable metallic stent with a 6-fr delivery system for unresectable malignant hilar biliary obstruction: A feasibility study. J Hepatobiliary Pancreat Sci. 2015;22:151–5. doi: 10.1002/jhbp.173. [DOI] [PubMed] [Google Scholar]
- 27.Yamada M, Ogura T, Kamiyama R, et al. EUS-guided antegrade biliary stenting using a novel fully covered metal stent (with video) J Gastrointest Surg. 2019;23:192–8. doi: 10.1007/s11605-018-3914-7. [DOI] [PubMed] [Google Scholar]
- 28.Shah RJ, Smolkin M, Yen R, et al. A multicenter, U.S. experience of single-balloon, double-balloon, and rotational overtube-assisted enteroscopy ERCP in patients with surgically altered pancreaticobiliary anatomy (with video) Gastrointest Endosc. 2013;77:593–600. doi: 10.1016/j.gie.2012.10.015. [DOI] [PubMed] [Google Scholar]
- 29.Skinner M, Popa D, Neumann H, et al. ERCP with the overtube-assisted enteroscopy technique: A systematic review. Endoscopy. 2014;46:560–72. doi: 10.1055/s-0034-1365698. [DOI] [PubMed] [Google Scholar]
- 30.Itoi T, Sofuni A, Tsuchiya T, et al. Endoscopic ultrasonography-guided transhepatic antegrade stone removal in patients with surgically altered anatomy: Case series and technical review (with videos) J Hepatobiliary Pancreat Sci. 2014;21:E86–93. doi: 10.1002/jhbp.165. [DOI] [PubMed] [Google Scholar]
- 31.Iwashita T, Nakai Y, Hara K, et al. Endoscopic ultrasound-guided antegrade treatment of bile duct stone in patients with surgically altered anatomy: A multicenter retrospective cohort study. J Hepatobiliary Pancreat Sci. 2016;23:227–33. doi: 10.1002/jhbp.329. [DOI] [PubMed] [Google Scholar]
- 32.Nakai Y, Isayama H, Koike K. Two-step endoscopic ultrasonography-guided antegrade treatment of a difficult bile duct stone in a surgically altered anatomy patient. Dig Endosc. 2018;30:125–7. doi: 10.1111/den.12965. [DOI] [PubMed] [Google Scholar]
- 33.Shimatani M, Mitsuyama T, Takaoka M, et al. Role of two-step endoscopic ultrasonography-guided antegrade treatment as an option for bile duct stones in patients with surgically altered anatomy. Dig Endosc. 2018;30:50–1. doi: 10.1111/den.12981. [DOI] [PubMed] [Google Scholar]
- 34.Hosmer A, Abdelfatah MM, Law R, et al. Endoscopic ultrasound-guided hepaticogastrostomy and antegrade clearance of biliary lithiasis in patients with surgically-altered anatomy. Endosc Int Open. 2018;6:E127–30. doi: 10.1055/s-0043-123188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miranda-García P, Gonzalez JM, Tellechea JI, et al. EUS hepaticogastrostomy for bilioenteric anastomotic strictures: A permanent access for repeated ambulatory dilations? Results from a pilot study. Endosc Int Open. 2016;4:E461–5. doi: 10.1055/s-0042-103241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weilert F, Binmoeller KF, Marson F, et al. Endoscopic ultrasound-guided anterograde treatment of biliary stones following gastric bypass. Endoscopy. 2011;43:1105–8. doi: 10.1055/s-0030-1256961. [DOI] [PubMed] [Google Scholar]
- 37.Iwashita T, Yasuda I, Doi S, et al. Endoscopic ultrasound-guided antegrade treatments for biliary disorders in patients with surgically altered anatomy. Dig Dis Sci. 2013;58:2417–22. doi: 10.1007/s10620-013-2645-6. [DOI] [PubMed] [Google Scholar]
- 38.James TW, Fan YC, Baron TH. EUS-guided hepaticoenterostomy as a portal to allow definitive antegrade treatment of benign biliary diseases in patients with surgically altered anatomy. Gastrointest Endosc. 2018;88:547–54. doi: 10.1016/j.gie.2018.04.2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Prachayakul V, Aswakul P. Successful endoscopic treatment of iatrogenic biloma as a complication of endosonography-guided hepaticogastrostomy: The first case report. J Interv Gastroenterol. 2012;2:202–4. doi: 10.4161/jig.23750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Paik WH, Park DH, Choi JH, et al. Simplified fistula dilation technique and modified stent deployment maneuver for EUS-guided hepaticogastrostomy. World J Gastroenterol. 2014;20:5051–9. doi: 10.3748/wjg.v20.i17.5051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hara K, Yamao K, Mizuno N, et al. Endoscopic ultrasonography-guided biliary drainage: Who, when, which, and how? World J Gastroenterol. 2016;22:1297–303. doi: 10.3748/wjg.v22.i3.1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Umeda J, Itoi T, Tsuchiya T, et al. A newly designed plastic stent for EUS-guided hepaticogastrostomy: A prospective preliminary feasibility study (with videos) Gastrointest Endosc. 2015;82:390–6. doi: 10.1016/j.gie.2015.02.041. [DOI] [PubMed] [Google Scholar]
- 43.Tonozuka R, Mukai S, Tsuchiya T, et al. Recanalization after biliojejunostomy by use of a new digital per-oral cholangioscope through the hepaticogastrostomy route. VideoGIE. 2016;1:63–5. doi: 10.1016/j.vgie.2016.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tanaka R, Mukai S, Itoi T, et al. New digital cholangioscopy-guided removal of a transpapillary plastic stent through the hepaticogastrostomy route. Gastrointest Endosc. 2016;84:371. doi: 10.1016/j.gie.2016.03.783. [DOI] [PubMed] [Google Scholar]
- 45.Tanaka R, Itoi T, Honjo M, et al. New digital cholangiopancreatoscopy for diagnosis and therapy of pancreaticobiliary diseases (with videos) J Hepatobiliary Pancreat Sci. 2016;23:220–6. doi: 10.1002/jhbp.328. [DOI] [PubMed] [Google Scholar]
- 46.Wang K, Zhu J, Xing L, et al. Assessment of efficacy and safety of EUS-guided biliary drainage: A systematic review. Gastrointest Endos c. 2016;83:1218–27. doi: 10.1016/j.gie.2015.10.033. [DOI] [PubMed] [Google Scholar]