Abstract
We report a case of trifocal choroidal melanoma (three separate tumors) in a 48-year-old Caucasian female who had been followed for oculodermal melanocytosis since childhood. At presentation, no tumor was present and annual examination was advised. Seventeen years later, three choroidal melanocytic lesions were detected in the right eye. Growth of each was documented, enucleation was performed, and histopathology revealed three independent choroidal melanomas. The patient developed extensive liver and bone metastases and subsequently died. Oculodermal melanocytosis is a risk factor for the development of uveal melanoma and a potential marker for worse prognosis. Careful long-term follow-up is required.
Keywords: Choroid, melanocytosis, melanoma, multifocal, trifocal
Congenital oculodermal melanocytosis is a well-known condition characterized by patchy periocular cutaneous, episcleral, uveal, orbital, meningeal, and palate pigmentation.[1] This condition can lead to uveal melanoma, estimated to occur 1 in 400 affected Caucasians,[2] and melanoma of the ipsilateral orbit and meninges.[1] Patients with ocular melanocytosis and uveal melanoma typically manifest a more fulminant disease with twice greater risk for metastasis compared to those without melanocytosis.[3]
Typically, choroidal melanoma manifests as a unilateral and unifocal tumor.[4] Honavar et al. reported in 2002 that only 19 cases of multifocal uveal melanoma had been recorded over approximately 120 years and nearly all were bifocal (two melanomas in an eye).[4] Trifocal melanoma is exceedingly rare. A PubMed search for <trifocal><melanoma><eye> and <uvea> revealed only one published case of a patient displaying bifocal melanoma in an eye and unifocal melanoma in the contralateral eye.[5] Multifocal uveal melanoma is typically associated with ocular melanocytosis, but can be related to BAP-1 cancer predisposition syndrome.[6,7] It has been estimated that Caucasian individuals with unilateral multifocal melanoma have a thousandfold greater chance of demonstrating ocular melanocytosis.[4] To our knowledge, we describe the first case of three separate melanomas in an eye in a patient with underlying ocular melanocytosis since childhood.
Case Report
In July 1986, a 48-year-old Caucasian female was referred for evaluation of pigmentation on both eyes (OU), which was present since childhood [Fig. 1]. Best corrected visual acuity was 20/20 OU and intraocular pressures were normal OU. Full-face evaluation revealed bilateral blue dermal pigmentation on the temporal scalp and slight pigmentation on the palate was noted. Ophthalmic examination showed diffuse bilateral scleral pigmentation and choroidal darkening and thickening were noted OU. There was no sign of uveal melanoma. Oculodermal melanocytosis was diagnosed, and yearly follow-up was advised.
Figure 1.
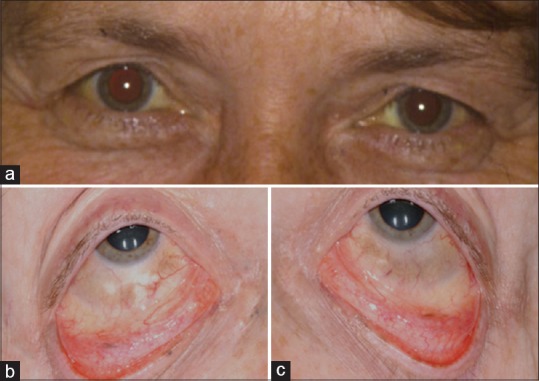
Bilateral scleral melanoctosis. (a) External photograph demonstrating minimal periocular dermal pigmentation, but there was dark blue temporal fossa pigmentation and there was diffuse episcleral melanocytosis of the right (b) and the left (c) eyes
The patient remained stable until October 2002, when a flat amelanotic lesion measuring 2 × 1.5 mm in basal diameter and 1.5 mm in thickness was noted superotemporally in the right eye (OD). In 2003, a new pigmented choroidal lesion was noted inferotemporally OD, measuring 3.5 × 3.5 mm in basal diameter and 2.5 mm in thickness. In 2005, a third pigmented lesion was found inferonasally OD, measuring 3 × 3 mm in basal diameter and 2.7 mm in thickness. Treatment was discussed, but patient preferred close follow-up [Fig. 2a]. Systemic work-up including physical examination, liver function tests, and CT scan of abdomen and chest were advised to rule out malignancy elsewhere in the body.
Figure 2.
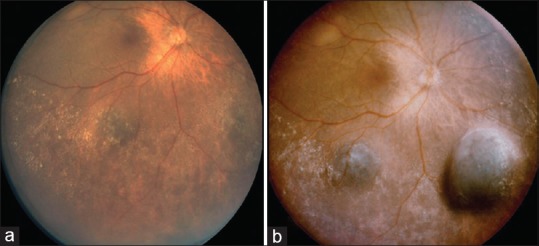
Slow enlargement of three melanomas in an eye with uveal melanocytosis. (a) In 2005, three thin choroidal tumors were noted superotemporally, inferotemporally, and inferonasally, all with bland features. (b) Eight months later, all three tumors showed enlargement
Eight months later, all three tumors enlarged [Fig. 2b], suggesting multifocal choroidal melanoma. Conservative management with plaque radiotherapy and thermotherapy were discussed, but the patient preferred enucleation. Genetic testing for cytogenetics and BAP-1 predisposition syndrome was not performed as it was not routinely available in our practice at the time of treatment.
Histopathology of the globe revealed congenital oculodermal melanocytosis and three distinct choroidal melanomas, including mixed cell type (inferotemporally and inferonasally) and low-grade spindle cell type (superotemporally) [Fig. 3]. Oculodermal melanocytosis with scattered dendritic melanocytes in the epibulbar surface, moderate amount of pigment in the iris and ciliary body stroma, thickened and intensely pigmented choroidal stroma, and discrete foci of melanocytes in the posterior sclera was confirmed on histopathology.
Figure 3.
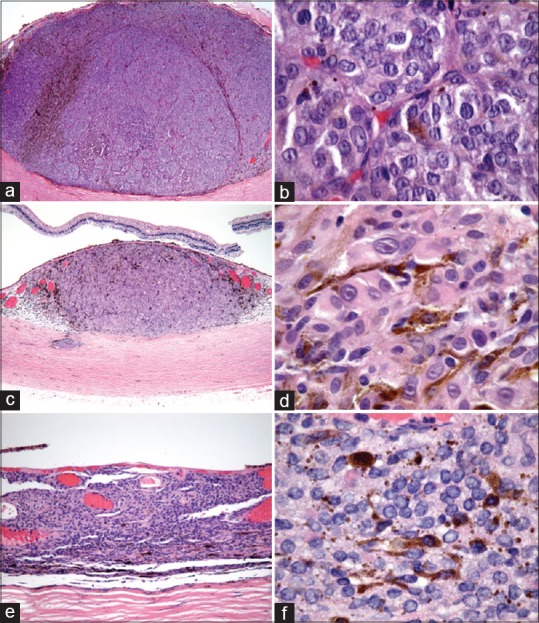
Histopathology of each tumor. (a and b) The inferonasal melanoma demonstrated mixed cell type and 14 mitotic figures in 40 high-power fields (HPF). (c and d) The inferotemporal melanoma revealed mixed cell type and 1 mitotic figure in 40 HPF. (e and f) The superotemporal melanoma demonstrated mixed cell, predominantly spindle type and no mitotic figure in 40 HPF
In December 2008, metastatic melanoma to the liver and later bone was detected, leading to death within one month.
Discussion
Congenital oculodermal melanocytosis is characterized by excessive melanocytes in the periocular region involving the skin, sclera, uvea, orbit, meninges, palate, and/or tympanic membrane.[1,3] This condition carries risk for the development of ipsilateral glaucoma, related to trabecular meshwork pigmentation, as well as uveal melanoma, related to uveal pigmentation.[1] It has been estimated that the lifetime risk for a single uveal melanoma in an, otherwise, healthy Caucasian is 1 in 13,000, compared to 1 in 400 in a Caucasian with ocular melanocytosis.[2] The calculated rate of two melanomas in a Caucasian with melanocytosis would be 1 in 160,000 and three melanomas in the same scenario would be 1/64,000,000, presuming all are independent of each other.[2,4]
Recent data have suggested that ocular melanocytosis, a sporadic condition, is related to a somatic mutation of GNAQ, a heterotrimeric G protein alpha subunit.[8] This mutation is found in blue nevus (83%), uveal melanoma (46%), and oculodermal melanocytosis (6%).[8] Thomas et al. studied skin biopsies of cutaneous melanocytosis in six cases and found GNAQ/GNA11 mutations in three (50%).[9] Tse et al. reported transformation of dermal melanocytosis into melanoma and documented both GNAQ and BAP-1 mutations.[10] Our patient did not have genetic testing as these associations were discovered after her management, but we speculate that GNAQ/GNA11 somatic mutation could have been present.
Outcomes regarding metastases in patients with choroidal melanoma arising from oculodermal melanocytosis has revealed a 1.6 times greater metastatic risk compared to individuals without oculodermal melanocytosis.[3] By Kaplan–Meier analysis, metastatic risk in those with melanoma and melanocytosis was 2% at one year, 27% at five years, and 48% at ten years.[3] Our patient developed metastatic disease to the liver three years following therapy for the trifocal melanoma.
A review of the published literature has revealed one case of trifocal melanoma affecting two eyes and no cases in an eye.[5] In the bilateral case, Lois et al. described a patient without ocular melanocytosis who developed trifocal melanoma involving both eyes in sequential fashion. Two years after treatment, the patient remained alive without metastatic disease. Without evidence of melanocytosis, this patient could have had underlying BAP1 tumor predisposition syndrome or a yet undiscovered germline cancer mutation.[6,7]
Conclusion
In conclusion, our case illustrates an exceedingly rare combination of ocular melanocytosis with three independent melanomas in an eye, which has not been previously described. Interestingly, the patient was followed for 16 years before the choroidal lesions were noted and subsequently developed into melanoma over 2 years. We advise all patients with oculodermal melanocytosis to have twice yearly evaluation and prompt intervention if malignancy is detected.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Shields JA, Shields CL. Intraocular Tumors: An Atlas and Textbook. 3rd ed. Philadelphia (PA): Lippincott, Williams & Wilkins; 2016. [Google Scholar]
- 2.Singh AD, De Potter P, Fijal BA, Shields CL, Shields JA, Elston RC. Lifetime prevalence of uveal melanoma in white patients with oculo (dermal) melanocytosis. Ophtahlmology. 1998;105:195–8. doi: 10.1016/s0161-6420(98)92205-9. [DOI] [PubMed] [Google Scholar]
- 3.Shields CL, Kaliki S, Livesey M, Walker B, Garoon R, Bucci M, et al. Association of ocular and oculodermal melanocytosis with the rate of uveal melanoma metastasis. Analysis of 7872 consecutive eyes. JAMA Ophthalmol. 2013;131:993–1003. doi: 10.1001/jamaophthalmol.2013.129. [DOI] [PubMed] [Google Scholar]
- 4.Honavar SG, Shields CL, Singh AD, Demirci H, Rutledge BK, Shields JA, et al. Two discrete choroidal melanomas in an eye with ocular melanocytosis. Surv Opthalmol. 2002;47:36–41. doi: 10.1016/s0039-6257(01)00281-8. [DOI] [PubMed] [Google Scholar]
- 5.Lois N, Shields CL, Shields JA, De Potter P, Ramsey MS. Trifocal uveal melanoma. Am J Ophthalmol. 1997;124:848–50. doi: 10.1016/s0002-9394(14)71708-8. [DOI] [PubMed] [Google Scholar]
- 6.Rao R, Pointdujour-Lim R, Ganguly A, Shields CL. Multifocal choroidal melanoma in a patient with germ line BRCA-associated protein 1 mutation. Retin Cases Brief Rep. 2016;12:1–4. doi: 10.1097/ICB.0000000000000432. [DOI] [PubMed] [Google Scholar]
- 7.Masoomian B, Shields JA, Shields CL. Overview of BAP1 cancer predisposition syndrome and the relationship to uveal melanoma. J Curr Ophthalmol. 2018;30:102–9. doi: 10.1016/j.joco.2018.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Van Raamsdonk CD, Bezrookove V, Green G, Bauer J, Gaugler L, O’Brien JM, et al. Frequent somatic mutations of GNAQ in uveal melanoma and blue naevi. Nature. 2009;457:599–602. doi: 10.1038/nature07586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thomas AC, Zeng Z, Riviere JB, O’Shaughnessy R, Al-Olabi L, St-Onge J, et al. Mosaic activating mutations in GNA11 and GNAQ are associated with phakomatosis pigmentovascularis and extensive dermal melanocytosis. J Invest Dermatol. 2016;136:770–8. doi: 10.1016/j.jid.2015.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tse JY, Walls BE, Pomerantz H, Yoon CH, Buchbinder EI, Werchniak AE, et al. Melanoma arising in a nevus of Ito: Novel genetic mutations and a review of the literature on cutaneous malignant transformation of dermal melanocytosis. J Cut Pathol. 2016;43:57–63. doi: 10.1111/cup.12568. [DOI] [PubMed] [Google Scholar]


