Abstract
Circumscribed choroidal hemangioma is a benign vascular tumor which presents in middle-aged adults with progressive diminution of vision, metamorphopsia, floaters, and visual field defects. Diagnosis is based on the characteristic clinical features. It is an orange-red, usually solitary, tumor situated in the posterior pole. The visual symptoms are because of the associated subretinal fluid, cystoid macular edema, and, in long-standing cases, retinal pigment epithelium changes, subretinal fibrosis and retinoschisis. It must be distinguished from the more ominous amelanotic melanoma and choroidal metastasis. Diagnostic tools such as ultrasound, fundus fluorescein angiography, indocyanine green angiography, and optical coherence tomography are helpful in cases with diagnostic dilemma. Treatment is indicated in symptomatic cases. The management of choroidal hemangioma has evolved over the years beginning with laser photocoagulation to transpupillary thermotherapy, photodynamic therapy, plaque brachytherapy and external beam radiotherapy. No one therapeutic option holds superiority over the other. In this article, we review the epidemiology, clinical manifestations and treatment of the circumscribed variant of choroidal hemangioma.
Keywords: Brachytherapy, choroidal hemangioma, photodynamic therapy, subretinal fluid, transpupillary thermotherapy
Choroidal hemangioma is a congenital, benign vascular tumor. It is of two types, based on the extent of involvement. Circumscribed choroidal hemangiomas (CCHs) are well-demarcated solitary lesions, usually situated posterior to the equator, while diffuse choroidal hemangiomas have a splashed ketchup appearance with ill-defined thickening of the choroid involving more than one zone (macula, macula to equator, equator to ora) or quadrant. Choroidal hemangioma warrants treatment when it is associated with visual symptoms and exudative retinal detachment.
Various treatment modalities have been used beginning with laser photocoagulation. Photodynamic therapy (PDT) and transpupillary thermotherapy (TTT) have been successful in treating hemangiomas but have their own set of disadvantages. More recently, radiation in the form of external beam radiotherapy (EBRT) and episcleral brachytherapy have paved the way for the management with preservation and improvement of vision.
In this report, we present the clinical manifestations of a CCH, the various tests available to aid in the diagnosis, and the modalities of management for treating this tumor.
Epidemiology
CCH is a hamartoma that may be present at birth. Majority of the patients manifest symptoms and present, however, in adulthood, in the fourth to sixth decades.[1] It is sporadic in nature and there is a slight male predominance.[2,3,4,5] In a review of 200 consecutive patients with CCH, the mean age of presentation was 47 years (range 4–81 years).[2] In a study of clinical and topographical features of CCH in 113 patients, the male-to-female ratio was 1.75.[3] The mean age in the same study was 58 years (range 11–93 years).[3] In a recent study of 238 patients with CCH between 2002 and 2018, the mean age was 53.8 years and 60% of the patients were male.[6] It is more common among Caucasians.[6] It is known that patients with diffuse hemangiomas have other features of Sturge–Weber syndrome. Shields et al. reported four patients with circumscribed hemangioma who had facial nevus flameus and other manifestations of Sturge–Weber syndrome. In addition, one patient had neurofibromatosis and there were five patients with systemic mucosal or remote cutaneous hemangiomas.[2] Systemic hypertension is seen in 33.8% of the patients with CCH.[6]
The exact incidence is not known because only the symptomatic or incidentally diagnosed cases come to light. It is estimated that there is a case of CCH for every 15 cases of choroidal melanoma.[7]
Clinical Features
Patients present with progressive blurring of vision, metamorphopsia, field defects or floaters. Sometimes it may be detected incidentally. Table 1 shows a list of the common symptoms that patients complain of.
Table 1.
Main symptoms of patients with circumscribed choroidal hemangioma
| Symptom | % |
|---|---|
| Blurred vision | 70-80 |
| Metamorphopsia | 3-10 |
| Visual field defect | 7 |
| Photopsia | 1-4 |
| Floaters | 2 |
| Progressive hypermetropia | 1 |
| Ocular pain | 1 |
| No symptoms | 6 |
Symptoms are often subtle, and it can be some time before the patient visits an ophthalmologist. The mean duration of symptoms in the study by Shields et al. was 28 months (range 0–420 months).[2] It is not uncommon for an ophthalmologist to diagnose a choroidal hemangioma during routine examination. Visual symptoms are due to subfoveal fluid and hyperopic shift. Macular elevation/tilting, cystoid macular edema, retinal pigment epithelium (RPE) alteration and photoreceptor degeneration are the other causes of vision loss.[8] Visual acuity can vary from 20/20 to hand motions. Poor initial visual acuity (20/200 or worse) has been shown to be associated with poor final visual acuity (P < 0.0001).[2]
The clinical features of CCH are quite typical. In spite of this, only about 30% of the cases are accurately suspected at the time of referral to an ocular oncologist. This is possibly because the other intraocular tumors, particularly melanoma and metastasis, can mimic a hemangioma due to their myriad of atypical features. Table 2 gives the common referral diagnoses.
Table 2.
Common referral diagnoses for a circumscribed choroidal hemangioma
| Referral diagnosis | % |
|---|---|
| Choroidal hemangioma | 29-33 |
| Unspecified choroidal tumor | 31 |
| Choroidal melanoma | 12-29 |
| Central serous chorioretinopathy | 5-8 |
| Choroidal metastasis | 3-9 |
| Retinal detachment | 6 |
| Choroidal nevus | 2-4 |
| Macular edema | 3 |
| Choroidal granuloma | 1 |
| Vasoproliferative retinal tumor | 1 |
| Optic neuritis | 1 |
| Retinoblastoma | 1 |
| Choroidal osteoma | 1 |
| Age-related macular degeneration | 1-6 |
| High hypermetropia | 1 |
| No diagnosis | 14 |
Intraocular pressure is usually normal. It can be raised in patients with neovascular glaucoma secondary to extensive retinal detachment. These patients have a painful eye with poor visual prognosis. They remain the only cases which are eligible for enucleation for choroidal hemangioma. Anterior segment examination is normal. Rarely, dilated episcleral vessels (4%), heterochromia irides (1%) and iris neovascularisation (1%) may be present which add to the diagnostic dilemma.[2]
Fundus examination reveals a well-defined, orange-red colored mass similar to the choroid itself [Fig. 1]. The base of the tumor can be pigmented, formed by the rim of compressed choroid.[9,10] It is solitary and unilateral.[3] Bilateral cases of CCH have been reported.[11,12,13,14] The height of the hemangioma is usually less than 5 mm and rarely exceeds 6 mm.[3] These tumors generally have a diameter-to-height ratio >2.[3] Macula is the most common location (67%) and the remaining are situated in the posterior pole between the macula and equator, superior and nasal being the common sectors involved, 11% and 14% of the times, respectively.[2] Krohn et al. characterized the topographical distribution of CCHs.[3] They found superotemporal quadrant close to the macula to be the most common location with temporal quadrant (66%) and superior hemisphere (60%) being more frequently involved than nasal quadrant (34%) and inferior hemisphere (45%).[3]
Figure 1.
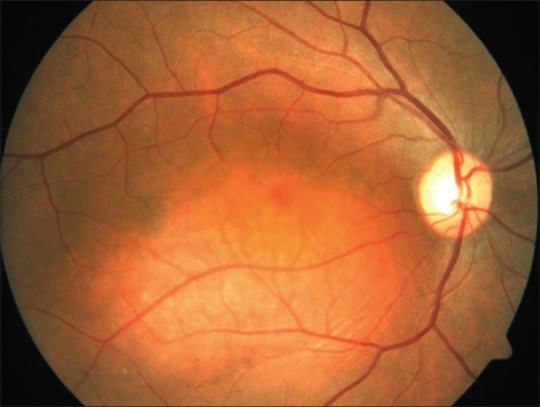
Fundus photograph of a 26-year-old gentleman with circumscribed choroidal hemangioma showing a well-defined orange-red mass situated in the posterior pole involving the fovea
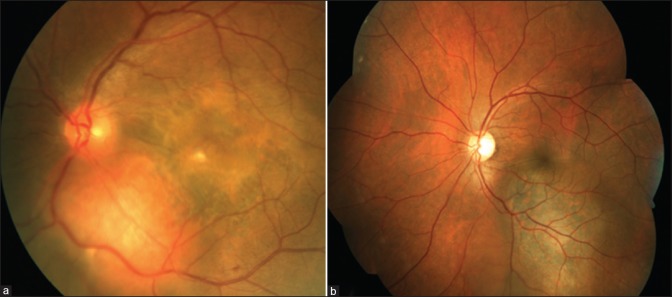
Subretinal fluid (SRF) is found in 80% of the cases over and around the tumor.[2] Presence of SRF is associated with younger age at diagnosis (P = 0.0002), a low basal diameter-to-height ratio (P = 0.0004), nasal location of tumor (P = 0.006), and close proximity to the disc (P = 0.004).[3] Retinal pigment epithelial hyperplasia (33%), fibrous metaplasia (20%), and retinoschisis (5%) are secondary changes seen in the posterior segment. Orange pigments and drusen are extremely rare in a hemangioma and are helpful in distinguishing from choroidal melanoma [Fig. 2]. Macular edema (17%), retinal and subretinal exudates (7%), and, in long-standing cases, epiretinal membrane (7%) and choroidal neovascular membrane (2%) are the other findings and contribute to visual symptoms.[2]
Figure 2.
Fundus photographs of (a) circumscribed choroidal hemangioma with subfoveal fluid, fibrous metaplasia over the fovea and (b) choroidal melanoma with overlying orange pigments
Differential Diagnosis
Differential diagnosis includes choroidal melanoma especially the amelanotic variant. Amelanotic melanoma has a yellow-tan color with subtle pigmentation, appreciable intrinsic vascularity, and overlying drusen. The basal diameter-to-height ratio is <2 in about 50% of the cases.[15] Patients are older and the retinal detachment is usually more extensive and bullous. Choroidal metastasis is creamy yellow, plateau, or dome-shaped and can be bilateral and multifocal. Orange-colored choroidal metastatic lesions are seen in patients with carcinoid tumor, renal cell carcinoma, and thyroid carcinoma.[9,16] Central serous chorioretinopathy typically occurs in middle-aged adults and examination shows an elevated area in the macula with SRF. It can regress spontaneously or can become chronic. Posterior nodular scleritis can also simulate a tumor at the posterior pole but has associated signs and symptoms of inflammation, pain, anterior scleritis, and vitreous cells.[17] Various ancillary tests are there which help in cases which are difficult to diagnose clinically.
Investigations
On ultrasound, hemangiomas are dome-shaped and have high internal reflectivity on A-scan and acoustic solidity similar to the surrounding choroid on B-scan. The echogenic character is similar to the normal choroid[2] [Fig. 3]. Melanoma will classically show moderate to low internal reflectivity with acoustic hollowing. On fluorescein angiography, hemangioma shows varying degrees of hyperfluorescence in all phases. There is early lacy mild hyperfluorescence in the prearterial or early arterial phase and diffuse intense hyperfluorescence in the late phase[2] [Fig. 4]. Indocyanine green (ICG) angiography shows early rapid filling with extreme hyperfluorescence in the first minute and a “washout” phenomenon with relative hypofluorescence compared with the surrounding normal choroid by 20 min[18,19,20] [Fig. 5]. The choroidal filling in fluorescein angiography and ICG is slower and less intense in both melanoma and metastasis.[18] On magnetic resonance imaging, it appears hyperintense to vitreous on T1 and hyper-isointense on T2 with contrast enhancement. Melanoma, on the other hand, is hyperintense on T1 but hypointense on T2-weighted images.[21,22,23]
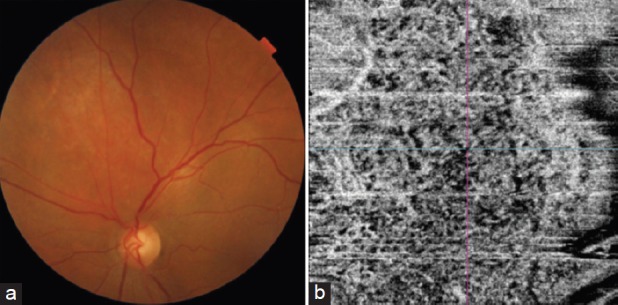
Figure 3.
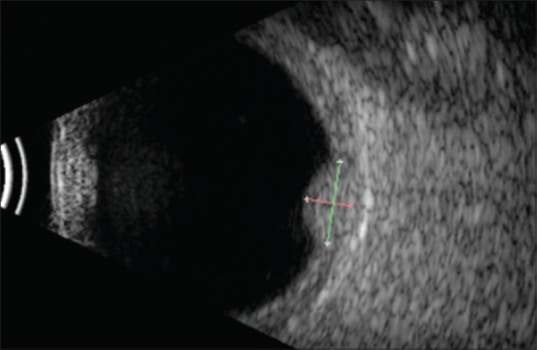
Ultrasound B-scan of a circumscribed choroidal hemangioma showing an acoustically solid dome-shaped mass with echogenicity like that of the surrounding normal choroid
Figure 4.
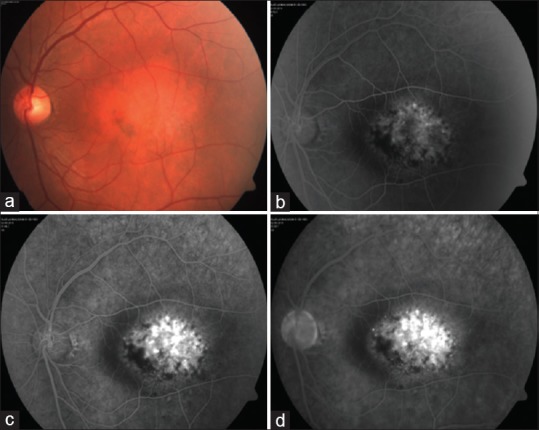
(a) Fundus photograph of a macular circumscribed choroidal hemangioma and the fundus fluorescein angiography showing (b) early lacy hyperfluorescence (30 s), (c) intense hyperfluorescence (1.42 min) that persists in (d) the late phase (15 min)
Figure 5.
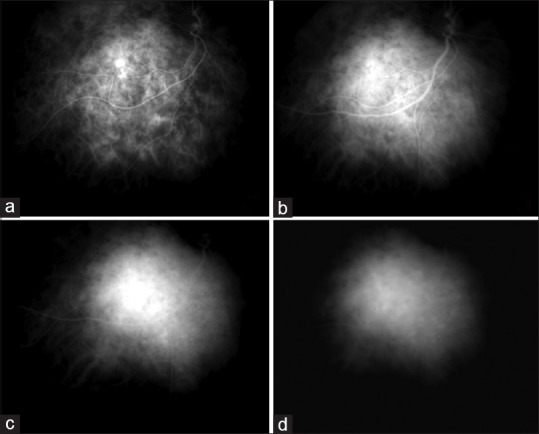
ICG angiography in a case of circumscribed choroidal hemangioma. The arterial phase shows filling of intratumoral vessels on a hypofluorescent tumoral background (a). During the venous phase, the tumor reaches maximal intensity of fluorescence (b), with superimposed hyper- and hypofluorescent spots. The late phases (c and d) show a hypofluorescent lesion with sparse hyperfluorescent caverns. Early hyperfluorescence and late hyporfluorescence on ICG is termed “washout phenomenon,” typical of choroidal hemangioma
Newer testing modalities include enhanced-depth imaging (EDI) optical coherence tomography (OCT) and OCT-angiography (OCT-A). EDI-OCT of CCH shows a smooth choroidal mass with gentle slope. It helps to understand the pathological changes happening in choroid and different layers of retina with histopathology-like sections. This is important because we do not have many histopathological studies on hemangioma since the one done in 1976 by Witschel and Font.[24,25,26] They described CCH as a solitary, sharply demarcated tumor causing compression of adjacent choroidal melanocytes and lamellae.[24] EDI-OCT images display the expansion of medium-and large-sized choroidal vessels without compression of choriocapillaris and an intact Bruch's membrane. The retinal abnormalities that are seen include SRF, lipofuscin deposition, irregularity and thinning of RPE, absence or irregularity of the ellipsoid layer, absent external limiting membrane, disruption of outer nuclear and outer plexiform layer, irregularity of inner nuclear layer, and structural loss or edema of inner plexiform layer.[27]
OCT-angiography is a noninvasive modality that shows a dense irregular vascular network in the choriocapillary and deeper choroidal layers which appears larger than the surrounding normal choroidal vessels [Fig 6]. It is a rapid tool which can be used to monitor treatment response. It is based on split-spectrum decorrelation angiography. In OCT-A, the tumor vessel area is defined as percentage area occupied by large vessels. The flow area is measured by summing the pixel area with the active vascular flow. Cennamo et al. followed up seven patients treated with Ruthenium-106 plaque brachytherapy for a year. Intratumor OCT-angiography showed statistically significant reduction in vessel and flow areas (P = 0.006; 0.002) and resolution of SRF and macular edema.[29] In a study by Chawla et al., OCT-A of CCH before and after laser photocoagulation showed laser-induced damage to the overlying choriocapillaris with no loss or damage to the medium-sized vessels of the tumor itself.[30] This questions the basic rationale of using laser photocoagulation to treat hemangiomas.
Figure 6.
(a) Fundus photograph of the left eye showing circumscribed choroidal hemangioma superior to the disc with subretinal fluid at the macula and (b) optical coherence tomography angiography showing a dense vascular network in the choriocapillary layer. (figure reprinted with permission from Dr Mahesh Shanmugam, Konana VK, Shanmugam PM, Ramanjulu R, Mishra KD, Sagar P. Optical coherence tomography angiography features of choroidal hemangioma. Indian journal of ophthalmology. 2018 Apr;66(4):581.)[28]
Management
Asymptomatic cases of CCH are kept under observation. The main aim of treatment is to resolve the subfoveal fluid and macular edema which is causing diminution of vision.[2] The decrease in size of the tumor is an additional outcome but not the primary goal. Various management modalities have been advocated over the years.
Laser photocoagulation is effective in treating choroidal hemangiomas[2,5,31,32,33,34] with resolution of SRF reported in 62%–100% of the cases. Xenon arc lasers were replaced by argon laser over the years. Scatter laser photocoagulation technique is used with spot size of 200–500 μm, duration of 0.5–1.0 s, and moderately intense power. The laser spots are placed one-half to one burn-width apart to produce a white color change.[35] The entire surface of the tumor is treated with laser and results in retinal and choroidal scarring which can itself hamper the patient's visual recovery. The SRF responds well but the tumor does not reduce in size. Recurrence of SRF has been observed in more than 50% of the cases treated with laser photocoagulation. Anand et al. reported the reaccumulation of SRF in 40% of the cases treated with laser photocoagulation.[36] Retreatments are necessary and this adds to the scarring. Damage to the retinal structures such as the nerve fiber layer results in visual field defect.[37] Laser photocoagulation should not be used for subfoveal hemangiomas or those with extensive SRF.
TTT is another method that results in almost 100% resorption of SRF with a decrease in tumor height by 42% as described by Arumi et al.[38] It uses a diode laser with a wavelength of 810 nm, with broad beam and long exposure time with deeper penetration and causes hyperthermia and occlusion of the blood vessels within the tumor. TTT is performed under local (retrobulbar) or topical anesthesia with either a slit-lamp biomicroscopy delivery system or using a laser indirect ophthalmoscope. The spot size varies between 2 and 3 mm and the initial power setting is 200–300 mW. The power can be gradually increased in increments of 50–100 mW till a light grey color change is produced over the tumor surface during the latter half of a 1 minute application period. The duration of each spot is 1 minute and the entire tumor surface is covered in an overlapping fashion with exposure time varying depending on the size of the tumor.[35] TTT is repeated at 2–3 months interval if the subfoveal fluid persists. Treatment can be stopped once the fluid resolves, and tumor regression continues for many months after TTT.
ICG-enhanced TTT is also being used as an alternative to PDT. It is more easily available, cost is less, and being a weak photosensitizer, strict light protection measures are not required after the procedure. It acts by both the photodynamic and thermal effects and has been found to be effective in causing resolution of SRF, tumor shrinkage, and improving visual acuity.[39]
TTT produces heat-induced sclerosis of the vascular channels.[35] Histopathological studies done on TTT-treated eyes enucleated for choroidal melanoma showed thrombosis of the tumor vessels and cytolysis of tumor cells and vascular endothelium.[40] Connolly et al. studied the effect of TTT on normal retina in enucleated specimen of eyes with choroidal melanoma. They found that low- (430 mW) or medium- (530 mW) dose TTT produces mild or no change on the outer sensory retina but high-dose TTT (630 mW) can produce sensory retinal damage especially in heavily pigmented fundus (racial or with choroidal melanoma).[41]
TTT should be used to treat CCHs with anterior edge posterior to the equator, largest base diameter <10 mm, tumor thickness <4 mm, and with shallow overlying SRF.[35] Tumors greater than these dimensions are associated with extensive, exudative retinal detachment which hamper in the effective visualisation required for focussing the laser beam. Such tumors are best treated with radiotherapy.[35]
Two situations need special consideration while treating a hemangioma with TTT – juxtapapillary and subfoveal tumors. TTT done adequately for a tumor touching the optic disc can rarely cause thermal papillopathy (2.5%).[42] With such lesions, there is a higher risk of nerve fiber layer defect. Inadequate treatment to avoid these complications may lead to treatment failure. If symptomatic juxtapapillary hemangiomas are treated with TTT, sparing of the disc margin is recommended.[35] TTT is avoided for subfoveal CCH because of the risk of damage to the sensory retina. Extrafoveal portions of the tumor may be treated.43] PDT is a better option for the management of such cases because of its selective effect on abnormal blood vessels while sparing the normal choriocapillaris and sensory retina.[44,45] Tumors with increased thickness, subretinal fibrosis, and those treated previously with laser photocoagulation may not respond to TTT as these prevent effective uptake of heat within the tumor.[35,43] TTT is associated with complications such as damage to the RPE, branch retinal vein occlusion, recurrent macular edema, scarring and preretinal fibrosis.[46] TTT can also lead to retinal tears, retinal traction or hemorrhage. Chorioretinal atrophy and visual field loss are seen, especially in cases which have been retreated with TTT. Focal iris atrophy can happen if the broad laser beam hits a partially dilated pupil.
PDT with verteporfin causes selective occlusion of vessels with minimal collateral retinal damage.[35,47] The photochemical binds to low-density lipoproteins in the endothelium of the tumor. The laser, which is subsequently applied, affects only the areas that are bound to the photochemical. The standard dose used is 6 mg/m2 of the intravenous verteporfin infused over 10 min (with an added 5 min before laser activation). A bolus infusion over 1 min has also been used to reduce the washout.[48] The laser settings have varied in literature. The standard setting is 50 J/cm2 power, 600 mW/cm2 fluence, and 83 s duration.[49] A single spot and for larger tumors, multiple, nonoverlapping spots are used. Double-duration PDT over 166 s with bolus infusion and double laser power of 100 J/cm2 with full fluence (600 mW/cm2) have been used.[50,51,52,53,54] Double power is efficient in cases requiring retreatment because of increased thickness of the lesions. A double duration (166 s) with standard power setting of 50 J/cm2 has been used with the rationale of slower blood flow through choroidal hemangiomas without increasing the risk of extensive choroidal atrophy or ischemia because of double power.[49] This setting of double duration with standard power was found to have better improvement in visual acuity.[49] Low-fluence PDT using 25 J/cm2 has been tried for central serous chorioretinopathy but its role in choroidal hemangioma is yet to be established. Fluorescein angiography of CCH cases treated with PDT shows nonperfusion, reduced leakage, and focal choroidal atrophy.[48] PDT can be repeated upto four times for persistent SRF at 3 monthly intervals.[49]
Studies have shown excellent results with complete regression of tumor, rapid resorption of SRF, and favorable visual outcomes. It can also be performed as an outpatient procedure under topical anesthesia. The main problem is that about 15% of the cases require more than one session of PDT. Persistent choroidal ischemia and atrophy, and visual field defects can develop with focal overtreatment.[38,47,48,54,55] A case of polypoidal choroidal vasculopathy following PDT for CCH has also been reported.[56] It depends on visualization of the tumor to aim the beam and is difficult to perform in tumors lying beneath large serous retinal detachment. It is also not possible to perform PDT in large tumors situated anteriorly.[57]
Radiation therapy is coming up in a big way for the management of choroidal hamenagiomas. It is preferred in subfoveal hemangiomas and those with extensive bullous retinal detachments. EBRT is used for diffuse choroidal hemangiomas with large retinal detachment. Schilling described the usefulness of EBRT in the treatment of 22 cases of CCH with no complications related to radiation.[58] Recent advances in the form of beam rotation in stereotactic modalities and Bragg peak in proton beam radiotherapy have greatly reduced stray radiation, and therefore the complications of external radiotherapy making it almost comparable to brachytherapy. Kivelä et al., Levy-Gabriel et al., and Kong et al. have demonstrated encouraging results of stereotactic radiotherapy, proton beam therapy, and gamma knife radiosurgery, respectively, for choroidal hemangiomas.[59,60,61] The major drawback of EBRT is the cost and its unavailability in all hospitals. There is also slow absorption of SRF, and normal tissues are exposed to radiation with higher risk of radiation-induced keratoconjunctivitis, cataract, radiation retinopathy, and optic neuropathy.[56,57,61,62] Proton beam radiotherapy uses charged particles and allows a homogeneous dose of radiation at the tumor but sparing the surrounding tissue.[46] The cost and limited availability are the major drawbacks.
Episcleral plaque brachytherapy offers the advantage that it can be available in any eye hospital, and of ease of handling and storage. Co-60, I-125, Pd-103, and Ru-106 are the various radioactive isotopes which have been used successfully with early, complete resolution of SRF, regression of tumor, and improvement in vision [Fig. 7]. It is also effective for eyes where other treatments have failed. It treats the tumor from its base and is closer to the choroid and therefore the site of pathology. This also reduces the amount of anterior segment complications from radiotherapy. The disadvantage is that it requires two surgeries, one for insertion of the plaque and the other for removal.[46] The apex dose used has varied in literature ranging from as high as 40–60 Gy with Cobalt-60 plaque to low dose of 25 Gy.[64,65]
Figure 7.
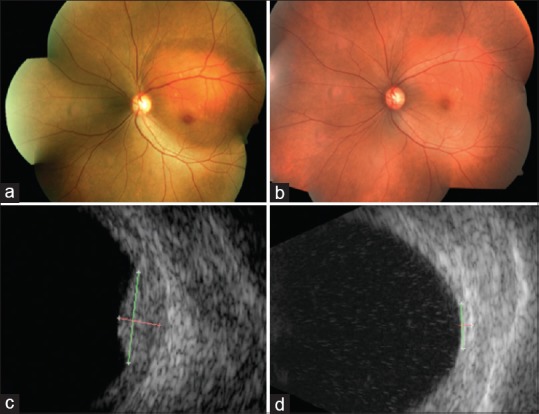
(a) Fundus photograph and (c) ultrasound of a 52-year-old lady with juxtapapillary circumscribed choroidal hemangioma of the left eye with surrounding subretinal fluid involving the fovea causing diminution of vision (20/400). She was treated with Ruthenium-106 plaque brachytherapy with apex dose of 40 Gy. Six months after the treatment, (b) the lesion had regressed as a placoid scar with (d) no measurable mass on ultrasound and with complete resolution of subretinal fluid and visual acuity of 20/40
Table 3 gives an overview of the studies on different treatment modalities and their main outcomes.
Table 3.
Studies showing the results of different treatment modalities for circumscribed choroidal hemangioma
| Author | Year | Sample size | Treatment | Follow-up months | Tumor regression | SRF resolved (%) | Visual acuity improved/stable (%) | Complications | Additional treatment |
|---|---|---|---|---|---|---|---|---|---|
| Jamison et al.[71] | 2018 | 17 | PDT | 36.5 | 17 in 1 year (100) | 10/17 (58.9) | CNV - 1 patient | 4 - retreatment due to increasing SRF, 2 - TTT | |
| Singh et al.[72] | 2004 | 10 | PDT | 7 | 10 | 10 (100) | 8/10 (80) | Choroidal atrophy - 2 | |
| Cennamo et al.[73] | 2016 | 25 | TTT | 49 | 25 | 25 (100) | 17/25 (85) | Preretinal fibrosis - 3, fibrovascular retinal fibrosis - 2, subfoveal detachment - 3, BRVO - 1 | Retrobulbar steroid injection for persistent SRF or intraretinal fluid - 4; re-TTT - 2-10%, 3-5% |
| Gunduz[35] | 2004 | 38 | TTT | 2-44 | 36/38 | 38 (100) | 26/38 (68.4) | CME - 3, preretinal fibrosis - 2, focal iris arophy - 3, retinal vascular occlusion - 1 | |
| Kivelä et al.[59] | 2003 | 5 | Low-dose (20 Gy) stereotactic RT | 34 | 5 | 5 (100) | 4/5 (80) | RPE mottling - 2 PSC - 1 | |
| Levy-Gabriel et al.[60] | 2009 | 71 | Proton beam (20 cobalt Gy equivalent), four fractions | 52 | 71 | 71 (100) | 37/71 (52) | Cataract - 28%, radiation maculopathy - 8% | |
| Mahdjoubi et al.[66] | 2018 | 43 | Hyperfractionated proton beam, eight fractions, 20 Gy relative biological effectiveness | 26 | 43 | 42 (97.6) | 37/43 (86) | Cataract - 3 | |
| Naseripour et al.[62] | 2017 | 21 | Ruthenium 106 plaque | 12 | 21 | 21 (100) | 19/21 (90.5) | Radiation retinopathy - 5, 24%, radiation papillopathy - 1, 5%, subretinal fibrosis - 2, 10% | |
| Lopez-Caballero et al.[8] | 2009 | 8 | I-125 plaque | 83 | 8 | 8 (100) | 6 (75) | Radiation retinopathy - 3, subretinal fibrosis - 1 |
PDT: photodynamic therapy; TTT: transpupillary thermotherapy; CNV: Choroidal neovascularization; SRF: subretinal fluid; BRVO: Branch Retinal Vein Occlusion; CME: Cystoid Macular Edema; RPE: retinal pigment epithelium; PSC: Posterior Subcapsular cataract
Intravitreal anti-VEGF injections are effective in resolving macular edema but they need to be repeated. These are usually combined with other therapies mentioned above.[35,46,66,67] In a series of three cases of choroidal hemangioma, Mandal et al. used the pan-VEGF inhibitor bevacizumab (1.25 mg, two doses, 6 weeks apart) and showed complete resolution of subfoveal fluid. The injection was given as a secondary measure in two patients following the failure of reduction of SRF after TTT and laser photocoagulation, respectively, and as a primary treatment in the third patient who was treated with laser photocoagulation along with the second sitting of injection.[68] Combined anti-VEGF injection with PDT has been shown to result in rapid and sustained resolution of SRF for 4 years and marked improvement in visual acuity.[69] Sagong et al. have described the use of anti-VEGF for pretreatment of two cases of CCH followed by PDT after 1 week.[70] These cases demonstrate that anti-VEGF effectively attains the primary goal of treatment of choroidal hemangioma, that is, resolution of SRF. It acts as an adjuvant treatment to reduce the post-PDT inflammation that results in transient choroidal effusion, perifoveal hemorrhage, vascular occlusion, and polypoidal choroidal vasculopathy.[69] Finally, it can be used to reduce the SRF prior to PDT to allow better visualization.[70] However, because it does not act on the underlying pathology which is the tumor itself but only reduces its complication, it has to be combined with other modalities of treatment.
Enucleation is limited to painful blind eyes with neovascular glaucoma.[66]
In a review of 458 patients over a period of 51 years (pre-PDT era 1967–2001 and PDT era 2002–2018), the treatment of choroidal hemangioma shows a shift from argon laser photocoagulation (42.1% vs. 0.4%) and observation (48.6% vs. 47.6%) to PDT (0% vs. 43.8%), plaque radiotherapy (7% vs. 5.2%), and EBRT (1.4% vs 1.3%). Final visual acuity was better for patients in the PDT era (logMAR 1.28 vs. 0.51, P < 0.001).[6] Papastefanou et al. compared the results of lens-sparing EBRT (n = 23), plaque brachytherapy (n = 3), and PDT (n = 16).[49] There was no difference in the visual acuity gain between EBRT and PDT, but all three patients treated with plaque showed a decrease in visual acuity. Radiation-related complications were noted in 10 of 23 (44%) and 2 of 3 (67%) of the patients treated with EBRT and plaque, respectively.[49] Plaque brachytherapy still remains a preferred option of treatment because of its ease of administration, minimal collateral damage, and usefulness for a variety of hemangiomas in terms of location and amount of SRF.
Conclusion
CCH is a relatively rare tumor that becomes visually debilitating because of its location on the posterior pole and SRF exudation. The aim of each of the therapeutic options described above is to improve or preserve the visual acuity. While each of them have their advantages and drawbacks, it is difficult to prove the supremacy of one over the other in the absence of large-scale comparative studies between the different treatment modalities. In spite of complete resolution of SRF, the visual acuity would remain poor (<20/200) in more than 60% of the patients at 10 years.[2] This has improved over the recent years with the use of PDT and EBRT with 47%–75% of the patients achieving a vision of ≥20/40 depending on their initial visual acuity.[6] Correct diagnosis based on the clinical manifestations and ancillary tests, timely referral to an ocular oncologist and early initiation of treatment are essential for achieving a better visual outcome.73
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Mashayekhi A, Shields CL. Circumscribed choroidal hemangioma. Curr Opin Ophthalmol. 2003;14:142–9. doi: 10.1097/00055735-200306000-00006. [DOI] [PubMed] [Google Scholar]
- 2.Shields CL, Honavar SG, Shields JA, Cater J, Demirci H. Circumscribed choroidal hemangioma: Clinical manifestations and factors predictive of visual outcome in 200 consecutive cases. Ophthalmology. 2001;108:2237–48. doi: 10.1016/s0161-6420(01)00812-0. [DOI] [PubMed] [Google Scholar]
- 3.Krohn J, Rishi P, Frøystein T, Singh AD. Circumscribed choroidal haemangioma: Clinical and topographical features. Br J Ophthalmo. 2019;103:1448–1452. doi: 10.1136/bjophthalmol-2018-313388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schalenbourg A, Piguet B, Zografos L. Indocyanine green angiographic findings in choroidal hemangiomas: A study of 75 cases. Ophthalmologica. 2000;214:246–52. doi: 10.1159/000027499. [DOI] [PubMed] [Google Scholar]
- 5.Sanborn GE, Augsburger JJ, Shields JA. Treatment of circumscribed choroidal hemangiomas. Ophthalmology. 1982;89:1374–80. doi: 10.1016/s0161-6420(82)34635-7. [DOI] [PubMed] [Google Scholar]
- 6.Shields CL, Dalvin LA, Lim LA, Chang M, Udyaver S, Mazloumi M, et al. Circumscribed choroidal hemangioma: Visual outcome in the pre-photodynamic therapy (PDT) vs PDT eras in 458 cases. Ophthalmology Retina. 2019 doi: 10.1016/j.oret.2019.08.004. In Press. [DOI] [PubMed] [Google Scholar]
- 7.Jarrett WH, Hagler WS, Larose JH, Shields JA. Clinical experience with presumed hemangioma of the choroid: Radioactive phosphorus uptake studies as an aid in differential diagnosis. Trans Sect Ophthalmol Am Acad Ophthalmol Otolaryngol. 1976;81:862–70. [PubMed] [Google Scholar]
- 8.Lopez-Caballero C, Saornil MA, De Frutos J, Bianciotto C, Muiños Y, Almaraz A, et al. High-dose iodine-125 episcleral brachytherapy for circumscribed choroidal haemangioma. Br J Ophthalmol. 2010;94:470–3. doi: 10.1136/bjo.2009.160184. [DOI] [PubMed] [Google Scholar]
- 9.Shields JA, Shields CL. Atlas of Intraocular Tumors. Philadelphia, PA: Lippincott Williams & Wilkins; 1999. pp. 170–9. [Google Scholar]
- 10.Shields JA, Shields CL. Intraocular Tumors: A Text and Atlas. Philadelphia, PA: Saunders; 1992. pp. 239–60. [Google Scholar]
- 11.Schepens CL, Schwartz A. Intraocular tumors. I. Bilateral hemangioma of the choroid. AMA Arch Ophthalmol. 1958;60:72–83. [PubMed] [Google Scholar]
- 12.Perri P, Incorvaia C, Costagliola C, Parmeggiani F, Lamberti G, Paduano B, et al. Bilateral circumscribed haemangioma of the choroid not associated with systemic vascular syndrome. Br J Ophthalmol. 2001;85:1260–1. doi: 10.1136/bjo.85.10.1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tran HV, Schalenbourg A, Zografos L. Bilateral circumscribed choroidal hemangioma in an otherwise healthy individual. Retin Cases Brief Rep. 2007;1:149–52. doi: 10.1097/01.ICB.0000279646.84077.b4. [DOI] [PubMed] [Google Scholar]
- 14.Rahman W, Horgan N, Hungerford J. Circumscribed choroidal haemangioma mimicking chronic central serous chorioretinopathy. J Fr Ophtalmol. 2013;36:e37–40. doi: 10.1016/j.jfo.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 15.Krohn J, Frøystein T, Dahl O. Posterior uveal melanoma. Distribution of the sites of origin and patterns of tumour extent in the ocular fundus. Br J Ophthalmol. 2008;92:751–6. doi: 10.1136/bjo.2007.133025. [DOI] [PubMed] [Google Scholar]
- 16.Shields CL, Shields JA, Gross NE, Schwartz GP, Lally SE. Survey of 520 uveal metastases. Ophthalmology. 1997;104:1265–76. doi: 10.1016/s0161-6420(97)30148-1. [DOI] [PubMed] [Google Scholar]
- 17.Agarwal R, Lavric A, Resotri M, Pavesio C, Sagoo MS. Nodular posterior scleritis: Clinic-sonographic characteristics and proposed diagnostic criteria. Retina. 2015;0:1–10. doi: 10.1097/IAE.0000000000000699. [DOI] [PubMed] [Google Scholar]
- 18.Shields CL, Shields CL, De Potter P. Patterns of indocyanine green videoangiography of choroidal tumours. Br J Ophthalmol. 1995;79:237–45. doi: 10.1136/bjo.79.3.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arevalo JF, Shields CL, Shields JA, Hykin PG, De Potter P. Circumscribed choroidal hemangioma: Characteristic features with indocyanine green videoangiography. Ophthalmology. 2000;107:344–50. doi: 10.1016/s0161-6420(99)00051-2. [DOI] [PubMed] [Google Scholar]
- 20.Verbeek AM, Koutentakis P, Deutman AF. Circumscribed choroidal hemangioma diagnosed by ultrasonography. A retrospective analysis of 40 cases. Int Ophthalmol. 1995;96(19):185–9. doi: 10.1007/BF00133736. [DOI] [PubMed] [Google Scholar]
- 21.Mafee MF. Uveal melanoma, choroidal hemangioma, and simulating lesions. Role of MR imaging [review] Radiol Clin North Am. 1998;36:1083–99. doi: 10.1016/s0033-8389(05)70233-5. [DOI] [PubMed] [Google Scholar]
- 22.DePotter P, Shields JA, Shields CL, editors. MRI of the Eye and Orbit. Philadelphia, PA: Lippincott; 1995. pp. 55–92. [Google Scholar]
- 23.Stroszczynski C, Hosten N, Bornfeld N, Wiegel T, Schueler A, Foerster P, et al. Choroidal hemangioma: MR findings and differentiation from uveal melanoma. AJNR Am J Neuroradiol. 1998;19:1441–7. [PMC free article] [PubMed] [Google Scholar]
- 24.Witschel H, Font RL. Hemangioma of the choroid. A clinicopathologic study of 71 cases and a review of the literature. Surv Ophthalmol. 1976;20:415–31. doi: 10.1016/0039-6257(76)90067-9. [DOI] [PubMed] [Google Scholar]
- 25.Shields JA, Stephens RF, Eagle RC, Jr, Shields CL, De Potter P. Progressive enlargement of a circumscribed choroidal hemangioma. A clinicopathologic correlation. Arch Ophthalmol. 1992;110:1276–8. doi: 10.1001/archopht.1992.01080210094033. [DOI] [PubMed] [Google Scholar]
- 26.Spraul CW, Kim D, Fineberg E, Grossniklaus HE. Mushroom shaped choroidal hemangioma. Am J Ophthalmol. 1996;122:434–6. doi: 10.1016/s0002-9394(14)72076-8. [DOI] [PubMed] [Google Scholar]
- 27.Rojanaporn D, Kaliki S, Ferenczy SR, Shields CL. Enhanced depth imaging optical coherence tomography of circumscribed choroidal hemangioma in 10 consecutive cases. Middle East Afr J Ophthalmol. 2015;22:192. doi: 10.4103/0974-9233.150629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Konana VK, Shanmugam PM, Ramanjulu R, Mishra KD, Sagar P. Optical coherence tomography angiography features of choroidal hemangioma. Indian J Ophthalmol. 2018;66:581. doi: 10.4103/ijo.IJO_955_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cennamo G, Rossi C, Breve MA, Velotti N, Farella A, Liuzzi R, Cennamo G. Evaluation of vascular changes with optical coherence tomography angiography after ruthenium-106 brachytherapy of circumscribed choroidal hemangioma. Eye. 2018;18:1. doi: 10.1038/s41433-018-0100-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chawla R, Tripathy K, Sharma A, Vohra R. Swept source optical coherence tomography-angiography of choroid in choroidal hemangioma before and after laser photocoagulation. Indian J Ophthalmol. 2017;65:751. doi: 10.4103/ijo.IJO_974_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.31 Anand R, Augsburger JJ, Shields JA. Circumscribed choroidal hemangiomas. Arch Ophthalmol. 1989;107:1338–42. doi: 10.1001/archopht.1989.01070020408045. [DOI] [PubMed] [Google Scholar]
- 32.Bottoni F, Tervaert DC, Deutman AF. Fluorescein angiographic findings and results of laser treatment in circumscribed choroidal hemangioma. Int Ophthalmol. 1990;14:259–65. doi: 10.1007/BF00159861. [DOI] [PubMed] [Google Scholar]
- 33.Shields CL, Shields JA, Gross NE, Schwartz GP, Lally SE. Survey of 520 uveal metastases. Ophthalmology. 1997;104:1265–76. doi: 10.1016/s0161-6420(97)30148-1. [DOI] [PubMed] [Google Scholar]
- 34.Shields JA. The expanding role of laser photocoagulation for intraocular tumors. The 1993 H. Christian Zweng Memorial Lecture. Retina. 1994;14:310–22. doi: 10.1097/00006982-199414040-00004. [DOI] [PubMed] [Google Scholar]
- 35.Gunduz K. Transpupillary thermotherapy in the management of circumscribed choroidal hemangioma. Surv Ophthalmol. 2004;49:316–26. doi: 10.1016/j.survophthal.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 36.Anand R, Augsburger JJ, Shields JA. Circumscribed choroidal hemangiomas. Arch Ophthalmol. 1989;107:1338–42. doi: 10.1001/archopht.1989.01070020408045. [DOI] [PubMed] [Google Scholar]
- 37.Lanzetta P, Virgili G, Ferrari E, Menchini U. Diode laser photocoagulation of choroidal hemangioma. Int Ophthalmol. 1996;19:239–47. doi: 10.1007/BF00132693. [DOI] [PubMed] [Google Scholar]
- 38.Garcia-Arumi J, Ramsay LS, Guyara BC. Transpupillary thermotherapy for circumscribed choridal hemangiomas. Ophthalmology. 2000;107:351e6. doi: 10.1016/s0161-6420(99)00046-9. [DOI] [PubMed] [Google Scholar]
- 39.Tian C, Chen X, Cao J, Yang L. Application of ICG-enhanced thermocoagulation method and photodynamic therapy in circumscribed choroidal hemangioma. Oncol Lett. 2018;15:5760–6. doi: 10.3892/ol.2018.8056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Journee-de Korver JG, Oosterhius JA, de Wolff-Rouendaal D, Kemme H. Histopathological findings in human choroidal melanomas after transpupillary thermotherapy. Br J Ophthalmol. 1997;81:234–9. doi: 10.1136/bjo.81.3.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Connolly BP, Regillo CD, Eagle RC, Jr, Shields CL, Shields JA, Moran H. The histopathologic effects of transpupillary thermotherapy in human eyes. Ophthalmology. 2003;110:415–20. doi: 10.1016/S0161-6420(02)01561-0. [DOI] [PubMed] [Google Scholar]
- 42.Shields CL, Shields JA, Perez N, Singh AD, Cater J. Primary transpupillary thermotherapy for small choroidal melanoma in 256 consecutive cases. Outcomes and limitations. Ophthalmology. 2002;109:225–34. doi: 10.1016/s0161-6420(01)00902-2. [DOI] [PubMed] [Google Scholar]
- 43.Fuchs AV, Mueller AJ, Grueterich M, Ulbig MW. Transpupillary thermotherapy (TTT) in circumscribed choroidal hemangioma. Graefes Arch Clin Exp Ophthalmol. 2002;240:7–11. doi: 10.1007/s004170100350. [DOI] [PubMed] [Google Scholar]
- 44.Jurklies B, Anastassiou G, Ortmans S, Schüler A, Schilling H, Schmidt-Erfurth U, et al. Photodynamic therapy using verteporfin in circumscribed choroidal hemangioma. Br J Ophthalmol. 2003;87:84–9. doi: 10.1136/bjo.87.1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sheidow TG, Harbour JW. Photodynamic therapy for circumscribed choroidal hemangioma. Can J Ophthalmol. 2002;37:314–7. doi: 10.1016/s0008-4182(02)80035-7. [DOI] [PubMed] [Google Scholar]
- 46.Berry M, Lucas LJ. Circumscribed choroidal hemangioma: A case report and literature review. J Optometry. 2017;10:79–83. doi: 10.1016/j.optom.2015.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bioxandera A, Arumi J, Martinex-Castillo V, Encinas JL, Elizalde J, Blanco-Mateos G, et al. Prospective clinical trial evaluating the efficacy of photodynamic therapy for symptomatic circumscribed choroidal hemangioma. Ophthalmology. 2009;116:100–5. doi: 10.1016/j.ophtha.2008.08.029. [DOI] [PubMed] [Google Scholar]
- 48.Schmidt-Erfurth UM, Michels S, Kusserow C, Jurklies B, Augustin AJ. Photodynamic therapy for symptomatic choroidal hemangioma: Visual and anatomic results. Ophthalmology. 2002;109:2284e94. doi: 10.1016/s0161-6420(02)01454-9. [DOI] [PubMed] [Google Scholar]
- 49.Papastefanou VP, Plowman PN, Reich E, Pavlidou E, Restori M, Hungerford JL, et al. Analysis of long-term outcomes of radiotherapy and verteporfin photodynamic therapy for circumscribed choroidal hemangioma. Ophthalmol Retina. 2018;2:842–57. doi: 10.1016/j.oret.2017.12.002. [DOI] [PubMed] [Google Scholar]
- 50.Pilotto E, Urban F, Parrozzani R, Midena E. Standard versus bolus photodynamic therapy in circumscribed choroidal hemangioma: Functional outcomes. Eur J Ophthalmol. 2011;21:452e458. doi: 10.5301/EJO.2011.6263. [DOI] [PubMed] [Google Scholar]
- 51.51 Michels S, Michels R, Simader C, Schmidt-Erfurth U. Verteporfin therapy for choroidal hemangioma: A long-term follow-up. Retina. 2005;25:697e703. doi: 10.1097/00006982-200509000-00003. [DOI] [PubMed] [Google Scholar]
- 52.Blasi MA, Tiberti AC, Scupola A, Balestrazzi A, Colangelo E, Valente P, et al. Photodynamic therapy with verteporfin for symptomatic circumscribed choroidal hemangioma: Five-year outcomes. Ophthalmology. 2010;117:1630e1637. doi: 10.1016/j.ophtha.2009.12.033. [DOI] [PubMed] [Google Scholar]
- 53.Jurklies B, Anastassiou G, Ortmans S, Schüler A, Schilling H, Schmidt-Erfurth U, et al. Photodynamic therapy using verteporfin in circumscribed choroidal hemangioma. Br J Ophthalmol. 2003;87:84e89. doi: 10.1136/bjo.87.1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Porrini G, Giovannini A, Amato G, Ioni A, Pantanetti M. Photodynamic therapy of circumscribed choroidal hemangioma. Ophthalmology. 2003;110:674e680. doi: 10.1016/S0161-6420(02)01968-1. [DOI] [PubMed] [Google Scholar]
- 55.Robertson DM. Photodynamic therapy for choroidal hemangioma associated with serous retinal detachment. Arch Ophthalmol. 2002;120:1155e61. doi: 10.1001/archopht.120.9.1155. [DOI] [PubMed] [Google Scholar]
- 56.Tuncer S, Demirci H, Shields CL, Shields JA. Polypoidal choroidal vasculopathy following photodynamic therapy for choroidal hemangioma. Eur J Ophthalmol. 2009;19:159:62. doi: 10.1177/112067210901900127. [DOI] [PubMed] [Google Scholar]
- 57.Aizman A, Finger PT, Shabto U, Szechter A, Berson A. Palladium 103 (103Pd) plaque radiation therapy for circumscribed choroidal hemangioma with retinal detachment. Arch Ophthalmol. 2004;122:1652–6. doi: 10.1001/archopht.122.11.1652. [DOI] [PubMed] [Google Scholar]
- 58.Schilling H, Sauerwein W, Lommatzsch A, Friedrichs W, Brylak S, Bornfeld N, et al. Long-term results after low dose ocular irradiation for choroidal haemangiomas. Br J Ophthalmol. 1997;81:258e9. doi: 10.1136/bjo.81.4.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kivelä T, Tenhunen M, Joensuu T, Tommila P, Joensuu H, Kouri M. Stereotactic radiotherapy of symptomatic circumscribed choroidal hemangiomas. Ophthalmology. 2003;110:1977–82. doi: 10.1016/S0161-6420(03)00483-4. [DOI] [PubMed] [Google Scholar]
- 60.Levy-Gabriel C, Lumbroso-Le Rouic Li, Plancher C, Dendale R, Delacroix S, Asselain B, et al. Long-term results of low-dose proton beam therapy for circumscribed choroidal hemangiomas. Retina. 2009;29:170–5. doi: 10.1097/IAE.0b013e31818bccfb. [DOI] [PubMed] [Google Scholar]
- 61.Kong DS, Lee JI, Kang SW. Gamma knife radiosurgery for choroidal hemangioma. American journal of ophthalmology. 2007;144:319–22. doi: 10.1016/j.ajo.2007.03.052. [DOI] [PubMed] [Google Scholar]
- 62.Naseripour M, Maleki A, Astaraki A, Sedaghat A, Jaberi R, Lee S, et al. Ruthenium-106 brachytherapy in the treatment of circumscribed choroidal hemangioma. Retina. 2018;38:1024–30. doi: 10.1097/IAE.0000000000001616. [DOI] [PubMed] [Google Scholar]
- 63.Arepalli S, Shields CL, Kaliki S, Emrich J, Komarnicky L, Shields JA. Diffuse choroidal hemangioma management with plaque radiotherapy in 5 cases. Ophthalmology. 2013;120:2358–9. doi: 10.1016/j.ophtha.2013.07.058. [DOI] [PubMed] [Google Scholar]
- 64.64 Ritland JS, Eide N, Tausjo J. External beam irradiation therapy for choroidal hemangiomas. Visual and anatomic results after a dose of 20 to 25 Gy. Acta Ophthalmol Scand. 2001;79:184–6. doi: 10.1034/j.1600-0420.2001.079002184.x. [DOI] [PubMed] [Google Scholar]
- 65.65 Zografos L, Bercher L, Chamot L, Gailloud C, Raimondi S, Egger E. Cobalt-60 treatment of choroidal hemangiomas. Am J Ophthalmol. 1996;121:190–9. doi: 10.1016/s0002-9394(14)70584-7. [DOI] [PubMed] [Google Scholar]
- 66.66 Mahdjoubi A, Dendale R, Desjardins L, Lemaitre S, Lumbroso-Le Rouic L, Goudjil F, et al. Treatment of exudative circumscribed choroidal hemangioma: Efficacy of fractionated proton therapy (20 gray relative biological effectiveness in 8 fractions) Retina. 2019;39:692–9. doi: 10.1097/IAE.0000000000002002. [DOI] [PubMed] [Google Scholar]
- 67.Zeisberg A, Seibel I, Cordini D, Lakotka N, Willerding G, Moser L, et al. Long-term (4 years) results of choroidal hemangioma treated with proton beam irradiation. Graefes Arch Clin Exp Ophthalmol. 2014;252:1165–70. doi: 10.1007/s00417-014-2635-1. [DOI] [PubMed] [Google Scholar]
- 68.Mandal S, Naithani P, Venkatesh P, Garg S. Intravitreal bevacizumab (avastin) for circumscribed choroidal hemangioma. Indian J Ophthalmol. 2011;59:248. doi: 10.4103/0301-4738.81051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lasave AF, Serrano MA, Arevalo JF. Photodynamic therapy with verteporfin plus intravitreal bevacizumab for circumscribed choroidal hemangioma: 4 years of follow-up. Retin Cases Brief Rep. 2017 doi: 10.1097/ICB.0000000000000677. doi: 101097/ICB0000000000000677. [DOI] [PubMed] [Google Scholar]
- 70.Sagong M, Lee J, Chang W. Application of intravitreal bevacizumab for circumscribed choroidal hemangioma. Korean J Ophthalmol. 2009;23:127–31. doi: 10.3341/kjo.2009.23.2.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jamison A, Cauchi P, Gilmour DF. Photodynamic therapy for circumscribed choroidal haemangioma in a Scottish cohort. Ocul Oncol Pathol. 2018;4:322–30. doi: 10.1159/000486340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Singh AD, Kaiser PK, Sears JE, Gupta M, Rundle PA, Rennie IG. Photodynamic therapy of circumscribed choroidal haemangioma. Br J Ophthalmol. 2004;88:1414–8. doi: 10.1136/bjo.2004.044396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cennamo G, Breve MA, Rossi C, Romano MR, De Crecchio G, Cennamo G. Transpupillary thermotherapy as a primary treatment for circumscribed choroidal haemangioma. Acta Ophthalmol. 2016;94:e167–9. doi: 10.1111/aos.12810. [DOI] [PubMed] [Google Scholar]