Abstract
We present a rare case of a bilateral diffuse uveal melanocytic proliferation (BDUMP), which occurred secondary to recurrence of carcinoma of thyroid in a 79-year-old gentleman who was initially misdiagnosed to have age related macular degeneration and/or chronic central serous chorioretinopathy. In spite of being treated with anti-VEGF injection and photodynamic therapy there was progressive loss of vision. Multimodal imaging like autoflourescence, infrared imaging, fluorescein angiography, indocyanine angiography, and OCT angiography helped us in clinching the final diagnosis.
Keywords: Bilateral diffuse uveal melanocytic proliferation, infrared imaging, paraneoplastic syndrome, thyroid carcinoma
Bilateral diffuse uveal melanocytic proliferation (BDUMP) is a rare paraneoplastic neoplasm first described in 1966 by Machemer.[1] It is characterized by multiple, elevated pigmented chorioretinal lesions, rapidly progressive cataracts, and exudative retinal detachment.[2] The most common primary malignancy associated with BDUMP in females is urogenital carcinoma (71%) out of which 26% have ovarian cancer whereas in males lung carcinoma accounted to 51% of cases.[2] The most characteristic feature seen on fluorescein angiography (FA) is presence of early hyper florescence corresponding to multiple, circular retinal pigment epithelial atrophic areas resembling a giraffe-like pattern.[3] With increase life expectancy and better understanding the incidence of this disease is on rise in last 5 years (4.4 cases per year)[2] However, still in many cases there is delay in diagnosis and treatment as it can masquerade like age related macular degeneration (ARMD), chronic central serous chorioretinopathy (CSCR), polypoidal choroidal vasculopathy (PCV) and pigmented choriodal nevi.
Case Report
A 79-year-old gentleman complained of blurred vision in both the eyes of 1 month duration. He gave a significant past history of thyroid carcinoma diagnosed in 2008 for which he underwent multiple sessions of radiotherapy. Systemic history and general examination didn't show any signs of tumor recurrence. His best corrected visual acuity (BCVA) was 6/12, N10 and 6/9, N6 in right and left eye respectively. Anterior segment showed nuclear sclerosis of grade 2 in both the eyes. Intraocular pressure recorded was 18 mm HG in both the eyes. Fundus examination showed presence of multiple, irregular, yellowish lesions at posterior pole with retinal pigment epithelium (RPE) alterations in both the eyes [Fig. 1a and b]. Optical coherence tomography (OCT) at the initial visit showed outer retinal edema with presence of subretinal fluid (SRF) more in the right eye as compare to left eye [Fig. 1c and d]. The underneath RPE layer was irregular with presence of few hypereflective deposits. FA in the early phase showed presence of few hyper fluorescence areas without any leakage in the late phase [Fig. 1e and f]. A provisional diagnosis of neovascular ARMD was made for which he received intravitreal injection of ranibizumab in both the eyes subsequently. At 2 months follow-up, visual acuity reduced to 6/36 in the right eye whereas left eye maintained vision. At this visit indocyanine angiography (ICG) was done which showed areas of hypo and hypercyanscence with increased choroidal permeability [Fig. 1g and h]. OCT showed increased SRF with presence of irregular RPE layer with increased choroidal thickness. A presumptive diagnosis of CSCR was made and patient was advised photodynamic therapy (PDT) in right eye. At 1 month post PDT vision improved to 6/12 in right eye; OCT showed minimal reduction in amount of subfoveal SRF. However, at 2 months follow-up vision acuity reduced further to 6/24. As the patient was symptomatic and vision acuity was progressively deteriorating multimodal imaging using confocal scanning laser based retinal angiography was performed. FA at this stage showed multiple nummular hyperfluorescent area around the macula with few hypofluorescent areas corresponding to pigmented lesions [Fig. 2a, b, e and f]. ICG showed both hypo and hypercyanscence areas corresponding to RPE atrophic areas [Fig. 2i and j]. Autofluorescence (AF) [Fig. 2c and d] and Infra-red reflectance image (IR) [Fig. 2g and h] showed characteristic giraffe-like pattern of hypo and hyper lesions which delineated entire extent of lesion. OCT showed subfoveal SRF with irregular RPE layer with presence of subretinal deposits. OCT-A in the choriocapillarie layer showed signal void areas with obscuration of choroidal blood supply in the affected areas [Fig. 2k and l]. Electroretinogram (ERG) in both the eyes showed reduced a and b wave amplitudes with reduction in amplitude of 30 Hz flicker ERG.
Figure 1.
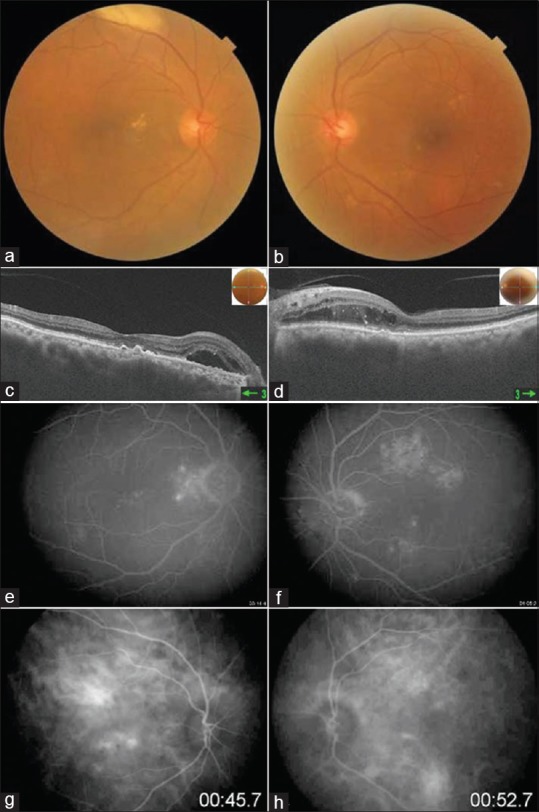
Colour fundus photo shows multiple yellowish drusen like exudates with RPE alterations (a and b). OCT shows irregular RPE layer with presence of subretinal hyperreflective lesions and outer retinal edema (c and d). FA shows multiple hyperfluorescence areas withoutany leakage in late phase (e and f). ICG shows hypo and hypercyanscence spots with dilated choroidal vasculature (g and h)
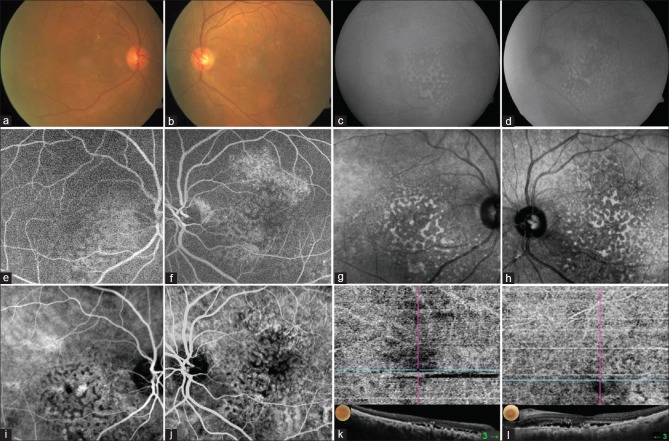
Figure 2.
Colour fundus photo shows multiple nummular pigmented RPE lesions at posterior pole (a and b). AF shows multiple hypoautofluorecent areas resembling giraffe-like pattern (c and d). FA shows multiple hypo as well as hyperfluorescent ares corresponding to RPE atrophy (e and f). IR imaging shows multiple hypo and hyperreflective lesion demonstrating entire extent of lesion (g and h). ICG shows multiple hyper and hypocyanscent spots corresponding to RPE lesions (i and j). OCTA in choriocapillary slab showed obscuration of choroidal vasculature with signal void areas (k and l)
A diagnosis of BDUMP was made and patient was referred to medical oncologist to look for recurrence of primary tumor. PET scan confirmed recurrence of medullary carcinoma of thyroid with distant metastatsis to lungs and kidneys.
In view of distant metastasis, systemic chemotherapy was started (tablet soranib 200 mg BD). Plasmapharesis option was suggested in view of progressive reduction in visual acuity however patient refused due to financial constraints. At last follow-up (2 months from diagnosis), his BCVA was 6/36, N24 and 6/18, N8 in right and left eye respectively. He was advised to review after 2 months; however the patient expired due to his long standing illness.
Discussion
BDUMP is a rare paraneoplastic disorder where about 44% of cases are diagnosed prior to diagnose of primary cancer and 44% of cases are diagnosed after or simultaneously with the presentation.[2] Multiple primary cancers have been reported to be associated with BDUMP including ovarian, lung, cervical, uterine, pancreatic, gallbladder, breast, gastric and colorectal cancer.[4] Most of the patients are legally blind within 1 year or die within first 3 years of initial presentation.[4,5] This can be attributed to delay in diagnosis of BDUMP as well as the end stage of primary cancer with distant metastasis where role of systemic chemotherapy and radiotherapy is very limited.
Till date there are around 60 case reports of BDUMP reported in literature.[2] The earliest clinical presentation of yellowish drusen-like exudates and subretinal fluid before the appearance of pigmented RPE lesion can often be misdiagnosed as ARMD[6] or CSCR[7] or PCV which can present with similar clinical picture. In our case ICG ruled out PCV due to absence of polypoidal lesions, however it did showed features resembling chronic CSCR which in spite of anti-VEGF injection and PDT kept on worsening. At this stage multimodal imaging particularly IR imaging and AF helped us in clinching the diagnosis which showed characteristic giraffe-like pattern. OCT-A showed presence of multiple hyporeflective areas which increased in size over last 9 months as it is converted to fulminant form. Shiraki A et al.[8] have reported OCT-A features with alteration in choroidal vasculature which might help to elucidate the specific prognosis/pathophysiology of BDUMP.
The possible etiology for various urogenital and lung has been postulated to chronic high levels of hepatocyte growth factor (HGF) which is a ligand for the tyrosine kinase receptor encoded by the met proto-oncogene.[6] In the eyes, RPE layer is the most sensitive for HGF receptor expression and hence increased levels of HGF promotes melanocyte and RPE proliferation and migration. In patients of BDUMP there is development of retinal autoantibodies to α-HGF.
In majority of cases treatment of primary malignancy may help in improvement of visual acuity. Plasmapheresis and to some extent local and systemic steroids have shown limited success in terms of improvement in visual acuity and serous retinal detachment.[9] In spite of all this treatment modalities the long term survival is poor as most of the patients die due to dissemination of their primary malignancy.
Conclusion
Our case highlights the importance of early diagnosis of BDUMP by proper history taking, multimodal imaging to differentiate it from ARMD and CSCR, and diagnosis of underlying systemic malignancy which should alert the clinician about the possible diagnosis of paraneoplastic syndrome.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Machemer R. On the pathogenesis of the flat malignant melanoma. Klin Monbl Augenheilkd. 1966;148:641–52. [PubMed] [Google Scholar]
- 2.Klemp K, Kiilgaard JF, Heegaard S, Nørgaard T, Andersen MK, Prause JU. Bilateral diffuse uveal melanocytic proliferation: Case report and literature review. Acta Ophthalmol. 2017;95:439–45. doi: 10.1111/aos.13481. [DOI] [PubMed] [Google Scholar]
- 3.Gass JD, Gieser RG, Wilkinson CP, Beahm DE, Pautler SE. Bilateral diffuse uveal melanocytic proliferation in patients with occult carcinoma. Arch Ophthalmol. 1990;108:527–33. doi: 10.1001/archopht.1990.01070060075053. [DOI] [PubMed] [Google Scholar]
- 4.O’Neal KD, Butnor KJ, Perkinson KR, Proia AD. Bilateral diffuse uveal melanocytic proliferation associated with pancreatic carcinoma: A case report and literature review of this paraneoplastic syndrome. Sur Ophthalmol. 2003;48:613–25. doi: 10.1016/j.survophthal.2003.08.005. [DOI] [PubMed] [Google Scholar]
- 5.Chahud F, Young RH, Remulla JF, Khadem JJ, Dryja TP. Bilateral diffuse uveal melanocytic proliferation associated with extraocular cancers: Review of a process particularly associated with gynecologic cancers. Am J Surg Pathol. 2001;25:212–8. doi: 10.1097/00000478-200102000-00009. [DOI] [PubMed] [Google Scholar]
- 6.Niffenegger JH, Soltero A, Niffenegger JS, Yang S, Adamus G. Prevalence of hepatocyte growth factor and autoantibodies to a-HGF as a new etiology for bilateral diffuse uveal melanocytic proliferation masquerading as neovascular age-related macular degeneration. J Clin Exp Ophthalmol. 2018:9. doi: 10.4172/2155-9570.1000740. doi: 10.4172/2155-9570.1000740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sahoo NK, Singh SR, Rajendran A, Shukla D, Chhablani J. Masqueraders of central serous chorioretinopathy. Surv Ophthalmol. 2019;64:30–44. doi: 10.1016/j.survophthal.2018.09.001. [DOI] [PubMed] [Google Scholar]
- 8.Shiraki A, Winegarner A, Hashida N, Nishi O, Nishi Y, Maruyama K, et al. Diagnostic evaluation of optical coherence tomography angiography and fundus autofluorescence in bilateral diffuse uveal melanocytic proliferation. Am J Ophthalmol Case Rep. 2018;11:32–4. doi: 10.1016/j.ajoc.2018.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mets RB, Golchet P, Adamus G, Anitori R, Wilson D, Shaw J, et al. Bilateral diffuse uveal melanocytic proliferation with a positive ophthalmoscopic and visual response to plasmapheresis. Arch Ophthalmol. 2011;129:1235–8. doi: 10.1001/archophthalmol.2011.277. [DOI] [PMC free article] [PubMed] [Google Scholar]