Abstract
A 60-year-old Caucasian female was referred for biopsy-proven amelanotic orbito-conjunctival melanoma. Map biopsies revealed residual invasive melanoma on the deep tarsal margin at the site of previous surgery. Repeat excisions were required after recurrence was detected following 3 months and 7 months. Positron emission tomography scan detected liver metastasis and additional orbito-conjunctival melanoma recurrence. Biomarker testing showed NRAS mutation without BRAF or c-KIT mutations and without PD-L1 expression. Systemic checkpoint inhibitor therapy was initiated with regression of both the orbito-conjunctival melanoma and liver metastasis. Invasive, non resectable orbito-conjunctival melanoma with liver metastasis can demonstrate a response to systemic checkpoint inhibitor therapy.
Keywords: Checkpoint inhibitor, conjunctiva, eye, melanoma, tumor
Conjunctival melanoma is a rare tumor with an incidence of 0.54 per million.[1] This malignancy is typically managed with wide surgical resection using the “no touch” technique.[1] In a study of 382 cases by Shields et al., an estimated 59% of treated cases showed recurrence or new growth after 10 years and 19% demonstrated metastases.[2] Factors predictive of metastasis and disease-related death included orbital invasion, often requiring exenteration. More recently, immune checkpoint inhibitors, a form of immunotherapy, have been associated with improved survival for various malignancies, including cutaneous melanoma.[3] Herein, we present a case of recurrent orbito-conjunctival melanoma with metastasis that responded to immune checkpoint inhibitors.
Case Report
A 60-year-old Caucasian female with a 30-pack-year smoking history and family history of cutaneous melanoma presented with a left upper tarsal conjunctival melanoma with orbital invasion, previously managed elsewhere with orbitotomy. Histopathology confirmed amelanotic melanoma arising from primary acquired melanosis and with positive margins.
On our examination, visual acuity was 20/20 in both eyes (OU) with a normal anterior segment in the right eye (OD). The left eye (OS) demonstrated well-healed biopsy sites on the upper tarsal conjunctiva with no visible mass. Funduscopic examination was normal OU. Given positive margins on the previous biopsy, repeat map biopsies, and extensive cryotherapy were performed. Histopathology revealed a small focus of residual melanoma that showed an NRAS mutation, but no BRAF or c-KIT mutations. PD-L1 expression was not detected.
Three months later, melanoma recurrence [Fig. 1a] was detected in the anterior orbit on magnetic resonance imaging (MRI) [Fig. 1b] and was managed surgically. Four months later, further recurrence was documented and histopathologically confirmed. Sentinel lymph node mapping and biopsy were negative for regional metastasis. Ocular stereotactic radiotherapy was planned, but later withheld owing to patient preference for vision preservation and presence of metastasis seen as a hypermetabolic liver lesion on positron emission tomography, confirmed with MRI [Fig. 2a].
Figure 1.
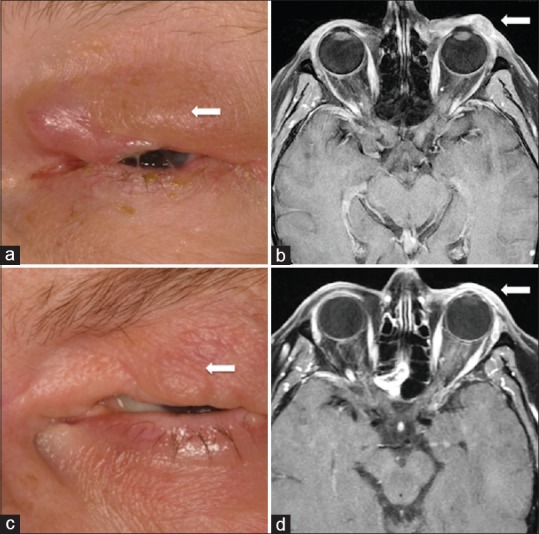
Recurrent orbito-conjunctival melanoma in a 60-year-old female appearing as (a) a palpable mass in the dermis and confirmed on (b) magnetic resonance imaging (MRI) as an enhancing mass along the superolateral aspect of the orbit. Following systemic immune checkpoint inhibitor therapy (c) the palpable mass resolved and the (d) MRI showed no evident tumor at 7 months
Figure 2.
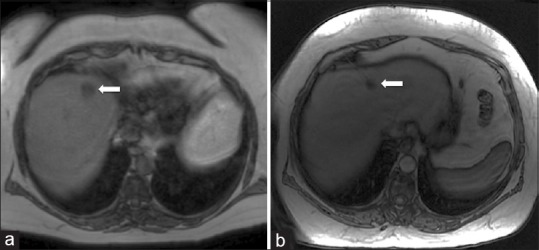
There was additional (a) liver metastasis on magnetic resonance imaging (MRI) appearing as low signal that (b) responded and remained regressed to systemic immune checkpoint inhibitor therapy at 2 years
Given the rapid pace of recurrence and with metastasis, systemic checkpoint inhibitor therapy was considered. Variable treatment efficacy and possible side effects were discussed. The patient agreed to proceed and was started with multi-agent ipilimumab (3 mg/kg) and nivolumab (1 mg/kg) for 2 cycles. Following two cycles of the combination, she experienced grade 2/3 hepatitis. She was then switched to single-agent nivolumab (240 mg every 2 weeks for 2 cycles and 480 mg every 4 weeks for 1 cycle). She then developed infusion reaction to nivolumab and was switched to single-agent pembrolizumab (200 mg every 3 weeks for 9 cycles). After 14 cycles of immunotherapy, the orbito-conjunctival melanoma [Fig. 1c and d] and liver metastasis [Fig. 2b] showed response on MRI and remained stable at 2 years.
Discussion
The immune system combats cancer by recognizing and destroying tumor cells.[3] Tumor cells, however, can evolve to evade immune recognition and killing.[3] Systemic immune checkpoint inhibitors cause increased activation of the immune system by targeting cytotoxic T-lymphocyte antigen-4, programmed death protein (PD-1), or programmed death ligand-1 (PD-L1), to release inhibition on T cell activity.[3,4] Thereby, these medications promote and augment the ongoing immunologic response against malignant cells.[3,4]
Treatment of advanced and metastatic melanoma is dependent on tumor subtype and genetic profile.[5] There are no current targeted therapies approved for NRAS-mutated cutaneous melanomas. However, immune checkpoint inhibitor therapies are considered first-line treatment for metastatic melanoma.[5] Given genetic similarities to cutaneous melanoma, immune-based treatments have been attempted for conjunctival melanoma with metastasis as well.[3,6] Sagiv et al. reported two cases of recurrent conjunctival melanoma with metastasis successfully treated with systemic PD-1 inhibitors (nivolumab and pembrolizumab).[6] Both patients experienced a reduction in tumor size (one with complete resolution) after 6 cycles of systemic therapy. Our patient, who presented with NRAS-positive, orbito-conjunctival melanoma, had similar results with a resolution of orbito-conjunctival mass and regression of liver lesion following 14 cycles of systemic immunotherapy, in spite the absence of PD-L1 expression by the tumor.
Conclusion
In summary, we present a case of recurrent orbito-conjunctival melanoma with metastasis that showed regression following systemic immune checkpoint inhibitor therapy. Larger studies with advanced and metastatic conjunctival melanoma are needed to assess long-term outcomes and potential predictors of response.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Support provided in part by the Eye Tumor Research Foundation, Philadelphia, PA (CLS), an unrestricted grant from Research to Prevent Blindness, Inc (LAD), and the Heed Ophthalmic Foundation (LAD). The funders had no role in the design and conduct of the study, in the collection, analysis, and interpretation of the data, and in the preparation, review or approval of the manuscript. Carol L. Shields, M.D. has had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Shields CL, Markowitz JS, Belinsky I, Schwartzstein H, George NS, Lally SE, et al. Conjunctival melanoma. Outcomes based on tumor origin in 382 consecutive cases. Ophthalmology. 2011;118:389–95. doi: 10.1016/j.ophtha.2010.06.021. [DOI] [PubMed] [Google Scholar]
- 2.Shields CL, Chien JL, Surakiatchanukul T, Sioufi K, Lally SE, Shields JA. Conjunctival tumors: Review of clinical features, risks, biomarkers, and outcomes. The 2017 J. Donald M. Gass Lecture. Asia Pac J Ophthalmol. 2017;6:109–20. doi: 10.22608/APO.201710. [DOI] [PubMed] [Google Scholar]
- 3.Dalvin LA, Shields CL, Orloff M, Sato T, Shields JA. Checkpoint inhibitor immune therapy: Systemic indications and ophthalmic side effects. Retina. 2018;38:1063–78. doi: 10.1097/IAE.0000000000002181. [DOI] [PubMed] [Google Scholar]
- 4.Barbee MS, Ogunniyi A, Horvat TZ, Dang TO. Current status and future directions of the immune checkpoint inhibitors ipilimumab, pembrolizumab, and nivolumab in oncology. Ann Pharmacother. 2015;49:907–36. doi: 10.1177/1060028015586218. [DOI] [PubMed] [Google Scholar]
- 5.Boespflug A, Caramel J, Dalle S, Thomas L. Treatment of NRAS-mutated advanced or metastatic melanoma: Rationale, current trials and evidence to date. Ther Adv Med Oncol. 2017;9:481–92. doi: 10.1177/1758834017708160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sagiv O, Thakar SD, Kandl TJ, Ford J, Sniegowski MC, Hwu WJ, et al. Immunotherapy with programmed cell death 1 inhibitors for 5 patients with conjunctival melanoma. JAMA Ophthalmol. 2018;136:1236–41. doi: 10.1001/jamaophthalmol.2018.3488. [DOI] [PMC free article] [PubMed] [Google Scholar]


