Abstract
In this case report, we demonstrate the use of optical coherence tomography angiography (OCTA) as a tool to evaluate intrinsic vasculature in a case of juxtapapillary melanoma which underwent ruthinium.106 plaque brachytherapy. In this case, OCTA could demonstrate a decrease in caliber and density of the intrinsic vasculature of the tumor post brachytherapy.
Keywords: Angiography, brachytherapy, juxtapapillary, melanoma, OCTA, response, tumor, vasculature
Uveal melanomas can be peripapillary (near but not touching the optic disc), juxtapapillary (touching the optic disc), or circumpapillary (surrounding the optic disc). Various treatment modalities for the treatment of juxtapapillary melanoma are plaque brachytherapy, proton beam radiotherapy, stereotactic radiotherapy, and enucleation.[1,2,3,4] The response of the tumor to the treatment is assessed by monitoring the tumor thickness, surface characteristics, tumor vascularity, ultrasonography, fluorescein angiography (FA), and optical coherence tomography (OCT).[5,6] Assessment of the pattern of intrinsic vasculature of the tumor in choroidal melanoma is possible by FA. This is challenging due to leakage and staining in late phases of FA. Optical coherence tomography angiography (OCTA) is an effective noninvasive tool which can be used in understanding the tumor vasculature. In this case report, we report OCTA-assisted analysis of intrinsic tumor vasculature of juxtapapillary melanoma post brachytherapy.
Case Report
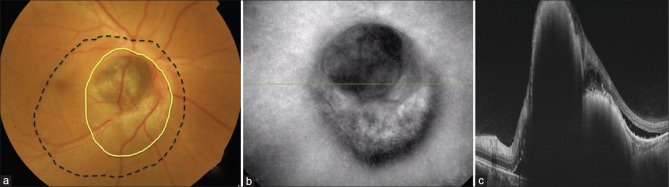
A 36-year-old male presented with complaints of blurring of vision and distortion of images in the right eye since 4 weeks. His best-corrected visual acuity in both eyes was 6/6, N6. Anterior segment examination of both eyes was normal. Fundus examination of the right eye showed an irregular brownish, elevated mass lesion over the disc and the papillomacular bundle and a U-shaped extension below the disc, measuring 3–4 disc diameter vertically and 2–3 disc diameter horizontally. There was subretinal fluid around the lesion, extending to the fovea [Fig. 1a]. The extent of the lesion was better appreciated with infrared reflectance imaging [Fig. 1b]. OCT of the lesion showed a bi-lobed lesion with a upper elevated lesion over the disc [Fig. 1c]. Ultrasonography showed a dome-shaped lesion located over the disc with high surface reflectivity and moderate internal reflectivity measuring 3.04 mm horizontally, 3.9 mm vertically, and thickness of 2.24 mm. According to the American Joint Committee on Cancer staging manual, the tumor stage was T1aN0M0.[7] OCTA was performed with swept-source OCTA (Topcon DRI OCT Triton; Topcon Corporation, Japan) 6 × 6 mm protocol over the tumor. Automated segmentation showed segmentation artifacts which could be overcome by manual segmentation [Fig. 2a and b]. OCT angiograph over the lesion demonstrated large retinal vessel over the tumor and the tumor intrinsic vasculature. The intrinsic choroidal vasculature was seen as fine interlacing vessels [Fig. 2c]. Magnetic resonance imaging with contrast showed an elevated lesion over the disc which was hyperintense on T1 and hypointense on T2 with minimal contrast enhancement. The tumor was treated with a ruthenium-106 (Ru-106) COB notched plaque (Eckert and Ziegler BEBIG GmbH, Berlin, Germany), tumor free margin of 2 mm, delivering 8500 cGy radiation to tumor apex for 5 days. The notch diameter was 5 mm and was adequate enough to accommodate the optic nerve. Intraoperatively, the adequacy of coverage of the plaque was confirmed by transillumination. The treatment was in accordance with the American Brachytherapy Society consensus guidelines for plaque brachytherapy of uveal melanoma.[8] On follow-up visits, at 3 and 6 months, OCTA was repeated over the tumor. The serial OCTA showed significant reduction in the density and caliber of interlacing choroidal vessels [Fig. 3a–c]. Fundus FA and indocyanine green angiography (ICGA) was done at 6 months of follow-up to assess the vascularity of the tumor [Fig. 4a and b].
Figure 1.
(a) Color photograph of right eye showing a brownish, elevated lesion over disc with U-shaped extension below the disc(yellow line). Blood vessels over the lesion can be seen dipping into the tumor. Subretinal fluid was noted around the lesion which involved the fovea (black dotted line). (b) Infrared reflectance image of right eye showing the extent of the lesion. (c) Optical coherence tomography of right eye disc showing a bilobed elevated lesion with high surface reflectivity with back shadowing. Subretinal fluid can be seen adjacent to the lesion
Figure 2.
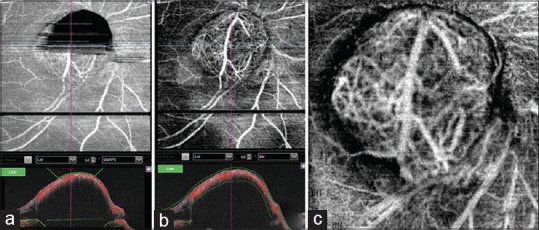
(a) Optical coherence tomography angiography (OCTA) after automatic segmentation. Upper half of the lesion over the disc appears black due to segmentation artifact. (b) OCTA of the same lesion after manual segmentation. (c) OCTA 6 × 6 mm protocol showing large retinal vessel over the tumor with its branches entering the tumor with interlacing of tumor vasculature
Figure 3.
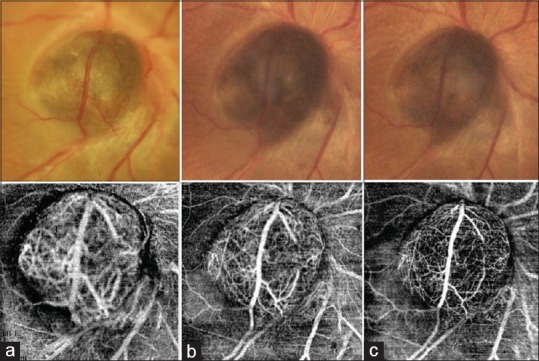
Serial color photograph and corresponding 6 × 6 mm OCTA scans at (a) presentation, (b) 3 months after plaque brachytherapy, and (c) 6 months after plaque brachytherapy. Serial photographs show significant decrease in size, color, and caliber of the overlying vessel. Prominent vascular branches seen at presentation could not be noted after brachytherapy indicating decrease in the vascularity of the tumor. Serial OCTA of the lesion demonstrated significant decrease in the caliber and density of the intrinsic vasculature of the tumor
Figure 4.
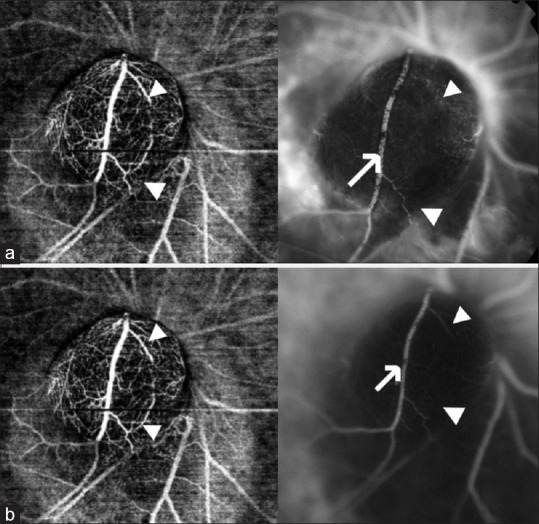
(a) OCTA at 6 months of follow-up with corresponding fluorescein angiography (FA). Note that the vessel seen on OCTA is not seen on FA (white arrow head) and large vessel traversing the tumor is partially obscured by pigment clumps on FA (white arrow). Leakage can be noted around the lesion. (b) OCTA and corresponding indocyanine green angiography (ICG) at 6 months of follow-up. The vascular branches and the intrinsic vasculature were better visualized with OCTA when compared with ICG (white arrow head). Vessel traversing the tumor was segmentally obscured by overlying pigment clumps on ICG (white arrow)
Discussion
Tumor regression post brachytherapy can be assessed by clinical examination, ultrasound, and imaging modalities such as fundus photography. Tumor regression post radiation therapy can be assessed by monitoring tumor thickness, changes in surface characteristics, tumor vascularity, ultrasonography, FA, and OCT features.[5,6] Post brachytherapy, almost half of the tumors show 50% decrease in thickness.[5]
Maheshwari and Finger noted that partial reduction or complete elimination of intrinsic tumor vascularity was found to be the most consistent finding related to tumor regression. Appearance of new tumor vessels is suggestive of tumor recurrence.[6]
The intrinsic vasculature of the tumor can be visualized by FFA and ICGA. Assessment of tumor vasculature by these imaging techniques in choroidal melanoma is challenging due to dye leakage. Literature on regression of intrinsic tumor vasculature in choroidal melanoma post brachytherapy is sparse.[5,6,9] Maheshwari and Finger studied 170 consecutive patients treated with palladium-103 eye plaue radiation for choroidal melanoma. Of the 120 that demonstrated intrinsic vascularity, 10% (n = 12/120) had decreased tumor-related vascularity and 90% (n = 108/120) showed complete resolution.[6]
Chaugule and Finger studied regression patterns of iris melanoma after palladium-103 plaque brachytherapy.[9] They reported that at the last follow-up (mean follow-up of 5.2 years), 63% of the tumors showed decrease in intrinsic vascularity and 37% had absence of intrinsic vascularity and none had persistent intrinsic vascularity. This implies that all cases for iris melanoma had regression of internal vasculature post brachytherapy either partially or completely.
OCTA can be an useful noninvasive tool in assessing the intrinsic vascularity of choroidal melanoma.[10] Serial OCTA at each visit post brachytherapy can help us understand the response to treatment and also help us identify local recurrence. The advantages of using OCTA in assessing tumor vasculature are that it helps us visualize the vasculature better, it is noninvasive, and endlessly repeatable. Unlike FFA where the visualization of vasculature can be compromised by dye leakage, OCTA provides better visualization of intrinsic vascularity. In our case, few superficial vessels which were seen on OCTA could not be seen on FFA and ICGA. This was because of the pigment clumping over the blood vessels, which result in blocked fluorescence.
The interpretation of OCTA can be limited by motion and segmentation artifacts. Segmentation artifact results from error in the segmentation of the retinal or choroidal layers leading to a deviation of the slab.[11] This can be overcome by manual segmentation as demonstrated in our case. The limited area of image acquisition and difficulty in capturing OCTA of peripherally located tumors can be a shortcoming. This can be overcome to certain extent using OCTA machines with the capability of excursion.
So far, to our knowledge, ours is the first study demonstrating regression of the intrinsic vasculature of the juxtapapillary melanoma post brachytherapy therapy on OCTA.
Conclusion
OCTA can be a useful noninvasive tool to assess intrinsic tumor vasculature before and after brachytherapy.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Shields CL, Shields JA. Recent developments in the management of choroidal melanoma. Curr Opin Ophthalmol. 2004;15:244–51. doi: 10.1097/01.icu.0000120713.35941.e4. [DOI] [PubMed] [Google Scholar]
- 2.Gragoudas ES, Goitein M, Seddon J, Verhey L, Munzenrider J, Urie M, et al. Preliminary results of proton beam irradiation of macular and paramacular melanomas. Br J Ophthalmol. 1984;68:479–85. doi: 10.1136/bjo.68.7.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Emara K, Weisbrod DJ, Sahgal A, McGowan H, Jaywant S, Michaels H, et al. Stereotactic radiotherapy in the treatment of juxtapapillary choroidal melanoma. Int J Radiat Oncol Biol Phys. 2004;59:94–100. doi: 10.1016/j.ijrobp.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 4.Weinhaus RS, Seddon JM, Albert DM, Gragoudas ES, Robinson N. Prognostic factor study of survival after enucleation for juxtapapillary melanomas. Arch Ophthalmol. 1985;103:1673–7. doi: 10.1001/archopht.1985.01050110067027. [DOI] [PubMed] [Google Scholar]
- 5.Schachat AP. Ryan's retina. In: Boldt CH, Houston SK, Markoe AM, Murray TG, editors. Brachytherapy for Choroidal Melanoma. 6th ed. Elsevier; 2018. pp. 2566–81. [Google Scholar]
- 6.Maheshwari A, Finger PT. Regression patterns of choroidal melanoma: After palladium-103 (103Pd) plaque brachytherapy. Eur J Ophthalmol. 2018;28:722–30. doi: 10.1177/1120672118776146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Amin MB, Edge S, Greene F, Byrd DR, Brookland RK, Washington MK, et al. AJCC Cancer Staging Manual. 8th ed. New York, NY: Springer; 2017. Uveal melanoma; pp. 805–17. [Google Scholar]
- 8.American Brachytherapy Society—Ophthalmic Oncology Task Force. The American brachytherapy Society consensus guidelines for plaque brachytherapy of uveal melanoma and retinoblastoma. Brachytherapy. 2014;13:1–14. doi: 10.1016/j.brachy.2013.11.008. [DOI] [PubMed] [Google Scholar]
- 9.Chaugule SS, Finger PT. Regression patterns of iris melanoma after palladium.103 (103Pd) plaque brachytherapy. Ophthalmology. 2017;124:1023. doi: 10.1016/j.ophtha.2017.02.015. 30. [DOI] [PubMed] [Google Scholar]
- 10.Ghassemi F, Mirshahi R, Fadakar K, Sabour S. Optical coherence tomography angiography in choroidal melanoma and nevus. Clin Ophthalmol. 2018;12:207–14. doi: 10.2147/OPTH.S148897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ghasemi Falavarjani K, Al-Sheikh M, Akil H, Sadda SR. Image artefacts in swept-source optical coherence tomography angiography. Br J Ophthalmol. 2017;101:564–68. doi: 10.1136/bjophthalmol-2016-309104. [DOI] [PubMed] [Google Scholar]