Abstract
Achondroplasia has an effect on intracartilaginous ossification during the development of the spine resulting in a narrow spinal canal. This abnormal anatomy could make an achondroplastic patient tend to have spinal canal stenosis. We reported a case of congenital spinal canal stenosis with achondroplasia combined with ossified ligamentum flavum (OLF) at the thoracolumbar and lumbar spine, which was treated by decompressive surgery. We reported a 52-year-old Thai male with achondroplasia presented with progressive myelopathy and neurogenic claudication due to spinal canal stenosis. Spinal canal stenosis was observed at T10/11 and L1–L5 and OLF at T10/11 through L5 varying in size. Laminectomy and removal of the OLF were performed at T11 and L1–L5. The patient's neurological symptom improved after the surgery. He could walk with a walker at the time of 6-month follow-up postoperatively. In this report, we describe a rare case of achondroplasia with OLF presenting with progressive myelopathy and claudication symptoms from multiple levels of spinal canal stenosis. Laminectomy, removal of the ossified ligament, and fusion with instrumentation resulted in the improvement of the patient's neurological symptoms and function.
Keywords: Achondroplasia, ossification of ligamentum flavum, ossified ligamentum flavum, spinal canal stenosis
Introduction
Achondroplasia is an autosomal dominant genetic disease that affects the fibroblast growth factor receptor 3 (FGFR3) gene but also can be found as a new mutation in certain populations.[1] The effect on intracartilaginous ossification during the development of the spine can result in an unusual anatomy such as thoracolumbar kyphosis, short pedicles, and a progressive decrease in the interpedicular distance in a craniocaudal direction, resulting in a narrow spinal canal.[2,3] Other findings include an underdeveloped and narrow sacrum. The iliac wings are also located relatively higher, and the L5 vertebra is deeply sunk below the iliac wings.[4,5]
This abnormal anatomy could make an achondroplastic patient tend to have spinal stenosis so that the symptoms would present earlier than the normal population at the third to fourth decade of life.[6] Patients with spinal canal stenosis will have symptoms such as intermittent claudication, nerve root compression, and paraplegia, depending on the level of stenosis. Although canal stenosis at the lumbar region is commonly found in the normal population, the imaging of the whole spine is recommended to screen the other regions for pathology in the achondroplasia patient, as cervical, thoracic, and thoracolumbar spinal stenosis are also commonly found in these patients.[7]
There are multiple causes of spinal stenosis such as degenerative discs, posterior osteophytes, facet hypertrophy, and hypertrophic ligamentum flavum. The ossified ligamentum flavum (OLF) is a rare condition that is reported in the Asian and Caucasian populations.[8] The developmental mode of OLF was confirmed to be mainly endochondral ossification, which is also controlled by the FGFR3 gene.[9,10] The frequency of intraoperative dural tear is accompanied by dural ossification, which makes the surgical decompression more technically demanding.[10,11]
The gold standard of treatment of spinal canal stenosis is to address the level of pathology and early decompression of the neural elements; this includes avoiding complications such as incidental durotomy and spinal cord injury during decompression. Surgical planning, instrumentation, and patient counseling about complications are important prior to performing the operation.
This article reports an achondroplasia patient with thoracolumbar and lumbar spinal canal stenosis caused by abnormal anatomy and OLF.
Case Report
A 52-year-old Thai male with achondroplasia presented with 2 years of leg pain and intermittent claudication of both legs [Figure 1]. A year after, he was unable to walk due to progressive weakness and numbness of both legs. Physical examination revealed a significantly decreased range of motion on his back. Manual muscle testing revealed bilateral generalized muscle weakness on both lower extremities. Manual muscle testing revealed motor muscle power grade 3 of the hip flexors and knee extensors, and grade 4 for ankle dorsiflexion, big toe extension and ankle plantar flexion. Bilateral paresthesia was found below the L1 level; both legs had hyperreflexia with Babinski and clonus signs which were positive In addition, there are no muscle weakness and sensory abnormalities on both the upper extremities. Scapulohumeral reflex (Shimizu), Tromner reflex, and Hoffmann reflex were negative. These findings indicated that the pathology level of spinal canal stenosis is cephalad level of L1–L2.
Figure 1.
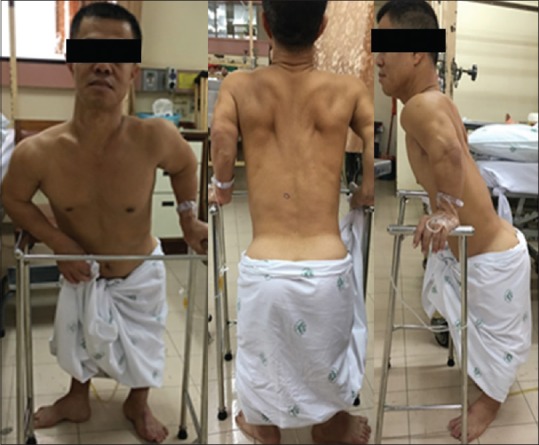
Preoperative image of the patient with typical appearance of achondroplasia with the stooped posture
A plain radiograph of the lumbosacral spine in the posteroanterior view of the thoracolumbar spine showed a large pedicle cortex outline and a progressive decrease of the interpedicular distance from the cephalad to the caudate [Figures 2 and 3]. A plain radiograph of the lumbosacral spine in the lateral view also showed large pedicles that were short in length, and the canal was measured to be <13 mm. We also found a minimal wedge deformity of the L1 and L2 vertebra. The OLF was seen in the intervertebral foramen of L3 and L4. Computer tomography (CT) showed OLF varying in size located at the T10/11 and L1–L5 levels [Figure 4], and CT screening of the whole spine including the cervical spine and thoracic spine revealed no OLF [Figure 5]. Sagittal and axial T2-weighted magnetic resonance imaging of the lumbar spine showed that the thecal sac was severely compressed at T11 posterolaterally by the OLF and severely compressed at L1–L5 by both the degenerative disc anteriorly and the OLF posteriorly [Figure 6].
Figure 2.
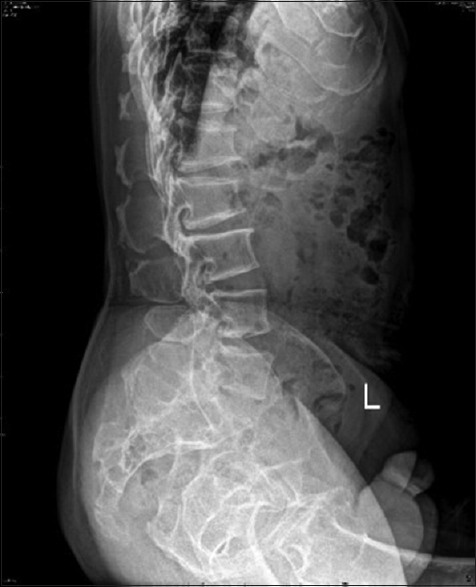
Plain radiograph of the lumbosacral spine in the lateral view revealed short pedicles and ossified ligamentum flavum in the intervertebral foramen of L3 and L4
Figure 3.
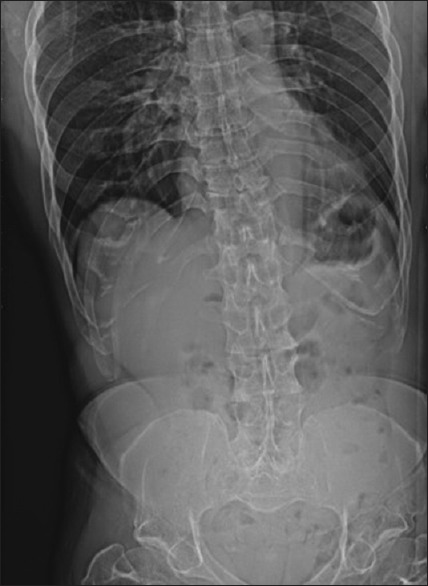
Plain radiograph of the lumbosacral spine in the posteroanterior view revealed a progressive narrowing of the interpedicular distance
Figure 4.
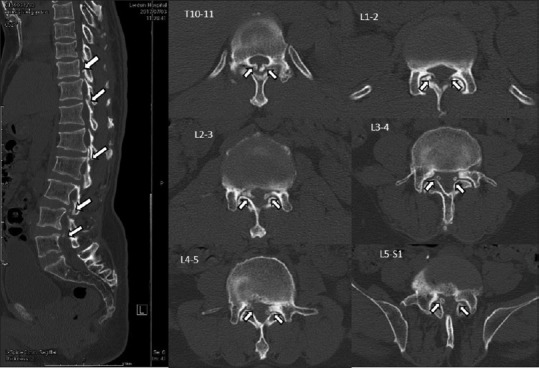
Preoperative computer tomography parasagittal view revealed an ossified ligamentum flavum at T10/11 through L5 varying in size. The axial view revealed ossified ligamentum flavum at the sublamina and facets, causing central and lateral recess stenosis of T10 and L1–L5
Figure 5.
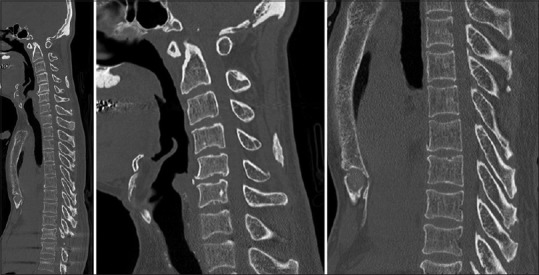
Preoperative computer tomography screening of the whole spine including cervical spine and thoracic spine revealed no ossified ligamentum flavum
Figure 6.
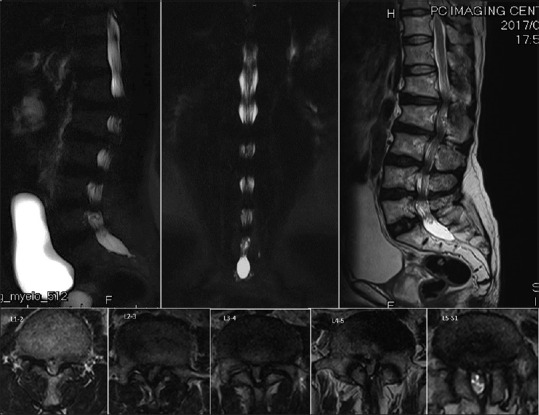
Preoperative magnetic resonance imaging revealed markedly spinal canal stenosis from T10/11 and L1–L2 to L5/S1. The sagittal view showed multiple lumbar disc degeneration from L1/2 to L5/S1 with mild posterior bulging. The axial view revealed severe central and lateral recess stenosis
The operation was performed after proper surgical preparation, which included the patient and family education regarding a higher than usual chance of perioperative complications. The patient was in the prone position on a Jackson spinal table. Hypotensive anesthesia was used during the operation. Pedicular screws were inserted by the freehand technique with fluoroscope guidance from T10 to S1. Laminectomy and removal of the OLF were performed at T11 and L1–L5 using a high-speed burr and a Kerrison rongeur [Figure 7]. During surgical decompression, there was an incidental durotomy at L1/2. The dura mater was repaired with nonabsorbable sutures, fibrin sealant, and a fat pad graft which were taken from subcutaneous fat. After all pathologies were addressed, the surgeon rechecked the repaired dura and small-size tear by direct visualization with the Valsalva maneuver with assistance from the anesthesiologist.
Figure 7.
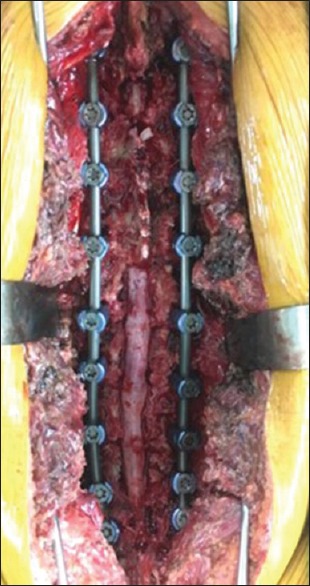
Intraoperative picture after decompression
After initial postoperative care, the plain radiograph of the lumbosacral spine was taken which revealed a good instrument position [Figure 8]. The patient was able to sit and stand with gait aid by the day 7. Bilateral leg pain dramatically improved immediately after the operation. At 6 months postoperatively, the patient's motor power gradually improved to Grades 3–4 at L2–L3 bilaterally and Grades 4–5 at L4–S1. The patient also had a decrease of numbness in both the legs. He was independently walking with a gait aid.
Figure 8.
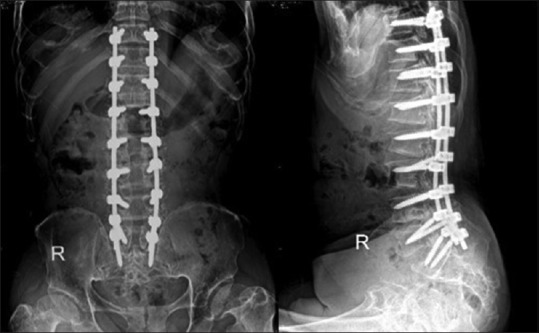
Postoperative plain radiograph of the lumbosacral spine after decompression and posterior spinal fusion and instrumentation from T10 to S1
Discussion
In spinal canal stenosis in an achondroplastic patient, the causes to compromise the canal are not different from the normal population. These causes include degenerative discs, posterior osteophytes, facet hypertrophy, or ligamentum flavum, and another contributing cause is thoracolumbar kyphosis from wedge-shaped vertebra. However, these patients are more susceptible to spinal canal stenosis because of their abnormal anatomy of the spine, despite having such subtle pathology.[12,13]
In an achondroplastic patient, there are abnormalities of intracartilaginous ossification, resulting from the mutation of the FGFR3 gene. Ossification of the ligamentum flavum also occurs through intracartilaginous ossification. This may have some correlation, but the mechanism is still unknown. Previously, in the literature, there have only been eight achondroplastic cases presenting with spinal stenosis and OLF reported, with the age ranging from 18 to 58 years, and all patients were the Japanese.[14,15,16,17,18,19] The levels of pathology were mostly reported to be thoracic and thoracolumbar, and only one study reported pathology at the lumbar region. To the best of our knowledge, this is the first study that reported OLF at the thoracolumbar and lumbar regions. Myelopathy at the thoracic and thoracolumbar level was commonly found in achondroplastic patients in contrast to lumbar spinal stenosis in the normal population. Early surgical decompression is the key to gain better neurologic recovery and pain improvement in achondroplastic patients with spinal canal stenosis, especially with thoracic or thoracolumbar stenosis [Table 1].
Table 1.
Previous case report and this study
| Study | Sex/age | Level of pathology | Neurological status | Laminectomy | Improvement |
|---|---|---|---|---|---|
| Takano et al., 1987[14] | Male/36 | T4-L5 | Myelopathy below T4 | T5-S1 | No |
| Takano et al., 1987[14] | Male/58 | T8-T11 | Myelopathy below T10 | T11-S1 | Yes |
| Kataoka 1990[15] | Male/52 | T8-T11 | Myelopathy below L1 | T7-L5 | No |
| Nakahashi et al., 1991[16] | Male/19 | T4/5, T10-T12 | Myelopathy below T4 | T4-T5 | Yes |
| Baba et al., 1992[17] | Male/19 | T4/5, T10-T12 | Myelopathy below T4 | T4-T6 | Yes |
| Imamura et al., 1997[18] | Female/19 | T9-T12 | Intermittent claudication | T8-L2 | Yes |
| Suzuki et al., 2008[19] | Male/53 | T9-T12 | Myelopathy below L1 | T9-L1 | Yes |
| Saito et al., 2014[20] | Female/75 | L1-L4 | Intermittent claudication | L1-L5 | Yes |
| This study | Male/52 | T10-L5 | Intermittent claudication plus myelopathy below T10 | T11, L1-L5 | Yes |
Length of fusion and the lowest instrumented level after decompression must also be considered differently in the achondroplastic patient because of the anatomically deep sinking of L5 below the intercristal line that makes the lumbosacral junction quite stable after the fusion.[21] The S2 and iliac instrumentation might not be needed in these patients, and this would result in a smaller surgical wound and less instrumented complications.
The incidence of incidental durotomy during lumbar decompression varies widely among different authors (1%–17%) depending on many factors such as surgeon experience, type, and complexity of the surgery, particularly for revision surgery.[22] Dural ossification is a common finding in OLF (40%)[8] and could lead to iatrogenic tears of the dura during decompression in achondroplastic patients with spinal stenosis. According to Sun et al., the incidence of dural tears and cerebrospinal fluid leakage in OLF patients was 32%.[23] Therefore, a CT evaluation to identify an OLF and dural ossification is recommended. In the surgical technique aspect, it is recommended to use a high-speed burr to gradually thin the lamina before using the Kerrison rongeur to remove the bone, then to recheck the small size dural penetration and promptly repair the dura tear. These steps are necessary, and the skill of the surgeon must be considered. Patient and family education in case of severe perioperative complications such as spinal cord injury, dural tears, and worsened motor function after surgery is also very important.
Written informed consent was obtained from the patient for publication of this case report and any accompanying images.
Conclusion
In this report, we described a rare case of achondroplasia with OLF presenting with progressive myelopathy and claudication symptoms from multiple levels of spinal canal stenosis. Laminectomy, removal of the ossified ligament, and fusion with instrumentation resulted in the improvement of the patient's neurological symptoms and function.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient has given his consent for his images and other clinical information to be reported in the journal. The patient understands that name and initials will not be published and due efforts will be made to conceal identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Shiang R, Thompson LM, Zhu YZ, Church DM, Fielder TJ, Bocian M, et al. Mutations in the transmembrane domain of FGFR3 cause the most common genetic form of dwarfism, achondroplasia. Cell. 1994;78:335–42. doi: 10.1016/0092-8674(94)90302-6. [DOI] [PubMed] [Google Scholar]
- 2.Srikumaran U, Woodard EJ, Leet AI, Rigamonti D, Sponseller PD, Ain MC. Pedicle and spinal canal parameters of the lower thoracic and lumbar vertebrae in the achondroplast population. Spine (Phila Pa 1976) 2007;32:2423–31. doi: 10.1097/BRS.0b013e3181574286. [DOI] [PubMed] [Google Scholar]
- 3.Lutter LD, Longstein JE, Winter RB, Langer LO. Anatomy of the achondroplastic lumbar canal. Clin Orthop Relat Res. 1977;126:139–42. [PubMed] [Google Scholar]
- 4.Bailey JA., 2nd Orthopaedic aspects of achondroplasia. J Bone Joint Surg Am. 1970;52:1285–301. [PubMed] [Google Scholar]
- 5.Caffey J. Achondroplasia of pelvis and lumbosacral spine; some roentgenographic features. Am J Roentgenol Radium Ther Nucl Med. 1958;80:449–57. [PubMed] [Google Scholar]
- 6.Fortuna A, Ferrante L, Acqui M, Santoro A, Mastronardi L. Narrowing of thoraco-lumbar spinal canal in achondroplasia. J Neurosurg Sci. 1989;33:185–96. [PubMed] [Google Scholar]
- 7.Hamamci N, Hawran S, Biering-Sørensen F. Achondroplasia and spinal cord lesion. Three case reports. Paraplegia. 1993;31:375–9. doi: 10.1038/sc.1993.62. [DOI] [PubMed] [Google Scholar]
- 8.Muthukumar N. Dural ossification in ossification of the ligamentum flavum: A preliminary report. Spine (Phila Pa 1976) 2009;34:2654–61. doi: 10.1097/BRS.0b013e3181b541c9. [DOI] [PubMed] [Google Scholar]
- 9.Zhou ZQ, Ota S, Deng C, Akiyama H, Hurlin PJ. Mutant activated FGFR3 impairs endochondral bone growth by preventing SOX9 downregulation in differentiating chondrocytes. Hum Mol Genet. 2015;24:1764–73. doi: 10.1093/hmg/ddu594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rousseau F, Bonaventure J, Legeai-Mallet L, Pelet A, Rozet JM, Maroteaux P, et al. Mutations in the gene encoding fibroblast growth factor receptor-3 in achondroplasia. Nature. 1994;371:252–4. doi: 10.1038/371252a0. [DOI] [PubMed] [Google Scholar]
- 11.Yang Z, Xue Y, Zhang C, Dai Q, Zhou H. Surgical treatment of ossification of the ligamentum flavum associated with dural ossification in the thoracic spine. J Clin Neurosci. 2013;20:212–6. doi: 10.1016/j.jocn.2012.02.028. [DOI] [PubMed] [Google Scholar]
- 12.Kahanovitz N, Rimoin DL, Sillence DO. The clinical spectrum of lumbar spine disease in achondroplasia. Spine (Phila Pa 1976) 1982;7:137–40. doi: 10.1097/00007632-198203000-00008. [DOI] [PubMed] [Google Scholar]
- 13.Epstein JA, Malis LI. Compression of spinal cord and cauda equina in achondroplastic dwarfs. Neurology. 1955;5:875–81. doi: 10.1212/wnl.5.12.875. [DOI] [PubMed] [Google Scholar]
- 14.Takano T, Takano H, Kato Y, Yamashita S, Tsuji H. Four cases of achondroplastic spinal stenosis: Long term results. Cent Jpn J Orthop Surg Traumatol. 1987;32:1416–8. [Google Scholar]
- 15.Kataoka O. A case of achondroplasia occurred palaplasis after trauma. Spinal Surg. 1990;1:346–50. [Google Scholar]
- 16.Nakahashi K, Baba H, Takahasi K, Kawahara N, Kikuchi Y, Tomita K, et al. Achondroplasia with ossification of yellow ligament of the thoracic spine: Report of a case. Orthop Surg Traumatol. 1991;34:397–400. [Google Scholar]
- 17.Baba H, Imura S, Tomita K. Achondroplasia with spinal cord or cauda equina symptoms: Report of three cases. Orthop Surg. 1992;43:47–52. [Google Scholar]
- 18.Imamura T, Oga M, Tamaru T, Arima J, Ikuta K, Esaki Y, et al. A case of achondroplasia with ossification of the yellow ligament. Orthop Traumatol. 1997;46:1227–32. [Google Scholar]
- 19.Suzuki K, Kanamori M, Nobukiyo M. Ossification of the thoracic ligamentum flavum in an achondroplastic patient: A case report. J Orthop Surg (Hong Kong) 2008;16:392–5. doi: 10.1177/230949900801600327. [DOI] [PubMed] [Google Scholar]
- 20.Saito K, Miyakoshi N, Hongo M, Kasukawa Y, Ishikawa Y, Shimada Y, et al. Congenital lumbar spinal stenosis with ossification of the ligamentum flavum in achondroplasia: A case report. J Med Case Rep. 2014;8:88. doi: 10.1186/1752-1947-8-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.MacGibbon B, Farfan HF. A radiologic survey of various configurations of the lumbar spine. Spine (Phila Pa 1976) 1979;4:258–66. doi: 10.1097/00007632-197905000-00013. [DOI] [PubMed] [Google Scholar]
- 22.Kalevski SK, Peev NA, Haritonov DG. Incidental dural tears in lumbar decompressive surgery: Incidence, causes, treatment, results. Asian J Neurosurg. 2010;5:54–9. [PMC free article] [PubMed] [Google Scholar]
- 23.Sun J, Zhang C, Ning G, Li Y, Li Y, Wang P, et al. Surgical strategies for ossified ligamentum flavum associated with dural ossification in thoracic spinal stenosis. J Clin Neurosci. 2014;21:2102–6. doi: 10.1016/j.jocn.2014.02.027. [DOI] [PubMed] [Google Scholar]


