Abstract
Objective
The present meta-analysis was conducted to compare the efficacy and safety of intravenous application of tranexamic acid (TXA) with placebo in patients with hip fracture undergoing hip surgeries.
Methods
PubMed, EMBASE and Cochrane Library were searched from inception until March 2018. A combined searching strategy of subject words and random words was adopted. Only randomized clinical trials were included. The comparisons regarding transfusion rate, total blood loss, intraoperative blood loss, postoperative blood loss, postoperative haemoglobin and postoperative thromboembolic complications were conducted. The meta-analysis was performed using Review Manager 5.3, and the bias evaluation was based on the Cochrane Handbook 5.1.0.
Results
Ten randomized controlled trials published from 2007 to 2018 were included in the meta-analysis. The results showed that there were significant differences in the two groups concerning transfusion rate of allogeneic blood [risk ratio (RR) = 0.66, 95% confidence interval (CI): 0.56 to 0.78, P = 0.003], total blood loss [mean difference (MD) = −273.00, 95% CI: −353.15 to −192.84, P < 0.00001], intraoperative blood loss (MD = −76.63, 95% CI: −139.55 to −13.71, P = 0.02), postoperative blood loss (MD = −125.29, 95% CI: −221.96 to −28.62, P = 0.01) and postoperative haemoglobin (MD = 0.80, 95% CI: 0.38 to 1.22, P = 0.0002). Nonsignificant differences were found in the incidence of thromboembolic events (RR = 1.38, 95% CI: 0.74 to 2.55, P = 0.31).
Conclusions
This meta-analysis of the available evidence implies that the intravenous route of TXA shows an ability to reduce transfusion requirements and total blood loss, not increasing the incidence of thromboembolic events in patients undergoing hip surgeries.
The translational potential of this article
The result of this meta-analysis shows that the utilization of intravenous TXA in patients with hip fracture undergoing hip surgeries possesses great potential in reducing blood loss and allogeneic blood transfusion safely.
Keywords: Allogeneic blood transfusion, Blood loss, Hip fracture, Meta-analysis, Thromboembolic events, Tranexamic acid
Abbreviations: Randomized controlled trial, RCT; Tranexamic acid, TXA; haemoglobin, Hb; total hip arthroplasty, THA; total knee arthroplasty, TKA; deep vein thrombolism, DVT; pulmonary embolism, PE; cerebrovascular accident, CVA; mean difference, MD; risk ratio, RR; standard deviation, SD; confidence interval, CI
Introduction
Owing to decreased bone mineral density in elderly people, most hip fractures are caused by just a fall [1]. The incidence of hip fracture is increasing concurrently with the ageing of the population. It has been estimated that in many countries, the number of hip fractures will rise from 1.7 million in 1990 to 6.3 million in 2050 [2].
Hip fractures are associated with substantial blood loss which can lead to postoperative anaemia [3]. It was demonstrated that the hidden blood loss could reach about 1500 ml, and the mean examined haemoglobin (Hb) decrease from admission to postoperation is 31 g/L for intertrochanteric fractures and 18 g/L for intracapsular fractures [3], [4]. Postoperative anaemia in patients with hip fracture may lead to decreased ambulation, reduced functional independence and reduced walking distance on discharge [5], [6]. Patients with anaemia have a lower recovery rate of physical disability than those without anaemia [7], [8]. Thus, loads of techniques have been applied for blood management, including administration of tourniquet, thromboplastic agents, topical freezing saline, deliberate hypotension, fibrinolytic inhibitors, blood transfusion and autologous donation [9], [10]; of which, the allogeneic blood transfusion is the most commonly used strategy. In a previous study, the transfusion rate of allogeneic blood could reach as high as 84% [11]. However, allogeneic blood transfusion may increase the risk of adverse effects such as virus infection, immune response and cardiovascular dysfunction and even death, causing financial burden and a potential threat for patients [12], [13], [14], [15], [16], [17], [18], [19]. Meanwhile, the current medical care standard recommends conservative and limited utilization of blood products [20], [21], which underlines the need for better control of bleeding.
It was reported that the blood loss caused by fibrinolysis accounts for approximately 60% of total blood loss in total hip arthroplasty (THA) [22]. The body naturally inhibits fibrinolysis by 24 h after surgery, but antifibrinolytics such as tranexamic acid (TXA) may block the activation of plasminogen to plasmin earlier and therefore decrease perioperative blood loss [23], [24]. Thus, it was worthy of consideration to administer antifibrinolytic agents to manage blood loss within or after hip surgeries. Antifibrinolytic agents include aprotinin, TXA and epsilon-aminocaproic acid [25], [26].
TXA is a synthetic amino acid that carries out its effects through an antifibrinolytic action. It stabilizes formed clots and prevents the degradation of fibrin by reversibly inhibiting the lysine binding site on plasminogen. It can destroy plasminogen's linkage with fibrin to become plasmin, which normally creates a fibrinolytic effect and dissolves clots [20], [27], [28]. Through reducing fibrinolysis, it helps to decrease bleeding and blood loss resulting from the trauma of surgery and the release of tissue plasminogen activator [23], [29].
To our knowledge, loads of former studies have reported that the intravenous route of TXA would reduce blood loss and transfusion requirements in THA. However, researchers have not reached a consensus on the efficiency and safety of intravenous administration of TXA during hip fracture surgeries. Therefore, the present systematic review and meta-analysis was conducted to compare the efficacy and safety of intravenous application of TXA with placebo in patients with hip fracture undergoing hip surgeries.
Materials and methods
Search strategy
PubMed, EMBASE and Cochrane Library were searched by 2 reviewers from inception until March 2018. A combined searching strategy of subject words and random words was adopted. The key words “hip fracture”, “femoral neck fracture”, “intertrochanteric fracture”, “hip surgery” and “tranexamic acid” were used in combination. The concrete searching strategy for PubMed is as follows (((((((((((((((((Femur Neck Fractures) OR Fractures, Femur Neck) OR Fractures, Femoral neck) OR Femoral neck fractures) OR Fractures, Subtrochanteric) OR Subtrochanteric Fractures) OR Fractures, Intertrochanteric) OR Intertrochanteric fractures) OR Fractures, Trochanteric) OR Trochanteric Fractures) OR fractures, Pertrochanteric) OR Pertrochanteric fractures) OR Fractures, Hip) OR hip fractures) OR hip surgery) OR "Femoral neck fractures"[Mesh]) OR "Hip Fractures"[Mesh]) AND ((((((((((("Tranexamic Acid"[Mesh]) OR Tranexamic Acid) OR AMCHA) OR t-AMCHA) OR AMCA) OR Anvitoff) OR Cyklokapron) OR Ugurol) OR KABI 2161) OR Amchafibrin) OR Exacyl). Randomized controlled trials (RCTs) that compared TXA with placebo or nonuse of TXA for reducing transfusion rate or blood loss or Hb decrease in patients undergoing hip surgeries after hip fractures were included. The language of publications was limited to English. Reference lists of all eligible studies and relevant reviews were manually searched for additional studies.
Eligibility criteria
The inclusion criteria were as follows:
-
1)
Patients: patients undergoing hip surgery after hip fracture.
-
2)
Intervention: intravenous TXA applied preoperatively or postoperatively.
-
3)
Comparison: comparing TXA with placebo.
-
4)
Outcomes: the outcomes concerning efficacy including transfusion rate, total blood loss (both intraoperative and postoperative blood loss), intraoperative blood loss, postoperative blood loss and postoperative Hb; the outcomes concerning safety including postoperative thromboembolic complications including deep vein thrombosis, pulmonary embolism and cerebrovascular accident. The transfusion rate and total blood loss were set as the primary outcomes.
-
5)
Type of studies: only randomized controlled clinical trial was included.
The exclusion criteria were as follows:
-
1)
Patients: patients suffered from other fractures or multiple fractures or undergoing selective total hip fractures or hemiarthroplasty.
-
2)
Intervention: oral or topical utilization of TXA.
-
3)
Comparison: not comparing TXA with placebo.
-
4)
Outcomes: including neither transfusion rate nor total blood loss.
-
5)
Type of studies: those studies that are not RCTs or whose concrete description could not be extracted, letters and comments.
Data extraction
Two reviewers independently scanned the titles and abstracts of potentially included studies. Once the studies met the inclusion criteria, full texts of articles were reviewed thoroughly. The data concerning the studies, patient characteristics, outcomes and follow-up were extracted independently by the two reviewers. Disagreements about the data mention previously were resolved by discussion or consulting a senior reviewer. The data in other forms were converted to mean ± standard deviation, according to the Cochrane handbook. For those data not reported numerically, we used “Get Data” software (Get Data, Suzhou, China) to extract them from published figures.
Quality of included studies
Two independent reviewers assessed the risk of bias of every clinical randomized trial according to the rules of the Cochrane Handbook 5.1.0. A ‘risk of bias’ table was created including the following 7 items: random sequence generation, allocation concealment, blinding, incomplete outcome data, free of selective reporting and other bias. All the items were described as “low risk of bias”, “unclear risk of bias”, or “high risk of bias”.
Statistical analysis
The meta-analysis was conducted using Review Manager 5.3 software (Cochrane Collaboration, Oxford, UK). For dichotomous data, the risk ratio (RR) with 95% confidence interval (CI) was calculated (the transfusion rate and the occurrence of thromboembolic events), and for continuous outcomes (total blood loss, perioperative blood loss, postoperative blood loss and postoperative Hb), the mean difference (MD) was calculated. Statistical heterogeneity was assessed with the P and I values using the standard Chi-square test. When I2>50%, or P < 0.1, significant heterogeneity was indicated, and a random effects model was applied for the meta-analysis. Otherwise, a fixed effects model was used.
Quality of the included studies
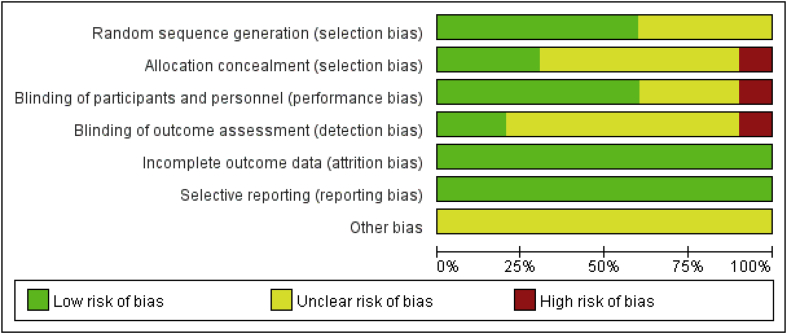
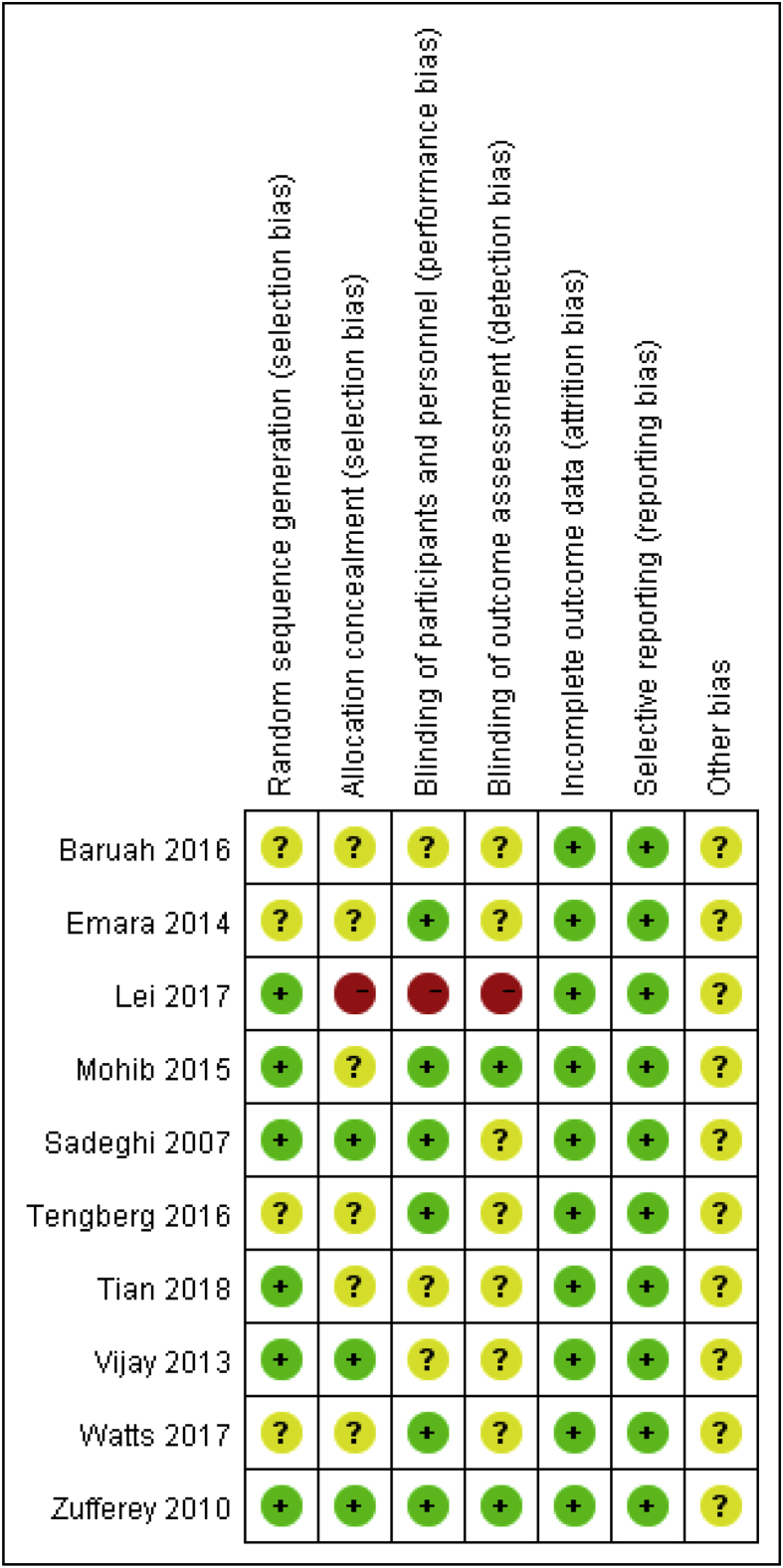
Risk of bias in the included studies is shown in Figure 1, Figure 2. For every bias item, the risk is presented as the percentage across all included studies, which indicates the proportion of different levels of risk of bias for each item. Among the included studies, three studies were randomized by computer-generated numbers [31], [32], [33], one was randomized by opaque sealed envelope technique [34], one was randomized by random number technique [37] and the remaining one did not report the method of random sequence generation. Three studies reported allocation concealment [32], [34], [35]. Double blinding was reported in 6 studies [11], [30], [31], [32], [35], [36]. In two studies, the blinding of outcome assessors were reported [31], [32]. No intention-to-treat analysis was performed in any of the RCTs; therefore, a potential risk of type II statistical error existed.
Figure 1.
Risk of bias graph.
Figure 2.
Risk of bias summary.
Results
Search results
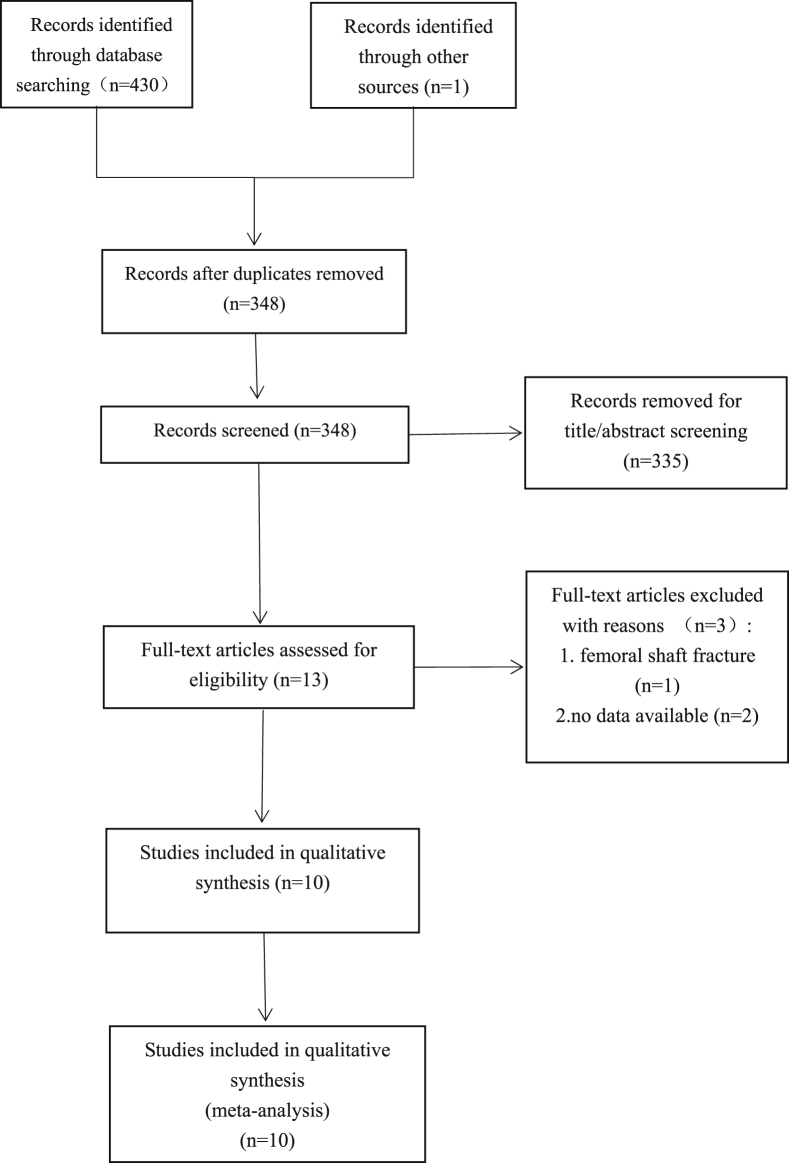
A total of 430 studies were identified from the search of the databases, and one study was identified through other sources. A total of 348 studies remained when the duplicates were removed. Then, the titles and abstracts of the 348 citations were scanned to exclude those which did not meet the inclusion criteria. As a consequence, 335 citations were excluded. Next, the 13 remaining studies were carefully full text reviewed to recognize those that could reach the inclusion criteria. At last, 10 RCTs [11], [30], [31], [32], [33], [34], [35], [36], [37], [38] published between 2007 and 2018 were included in the meta-analysis (Fig. 3).
Figure 3.
Preferred reporting items for systematic reviews and meta-analyses (PRISMA) flow diagram of literature selection.
The general characteristic of the included studies
In over half of the studies (6 of 10), the average age of the participants was more than 65 years [11], [30], [31], [32], [33], [37]. In 2 specific studies, the average age was more than 80 years [30], [32]. In 6 studies, the male/female ratios were lower than 1.0. In those studies where the average age was more than 65 years, the male/female ratios were lower than 1.0. The fracture types of the included studies were restricted to hip fractures: femoral neck fractures, intertrochanteric fractures and subtrochanteric fractures. One study may include one or more fracture types. The surgery procedure varied with the difference of the fracture types. For femoral neck fractures, THA or hemiarthroplasty was the most common surgical procedure. For intertrochanteric fractures, dynamic hip screw or intramedullary nail was commonly chosen. As to the duration of the surgeries, compared with THA and hemiarthroplasty, dynamic hip screw and intramedullary nail took about half the time (Table 1).
Table 1.
The general characteristic of the included studies.
| Study | Year | Sample size I/C | Mean age I/C | Fracture type | Surgical methods | Male/female ratio I/C | Tranexamic acid intervention | Control | Prophylactic antithrombotic | Transfusion criteria |
|---|---|---|---|---|---|---|---|---|---|---|
| Sadeghi [35] | 2007 | 32/35 | 51.8/44.4 | Intracapsular and extracapsular fractures | Hemiarthroplasty, plating and nailing | 1.13/2.18 | 15 mg/kg at induction of anaesthesia | Normal saline | NM | NM |
| Emara [36] | 2014 | 20/20 | 56.5/55 | Hip fractures | Hemiarthroplasty | 1.5/1.0 | 10 mg/kg before skin incision followed by 5 mg(kg• h) until the end of the surgery | Normal saline | Clexane | NM |
| Zufferey [32] | 2010 | 57/53 | 81/82 | Femoral neck fractures, intertrochanteric fractures, subtrochanteric fractures | THA, hemiarthroplasty, DHS, IMN | 0.20/0.08 | 15 mg/kg before surgery and 15 mg/kg 3 h later | Placebo | Fondaparinux | 9 g/dl |
| Vijay [34] | 2013 | 45/45 | 48.8/49.3 | Hip fractures | ORIF, hemiarthroplasty, THA | 0.29/0.29 | 500 mg before surgery, 1 mg/(kg• h) following until the completion of surgery | Normal saline | NM | Reduction in haemoglobin exceeding 25% of the preoperative level |
| Baruah [38] | 2016 | 30/30 | 57.7/55.3 | Trochanteric fractures | DHS | 4.0/5.0 | 15 mg/kg before surgery | Normal saline | NM | Hb < 8.5 g/dL or Hct<27% |
| Tengberg [11] | 2016 | 33/39 | 79.8/75.0 | Unstable trochanteric fractures | IMN | 0.27/0.56 | 1 g before surgery, 3 g the following 24 h | NM | Fragmin | Hb < 9.67 g/dL |
| Mohib [31] | 2015 | 50/50 | 69/70 | Intertrochanteric fractures | NM | 0.72/0.92 | 15 mg/kg before surgery, 15 mg/kg 3 h later | Normal saline | Enoxaparin | Hb < 7 g/dL |
| Watts [30] | 2017 | 69/69 | 81.0/82.2 | Femoral neck fractures | THA, hemiarthroplasty | 0.44/0.47 | 15 mg/kg before surgery, 15 mg/kg at wound closure | Normal saline | Low-molecular-weight heparin | Hb < 7 g/dL, Hb<8 g/dL in the presence of persistent symptoms |
| Lei [37] | 2017 | 37/40 | 77.8/79.2 | Intertrochanteric fractures | PFNA | 0.16/0.21 | 1 g before surgery | Normal saline | NM | Preoperative Hb < 9 g/dL |
| Tian [33] | 2018 | 50/50 | 77.7/79.3 | Intertrochanteric fractures | PFNA | 0.61/0.38 | 10 mg/kg 10 min preoperatively and 10 mg/kg 5 h postoperatively | No tranexamic acid | Low-molecular-weight heparin | Postoperative Hb < 9 g/dL |
C = control group; DHS = dynamic hip screw; Hb = haemoglobin; Hct = haematocrit; I = intravenous tranexamic acid group; IMN = intramedullary nail; NM = not mentioned; ORIF = open reduction and internal fixation; PFNA = proximal femoral nail antirotation; THA = total hip arthroplasty.
Meta-analysis
Transfusion rate
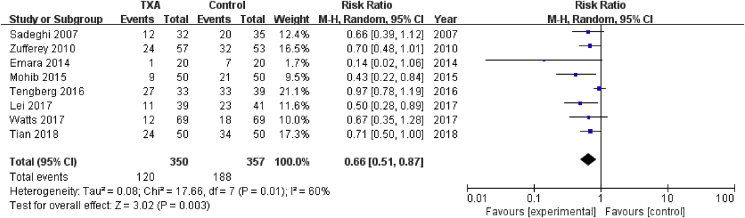
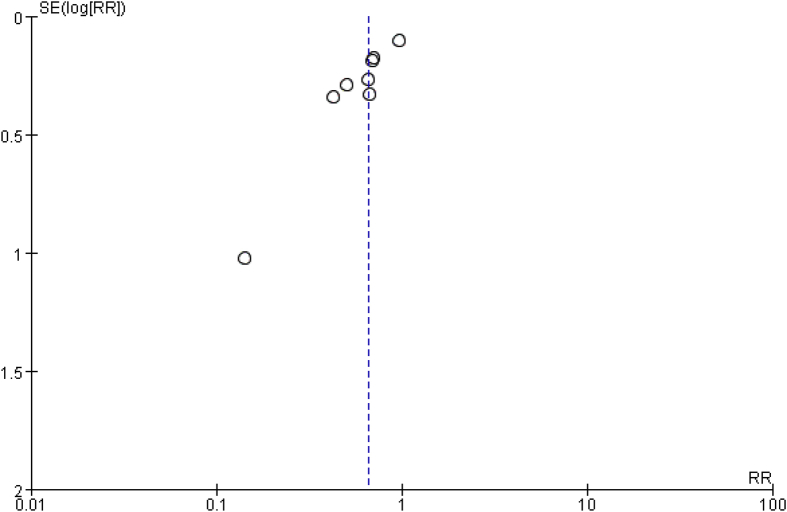
Eight studies involving 707 patients reported requirements of blood transfusion. A random effects model was applied because significant heterogeneity was found among these studies (P = 0.01, I2 = 0.60%). A significant difference was detected in transfusion rate between the two groups (RR = 0.66, 95% CI: 0.56 to 0.78, P = 0.003, Fig. 4). The funnel plot for transfusion rate of allogeneic blood was used to evaluate publication bias. The funnel plot showed little asymmetry, which suggested publication bias for transfusion rate of allogeneic blood meta-analysis (Fig. 5).
Figure 4.
Forest plot for transfusion rate of allogeneic blood. M-H=Mantel-Haenszel, CI = confidence interval; TXA = tranexamic acid.
Figure 5.
Funnel plot for transfusion rate of allogeneic blood. RR = risk ratio.
Total blood loss
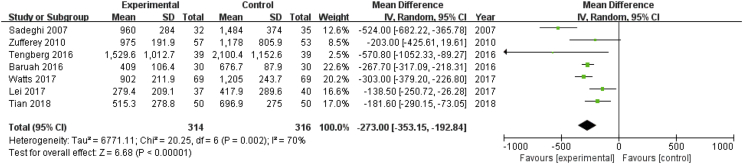
Seven studies including 630 patients reported total blood loss after hip fracture surgery. A random effects model was applied because significant heterogeneity was found among these studies (P = 0.002, I2 = 70%). A significant difference was detected in total blood loss between the two groups (MD = −273.00, 95% CI: −353.15 to −192.84, P < 0.00001, Fig. 6).
Figure 6.
Forest plot for total blood loss. IV=Inverse Variance, CI = confidence interval; SD = standard deviation.
Intraoperative blood loss
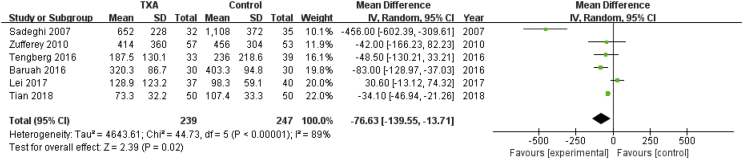
Six studies involving 486 patients reported intraoperative blood loss. A random effects model was applied because significant heterogeneity was found among these studies (P < 0.00001, I2 = 89%). A significant difference was detected in intraoperative blood loss between the two groups (MD = −76.63, 95% CI: −139.55 to −13.71, P = 0.02, Fig. 7).
Figure 7.
Forest plot for intraoperative blood loss. IV=Inverse Variance, CI = confidence interval; SD = standard deviation.
Postoperative blood loss
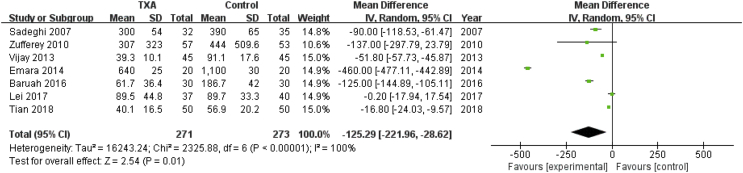
Seven studies involving 544 patients reported postoperative blood loss. A random effects model was applied because significant heterogeneity was found among these studies (P < 0.00001, I2 = 100%). A significant difference was detected in postoperative blood loss between the two groups (MD = −125.29, 95% CI: −221.96 to −28.62, P = 0.01, Fig. 8).
Figure 8.
Forest plot for postoperative blood loss. IV=Inverse Variance, CI = confidence interval; SD = standard deviation.
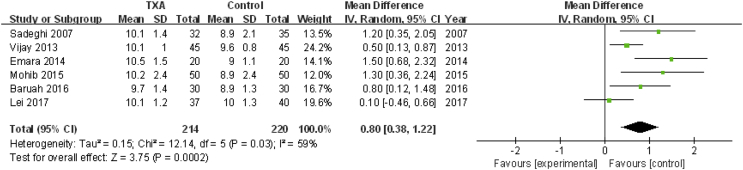
Postoperative Hb
Six studies involving 434 patients reported postoperative Hb concentration. A random effects model was applied because significant heterogeneity was found among these studies (P = 0.03, I2 = 59%). A significant difference was detected in postoperative Hb between the two groups (MD = 0.80, 95% CI: 0.38 to 1.22, P = 0.0002, Fig. 9).
Figure 9.
Forest plot for postoperative haemoglobin concentration. IV=Inverse Variance, CI = confidence interval; SD = standard deviation; TXA = tranexamic acid.
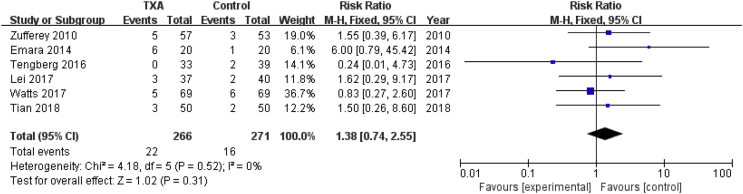
Thromboembolic events
Six studies involving 537 patients reported the incidence of thromboembolic events. A fixed effects model was applied because no significant heterogeneity was found among these studies (P = 0.52, I2 = 0%). Nonsignificant difference was detected in the incidence of thromboembolic events between the two groups (RR = 1.38, 95% CI: 0.74 to 2.55, P = 0.31, Fig. 10).
Figure 10.
Forest plot for incidence of thromboembolic events. M-H=Mantel-Haenszel, CI = confidence interval; TXA = tranexamic acid.
Sensitivity analysis
In the meta-analysis, significant heterogeneity was observed in the outcomes “transfusion rate” and “total blood loss”. Thus, we exclude 1 study to evaluate the influence of the deleted study on the overall result. When the data of the study by Tengberg et al. were removed, the result showed no significant heterogeneity (P = 0.55, I2 = 0%), and a significant difference was detected in transfusion rate between the two groups (RR = 0.60, 95% CI: 0.49 to 0.73, P < 0.00001). This may result from the highest transfusion threshold of 9.67 g/dl, leading to the highest transfusion rate of more than 80%. As regard to total blood loss, when the study by Sadeghi was removed, the result showed no significant heterogeneity (P = 0.09, I2 = 47%), with no change of the result of a significant of decrease in total blood loss. The difference of the fracture types, surgery methods and the calculation method of total blood loss may contribute to the heterogeneity.
Discussion
Although TXA was discovered more than 50 years ago, the clinical utilization of TXA to reduce blood loss and transfusion requirements has only been popularized in the past decade. It was not even included in the 350 essential medicines recommended by the World Health Organization(WHO) until 2013 [39]. As a consequence, the debate concerning the efficacy and safety of the intravenous utilization of TXA in hip fracture surgeries is ongoing. The aim of the current systematic review and meta-analysis of RCTs was to assess the efficacy and safety of intravenous administration of TXA alone in patients who suffered hip fracture undergoing hip surgeries.
Main findings
Based on the pooled 10 RCTs, the most important finding of this meta-analysis is that intravenous administration of TXA can significantly reduce the transfusion rate of allogeneic blood (RR = 0.64, 95% CI: 0.45 to 0.89, P = 0.009) and total blood loss (MD = −292.56, 95% CI: −383.53 to −201.59, P < 0.00001). This result was identified with a previous meta-analysis [42] focussing on the utilization of TXA in inpatients with hip fracture and another meta-analysis including studies conducted in patients undergoing THA and total knee arthroplasty (TKA) [43]. Compared with the former meta-analysis [42], the current one included the most recently published randomized studies in the last 2 years, while the Chinese Biomedical Literature Database was not searched resulting in few studies concerning hidden blood loss, which was not analyzed in this meta-analysis.
Although a few studies have also shown that TXA would increase the risk of thromboembolic events, including deep vein thrombolism and pulmonary embolism, our meta-analysis implies that intravenous TXA will not significantly increase the incidence of thromboembolic events (RR = 0.02, 95% CI: −0.03 to 0.07, P = 0.35). No significant heterogeneity was detected in the studies. The outcome had similar results with those of former studies [42]. Benoni et al. demonstrated that TXA inhibits fibrinolysis in the wound but not in general circulation, not inducing a prothrombotic state [48]. Most included studies excluded patients with a history of venous thromboembolism. As a consequence, most of the current available data would likely support safety of TXA in healthy patients rather than in those with higher risk of thromboembolic events. However, in a recent retrospective study by Sabbag et al., patients with a history of deep vein thrombosis(VTE) had a low risk of recurrent VTE (2%) after contemporary THA and TKA, and it was found that transfusion rate was not increased with the use of intravenous TXA [49]. More RCTs are required to verify the safety of intravenous TXA in this patient group.
Heterogeneity
Significant heterogeneity was observed in the outcomes “transfusion rate” and “total blood loss”. This may result from the difference in the fracture type and the transfusion standard. In the concluded 10 studies, two studies calculated hidden blood loss [11], [33]. In the two specific studies, hidden blood loss could reach more than 400 ml. Therefore, we extracted the calculated blood loss as the total blood loss for the two studies, whereas for the other studies, we took intraoperative bleeding and postoperative drainage as the total blood loss. This also was a source of the heterogeneity. It would be better for further studies to observe the same strategy for blood loss calculation.
Dosage and timing
In the former studies, the dosage of TXA was various. According to the pharmacokinetic profile of TXA, the optimal plasma concentration to TXA has been reported between 10 and 20 μg/ml in older in vitro studies [44]. To maintain a concentration of 20 μg/ml, combination administration of TXA was recommended, that is, administering a 10 mg/kg loading dose, followed by 5 mg/kg/h of infusion [44]. In the pooled studies, 7 studies adopted the administration of a loading dose combined with a following dose [11], [30], [31], [32], [33], [34], [36], whereas in the other 3, a single bolus was administered [35], [37], [38]. In the study by Sadeghi [35], a single bolus dose of 15 mg/kg was administered intravenously at induction of anaesthesia, leading to a decrease in total blood loss by 524 ml (p = 0.001), a lower infusion rate (37 vs 57, p = 0.04) and higher postoperative Hb level (10.1 g/dl vs 8.9 g/dl, p < 0.05). Baruah et al. [38] recommended the same dosage before surgery incision with similar results. In the study by Lei et al. [37], patients in the tranexamic group received 1 g of intravenous TXA before surgery, resulting in a great decline in transfusion rate by about 28% and a decrease in estimated total blood loss and estimated hidden total blood loss in patients undergoing proximal femoral nail antirotation for intertrochanteric fractures. In the study by Mohib et al [31], patients in the intervention TXA group received two doses of 15 mg/kg body weight of TXA, whereas those in the control group received two doses of 15 mg/kg body weight of placebo which was normal saline, intravenously. Through the study, they confirmed that the combination of a first intravenous dosage of 15 mg/kg and a subsequent dose of 15 mg/kg is effective in reducing blood loss and helps maintain a higher postoperative Hb level. Although Zufferey et al. [32] found no significant difference in total blood loss and postoperative infusion rate using the same dosage as Mohib et al., the decrease of total blood loss is 203 ml, and the postoperative infusion rate followed from 60.4% to 42.1%. Watts et al. also randomized patients into tranexamic groups receiving 2 doses of 15 mg/kg of TXA, one dose before incision and the second at wound closure, and the outcomes imply a tendency for decreased transfusion rate (17% vs 26%, P = 0.22) and decreased calculated total blood loss (902 vs 1205, P = 0.005, on the 3rd postoperative day) compared with the placebo group. In the other 2 studies using 2 intravenous doses [34], [36], despite the different doses of TXA, statistically significant differences were found in postoperative blood loss, transfusion rate and postoperative Hb. Future work should pay attention to the optimal plasma concentration and the corresponding intravenous administration of TXA with the greatest effect on the reduction of blood loss, meanwhile not increasing the risks of thromboembolic events.
Other administration methods of TXA
In addition, the topical TXA has also been studied in hip surgeries. In the study by Emara et al [36], for one group with topical use of TXA, 1.5 g of TXA was poured into the surgical field and left for 5 min before suction. As a consequence, postoperative blood loss showed a significant reduction from 1100 ml to 625 ml (compared with patients who received no TXA, p < 0.05). However, in another 2 articles [40], [41], the patients in the intervention group received 3 g of TXA subfascially administrated around the fracture site at the end of the surgical procedure or subfascial and intramuscular infiltration of 2 g of TXA before wound closure, and the results showed no significant difference in postoperative blood transfusion and total blood loss. In a meta-analysis, Zhang et al. [45] demonstrated that topical or IV administration of TXA leads to a statistically significant reduction in transfusion rates in total hip replacement and no significant increase in thromboembolic events. To our knowledge, oral administration of TXA among hip surgery patients has not been studied. But in the study by Lee et al [46] and Gandhi et al [47] in terms of total hip replacement, an oral dosage of TXA was used, leading to a significant decrease in blood loss and no significant cases of thromboembolic events. Therefore, future work should concentrate on the safety and efficacy of topical or oral or combined TXA administration in hip surgeries.
Economic benefits
Evangelista et al. reported that TXA can decrease transfusion rates for THA from 22.7% to 11.9% and transfusion rates for TKA from 19.4% to 7.0%. The average direct hospital cost reduction for THA and TKA was $3083 and $2582, respectively. Implementation of an intravenous TXA protocol significantly reduced transfusion in a safe and cost-effective manner [50]. Demos et al. [51] also implied an economic impact of cost reduction on THA patients with intravenous TXA. DiBlasi et al. [52] found that topical TXA costs significantly more than IV TXA. This may be supportive evidence for intravenous utilization of tranexamic.
Strengths and limitations of this meta-analysis
The present meta-analysis has strengths over the previous systematic reviews because it contains more RCTs and more severe inclusion and exclusion criteria. There are 6 main limitations to this meta-analysis. First, only 10 relevant studies were included, and the sample size of each study was relatively small. Second, the variation in doses of the single bolus dose or a loading dose combined with a following dose between the studies might cause heterogeneity. Third, differences in transfusion criteria may have influenced the transfusion rate. Fourth, the diversity of the type of fractures and surgeries might cause heterogeneity. Fifth, in terms of blood loss, different assessment methods might affect the accuracy. Finally, in almost all studies, data reporting was incomplete.
Conclusions
This meta-analysis of the available evidence implies that the intravenous route of TXA administration shows an ability to reduce transfusion requirements and total blood loss, not increasing the incidence of thromboembolic events in patients undergoing hip surgeries. Nevertheless, because of the great variation of the included studies, more large-sample and high-quality RCTs are demanded for further verification of the efficacy and safety of the intravenous routine of TXA in hip fracture surgeries.
Conflict of interest
The authors have no conflicts of interest to disclose in relation to this article.
Acknowledgements
This work was supported by the National Natural Science Foundation of China [81572187]; National Natural Science Foundation of China for Young Scholars [81201422]; Natural Science Foundation of Jiangsu Province for Young Scholars [BK2012334]; “Summit of the Six Top Talents” Program of Jiangsu Province [2013-WSW-054]; Wuxi City Science and Technology Development, Medical and Public Health Technology Research and Development Project Funding [WX18IIAN442]; Key Medical Talent's Project in Science and Education of Jiangsu Province [ZDRCA2016083] and Soft Science Research Projects of Nanjing Science and Technology Commission [2016ZD014].
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jot.2019.03.007.
Contributor Information
Hui Chen, Email: chhdxl@126.com.
Yun-feng Rui, Email: ruiyunfeng@126.com.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.LeBlanc K.E., Muncie H.J., LeBlanc L.L. Hip fracture: diagnosis, treatment, and secondary prevention. Am Fam Physician. 2014;89(12):945–951. [PubMed] [Google Scholar]
- 2.Gullberg B., Johnell O., Kanis J.A. World-wide projections for hip fracture. Osteoporos Int. 1997;7:407. doi: 10.1007/pl00004148. [DOI] [PubMed] [Google Scholar]
- 3.Foss N.B., Kehlet H. Hidden blood loss after surgery for hip fracture. J Bone Joint Surg Br. 2006;88:1053. doi: 10.1302/0301-620X.88B8.17534. [DOI] [PubMed] [Google Scholar]
- 4.Khan A.M., Mushtaq N., Giannakas K., Sochart D.H., Andrews J.G. Cross-match protocols for femoral neck fractures--finding one that can work. Ann R Coll Surg Engl. 2004;86:11. doi: 10.1308/003588404772614614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lawrence V.A., Silverstein J.H., Cornell J.E., Pederson T., Noveck H., Carson J.L. Higher Hb level is associated with better early functional recovery after hip fracture repair. Transfusion. 2003;43:1717. doi: 10.1046/j.0041-1132.2003.00581.x. [DOI] [PubMed] [Google Scholar]
- 6.Halm E.A., Wang J.J., Boockvar K., Penrod J., Silberzweig S.B., Magaziner J. The effect of perioperative anemia on clinical and functional outcomes in patients with hip fracture. J Orthop Trauma. 2004;18:369. doi: 10.1097/00005131-200407000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maraldi C., Ble A., Zuliani G., Guralnik J.M., Mussi C., Fellin R. Association between anemia and physical disability in older patients: role of comorbidity. Aging Clin Exp Res. 2006;18:485. doi: 10.1007/BF03324848. [DOI] [PubMed] [Google Scholar]
- 8.Foss N.B., Kristensen M.T., Kehlet H. Anaemia impedes functional mobility after hip fracture surgery. Age Ageing. 2008;37:173. doi: 10.1093/ageing/afm161. [DOI] [PubMed] [Google Scholar]
- 9.Friedman B.A. An analysis of surgical blood use in United States hospitals with application to the maximum surgical blood order schedule. Transfusion. 1979;19:268. doi: 10.1046/j.1537-2995.1979.19379204208.x. [DOI] [PubMed] [Google Scholar]
- 10.Conteduca F., Massai F., Iorio R., Zanzotto E., Luzon D., Ferretti A. Blood loss in computer-assisted mobile bearing total knee arthroplasty. A comparison of computer-assisted surgery with a conventional technique. Int Orthop. 2009;33:1609. doi: 10.1007/s00264-008-0651-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tengberg P.T., Foss N.B., Palm H., Kallemose T., Troelsen A. Tranexamic acid reduces blood loss in patients with extracapsular fractures of the hip: results of a randomised controlled trial. Bone Joint Lett J. 2016;98-B:747. doi: 10.1302/0301-620X.98B6.36645. [DOI] [PubMed] [Google Scholar]
- 12.Chiarugi M., Buccianti P., Disarli M., Galatioto C., Cavina E. Effect of blood transfusions on disease-free interval after rectal cancer surgery. Hepato-Gastroenterology. 2000;47:1002. [PubMed] [Google Scholar]
- 13.Marik P.E., Corwin H.L. Efficacy of red blood cell transfusion in the critically ill: a systematic review of the literature. Crit Care Med. 2008;36:2667. doi: 10.1097/CCM.0b013e3181844677. [DOI] [PubMed] [Google Scholar]
- 14.Izuel R.M., Garcia E.J., Gomez-Barrera M., Cuenca E.J., Abad S.R., Rabanaque H.M. [Relationship between allogeneic blood transfusion, iron deficiency and nosocomial infection in patients with hip fracture] Med Clin. 2008;131:647. doi: 10.1157/13128722. [DOI] [PubMed] [Google Scholar]
- 15.Zhang H., Chen J., Chen F., Que W. The effect of tranexamic acid on blood loss and use of blood products in total knee arthroplasty: a meta-analysis. Knee Surg Sports Traumatol Arthrosc. 2012;20:1742. doi: 10.1007/s00167-011-1754-z. [DOI] [PubMed] [Google Scholar]
- 16.Huang F., Wu D., Ma G., Yin Z., Wang Q. The use of tranexamic acid to reduce blood loss and transfusion in major orthopedic surgery: a meta-analysis. J Surg Res. 2014;186:318. doi: 10.1016/j.jss.2013.08.020. [DOI] [PubMed] [Google Scholar]
- 17.Bilgili M.G., Ercin E., Peker G., Kural C., Basaran S.H., Duramaz A. Efficiency and cost analysis of cell saver auto transfusion system in total knee arthroplasty. Balkan Med J. 2014;31:149. doi: 10.5152/balkanmedj.2014.13267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Appassakij H., Promwong C., Rujirojindakul P., Wutthanarungsan R., Silpapojakul K. The risk of blood transfusion-associated Chikungunya fever during the 2009 epidemic in Songkhla Province, Thailand. Transfusion. 2014;54:1945. doi: 10.1111/trf.12575. [DOI] [PubMed] [Google Scholar]
- 19.Dan M., Liu D., Martos S.M., Beller E. Intra-operative blood salvage in total hip and knee arthroplasty. J Orthop Surg (Hong Kong) 2016;24:204. doi: 10.1177/1602400217. [DOI] [PubMed] [Google Scholar]
- 20.Aguilera-Roig X., Jordan-Sales M., Natera-Cisneros L., Monllau-Garcia J.C., Martinez-Zapata M.J. [Tranexamic acid in orthopedic surgery] Rev Española Cirugía Ortopédica Traumatol. 2014;58:52. doi: 10.1016/j.recot.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 21.Carson J.L., Stanworth S.J., Roubinian N., Fergusson D.A., Triulzi D., Doree C. Transfusion thresholds and other strategies for guiding allogeneic red blood cell transfusion. Cochrane Database Syst Rev. 2016;10:D2042. doi: 10.1002/14651858.CD002042.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee Q.J., Chang W.Y., Wong Y.C. Blood-sparing efficacy of oral tranexamic acid in primary total hip arthroplasty. J Arthroplast. 2017;32:139. doi: 10.1016/j.arth.2016.06.058. [DOI] [PubMed] [Google Scholar]
- 23.Wind T.C., Barfield W.R., Moskal J.T. The effect of tranexamic acid on transfusion rate in primary total hip arthroplasty. J Arthroplast. 2014;29:387. doi: 10.1016/j.arth.2013.05.026. [DOI] [PubMed] [Google Scholar]
- 24.Rajesparan K., Biant L.C., Ahmad M., Field R.E. The effect of an intravenous bolus of tranexamic acid on blood loss in total hip replacement. J Bone Joint Surg Br. 2009;91:776. doi: 10.1302/0301-620X.91B6.22393. [DOI] [PubMed] [Google Scholar]
- 25.Li G., Sun T.W., Luo G., Zhang C. Efficacy of antifibrinolytic agents on surgical bleeding and transfusion requirements in spine surgery: a meta-analysis. Eur Spine J. 2017;26:140. doi: 10.1007/s00586-016-4792-x. [DOI] [PubMed] [Google Scholar]
- 26.Sun X., Dong Q., Zhang Y.G. Intravenous versus topical tranexamic acid in primary total hip replacement: a systemic review and meta-analysis. Int J Surg. 2016;32:10. doi: 10.1016/j.ijsu.2016.05.064. [DOI] [PubMed] [Google Scholar]
- 27.Abbasi H., Behdad S., Ayatollahi V., Nazemian N., Mirshamsi P. Comparison of two doses of tranexamic acid on bleeding and surgery site quality during sinus endoscopy surgery. Adv Clin Exp Med. 2012;21:773. [PubMed] [Google Scholar]
- 28.Dunn C.J., Goa K.L. Tranexamic acid: a review of its use in surgery and other indications. Drugs. 1999;57:1005. doi: 10.2165/00003495-199957060-00017. [DOI] [PubMed] [Google Scholar]
- 29.Kazemi S.M., Mosaffa F., Eajazi A., Kaffashi M., Daftari B.L., Bigdeli M.R. The effect of tranexamic acid on reducing blood loss in cementless total hip arthroplasty under epidural anesthesia. Orthopedics. 2010;33:17. doi: 10.3928/01477447-20091124-30. [DOI] [PubMed] [Google Scholar]
- 30.Watts C.D., Houdek M.T., Sems S.A., Cross W.W., Pagnano M.W. Tranexamic acid safely reduced blood loss in hemi- and total hip arthroplasty for acute femoral neck fracture: a randomized clinical trial. J Orthop Trauma. 2017;31:345. doi: 10.1097/BOT.0000000000000837. [DOI] [PubMed] [Google Scholar]
- 31.Mohib Y., Rashid R.H., Ali M., Zubairi A.J., Umer M. Does tranexamic acid reduce blood transfusion following surgery for inter-trochanteric fracture? A randomized control trial. J Pak Med Assoc. 2015;65:S17. [PubMed] [Google Scholar]
- 32.Zufferey P.J., Miquet M., Quenet S., Martin P., Adam P., Albaladejo P. Tranexamic acid in hip fracture surgery: a randomized controlled trial. Br J Anaesth. 2010;104:23. doi: 10.1093/bja/aep314. [DOI] [PubMed] [Google Scholar]
- 33.Tian S., Shen Z., Liu Y., Zhang Y., Peng A. The effect of tranexamic acid on hidden bleeding in older intertrochanteric fracture patients treated with PFNA. Injury. 2018;49:680. doi: 10.1016/j.injury.2018.01.026. [DOI] [PubMed] [Google Scholar]
- 34.Vijay B.S., Bedi V., Mitra S., Das B. Role of tranexamic acid in reducing postoperative blood loss and transfusion requirement in patients undergoing hip and femoral surgeries. Saudi J Anaesth. 2013;7:29. doi: 10.4103/1658-354X.109803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sadeghi . 2007. Does a single bolus dose of tranexamic acid reduce blood loss and transfusion requirements during hip fracture surgery? A prospective randomized double blind study in 67 patients. [Google Scholar]
- 36.Emara W.M., Moez K.K., Elkhouly A.H. Topical versus intravenous tranexamic acid as a blood conservation intervention for reduction of post-operative bleeding in hemiarthroplasty. Anesth Essays Res. 2014;8:48. doi: 10.4103/0259-1162.128908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lei J., Zhang B., Cong Y., Zhuang Y., Wei X., Fu Y. Tranexamic acid reduces hidden blood loss in the treatment of intertrochanteric fractures with PFNA: a single-center randomized controlled trial. J Orthop Surg Res. 2017;12:124. doi: 10.1186/s13018-017-0625-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Baruah R.K., Borah P.J., Haque R. Use of tranexamic acid in dynamic hip screw plate fixation for trochanteric fractures. J Orthop Surg (Hong Kong) 2016;24:379. doi: 10.1177/1602400322. [DOI] [PubMed] [Google Scholar]
- 39.World Health Organization (WHO) WHO model list of essential medicines. 2017. Available at: http://www.who.int/medicines/publications/essentialmedicines/en/ [DOI] [PubMed]
- 40.Drakos A., Raoulis V., Karatzios K., Doxariotis N., Kontogeorgakos V., Malizos K. Efficacy of local administration of tranexamic acid for blood salvage in patients undergoing intertrochanteric fracture surgery. J Orthop Trauma. 2016;30:409. doi: 10.1097/BOT.0000000000000577. [DOI] [PubMed] [Google Scholar]
- 41.Virani S.R., Dahapute A.A., Panda I., Bava S.S. Role of local infiltration of tranexamic acid in reducing blood loss in peritrochanteric fracture surgery in the elderly population. Malays Orthop J. 2016;10:26. doi: 10.5704/MOJ.1611.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang P., He J., Fang Y., Chen P., Liang Y., Wang J. Efficacy and safety of intravenous tranexamic acid administration in patients undergoing hip fracture surgery for hemostasis: a meta-analysis. Medicine (Baltim) 2017;96:e6940. doi: 10.1097/MD.0000000000006940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wei Z., Liu M. The effectiveness and safety of tranexamic acid in total hip or knee arthroplasty: a meta-analysis of 2720 cases. Transfus Med. 2015;25:151. doi: 10.1111/tme.12212. [DOI] [PubMed] [Google Scholar]
- 44.Goobie S.M., Meier P.M., Sethna N.F., Soriano S.G., Zurakowski D., Samant S. Population pharmacokinetics of tranexamic acid in paediatric patients undergoing craniosynostosis surgery. Clin Pharmacokinet. 2013;52:267. doi: 10.1007/s40262-013-0033-1. [DOI] [PubMed] [Google Scholar]
- 45.Zhang P., Liang Y., Chen P., Fang Y., He J., Wang J. Intravenous versus topical tranexamic acid in primary total hip replacement: a meta-analysis. Medicine (Baltim) 2016;95:e5573. doi: 10.1097/MD.0000000000005573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee Q.J., Chang W.Y., Wong Y.C. Blood-sparing efficacy of oral tranexamic acid in primary total hip arthroplasty. J Arthroplast. 2017;32:139. doi: 10.1016/j.arth.2016.06.058. [DOI] [PubMed] [Google Scholar]
- 47.Gandhi R., Evans H.M., Mahomed S.R., Mahomed N.N. Tranexamic acid and the reduction of blood loss in total knee and hip arthroplasty: a meta-analysis. BMC Res Notes. 2013;6:184. doi: 10.1186/1756-0500-6-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Benoni G., Lethagen S., Fredin H. The effect of tranexamic acid on local and plasma fibrinolysis during total knee arthroplasty. Thromb Res. 1997;85:195. doi: 10.1016/s0049-3848(97)00004-2. [DOI] [PubMed] [Google Scholar]
- 49.Sabbag O.D., Abdel M.P., Amundson A.W., Larson D.R., Pagnano M.W. Tranexamic acid was safe in arthroplasty patients with a history of venous thromboembolism: a matched outcome study. J Arthroplasty. 2017;32:S246. doi: 10.1016/j.arth.2017.02.008. [DOI] [PubMed] [Google Scholar]
- 50.Evangelista P.J., Aversano M.W., Koli E., Hutzler L., Inneh I., Bosco J. Effect of tranexamic acid on transfusion rates following total joint arthroplasty: a cost and comparative effectiveness analysis. Orthop Clin N Am. 2017;48:109. doi: 10.1016/j.ocl.2016.12.001. [DOI] [PubMed] [Google Scholar]
- 51.Demos H.A., Lin Z.X., Barfield W.R., Wilson S.H., Robertson D.C., Pellegrini V.J. Process improvement Project using tranexamic acid is cost-effective in reducing blood loss and transfusions after total hip and total knee arthroplasty. J Arthroplasty. 2017;32:2375. doi: 10.1016/j.arth.2017.02.068. [DOI] [PubMed] [Google Scholar]
- 52.DiBlasi J.F., Smith R.P., Garavaglia J., Quedado J., Frye B.M., Dietz M.J. Comparing cost, efficacy, and safety of intravenous and topical tranexamic acid in total hip and knee arthroplasty. Am J Orthop (Belle Mead NJ) 2016;45:E439. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.