Abstract
Background/objective
Osteoarthritis (OA) is the most common joint disorder. Angiogenesis and synovial hyperplasia are important factors in the development of OA. Previous studies demonstrated that bevacizumab, an antibody against vascular endothelial growth factor in angiogenesis for cancer treatment, might be a potential candidate for the treatment of OA. However, experimental studies were lacking in whether bevacizumab would be able to attenuate the severity of OA. In this study, we used normal New Zealand rabbits and a rabbit knee immobilization model of OA, to investigate the toxicity and efficacy of bevacizumab.
Methods
In the safety test of bevacizumab, sixteen rabbits were randomly divided into 2 groups: control group and bevacizumab group (n = 8 per group). We evaluated the blood chemistry and histology of normal rabbit joints after bevacizumab treatment. In the efficacy test of bevacizumab, thirty-two rabbits were used for establishing OA model and then randomly divided into 4 groups: bevacizumab group, sodium hyaluronate (SH) group, triamcinolone acetonide (TA) group and control group (n = 8 per group). We used histological evaluations and immunohistochemistry to examine the responses to bevacizumab treatment in a rabbit model of knee immobilization-induced OA.
Results
Bevacizumab treatment did not show any adverse effects histologically on normal joints. Blood tests and Mankin's score of cartilage revealed no significant difference between the bevacizumab and control groups (p > 0.05). The bevacizumab, SH, and TA groups attenuated articular cartilage degeneration and showed less synovial hyperplasia compared to the control group macroscopically and histologically, while the effect of the bevacizumab group was most obvious (p < 0.05). Immunohistochemistry revealed significantly lower vascular endothelial growth factor (VEGF) expression in the synovium and matrix metalloproteinase-1 (MMP-1) in the cartilage in the bevacizumab, SH, and TA groups compared to the control group (p < 0.05), while the expression of VEGF and MMP-1 in the bevacizumab group was the lowest among the four groups (p < 0.05).
Conclusions
Intra-articular injection of 4-mg bevacizumab in rabbit knees did not show adverse effects. The bevacizumab treatment prevented joint inflammation in terms of inhibition of reduced angiogenesis, inhibited synovial proliferation, and reduced VEGF and MMP-1 expression. Compared with SH and TA, bevacizumab protected the cartilage and produced a better therapeutic effect on primary knee OA in rabbits, which imply that bevacizumab, an anticancer drug, may become a potentially effective drug for the treatment of OA.
The translational potential of this article
Our study confirmed the therapeutic effect of bevacizumab on rabbit primary knee OA. This study demonstrated that bevacizumab may have clinical implications and contribute to the development of new OA treatments.
Keywords: Angiogenesis, Bevacizumab, Cartilage, Knee osteoarthritis, Synovium hyperplasia, VEGF
Introduction
Osteoarthritis (OA) is the most common joint disorder, and it imposes a tremendous burden on healthcare systems worldwide [1], [2]. OA is characterized by the degeneration of articular cartilage, synovial hyperplasia, osteophyte formation, and subchondral bone injury [3]. OA leads to stiffness and dysfunction of the affected joints. Clinical treatment relieves pain, corrects deformity, and improves or restores joint function to improve the quality of life [4]. Angiogenesis is closely related to the degree of synovial hyperplasia during the development of OA. Angiogenesis affects the innervation of articular cartilage, which produces pain in patients with OA. Blood vessels from the subchondral bone and synovial membrane invade the articular cartilage during the late stage of OA, which results in the ossification of articular cartilage and osteophyte formation. Vascular endothelial growth factor (VEGF) is essential in angiogenesis, and an angiogenesis inhibitor may be an effective treatment for OA [5].
Pegaptanib sodium, ranibizumab, and bevacizumab are the primary anti-VEGF drugs for clinical applications, especially for cancer treatment. Bevacizumab (commercial name Avastin) is a specific VEGF inhibitor that binds most active VEGF and nullifies the biological activity of endogenous VEGF [6]. The half-life of bevacizumab in normal blood circulation is 21.3 days [7]; this enables target therapeutic bevacizumab levels to be maintained with a range of administration schedules (such as once every 2 or 3 weeks) [8]. The approved dose of bevacizumab is 5 mg/kg, and the clinical interval is required to be longer than 2 weeks [9]. Bevacizumab is primarily used in ophthalmic clinical and a systemic antitumour therapy.
Intravenous bevacizumab (40 mg/kg) and an intra-articular injection of 25 mg bevacizumab resulted in significant cartilage protein expression and cartilage regeneration in an OA rabbit model of traumatic knee arthritis [10]. Lee et al. [11] reported significant cartilage regeneration via the injection of 2 mg/kg of bevacizumab into the articular cavity in a rabbit OA model. The dose of bevacizumab in the vitreous cavity was 1–1.25 mg in animal experiments, and some toxic effect appeared at doses higher than 5.0 mg [12], [13]. The nontoxic dose of bevacizumab for the retina and optic nerve is 2.5 mg in animals. Manzano RP et al. [12] investigated the retinal toxicity of intravitreal injections of 0.5 mg, 1.0 mg, 2.5 mg, and 5.0 mg bevacizumab in rabbits. Inflammatory cells appeared in the vitreous cavity of the 5.0-mg dose group, and the b-wave amplitude of the retinal electrogram decreased. Based on the safe dose of bevacizumab injection into the vitreous cavity of rabbit eyes, the present study firstly investigated the safety of 4 mg/kg bevacizumab in the normal rabbit knee joint and investigated whether bevacizumab administration attenuated OA progression in a well-established rabbit model induced by knee immobilization. This investigation might imply that bevacizumab could become a potentially useful drug for the treatment of patients with OA.
Materials and methods
Animals
It is generally assumed that rabbit cartilage would be matured and stop growing at six months of age [14]. Adult New Zealand white rabbits, aged 24 weeks and weighing 2.0–2.5 kg, were used in this study. Rabbits were purchased from a professional breeder (dilepu, Xi'an, China) and housed at the Experimental Animal Center in Shenzhen PKU-HKUST Medical Center with regular ventilation in an environmentally controlled room under a 12-h light/12-h dark cycle. All rabbits had free access to food and sterile water. All experiments were approved by the Animal Research Ethics Committee of Peking University Shenzhen Hospital.
Primary reagents
Bevacizumab was purchased from Roche, Basel, Switzerland. Sodium hyaluronate was purchased from Freda, Shandong, China. Triamcinolone was purchased from Jida, Yunnan, China. Sodium chloride injections (0.9%) were purchased from SSY, Shijiazhuang, China. Haematoxylin was purchased from Sojubio, Guangzhou, China. Eosin Y was purchased from Panera, Guangzhou, China. The AB-PAS staining kit was purchased from Huanyu Golden Eagle, Beijing, China. The VEGF antibody was purchased from Abcam, Cambridge, MA, USA. The matrix metalloproteinase-1 (MMP-1) antibody was purchased from Bioss, Beijing, China.
Safety test of bevacizumab
Sixteen New Zealand rabbits were randomly divided into 2 groups and injected with 0.4-ml normal saline or 0.4 ml of 4-mg bevacizumab (in 0.4 ml) into the articular cavity. The injections were repeated 3 weeks later. Rabbits were free to move after injections, and the behaviour of the entire animal was observed: spirit, activity, gait, fur, appetite, faeces, and so on. Rabbits were sacrificed after 6 weeks using overdose anaesthesia. Body weight was recorded prior to euthanasia, and 5-ml blood was collected from the ear vein for haematological examination and biochemical tests. The cartilage was collected from the medial femoral condyle, and synovium was collected between the patella and femur.
Efficacy test of bevacizumab
Thirty-two healthy New Zealand rabbits were used for establishing the OA model. The left knee of the experimental animal was fixed in a straightened position using plaster from 3 cm under the ankle joint to 1.5 cm under the groin [15], [16], [17], [18]. Animals were placed in cages to move and eat freely. The plaster was removed after 5 weeks, and the rabbits were randomly divided into 4 groups: bevacizumab group, SH group, TA group, and control group (n = 8 per group). The bevacizumab group received 0.4 ml of 4-mg bevacizumab twice, with a 3-week interval. The SH group received 0.4-ml intra-articular injections once weekly for 6 weeks. The TA group received 0.4-ml intra-articular injections of 1-mg diluted TA. The control group received 0.4 ml of normal saline twice with a 3-week interval. Rabbits were sacrificed after 6 weeks of treatment using overdoes anaesthesia. The cartilage from the medial femoral condyle and synovium between the patella and femur were collected for below described analysis.
Gross morphology
The pathological changes of the joint surface in the femur were observed under a dissecting microscope, and the specimens were scored according to the following principles: 0 points, the articular surface was smooth with normal colouring; 1 point, the articular surface was rough with small cracks and a dark colour; 2 points, the articular surface was eroded, and the defects reached the cartilage middle layer; 3 points, the articular surface was ulcerated, and the defects reached the cartilage deep layer; 4 points, the cartilage was stripped, and the subchondral bone was exposed [19].
Histological examination and Mankin's scores
Synovium specimens were fixed in 10% formaldehyde for 24 h, subjected to conventional dehydration, and embedded in paraffin. Serial 5-μm sections were obtained. Cartilage specimens were fixed in 10% paraformaldehyde, decalcified in 15% ethylenediaminetetraacetic acid for 10 weeks, and embedded in paraffin. Serial 5-μm sections were obtained. Synovium and cartilage sections were stained using haematoxylin-eosin (H&E). Tissue sections were treated with haematoxylin for 15 min, rinsed with water for 1 min, and immersed in static water for 5 min. Sections were treated with 0.5% eosin for 3 min and rinsed with water. Cartilage sections were stained using AB-PAS. Tissue sections were treated with 1% Alcian blue-3% acetic acid for 40 min, 1% periodic acid for 10 min, Schiff liquid dye for 30 min, and campeachy. Eight sections at the same location were randomly selected from each group, and the AB-PAS staining was observed under the microscope. The results were scored according to Mankin's scoring method, and the mean value was taken for statistical analysis. Modified Mankin's scores were calculated based on cartilage structure, chondrocytes, AB-PAS staining, and the integrity of the tidal line [20].
Immunohistochemical analysis
Cartilage specimens were fixed in 10% paraformaldehyde, decalcified in 15% ethylenediaminetetraacetic acid for 10 weeks, and embedded in paraffin. Serial 5-μm sections were obtained. Sections were baked for 3 min, rinsed twice with water, and subjected to antigen retrieval. Sections were washed 3 times with phosphate buffer saline (PBS), treated with a peroxidase blocker, and washed 3 times in PBS. Sections were treated with normal goat serum and incubated with VEGF and MMP-1 antibodies. Sections were washed 3 times using PBS and incubated with secondary antibodies. Sections were treated with an streptavidin-peroxidase (SP) solution followed by 2 drops of fresh 3,3′-diaminobenzidine (DAB) solution. The brown yellow area indicated positive staining. The number of positive cells was manual calculated under an Olympus microscope (Olympus, Tokyo, Japan). In the immunohistochemical analysis, 8 sections were selected from the same location in each group, and 5 fields were randomly selected for each section. The percentage of MMP-1 and VEGF positive cells was counted, and the mean value was taken. The expression levels of MMP-1 and VEGF were expressed by the percentage of positive cells in the total number of cells.
Statistical analysis
All data are expressed as mean ± standard deviation. The statistical software package SPSS 19.0 (IBM Corp., Armonk, NY, USA) was used for analysis. Independent-sample t-test was used to compare two group-design experiments. One-way analysis of variance was used to compare the effects of multiple group design experiments. P values less than 0.05 were considered statistically significant.
Results
Safety test of bevacizumab
Results of blood examination
Blood cell counts and biochemical markers were not significantly different between the bevacizumab group and the normal control group after 6 weeks (p > 0.05) (Table 1).
Table 1.
Blood cell count and biochemical test results of the experimental groups and the normal control group (mean ± SD).
| Groups | Num | White blood cell (WBC) | Red blood cell (RBC) | Platelet (PLT) | Aspartate aminotransferase (AST) | Blood urea nitrogen (BUN) |
|---|---|---|---|---|---|---|
| ( × 109) | ( × 1012) | ( × 109) | (U/L) | (mmol/L) | ||
| Normal control group | 8 | 8.00 ± 2.70 | 5.61 ± 0.42 | 276.50 ± 33.79 | 33.14 ± 7.01 | 8.43 ± 0.69 |
| Bevacizumab group | 8 | 7.82 ± 2.20 | 5.73 ± 0.11 | 267.25 ± 33.62 | 33.83 ± 4.92 | 9.09 ± 1.04 |
No statistical significance was detected among all parameters
Morphology
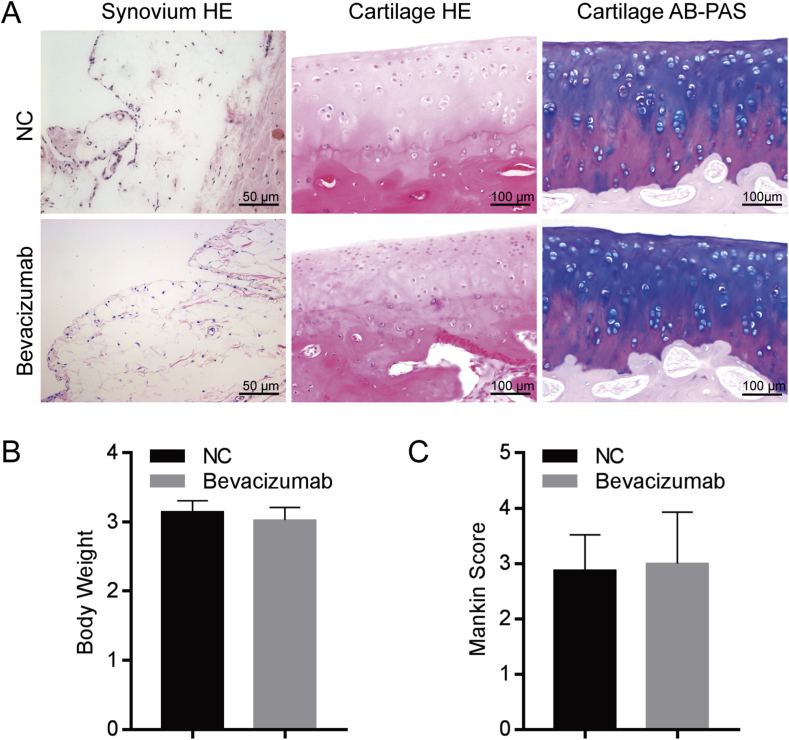
Animals in the bevacizumab and normal control groups survived and showed normal behaviour, good activity, good appetite, no depilation or skin infection, and no diarrhoea. There was no significant difference in body weight between the bevacizumab group and the normal control group up to animal sacrifice (p > 0.05) (Figure 1B).
Figure 1.
Safety test of bevacizumab. (A) H & E and AB-PAS staining of synovium and articular cartilage. The synovium and articular cartilage were normal in the NC group. The bevacizumab-treated group did not show cartilage degenerative changes. Scale bar, 50 μm (in left panels) and 100 μm (in middle and right panels). (B) Body weight did not show any statistical significant difference between the bevacizumab group and the normal control group. (C) Mankin's score showed no statistically significant difference between the bevacizumab group and the normal control group. n = 8 per group.
Histology and Mankin's scores
H&E staining of the synovium of the two groups revealed that the number of synovial cell layers in each group was 1–2, and no obvious synovial or vascular hyperplasia was observed (Figure 1A). Cartilage stained with H&E and AB-PAS revealed typical hyaline cartilage layers in the two groups, intact cartilage surface, normal-sized chondrocytes, and clear and complete tide lines in each group. The positive range of Alcian blue staining was similar between the bevacizumab group and the normal group. There was no significant difference in morphological structure between groups (Figure 1A). Mankin's score revealed no statistically significant difference between the bevacizumab group and the normal control group after 6 weeks of intra-articular injections (p > 0.05) (Figure 1C).
Efficacy of bevacizumab
Cartilage morphology
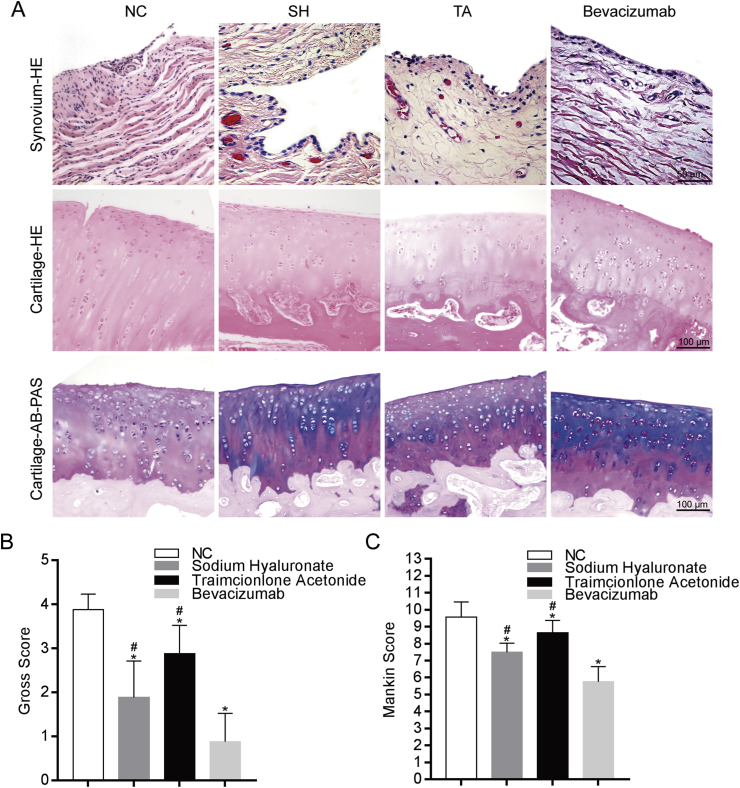
The articular surface of the knee joint in the bevacizumab group was rough with small cracks and grey in colour. The articular surface of chondrocytes in the SH group was eroded with a pale colour and small fissure damage. The articular surface of the TA group was obviously eroded with a pale colour and a small amount of cartilage defect. The articular surface of cartilage in the control group was grey with cartilage peeling and bone exposure under the cartilage. Cartilage scores of each group revealed that the bevacizumab group had scores lower than the SH, TA, and control group (p < 0.05), while the SH and TA groups had scores lower than the control group (p < 0.05) (Figure 2B).
Figure 2.
Histology evaluation of efficacy of bevacizumab. (A) H & E and AB-PAS staining of the synovium and articular cartilage. The NC group exhibited the most severe degenerative changes and synovium hyperplasia while the bevacizumab group shows fewer cartilage degenerative changes and less synovium hyperplasia. Scale bar, 50 mm (in top panels), 100 mm (in middle and bottom panels). (B) Gross scores show that cartilage degeneration in the NC group was the most severe one among the four groups while the bevacizumab group exhibited the least degenerative changes, indicating that bevacizumab treatment was effective to prevent cartilage damage; n = 8 per group. *p < 0.05, bevacizumab group, SH group, TA group compared to the NC group. #p < 0.05, bevacizumab group compared to the SH group and TA group. (C) Mankin scores showed that the cartilage degeneration in the NC group was the most severe one among the four groups while the bevacizumab group showed the least degenerative changes, indicating that bevacizumab treatment reduced the extent of cartilage damage; n = 8 per group. *p < 0.05, bevacizumab group, SH group, TA group compared to the NC group, #p < 0.05, bevacizumab group compared to the SH group and TA group.
Histology and Mankin's scores
H&E staining of the synovium of the bevacizumab group revealed that the synovial layer was composed of 2–3 layers of cells with few synovial cells and abundant loose connective tissue in the subsynovial layer with a small amount of fibrous tissue or vascular hyperplasia. H&E staining of the synovial of the SH and TA groups revealed that the synovial layer was composed of 3–5 layers of cells, and the number of cells in the subsynovial layer increased, indicating obvious vascular hyperplasia. Synovial layer cells increased significantly in the control group, vascular hyperplasia was obvious, and synovial layer cells were more numerous. Synovial villi formed in some parts, and the submembrane was thickened (Figure 2A).
H&E staining revealed a clear cartilage level, visible tidal line, and neatly arranged chondrocytes in the bevacizumab group. The levels of cartilage chondrocytes in the SH and TA groups were visible, the tidal line was visible, with less chondrocytes compared to the bevacizumab group. The cartilage surface of the control group was rough, and the integrity was impaired. Numerous cartilage cells in each layer were apoptotic, the number of cells was significantly reduced, with blurred and distorted tide line (Figure 2A).
AB-PAS staining revealed an intact cartilage structure with a deep and rich blue colour in the bevacizumab group. The SH and TA groups exhibited basically intact structure with few cells and a light blue colour. The surface of the control group was rough, and the cartilage layers were vague with a light staining (Figure 2A). Mankin's scores of the four groups were significantly different (p < 0.05) after 6 weeks of intra-articular injections (Figure 2C).
VEGF and MMP-1 expression
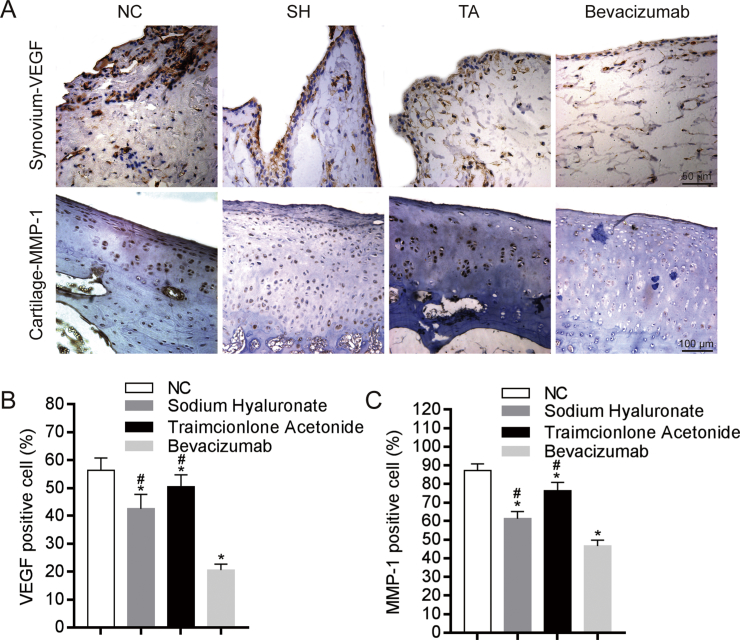
The percentages of VEGF-positive synoviocytes in the bevacizumab, SH, and TA groups were lower than those in the NC group (Figure 3A). The percentages of MMP-1–positive chondrocytes in the bevacizumab, SH, and TA groups were lower compared to the NC group (Figure 3A). The percentages of VEGF-positive or MMP-1–positive cells in the bevacizumab group were the lowest (p < 0.05) (Figure 3B).
Figure 3.
Influence of bevacizumab on VEGF and MMP-1 protein levels. VEGF and MMP-1 expression was analyzed by immunohistochemistry staining. (A) Representative images showing the immunostaining results of VEGF and MMP-1 in the synovium and articular cartilage regions of the four groups; Scale bar, 50 mm (in top panels), and 100 mm (in bottom panels). (B) Semiquantitative analyses of the percentage of VEGF positive cells (brown) in the synovium of the four groups. The results showed that bevacizumab treatment significantly reduced the number of VEGF-positive cells in the synovium compared to the NC group; n = 8 per group. *p < 0.05, bevacizumab group, SH group, TA group compared to the NC group, #p < 0.05, bevacizumab group compared to the SH group and TA group. (C) Semiquantitative analyses of the percentage of MMP-1 positive cells (brown) in the articular cartilage of the four groups. The results showed that bevacizumab treatment significantly reduced the MMP-1 positive cells in the articular cartilage compared to the NC group; n = 8 per group. *p < 0.05, bevacizumab group, SH group, TA group compared to the NC group, #p < 0.05, bevacizumab group compared to the SH group and TA group.
Discussion
The pathogenesis of OA is not fully understood. Recent studies suggest that cytokines play a key role in the process of OA and mediate the increased activity of MMPs, which lead to destruction of the cartilage matrix. Hyperplastic synoviocytes are the primary source of these cytokines. Many cytokines stimulate angiogenesis and synovial hyperplasia, which further aggravates the progression of OA [21]. Inflammation during OA leads to the activation of VEGF, synovial angiogenesis, and infiltration into the cartilage and the internal region of the meniscus [22]. The expression of VEGF is associated with a higher density of cartilage vessels [23], and VEGF plays an important role in the development of OA. Mabey et al. [24] demonstrated that VEGF was significantly different between patients with OA and patients in the control group. Local and systemic blood VEGF levels are important factors in primary bone and joint patients and are monitored as a biomarker. An increase in VEGF levels significantly promotes the pathological process of OA [25]. VEGF levels in the synovial fluid of patients with OA are related to radiation grading, ultrasound results, and functional status [26]. Further studies demonstrated that advanced glycation end products produce VEGF and inflammatory responses in elderly or diabetic patients via the receptor for advanced glycation end-products and nuclear factor-kappa B (RAGE-NF-κB) pathway in human chondrocytes [27]. VEGF plays an important role in the development of OA. Therefore, the regulation of VEGF is a future OA treatment [28]. Bevacizumab is a humanized monoclonal anti-VEGF antibody, which has been clinically used to inhibit tumour angiogenesis (approved in the USA in 2004). It was reported that bevacizumab could bind to VEGF, and thereby prevents the binding of VEGF to its receptor on the surface of endothelial cells and inhibits the biological activity of VEGF [29]. So we hypothesized that bevacizumab could delay the development of OA by inhibiting angiogenesis and synovial hyperplasia.
Bevacizumab was used in animal models of traumatic OA to inhibit articular cartilage degeneration and increase type II collagen fibre expression [30]. Bevacizumab exhibits other effects, such as inhibition of nerve growth factor [31], which reduces nerve growth and pain. However, excess bevacizumab also exhibited toxicity reported from animal experiments. Therefore, relevant safety experiments are needed for the introduction of bevacizumab to the knee joint space. Safety tests demonstrated no body weight loss or hair loss in rabbits after the administration of 4-mg bevacizumab to the joint cavity. There were no significant changes in gross anatomy or pathological observations. Blood tests revealed no significant changes compared with the normal group, which demonstrates that rabbit knee joint injection of 4-mg bevacizumab did not produce adverse reactions. These results laid the foundation for the safety of the rabbit knee joint injection of bevacizumab in the treatment of OA.
Mechanical stress plays a role in the structure and function of articular cartilage, and joint immobilization could influence the biomechanical, morphological, and biochemical properties of cartilage; the model shows how knee joint immobilized for a period of time presents the OA behaviour [16]. Traumatic synovitis caused by surgery is easy to affect cartilage and synovial biochemical metabolism, and it is not suitable to study the effects of drugs on the biochemical metabolism of OA. The rabbit model of OA induced by knee immobilization avoids the impact of traumatic synovitis caused by surgery, compared with other experimental OA models, and the knee immobilization model better simulated the natural process of OA pathogenesis. Besides, joint immobilization can safely and conveniently induce OA which is highly reproducible. This model exhibits obvious advantages in drug efficacy and therapeutic drug screening. Therefore, it is a good choice over the joint immobilization OA model to investigate this subject. Bevacizumab significantly inhibited VEGF expression in the synovium and inhibited the proliferation of blood vessels and synovicytes. MMP-1 expression in cartilage also decreased, which reduced cartilage erosion. HE staining revealed that the number of cells decreased in the control group, whereas the number of cells was higher in the bevacizumab group. HE revealed widespread apoptosis in cartilage, and AB-PAS staining revealed decreased matrix formation in the control group, and the bevacizumab group exhibited relatively more cartilage matrix. These results demonstrate that bevacizumab inhibits VEGF expression and exerts a certain effect on the treatment of OA. This finding is consistent with Nagai [10] and Soojung Lee [11].
SH and TA are the most commonly used intra-articular drugs. SH has been widely used domestically and abroad since the first report of the treatment of degenerative OA using an intra-articular injection of SH in 1971, and it achieved good therapeutic effects [32]. SH supplements or increases SH content in synovial fluid, increases the stickiness and lubrication of joint fluid and prevents the contact of inflammatory medium or cartilage matrix degrading enzyme with cartilage. However, persistent and irreversible damage to bone and cartilage remains [33]. Skin necrosis is a rare complication [34]. TA is a commonly used intra-articular drug that significantly reduces the secretion of interleukin-1 and tumour necrosis factor alpha from synovial tissue and inhibits the proliferation of capillaries and fibroblasts. A randomized controlled trial demonstrated that patients with OA who received hormone therapy exhibited increased the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) and the visual analogue scale (VAS) scores [35]. A 12-week observation of efficacy demonstrated that hormones alleviated joint pain and swelling in patients with OA without obvious side effects [36]. Other studies [37] demonstrated that the effect of intra-articular injection of hormones to treat OA was not clear after 1–6 weeks. Analysis at multiple time points indicated that the effect decreased overtime, and there was no evidence that the effect remained after 6 months of hormone injection or that long-term use could lead to cartilage or joint destruction [38]. These studies demonstrated the limitations of hormone intra-articular injection in the treatment of OA. TA in this study was a medium-effect glucocorticoid with strong and long-lasting anti-inflammatory and antiallergic effects, which rapidly eliminated swelling and pain, relieved symptoms, and blocked the activation and synthesis of MMP [39]. The results of the present study demonstrated that SH and TA also significantly reduced synoviocyte proliferation, reduced VEGF and MMP-1 production, delayed chondrocyte destruction, and delayed the development of OA compared to the control group.
Bevacizumab significantly inhibited VEGF expression in synovium and inhibited vascular proliferation and synoviocyte proliferation compared to the SH and TA groups, which indicated that fewer cytokines were released and play roles in inflammation. This result indicated that bevacizumab was superior to SH and TA in the inhibition of synovial proliferation and inflammation. The cartilage specimens of the SH and TA groups were slightly disordered, and Mankin's score was higher than that of the bevacizumab group, indicating their weaker effects on reducing the destruction of chondrocytes. The experimental results also demonstrated that SH and TA inhibited angiogenesis, which may be due to the reduction of cartilage damage and the reduction of synovial stimulation and inflammation [40]. However, this effect on vascular inhibition was smaller than bevacizumab, and bevacizumab may have a longer effect. In addition, according to the half-life of bevacizumab, the clinical interval is required to be longer than 2 weeks, while the clinical interval of TA or SH is once a week. Different injection times did not have an absolute effect on synovium, but they did reduce the risk of infection.
This study has some limitations: in addition to MMP-1, other OA markers including MMP-13 and IL-1β should also be used; the level of MMP-1 or VEGF should be tested by either real-time reverse transcription polymerase chain reaction (RT-PCR) or Western blot analysis. Future research should consider using a design to test multiple panels of OA markers at multiple time points in vivo and vitro study.
The present study evaluated the therapeutic effect of bevacizumab on rabbit primary knee OA. The results demonstrated that it effectively inhibited angiogenesis in the synovium of rabbit knee joint, inhibited synovial hyperplasia, and reduced cartilage degradation. It also inhibited the erosion of blood vessels on cartilage tissue and effectively protected the articular cartilage matrix. Intra-articular treatment is simple and directly targets the lesion at a low therapeutic dose. No side effects were found in this treatment up to now, and this may be a good option as intra-articular injection for OA treatment. Clinical trials should be tested to confirm the safety and efficacy of bevacizumab in OA. These results imply that bevacizumab may be a potentially useful drug for the treatment of patients with OA.
Funding/support
This work was supported by grants from the Shenzhen City Science and Technology Bureau of PR China (JCYJ20170307111755218 and JCYJ 20160428173412866). This study was also supported in part by grants from “San-Ming” Project of Medicine in Shenzhen (No. SZSM201612092 and SZSM201612078).
Conflicts of interest
The authors have no conflicts of interest relevant to this article.
References
- 1.Structured education and neuromuscular exercise program for hip and/or knee osteoarthritis: a health technology assessment. Ont Health Technol Assess Ser. 2018;18:1–110. [PMC free article] [PubMed] [Google Scholar]
- 2.Liu Y., Chang J.C., Hon C.C., Fukui N., Tanaka N., Zhang Z. Chromatin accessibility landscape of articular knee cartilage reveals aberrant enhancer regulation in osteoarthritis. Sci Rep. 2018;8:15499. doi: 10.1038/s41598-018-33779-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van den Bosch M.H.J. Inflammation in osteoarthritis: is it time to dampen the alarm-in this debilitating disease. Clin Exp Immunol. 2018;195:153–166. doi: 10.1111/cei.13237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sterzi S., Giordani L., Morrone M., Lena E., Magrone G., Scarpini C. The efficacy and safety of a combination of glucosamine hydrochloride, chondroitin sulfate and bio-curcumin with exercise in the treatment of knee osteoarthritis: a randomized, double-blind, placebo-controlled study. Eur J Phys Rehabil Med. 2016;52:321–330. [PubMed] [Google Scholar]
- 5.Bonnet C.S., Walsh D.A. Osteoarthritis, angiogenesis and inflammation. Rheumatology. 2005;44:7–16. doi: 10.1093/rheumatology/keh344. [DOI] [PubMed] [Google Scholar]
- 6.Grisanti S., Ziemssen F. Bevacizumab: off-label use in ophthalmology. Indian J Ophthalmol. 2007;55:417–420. doi: 10.4103/0301-4738.36474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miller K.D., Chap L.I., Holmes F.A., Cobleigh M.A., Marcom P.K., Fehrenbacher L. Randomized phase III trial of capecitabine compared with bevacizumab plus capecitabine in patients with previously treated metastatic breast cancer. J Clin Oncol. 2005;23:792–799. doi: 10.1200/JCO.2005.05.098. [DOI] [PubMed] [Google Scholar]
- 8.Iwamoto N., Hamada A., Shimada T. Antibody drug quantitation in coexistence with anti-drug antibodies on nSMOL bioanalysis. Anal Biochem. 2018;540-541:30–37. doi: 10.1016/j.ab.2017.11.002. [DOI] [PubMed] [Google Scholar]
- 9.Hurwitz H., Fehrenbacher L., Novotny W., Cartwright T., Hainsworth J., Heim W. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 10.Nagai T., Sato M., Kobayashi M., Yokoyama M., Tani Y., Mochida J. Bevacizumab, an anti-vascular endothelial growth factor antibody, inhibits osteoarthritis. Arthritis Res Ther. 2014;16:427. doi: 10.1186/s13075-014-0427-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee S., Nemeño J.G., Lee J.I. Repositioning bevacizumab: a promising therapeutic strategy for cartilage regeneration. Tissue Eng B Rev. 2016;22:341–357. doi: 10.1089/ten.TEB.2015.0300. [DOI] [PubMed] [Google Scholar]
- 12.Manzano R.P., Peyman G.A., Khan P., Kivilcim M. Testing intravitreal toxicity of bevacizumab (Avastin) Retina. 2006;26:257–261. doi: 10.1097/00006982-200603000-00001. [DOI] [PubMed] [Google Scholar]
- 13.Inan U.U., Avci B., Kusbeci T., Kaderli B., Avci R., Temel S.G. Preclinical safety evaluation of intravitreal injection of full-length humanized vascular endothelial growth factor antibody in rabbit eyes. Investig Ophthalmol Vis Sci. 2007;48:1773–1781. doi: 10.1167/iovs.06-0828. [DOI] [PubMed] [Google Scholar]
- 14.Yamamoto T., Wakitani S., Imoto K., Hattori T., Nakaya H., Saito M. Fibroblast growth factor-2 promotes the repair of partial thickness defects of articular cartilage in immature rabbits but not in mature rabbits. Osteoarthritis Cartilage. 2004;12:636–641. doi: 10.1016/j.joca.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 15.Videman T. Changes in compression and distances between tibial and femoral condyles during immobilization of rabbit knee. Arch Orthop Trauma Surg. 1981;98:289–291. doi: 10.1007/BF00378883. [DOI] [PubMed] [Google Scholar]
- 16.Zhou Q., Wei B., Liu S., Mao F., Zhang X., Hu J. Cartilage matrix changes in contralateral mobile knees in a rabbit model of osteoarthritis induced by immobilization. BMC Muscoskelet Disord. 2015;16:224. doi: 10.1186/s12891-015-0679-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wei B., Mao F., Guo Y., Yao Q., Tang C., Xu Y. Using 7.0T MRI T2 mapping to detect early changes of the cartilage matrix caused by immobilization in a rabbit model of immobilization-induced osteoarthritis. Magn Reson Imaging. 2015;33:1000–1006. doi: 10.1016/j.mri.2015.06.007. [DOI] [PubMed] [Google Scholar]
- 18.Lu W., Wang L., Yao J., Wo C., Chen Y. C5a aggravates dysfunction of the articular cartilage and synovial fluid in rats with knee joint immobilization. Mol Med Rep. 2018;18:2110–2116. doi: 10.3892/mmr.2018.9208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pelletier J.P., Jovanovic D., Fernandes J.C., Manning P., Connor J.R., Currie M.G. Reduced progression of experimental osteoarthritis in vivo by selective inhibition of inducible nitric oxide synthase. Arthritis Rheum. 1998;41:1275–1286. doi: 10.1002/1529-0131(199807)41:7<1275::AID-ART19>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 20.Li Y., Xiao W., Wu P., Deng Z., Zeng C., Li H. The expression of SIRT1 in articular cartilage of patients with knee osteoarthritis and its correlation with disease severity. J Orthop Surg Res. 2016;11:144. doi: 10.1186/s13018-016-0477-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goldring M.B. The role of cytokines as inflammatory mediators in osteoarthritis: lessons from animal models. Connect Tissue Res. 1999;40:1–11. doi: 10.3109/03008209909005273. [DOI] [PubMed] [Google Scholar]
- 22.Mapp P.I., Walsh D.A. Mechanisms and targets of angiogenesis and nerve growth in osteoarthritis. Nat Rev Rheumatol. 2012;8:390–398. doi: 10.1038/nrrheum.2012.80. [DOI] [PubMed] [Google Scholar]
- 23.Fransès R.E., McWilliams D.F., Mapp P.I., Walsh D.A. Osteochondral angiogenesis and increased protease inhibitor expression in OA. Osteoarthritis Cartilage. 2010;18:563–571. doi: 10.1016/j.joca.2009.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mabey T., Honsawek S., Saetan N., Poovorawan Y., Tanavalee A., Yuktanandana P. Angiogenic cytokine expression profiles in plasma and synovial fluid of primary knee osteoarthritis. Int Orthop. 2014;38:1885–1892. doi: 10.1007/s00264-014-2406-y. [DOI] [PubMed] [Google Scholar]
- 25.Yuan Q., Sun L., Li J.J., An C.H. Elevated VEGF levels contribute to the pathogenesis of osteoarthritis. BMC Muscoskelet Disord. 2014;15:437. doi: 10.1186/1471-2474-15-437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim H.R., Lee J.H., Kim K.W., Kim B.M., Lee S.H. The relationship between synovial fluid VEGF and serum leptin with ultrasonographic findings in knee osteoarthritis. Int J Rheum Dis. 2016;19:233–240. doi: 10.1111/1756-185X.12486. [DOI] [PubMed] [Google Scholar]
- 27.Chen Y.J., Chan D.C., Chiang C.K., Wang C.C., Yang T.H., Lan K.C. Advanced glycation end-products induced VEGF production and inflammatory responses in human synoviocytes via RAGE-NF-κB pathway activation. J Orthop Res. 2016;34:791–800. doi: 10.1002/jor.23083. [DOI] [PubMed] [Google Scholar]
- 28.Jansen H., Meffert R.H., Birkenfeld F., Petersen W., Pufe T. Detection of vascular endothelial growth factor (VEGF) in moderate osteoarthritis in a rabbit model. Ann Anat. 2012;194:452–456. doi: 10.1016/j.aanat.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 29.Lu J., Zhang H., Cai D., Zeng C., Lai P., Shao Y. Positive-feedback regulation of subchondral H-type vessel formation by chondrocyte promotes osteoarthritis development in mice. J Bone Miner Res. 2018;33:909–920. doi: 10.1002/jbmr.3388. [DOI] [PubMed] [Google Scholar]
- 30.Nagai T., Sato M., Kutsuna T., Kokubo M., Ebihara G., Ohta N. Intravenous administration of anti-vascular endothelial growth factor humanized monoclonal antibody bevacizumab improves articular cartilage repair. Arthritis Res Ther. 2010;12:R178. doi: 10.1186/ar3142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jee D., Lee W.K. Inhibitory effect of intravitreal injection of bevacizumab on nerve growth factor. Curr Eye Res. 2012;37:408–415. doi: 10.3109/02713683.2011.632108. [DOI] [PubMed] [Google Scholar]
- 32.Ong K.L., Anderson A.F., Niazi F., Fierlinger A.L., Kurtz S.M., Altman R.D. Hyaluronic acid injections in medicare knee osteoarthritis patients are associated with longer time to knee arthroplasty. J Arthroplast. 2016;31:1667–1673. doi: 10.1016/j.arth.2016.01.038. [DOI] [PubMed] [Google Scholar]
- 33.Ayhan E., Kesmezacar H., Akgun I. Intraarticular injections (corticosteroid, hyaluronic acid, platelet rich plasma) for the knee osteoarthritis. World J Orthoped. 2014;5:351–361. doi: 10.5312/wjo.v5.i3.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim W.B., Alhusayen R.O. Skin necrosis from intra-articular hyaluronic acid injection. J Cutan Med Surg. 2015;19:182–184. doi: 10.2310/7750.2014.14081. [DOI] [PubMed] [Google Scholar]
- 35.Dávila-Parrilla A., Santaella-Santé B., Otero-López A. Does injection site matter? A randomized controlled trial to evaluate different entry site efficacy of knee intra-articular injections. Bol Asoc Med P R. 2015;107:78–81. [PubMed] [Google Scholar]
- 36.Spolidoro P.N.O., Natour J., Machado F.S., de Oliveira H.A., Furtado R.N. Effectiveness of triamcinolone hexacetonide intraarticular injection in interphalangeal joints: a 12-week randomized controlled trial in patients with hand osteoarthritis. J Rheumatol. 2015;42:1869–1877. doi: 10.3899/jrheum.140736. [DOI] [PubMed] [Google Scholar]
- 37.Jüni P., Hari R., Rutjes A.W., Fischer R., Silletta M.G., Reichenbach S. Intra-articular corticosteroid for knee osteoarthritis. Cochrane Database Syst Rev. 2015:CD005328. doi: 10.1002/14651858.CD005328.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Raynauld J.P., Buckland-Wright C., Ward R., Choquette D., Haraoui B., Martel-Pelletier J. Safety and efficacy of long-term intraarticular steroid injections in osteoarthritis of the knee: a randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2003;48:370–377. doi: 10.1002/art.10777. [DOI] [PubMed] [Google Scholar]
- 39.Zille H., Paquet J., Henrionnet C., Scala-Bertola J., Leonard M., Six J.L. Evaluation of intra-articular delivery of hyaluronic acid functionalized biopolymeric nanoparticles in healthy rat knees. Bio Med Mater Eng. 2010;20:235–242. doi: 10.3233/BME-2010-0637. [DOI] [PubMed] [Google Scholar]
- 40.Gallo J., Raska M., Kriegova E., Goodman S.B. Inflammation and its resolution and the musculoskeletal system. J Orthop Translat. 2017;10:52–67. doi: 10.1016/j.jot.2017.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]