Abstract
Background/objective
Stem cell–based therapy has been applied to accelerate the revitalization of allograft tendon into a viable and functional tendon. Although many authors have proposed different methods to help the seeded stem cell distribution in the decellularized allograft, limited success has been achieved as tendon is a high dense connective tissue. We hypothesized that bone marrow stromal cells (BMSCs), seeded through the lateral slit, can regenerate the decellularized tendon (DCT) graft. The cell proliferation, cell viability, and tendon-specific gene expression are increased with the seeded cell density.
Methods
Eighty-seven flexor digitorum profundus tendons were equally and randomly divided into 6 treatment groups that were seeded with low-density (2 × 107 cells/mL) and high-density (5 × 107 cells/mL) BMSCs through lateral slits cultured for 2 and 4 weeks, DCT without cells, and fresh live tendons. Tendons were evaluated for cell distribution, cell proliferation, cell viability, gene expression of Collagen I and Collagen III, tenogenic markers, and MMPs.
Results
Histologic evaluation revealed BMSCs distributed from the lateral slit to the whole DCT. BMSCs were proliferated and kept viable in lateral slit decellularized tendon (LSDCT) in both seeded cell density groups after 2 and 4 weeks of culture. However, no significant differences in the cell proliferation between both cell density groups at 2 and 4 weeks of culture were observed. The lowest cell viability was found in the high-density group after 4 weeks of culture. BMSCs in LSDCT showed a significant tendency of higher gene expression of Collagen I, Collagen III, tenascin C, MMP2, MMP9, and MMP13 compared to normal tendons in both cell density groups at 2 and 4 weeks of culture.
Conclusion
BMSCs proliferated and remained viable after 2 and 4 weeks of culture with distribution throughout the lateral slits. Lateral slit preparation allows for the effective delivery and maintenance of mesenchymal cells with proliferation and generating a tenogenic behaviour of DCT in both the low and high cell densities in an in vitro model.
The translation potential of this article
Revitalizing the implanted decellularized allograft is important for clinical application. In this study, we demonstrated that the DCT, with lateral slits, could harbour the seeded stem cell and stimulate proliferation with collagen synthesis. This evidence was presented for clinical application of the lateral slit technique, in DCT grafts, which would repopulate the seeded BMSCs during tendon and ligament reconstruction.
Keywords: Bone marrow stromal cells, Decellularized graft, Flexor tendon, Tendon regeneration
Introduction
Tendon injuries are the most common hand injuries with a 10%–30% failure rate after flexor tendon repair because of postoperative complications, such as severe adhesions and repair rupture [1], [2], [3], [4], [5]. In this situation, the flexor tendon reconstruction with tendon graft is required to restore digit and hand function. Use of decellularized allograft not only provides sufficient resources and maintaining mechanical properties but also eliminates the donor site morbidity and host immunogenic rejection [6], [7], [8], [9]. Unfortunately, using decellularized allograft tendons resulted in the further delay of healing due to lack of living cells and slow migration of native cells.
Cell-based therapy has recently been studied to accelerate allograft revitalization in the early stages by directly seeding the cells into tendon. However, owing to the dense extracellular matrix structure, cell infiltration is typically limited to the allograft surface after implantation [10], [11], [12], [13], [14]. Several chemical agents and physical destruction methods have been proposed to break down the dense tendon matrix and improve cell infiltration in decellularized tendon (DCT) allografts. These methods would impair the tendon mechanical properties [15], [16], [17], [18]. Recently, Ozasa et al. [14] reported that a novel tendon slit technology was able to effectively deliver and maintain transplanted cells without interfering with the tendon's mechanical properties. However, because the multiple slits were made in a volardorsal direction, tendon friction increased substantially with the damaged tendon surface directly gliding against the pulley system [14]. Furthermore, the volardorsal slits were shallow because of the oval shape of the flexor tendon cross-section. More recently, a lateral tendon slit technique was introduced to seed bone marrow stromal cells (BMSCs) to enhance the revitalization of DCT graft [19]. The result revealed that the seeded BMSCs (2 × 107 cells/mL) remained viable and would repopulate in the DCT graft after 2 weeks of culture. However, the effects of different cell seeding density and culture time on cell distribution and revitalization in DCT after seeding through lateral slits need to be further investigated.
In this study, we investigated the effects of different cell densities and culture times on cell behaviour after seeding BMSCs through the lateral slits in DCT. We hypothesized that (1) BMSCs could distribute to the substance of the DCT in the early stage and retain viability and reducibility after seeding through lateral slits and (2) the greater the seeded cell density and culture time, the greater the cell viability and tendon-specific gene expression, possibly increasing allograft revitalization.
Materials and methods
All animal studies were approved by our Institutional Animal Care and Use Committee. A total of 87 fresh flexor digitorum profundus (FDP) tendons were harvested from 11 dogs (average age, 10 ± 1 months; average weight, 21.3 ± 3 kg). The second, third, fourth, and fifth FDP tendons were obtained from the forepaws of each dog. These FDP tendons were equally and randomly divided into six treatment groups, including fresh live tendon (positive control), lateral slits decellularized tendon (LSDCT) without cells (negative control), and two different BMSC seeding densities [low (L) and high (H)] and two culture times (2 weeks of culture [2] and 4 weeks of culture [4]) (LSDCT plus BMSCs 2L, 2H, 4L, 4H) (Table 1). Another three FDP tendons were used to evaluate LSDCT seeded with low-density BMSCs after 3 days of culture to investigate early seeding cell distribution.
Table 1.
Graft treatment and analysis.
| Group | Description | Analysis |
|||
|---|---|---|---|---|---|
| Histologic H&E |
Cell viability |
Gene expression | |||
| Live/death | DiI/DAPI | ||||
| A | Fresh live tendon | 3 | 3 | 3 | 6 |
| B | LSDCT without cells | 3 | 3 | 3 | |
| C | LSDCT with BMSCs in 2 × 105/slit 2 wk culture | 3 | 3 | 3 | 6 |
| D | LSDCT with BMSCs in 5 × 105/slit 2 wk culture | 3 | 3 | 3 | 6 |
| E | LSDCT with BMSCs in 2 × 105/slit 4 wk culture | 3 | 3 | 3 | 6 |
| F | LSDCT with BMSCs in 5 × 105/slit 4 wk culture | 3 | 3 | 3 | 6 |
BMSC = bone marrow stromal cell; DAPI = 4′, 6-diamidino-2-phenylindole; H&E = haematoxylin and eosin; LSDCT = lateral slits decellularized tendon.
Preparation of BMSCs
Bone marrow was harvested from mix-breed adult dogs, and the BMSCs were cultured as described in our previous study [14]. The suspended bone marrow was placed in 100-mm cell culture dishes and incubated at 37 °C in a 5% carbon dioxide (CO2) humidified incubator. Culture medium [minimal essential medium (MEM) with Earle's salts (GIBCO, Grand Island, NY, USA), 10% foetal bovine serum, and 1% antibiotics (Antibiotic-Antimycotic; GIBCO)] was changed every three days. When cultured cells reached 80%–90% confluence, they were detached with 0.25% trypsin and subcultured. The third-passage BMSCs were used for this study.
Multiple slit allograft preparation and cell seeding
After harvesting, 69 FDP tendons were immediately decellularized with freeze-thaw technique [20]. The tendon segments were immersed in liquid nitrogen for 2 min and then thawed in saline solution at 37 °C for 10 min. This procedure was repeated five times to kill residual cells in the tendon. Next, the DCT was lyophilized (Millrock Bench-Top Freeze Dryer; Millrock Technology, Inc, Kingston, NY, USA) for 24 h and then gas sterilized. Fifteen fresh tendons were defined as “live” and assessed immediately after harvesting.
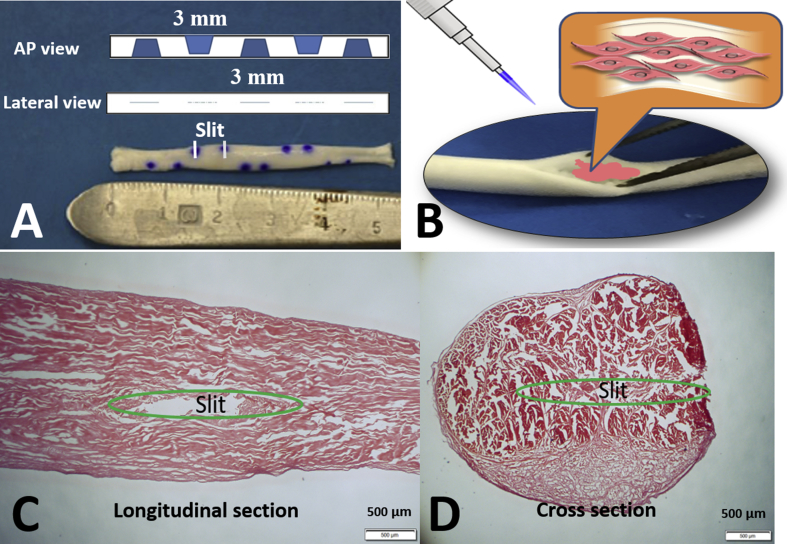
The DCTs were rehydrated in PBS for 24 h before use. Under sterile conditions, they were prepared with multiple lateral slits. A longitudinal slit was made in the lateral tendon surface with a #11 scalpel blade. Subsequent longitudinal slits were made along the length of the tendon at 3-mm intervals and staggered on both sides of the lateral surface of the tendon, totalling five slits per tendon (Fig. 1A). During this procedure, the blade penetrated the tendon core without penetrating the opposite tendon surface.
Figure 1.
(A) Multiple lateral slits in decellularized tendon; (B) cells seeded into the lateral slits using micropipette; (C and D) the slit position in the decellularized tendon (original magnification × 20, bar = 500 μm). The two blue dots on one side revealed the margin of the slit. AP view = anteroposterior view.
The BMSCs were seeded with low density (2 × 107 cells/mL) or high density (5 × 107 cells/mL) in medium with 10 μL of cell slurry using a micropipette and resulting in 2 × 105 cells or 5 × 105 cells in one slit (Fig. 1B). The cell densities were chosen on the basis of the study by Ozasa et al. [14] showing that 2 × 107 cells/mL cell density seeding in the DCT allograft reached 79% of total DNA content compared with normal tendon after 2 weeks of culture. After cell seeding, these DCTs were cultured for 2 or 4 weeks with MEM [10% foetal bovine serum, and 1% Antibiotic-Antimycotic (GIBCO)]. The cultured DCTs were analysed by histologic examination including cell viability with DiI/4′, 6-diamidino-2-phenylindole (DAPI), live/dead assay, and gene expression of Collagen I, Collagen III, tenomodulin (TNMD), tenascin C (TNC), and MMPs.
Histologic examination and cell viability
The fresh live tendon and LSDCT with and without seeded BMSCs were stained with haematoxylin and eosin to evaluate the cell distribution status.
Cell viability and tracking of the seeded cells were evaluated by labelling the BMSCs with Vybrant CM-DiI Cell-Labelling Solution (5-μL solution supplied per millilitre of cell suspension at 1 × 106 cells/mL; Molecular Probes, Eugene, OR, USA) before implantation. The labelled BMSCs were resuspended with MEM according to the assigned low (2 × 107 cells/mL) and high (5 × 107 cells/mL) cell density groups and seeded into the lateral slits of DCTs. After 2 or 4 weeks of tissue culture, the DCTs were fixed in 10% formalin at 4 °C for 24 h. Tendon segments were embedded in optical cutting temperature compound (Tissue-Tek, Sakura Finetek, Japan) and cut at 10 μm with a cryostat (Leica CM 1850, Wetzlar, Germany). The tendons were counterstained with DAPI (Vector Laboratories Inc, Burlingame, CA, USA) for nuclear staining and observed with a confocal microscope (LSM 780; Carl Zeiss, Oberkochen, Germany). The total cell number in a normal tendon and the total seeding cell (i.e., parent and daughter cells) number in the allograft were counted from 3 randomly selected areas (300 μm × 300 μm) in each of the three samples per study group. The result was analysed by ImageJ software (64-bit Java v. 1.6.0_24; NIH, Bethesda, MD, USA) and expressed as cell number per mm2.
Three graft tendons in each group (Table 1) were prepared to evaluate cell viability qualitatively labelled with Live/Dead Viability/Cytotoxicity Kit (Life Technologies, Grand Island, NY, USA) (7.7 mL Calcein AM and 15.4 mL ethidium homodimer-1 dissolved in 10 mL serum-free medium). Live cells were stained with Calcein AM showing green, and dead cells were stained with ethidium homodimer-1 showing red. Cell viability was calculated by the count of live cells divided by the count of live cells plus dead cells from 2 randomly selected areas (400 μm × 250 μm) in each of 3 samples per study group and expressed as percentage. The number of live or dead cells were measured using ImageJ software (64-bit Java v. 1.6.0_24; NIH).
Quantification of gene expression by real-time polymerase chain reaction
After 2 or 4 weeks of culture, six samples in the cell seeding group and the fresh normal tendon group were collected to assess cell gene expression by quantitatively measuring real-time polymerase chain reaction for Collagen I, Collagen III, TNMD, TNC, MMP2, MMP9, and MMP13. Primers are listed as supplementary data. The housekeeping gene for all experiments was the geomean of glyceraldehyde 3-phosphate dehydrogenase, l-lactate dehydrogenase A chain, and TATA-binding protein. The gene expression in the cell seeding group was showed as fold change by comparing with the expression of normal tendon that was set as 1.
Statistical analysis
The quantitative data of cell proliferation, cell viability, and gene expression within the BMSC-seeded group were tested by one-way analysis of variance (ANOVA). The Tukey–Kramer significant difference test was used as a post hoc test. The effects of seeded cell density and culture time on the cell proliferation and viability within the BMSC-seeded group were evaluated by two-way ANOVA. An independent t test was used to compare the normal tendon to each BMSC-seeded group. In all cases, p < .05 was set for statistical significance. The reported data in cell numbers and cell viability were presented as mean ± standard deviation. Gene expression data were reported as normalized fold change to control as mean ± standard error. All statistical analyses were conducted using SPSS software 20 (IBM, Rochester, MN, USA).
Results
Haematoxylin and eosin stain
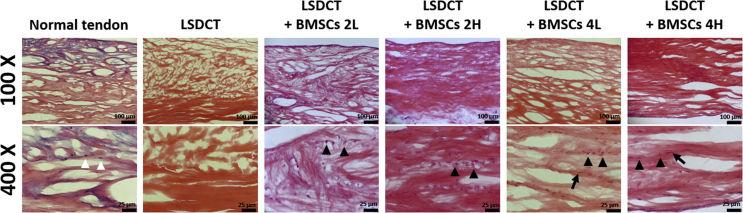
The position of the lateral slit in the DCT is shown in representative images with longitudinal and cross-sectional views (Fig. 1C and D). The LSDCT images showed more porosity and fewer residual nuclei compared to the normal tendon. Seeded BMSCs (black arrowheads) were spread from the lateral slit to the tendon matrix within the LSDCT graft. Distribution of seeded BMSCs in the LSDCT was similar to native tenocytes in the live normal tendon (white arrowheads) in both cell densities at 2 and 4 weeks of culture (Fig. 2).
Figure 2.
The H&E stain in the control and cell-seeded group. The distribution of seeded BMSCs (black arrowheads) in the LSDCT is similar to the tenocyte (white arrowheads) distribution pattern in the normal tendon. After four weeks of culture, the seeded BMSCs became spindle-like cells (arrows) infiltrated between collagen fibres. (Top row magnification × 100 with bar 100 μm; bottom row magnification × 400 with bar 25 μm).
BMSCs = bone marrow stromal cells; H&E = haematoxylin and eosin.
Cell viability assessment
DiI/DAPI assay
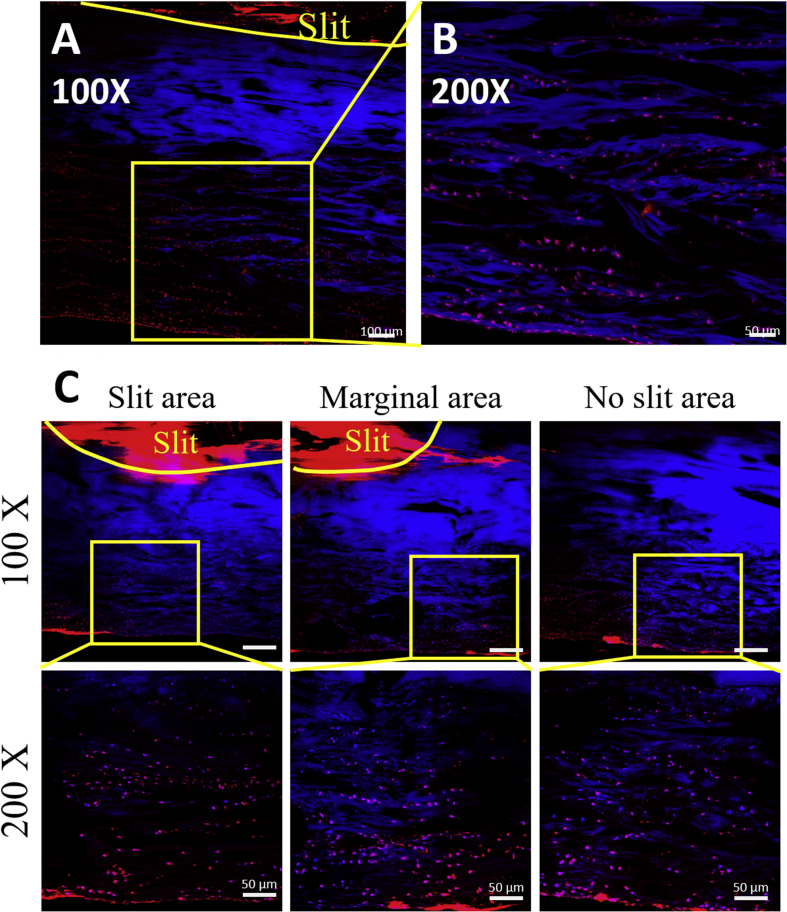
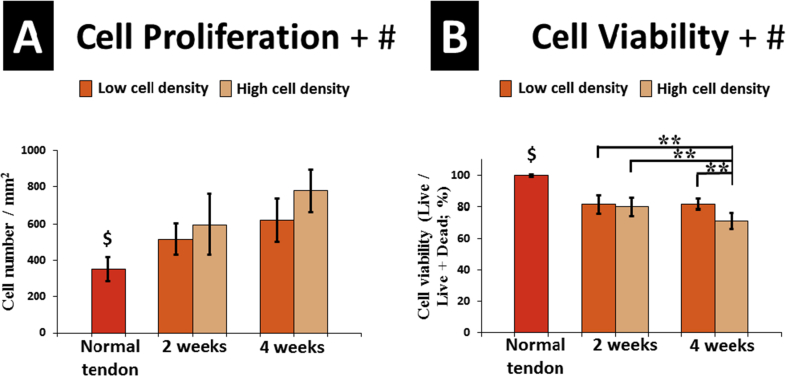
In the early implantation stage, seeded BMSCs were distributed through the tendon matrix after 3 days of culture (Fig. 3A and B). Based on the images of 2-week culture, we found seeded cells distributed not only beneath the slit area but also to the marginal and no-slit area (Fig. 3C). In addition, the DiI-labelled BMSCs migrated and distributed through the whole tendon from the slit in both cell density groups after 2 and 4 weeks of culture (Fig. 4). The BMSCs proliferated in the LSDCT, with increased total cells [implanted cells plus daughter cells (DiI stained)], at 4 weeks of culture as compared to the 2 weeks of culture in both the low and high cell density groups, but did not reach a significant difference. The total cell number was significantly affected by cell seeding density and culture time analysed by two-way ANOVA (Fig. 6A).
Figure 3.
Tracking BMSCs in LSDCT. (A and B) The DiI-stained BMSCs distributed through the slit to the surrounding area at 3 days after seeding (A, bar = 100 μm; B; bar = 50 μm). (C) The seeded BMSCs distributed from the slit area to the marginal and no-slit area after 2 weeks of culture. (Top row, bar = 100 μm; bottom row; bar = 50 μm).
BMSCs = bone marrow stromal cells.
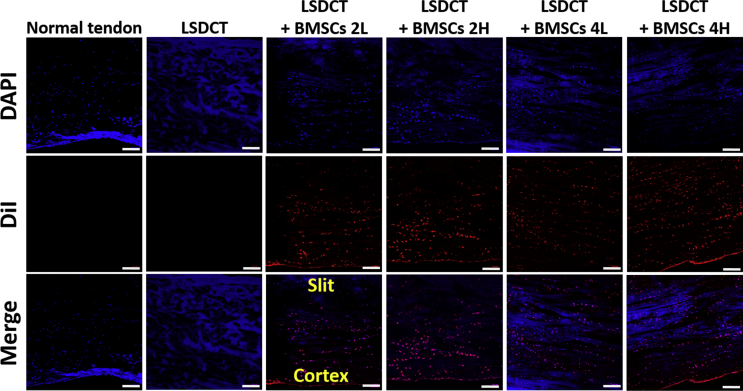
Figure 4.
Distribution of BMSCs in LSDCT treatment over time. BMSC distribution stained with DiI and DAPI in all cell-seeded groups. Seeded BMSCs (red) distributed to the whole tendon area in both low and high cell density and culture time groups (Bar = 100 μm). Normal tendon showed no BMSCs as a positive control group. LSDCT group revealed limited tendon cells and no seeded BMSCs as negative control.
BMSCs = bone marrow stromal cells; DAPI = 4′, 6-diamidino-2-phenylindole
Figure 6.
(A) Cell proliferation in normal tendon and LSDCT models. The total cell number [implanted cells plus daughter cells (DiI stained)] was more at the four weeks of culture compared with two weeks culture in both high and low cell density groups, but no significant difference was observed. Cell proliferation was significantly affected by cell density and culture time. (B) Cell viability was assessed by live/dead assay and presented with percentage of live/live + dead cells. The LSDCT + BMSC 4H group presented the lowest cell viability with significant difference to other BMSC-seeded groups. Both factors of cell density and culture time significantly affected the cell viability in the LSDCT. Error bars indicate ±1 standard deviation. $Significant difference (p < .05) between each BMSC-seeded group and normal tendon (Independent t test). ∗∗Very significant difference (p < .01) (one-way ANOVA). +Significant difference between seeded cell densities (two-way ANOVA). #Significant difference between culture times (two-way ANOVA).
ANOVA = analysis of variance; BMSC = bone marrow stromal cell.
Live/dead assay
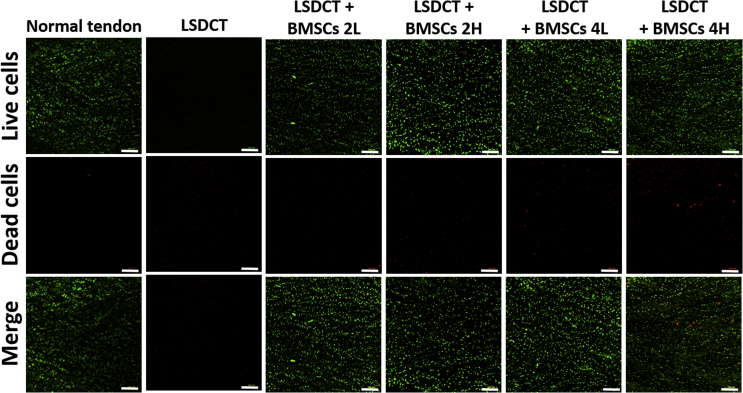
The normal tendon showed the lowest number of dead cells, and LSDCT showed no live cells. The LSDCT plus BMSC 4H group showed the highest number of dead cells and the lowest percent of live cells per total cells (Fig. 5). Cell viability assessed by live/dead assay showed that the percent of live cells in normal tendon was significantly higher than any of the cell-seeding groups. At 2 weeks of culture, the cell viability appeared higher in the low-density group than high-density group but did not reach a significant difference. The cell viability was significantly decreased in the LSDCT plus BMSC 4H group compared to other BMSC-seeded groups. Based on the result of two-way ANOVA, the cell viability was significantly affected by seeded cell density and culture time (Fig. 6B).
Figure 5.
Live/dead assay in all cell-seeded groups. Images represent the distribution of live (green) and dead (red) cells in different cell densities and culture time conditions (original magnification × 100 with bar = 200 μm). The LSDCT plus BMSC 4H group showed the highest number of dead cells. The normal tendon group that was defined as positive control showed few dead cells. The LSDCT group that was defined as negative control revealed no live cells and limited dead cells as negative control.
BMSC = bone marrow stromal cell.
Gene expression
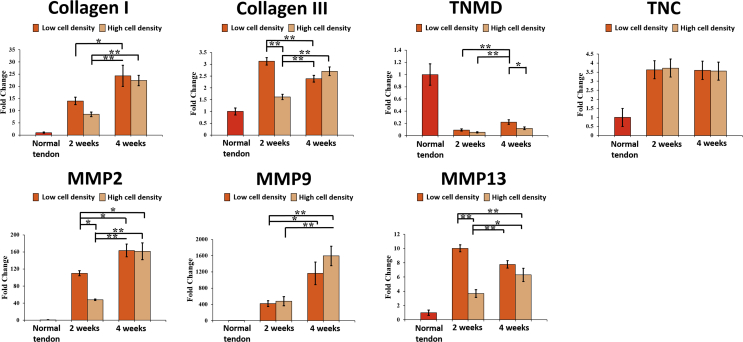
The results of gene expression were shown in Fig. 7. BMSCs in LSDCT showed a significant tendency of higher gene expression of Collagen I, Collagen III, tenascin C, MMP2, MMP9, and MMP13 compared to normal tendons in both cell density groups at 2 and 4 weeks of culture. Collagen I gene expression significantly increased at 4 weeks of culture than 2 weeks of culture in both cell density groups. The Collagen III gene expression, significantly different between low and high cell densities at 2 weeks of culture, decreased in the low cell density group and increased in the high cell density group at 4 weeks of culture.
Figure 7.
Gene expressions of Collagen I, Collagen III, TNMD, TNC, and MMPs in all study groups. The gene expression was shown as fold change by comparing with the normal tendon group that was set as 1. Error bars indicate mean ± standard error. ∗Significant difference (p < .05) and ∗∗very significant difference (p < .01) (one-way ANOVA).
TNMD = tenomodulin; TNC = tenascin C.
The TNMD (tendon marker [21], [22]) gene expression was increased from 2 to 4 weeks, and the expression in the low cell density group was significantly higher than that in the high cell density group at 4 weeks of culture (p < .05). TNC, a glycoprotein, is an important component of tendon extracellular matrix, which plays a role in collagen fibre orientation and alignment [23], [24], [25]. TNC gene expression was not significantly different among all implanted groups.
MMPs play an important role in collagen degradation and remodelling [26], [27], [28]. In this study, MMP gene expression varied among the groups. MMP2 and MMP9 gene expression was significantly increased at 4 weeks of culture than 2 weeks in the both density groups. The MMP13 gene expression declined in low cell density groups and increased in high cell density groups from 2 to 4 weeks of culture.
Discussion
Stem cell–based therapy has been applied to accelerate the revitalization of the allograft tendon into a viable and functional tendon [11], [14], [15], [16], [29], [30]. In the present study, we implanted BMSCs of different cell densities into the DCT through modified lateral slits with 2 and 4 weeks of culture using an in vitro canine tendon model. Our results revealed that seeded BMSCs distributed to the whole allograft tendon through the lateral slits as early as three days and extended to 2 and 4 weeks of culture. Furthermore, cell proliferation and viability results demonstrated that seeded BMSCs remain viable and proliferate with an increase in the cell number over time. The seeded BMSCs had significantly expressed Collagen I and III and TNC gene, indicating a tenogenic differentiation response. Although we have analysed MMP expression, the results were varied among groups. This may be related to the lack of remodelling phase in an ex vivo model without mechanical stimulation. However, in general, our results showed that MMP2 and MMP9, which are related to tendon matrix remodelling, increased in longer term culture at 4 weeks. Additionally, the divergent gene expression of MMP13 showed different MMP13 activity after different cell density seeding in LSDCT.
To enhance the revitalization of decellularized allograft, many authors have proposed different methods to help the seeded stem cell distribution in the decellularized allograft [14], [15], [16], [17], [18]. Woon et al. [17], [29] treated decellularized human flexor tendons with 5% peracetic acid and used a surface scoring technique to improve cell infiltration by increasing scaffold porosity. Ozasa et al. [14] used multiple short slits on the tendon surface in a volardorsal direction to improve BMSC penetration into the DCT. The surface scoring and slits could damage the tendon's gliding ability, which is an important tendon function, especially for flexor tendon reconstruction [14], [31], [32], [33], [34], [35]. In addition, the volardorsal slits limited the number of cells that could be seeded due to the shallow slit as the tendon's oval shape. In our group study, we created multiple lateral slits (radial-ulnar direction) to avoid damaging the tendon surface that directly glides against the pulley system (Fig. 1). This novel lateral slit also enlarged the slit pocket, thus providing a large area for cell seeding and migration. Based on the results of histologic evaluation and confocal images study, we demonstrated that the seeded BMSCs distributed from the lateral slit to the entire decellularized allograft tendon in the early implantation stage (Fig. 3A and B) and maintained viability for 2–4 weeks (Figure 4, Figure 5).
In our study, we prepared the DCT with repeated freeze-thaw procedures, which has been demonstrated to provide a stem cell matrix microenvironment that promotes implanted stem cell attachment, proliferation, and tenogenesis [13], [15], [20], [30]. Collagen I gene expression was significantly increased at 4 weeks of culture compared to 2 weeks of culture in both the low and high cell density groups, but no significant difference was detected in both the cell density groups at the same culture time. Collagen III gene expression was different between the two cell densities, which was significantly higher than the expression of normal tendon. From the results, it seemed that BMSC seeding upregulated collagen expression in LSDCT in both the low and high cell density groups. TNC gene expression in seeded BMSCs had a similar response as the collagen, MMP2, and MMP9 gene expression, which was significantly increased over time. The aforementioned findings indicated that transplanted cells are actively remodelling the extracellular matrix [27].
In our study, we seeded BMSCs with low and high cell densities—2 × 107 cells/mL and 5 × 107 cells/mL, respectively. From Figure 4, Figure 5, the total cell number in the DCT between low and high cell density groups was not compared to the ratio of the initial seeded cell number. However, the total cell number reflects not only the initial seeded cell density but also the cell proliferation rate and cell viability. In our study, the cell number increased with culture time in both cell density groups; however, no significant difference was detected between low- and high-density groups (Figure 4, Figure 6A). The cell viability was calculated by the ratio of the live cells divided by the total number of cells including both live and dead cells. Although the number of viable cells in the high-density group is higher than that in the low-density group, more dead cells were found in the high cell density group. The low-density seeding group presented a higher cell viability compared with high-density seeding group at 4 weeks of culture (p < .01; Figure 5, Figure 6B), and the LSDCT+BMSC 4H group presented with lowest cell viability, which indicates cell death (apoptosis or necrosis), may occur obviously in high-density cell transplantation for a relatively long period. These results showed that the low cell density seeding through the lateral slits is adequate to revitalize the DCT with similar proliferation rate but higher cell viability compared to high cell density group at 4 weeks of culture.
This study has some limitations. First, we only evaluated two cell densities and two culture times. Future work will investigate the seeded cell viability, gene expression, and tenogenic differentiation in LSDCT with various cell densities and longer culture times. Second, we did not mechanically evaluate these samples in this study. Our prior study indicates that no significant difference in mechanical characteristics among normal tendon, LSDCT, and LSDCT + BMSCs was observed after 2 weeks of culture in vitro [19]. Also, the mechanical evaluation of tendon samples under ex vivo situation does not reflect the actual physiological condition after tendon or ligament reconstruction. An in vivo investigation of the transplanted cell with tendon slits is needed to validate this novel technology for allograft tendon revitalization.
In conclusion, our results showed that the lateral slits provide a good environment to host cell seeding within the DCT. BMSCs distributed to the whole tendon in the early stage and remained viable with robust cell proliferation and matrix remodelling gene expression in the LSDCT after 2 and 4 weeks of culture. Furthermore, the low cell density seeding in LSDCT revealed similar results in cell proliferation and gene expression, but greater cell viability compared to the high cell density group. This study provides the evidence that the lateral slit technique in DCT graft is a viable option to repopulate the seeded BMSCs during tendon and ligament reconstruction, which can have a direct clinical application.
Conflicts of interest statement
The authors have no conflicts of interest relevant to this article.
Funding statement
This work was supported by a grant from National Institutes of Health/National Institute of Arthritis and Musculoskeletal and Skin Diseases (AR 057745) and Musculoskeletal Transplant Foundation (MTF). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author contributions
C.C.Lu, P.C.A., K.N.A., S.L.M., A.G., and C.F.Z. helped in conception and design of the study. C.C.Lu, T.Z., A.G., and C.F.Z. helped in acquisition of data. C.C.Lu, T.Z., P.C.A., K.N.A., S.L.M., A.G., and C.F.Z. helped in analysis or interpretation of data. C.C.Lu helped in drafting the manuscript. C.C.Lu, T.Z., P.C.A., K.N.A., S.L.M., A.G., and C.F.Z. helped in revising the manuscript critically for important intellectual content. C.C.Lu, T.Z., P.C.A., K.N.A., S.L.M., A.G., and C.F.Z helped in approval of the final version of the manuscript to be published.
Acknowledgments
This study was supported by a grant from National Institutes of Health / National Institute of Arthritis and Musculoskeletal and Skin Diseases (AR 057745) and Musculoskeletal Transplant Foundation (MTF).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jot.2019.05.002.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Gerbino P.G., 2nd, Saldana M.J., Westerbeck P., Schacherer T.G. Complications experienced in the rehabilitation of zone I flexor tendon injuries with dynamic traction splinting. J Hand Surg [Am] 1991;16(4):680–686. doi: 10.1016/0363-5023(91)90194-g. [DOI] [PubMed] [Google Scholar]
- 2.Tang J.B. Clinical outcomes associated with flexor tendon repair. Hand Clin. 2005;21(2):199–210. doi: 10.1016/j.hcl.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 3.de Putter C.E., Selles R.W., Polinder S., Panneman M.J., Hovius S.E., van Beeck E.F. Economic impact of hand and wrist injuries: health-care costs and productivity costs in a population-based study. J Bone Joint Surg Am. 2012;94(9):e56. doi: 10.2106/JBJS.K.00561. [DOI] [PubMed] [Google Scholar]
- 4.Dias J.J., Garcia-Elias M. Hand injury costs. Injury. 2006;37(11):1071–1077. doi: 10.1016/j.injury.2006.07.023. [DOI] [PubMed] [Google Scholar]
- 5.Hoxie S.C., Capo J.A., Dennison D.G., Shin A.Y. The economic impact of electric saw injuries to the hand. J Hand Surg [Am] 2009;34(5):886–889. doi: 10.1016/j.jhsa.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 6.Goertzen M.J., Clahsen H., Schulitz K.P. Anterior cruciate ligament reconstruction using cryopreserved irradiated bone-ACL-bone-allograft transplants. Knee Surg Sport Traumatol Arthrosc. 1994;2(3):150–157. doi: 10.1007/BF01467917. [DOI] [PubMed] [Google Scholar]
- 7.Mahirogullari M., Ferguson C.M., Whitlock P.W., Stabile K.J., Poehling G.G. Freeze-dried allografts for anterior cruciate ligament reconstruction. Clin Sports Med. 2007;26(4):625–637. doi: 10.1016/j.csm.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 8.Tejwani S.G., Shen W., Fu F.H. Soft tissue allograft and double-bundle reconstruction. Clin Sports Med. 2007;26(4):639–660. doi: 10.1016/j.csm.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 9.Webster D.A., Werner F.W. Mechanical and functional properties of implanted freeze-dried flexor tendons. Clin Orthop Relat Res. 1983;180:301–309. [PubMed] [Google Scholar]
- 10.Ozasa Y., Gingery A., Thoreson A.R., An K.N., Zhao C., Amadio P.C. A comparative study of the effects of growth and differentiation factor 5 on muscle-derived stem cells and bone marrow stromal cells in an in vitro tendon healing model. J Hand Surg [Am] 2014;39(9):1706–1713. doi: 10.1016/j.jhsa.2014.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pridgen B.C., Woon C.Y., Kim M., Thorfinn J., Lindsey D., Pham H. Flexor tendon tissue engineering: acellularization of human flexor tendons with preservation of biomechanical properties and biocompatibility. Tissue Eng C Methods. 2011;17(8):819–828. doi: 10.1089/ten.tec.2010.0457. [DOI] [PubMed] [Google Scholar]
- 12.Chong A.K., Riboh J., Smith R.L., Lindsey D.P., Pham H.M., Chang J. Flexor tendon tissue engineering: acellularized and reseeded tendon constructs. Plast Reconstr Surg. 2009;123(6):1759–1766. doi: 10.1097/PRS.0b013e3181a65ae7. [DOI] [PubMed] [Google Scholar]
- 13.Qin T.W., Sun Y.L., Thoreson A.R., Steinmann S.P., Amadio P.C., An K.N. Effect of mechanical stimulation on bone marrow stromal cell-seeded tendon slice constructs: a potential engineered tendon patch for rotator cuff repair. Biomaterials. 2015;51:43–50. doi: 10.1016/j.biomaterials.2015.01.070. [DOI] [PubMed] [Google Scholar]
- 14.Ozasa Y., Amadio P.C., Thoreson A.R., An K.N., Zhao C. Repopulation of intrasynovial flexor tendon allograft with bone marrow stromal cells: an ex vivo model. Tissue Eng Part A. 2014;20(3–4):566–574. doi: 10.1089/ten.tea.2013.0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ning L.J., Zhang Y.J., Zhang Y., Qing Q., Jiang Y.L., Yang J.L. The utilization of decellularized tendon slices to provide an inductive microenvironment for the proliferation and tenogenic differentiation of stem cells. Biomaterials. 2015;52:539–550. doi: 10.1016/j.biomaterials.2015.02.061. [DOI] [PubMed] [Google Scholar]
- 16.Omae H., Zhao C., Sun Y.L., An K.N., Amadio P.C. Multilayer tendon slices seeded with bone marrow stromal cells: a novel composite for tendon engineering. J Orthop Res. 2009;27(7):937–942. doi: 10.1002/jor.20823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Woon C.Y., Pridgen B.C., Kraus A., Bari S., Pham H., Chang J. Optimization of human tendon tissue engineering: peracetic acid oxidation for enhanced reseeding of acellularized intrasynovial tendon. Plast Reconstr Surg. 2011;127(3):1107–1117. doi: 10.1097/PRS.0b013e318205f298. [DOI] [PubMed] [Google Scholar]
- 18.Omi R., Gingery A., Steinmann S.P., Amadio P.C., An K.N., Zhao C. Rotator cuff repair augmentation in a rat model that combines a multilayer xenograft tendon scaffold with bone marrow stromal cells. J Shoulder Elb Surg. 2016;25(3):469–477. doi: 10.1016/j.jse.2015.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu J.H., Thoreson A.R., Gingery A., An K.N., Moran S.L., Amadio P.C. The revitalisation of flexor tendon allografts with bone marrow stromal cells and mechanical stimulation: an ex vivo model revitalising flexor tendon allografts. Bone Joint Res. 2017;6(3):179–185. doi: 10.1302/2046-3758.63.BJR-2016-0207.R1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ning L.J., Zhang Y., Chen X.H., Luo J.C., Li X.Q., Yang Z.M. Preparation and characterization of decellularized tendon slices for tendon tissue engineering. J Biomed Mater Res A. 2012;100(6):1448–1456. doi: 10.1002/jbm.a.34083. [DOI] [PubMed] [Google Scholar]
- 21.Alberton P., Dex S., Popov C., Shukunami C., Schieker M., Docheva D. Loss of tenomodulin results in reduced self-renewal and augmented senescence of tendon stem/progenitor cells. Stem Cells Dev. 2015;24(5):597–609. doi: 10.1089/scd.2014.0314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shukunami C., Oshima Y., Hiraki Y. Molecular cloning of tenomodulin, a novel chondromodulin-I related gene. Biochem Biophys Res Commun. 2001;280(5):1323–1327. doi: 10.1006/bbrc.2001.4271. [DOI] [PubMed] [Google Scholar]
- 23.Mackie E.J., Ramsey S. Expression of tenascin in joint-associated tissues during development and postnatal growth. J Anat. 1996;188(Pt 1):157–165. [PMC free article] [PubMed] [Google Scholar]
- 24.Riley G.P., Harrall R.L., Cawston T.E., Hazleman B.L., Mackie E.J. Tenascin-C and human tendon degeneration. Am J Pathol. 1996;149(3):933–943. [PMC free article] [PubMed] [Google Scholar]
- 25.Erickson H.P. Tenascin-C, tenascin-R and tenascin-X: a family of talented proteins in search of functions. Curr Opin Cell Biol. 1993;5(5):869–876. doi: 10.1016/0955-0674(93)90037-q. [DOI] [PubMed] [Google Scholar]
- 26.Ireland D., Harrall R., Curry V., Holloway G., Hackney R., Hazleman B. Multiple changes in gene expression in chronic human Achilles tendinopathy. Matrix Biol. 2001;20(3):159–169. doi: 10.1016/s0945-053x(01)00128-7. [DOI] [PubMed] [Google Scholar]
- 27.Oshiro W., Lou J., Xing X., Tu Y., Manske P.R. Flexor tendon healing in the rat: a histologic and gene expression study. J Hand Surg [Am] 2003;28(5):814–823. doi: 10.1016/s0363-5023(03)00366-6. [DOI] [PubMed] [Google Scholar]
- 28.Vu T.H., Werb Z. Matrix metalloproteinases: effectors of development and normal physiology. Genes Dev. 2000;14(17):2123–2133. doi: 10.1101/gad.815400. [DOI] [PubMed] [Google Scholar]
- 29.Woon C.Y., Farnebo S., Schmitt T., Kraus A., Megerle K., Pham H. Human flexor tendon tissue engineering: revitalization of biostatic allograft scaffolds. Tissue Eng Part A. 2012;18(23–24):2406–2417. doi: 10.1089/ten.TEA.2012.0152. [DOI] [PubMed] [Google Scholar]
- 30.Omae H., Sun Y.L., An K.N., Amadio P.C., Zhao C. Engineered tendon with decellularized xenotendon slices and bone marrow stromal cells: an in vivo animal study. J Tissue Eng Regenerat Med. 2012;6(3):238–244. doi: 10.1002/term.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ozasa Y., Amadio P.C., Thoreson A.R., An K.N., Zhao C. The effect of surface modification on gliding ability of decellularized flexor tendon in a canine model in vitro. J Hand Surg [Am] 2013;38(9):1698–1704. doi: 10.1016/j.jhsa.2013.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ji X., Reisdorf R.L., Thoreson A.R., Berglund L.R., Moran S.L., Jay G.D. Surface modification with chemically modified synovial fluid for flexor tendon reconstruction in a canine model in vivo. J Bone Joint Surg Am. 2015;97(12):972–978. doi: 10.2106/JBJS.N.01100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhao C., Sun Y.L., Ikeda J., Kirk R.L., Thoreson A.R., Moran S.L. Improvement of flexor tendon reconstruction with carbodiimide-derivatized hyaluronic acid and gelatin-modified intrasynovial allografts: study of a primary repair failure model. J Bone Joint Surg Am. 2010;92(17):2817–2828. doi: 10.2106/JBJS.I.01148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Galvez M.G., Crowe C., Farnebo S., Chang J. Tissue engineering in flexor tendon surgery: current state and future advances. J Hand Surg Eur. 2014;39(1):71–78. doi: 10.1177/1753193413512432. [DOI] [PubMed] [Google Scholar]
- 35.Zhao C., Hashimoto T., Kirk R.L., Thoreson A.R., Jay G.D., Moran S.L. Resurfacing with chemically modified hyaluronic acid and lubricin for flexor tendon reconstruction. J Orthop Res. 2013;31(6):969–975. doi: 10.1002/jor.22305. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.