Abstract
Background
Ovarian cancer has the highest ratio of mortality among gynecologic malignancies. Chemotherapy is one of the most common treatment options for ovarian cancer. However, tumor relapse in patients with advanced tumor stage is still a therapeutic challenge for its clinical management.
Main body
Therefore, it is required to clarify the molecular biology and mechanisms which are involved in chemo resistance to improve the survival rate of ovarian cancer patients. Cancer stem cells (CSCs) are a sub population of tumor cells which are related to drug resistance and tumor relapse.
Conclusion
In the present review, we summarized the recent findings about the role of CSCs in tumor relapse and drug resistance among ovarian cancer patients. Moreover, we focused on the targeted and combinational therapeutic methods against the ovarian CSCs.
Keywords: Cancer stem cell, Ovarian cancer, Isolation, Detection, Drug resistance
Background
Ovarian cancer is the seventh most common cancer and the fifth leading cause of cancer related deaths among women globally (15–20 per 100,000) [1]. Ovarian cancer is a heterogeneous malignancy with different clinical development. Such a large heterogeneity is the result of various biological processes that underlie different types of ovarian cancers. Contrary to the classic view that different ovarian cancer histotypes are caused by metaplastic changes of a single tissue, only a subset of epithelial ovarian cancers develops within the ovarian surface epithelium (OSE) [2]. Majority of tumors originate in non-ovarian areas [3]. Ovarian cancer has significant challenges due to its intrinsic molecular heterogeneity which is associated with different tumor histotypes [4]. Different types of ovarian tumors have different phenotypes, molecular biology, etiology, progression, and prognosis [5]. Ovarian cancer has two main histological sub types including surface epithelial stromal and sex cord stromal cells [6]. Surface epithelial cells (OSE) or intra epithelial carcinomas (STIC) are the most important origins of ovarian cancers. The epithelial type involves about 90% of ovarian tumors, and is categorized into genetically sustained with low grade serous and invasive genetically ephemeral with high grade serous [7]. The highest prevalence of ovarian cancer is observed in eastern Asian countries and central America [8]. Lifestyle changes have decreased the rate of mortality in western countries [9]. Epidemiological studies have shown that the contraceptive drugs, BRCA1–2 mutations, and multiple ovulations can be associated with ovarian cancer [10–12]. Most of the ovarian cancers are sporadic, which are developed by the accumulation of genetic aberrations [13]. The serous borderline tumors and low-grade serous adenocarcinoma are mostly characterized by the BRAF and K-RAS mutations [14]. However, there are various molecular patterns associated with the heterogeneous biology of ovarian cancer in the case of histopathology and malignancy potential [15]. Like the foci of aggressive high-grade serous ovarian carcinoma (HGSOC), the STIC lesions were proliferative, as measured by Ki67 and p53 immunohistochemistry (IHC). DNA sequencing also showed that the majority of STIC clonal lesions harbor the same TP53 mutation as the simultaneous HGSOC [16, 17]. HGSOC is mainly characterized by mutations in TP53, mutations in the homologous recombination DNA repair pathway, and an extensive range of copy number changes. One of the most communal copy number changes in ovarian cancer is amplification at the 19q12 locus [18].
Main text
Staging and prognosis
The prognosis of ovarian cancer is directly related to the stage of tumor and tumor cells remaining after resection. Exploratory laparotomy paves the way for tumor staging and debulking [19]. Having command on the spread pattern of ovarian cancer is highly required in appropriate radiological determining, diagnosis, and surgery [20]. Computed tomography (CT) is used for ovarian cancer staging before the surgery and also for the determination of tumor relapse [21]. MRI and multi detector CT are efficient methods for ovarian cancer staging [19]. New methods such as the proteomics patterns and bioinformatic tools are also used to detect ovarian cancer in the early stages [22]. It has been observed that the PET/CT method can detect restaging modality of the ovarian tumor with a higher efficiency compared to the CT method [23]. It has been shown that the WB-DWI/MRI method has more prognostic accuracy in primary, peritoneal, and distant ovarian tumors compared with CT and FDG-PET/CT methods [23].
Due to lack of efficient early detection methods in ovarian cancer, around 70% of cases are diagnosed in advanced stages with poor prognosis [24]. Surgical resection along with platinum-based chemotherapy is a standard treatment option for ovarian cancer. According to ovarian cancer surgery guidelines (ESGO 2017), the objective of early surgery is complete resection of the macroscopic tumors [24]. After surgery, patients will be undergoing the platinum/taxane treatment as the first-line chemotherapeutic modality [25]. However, early surgery is not possible for the patients with advanced stages of tumor (III, IV), since their other organs such as the intestine and liver are involved. In such cases, a neoadjuvant chemotherapy will be done before the interval debulking surgery (IDS) [25]. The bevacizumab and paclitaxel are also the first line treatment options. Despite the successful results of early treatment, the majority of patients may have tumor recurrence [26]. Second-line chemotherapy is the principle approach in treatment of recurrent ovarian cancer. Combinational treatment of platinum and other drugs will be used for patients who have partially or highly sensitive tumors with recurrence after 6–12 months or more than 12 months [27]. The angiogenesis inhibitors can also be used beside these treatment methods in ovarian cancer patients. Since, Vascular Endothelial Growth Factor (VEGF) is a key factor during vascular progression inside the tumor, its inhibition can be resulted in tumor elimination [28]. Poly (ADP-Ribose) polymerase (PARP) inhibitors have been also used in advanced ovarian cancer patients with BRCA2 and BRCA1 mutations [29].
Strategies for isolating and enriching CSCs
Cancer stem cells (CSCs) can be identified and isolated through different methods. Magnetic-activated cell storing method (MACS) and fluorescent-activated cell storing method (FACS) are efficient methods for isolation of CSCs from solid tumors based on cell surface or intra cellular markers [30]. MACS is a fast and easy method, but makes the separation in monoparameter form, whereas FACS is an expensive multiparameter isolation method [31]. Separation methods based on cell surface markers are commonly used for isolation of CSCs from the heterogeneous tumor cells. CD133 is one of the most common cell surface markers which is used for isolation of CSCs from various types of tumor cells such as breast cancer, glioblastoma, prostate cancer, colon cancer, and liver cancer [32]. It has been reported that the CD133 positive glioma stem cells (GSCs) were tumorigenic. The CD133+ and CD133- human lung cancer and mouse glioma cell lines were also tumorigenic with self-renewal and colonization abilities [33, 34]. Another study reported that the CD105 positive cells had higher CSC characteristics compared with CD105 positive cells following isolation using the MACS method [35]. CXCR4-positive cells sorted by FACS method had also higher ability in sphere formation and tumorigenesis in comparison with CXCR4-negative cells [36]. It has been shown that the CD133+/CD24+/CTR2+ cells had stem cell-like properties in renal cell carcinoma [37]. Several markers such as CD133, ALDH1/2, LY6A, LGR5, EpCAM, CD133, CD44, CD34, CD24, CD117, MyD88, and CDH1 were used for isolation of CSCs from the ovarian cell lines (Table 1) [38–47]. Another method of separating CSCs is based on Adlehyde dehydrogenase (ALDH) using Aldefluor method. This method allows single-cell imaging in monolayer cultures which can be a useful method in some cases. This method has higher stability and lower specificity compared with the cell surface methods [48]. Isolation based on ALDEFLUOR method from 6 ovarian cell lines and 8 ovarian cancer patients resulted in cells with higher sphere-formation ability, tumorigenicity, and invasiveness [49]. It has been also observed that the ALDH+CD133+ cells had a higher ability to create larger and faster tumors in xenograft mouse, and also create three dimensional sphere more efficiently compared with their negative counterparts in ovarian tumors [50]. Another method for separating CSCs is based on cell side population (SP) with the expression of ABC transporters using Hoechst 33342 dye-staining. In this method, SP cells exclude the Hoechst 33342 dye through a transporter. This is the mechanism to expel the chemotherapeutic drugs and creates resistance against chemotherapy [51]. This method is also used to isolate CSCs without a cell surface marker, however, it has lower specificity and purity and toxic effects on isolated cells compared with other methods. It was observed that the separated SP cells had high expression levels of CSC markers, ATP-binding cassette, ABCG2, nestin, and CD44 on SK-OV-3 ovarian cell line. These cells had a high self-renewal and proliferation ability [52].
Table 1.
Surface markers used to isolate ovarian cancer stem cells
| Surface marker | The distinctive feature of these cells | References |
|---|---|---|
| CD133+ | Higher clonogenic and proliferative potentials recapitulate the tumor characteristics in NOD/SCID mice | [33, 34] |
| CD44+ | Targeting CD44 by siRNA induced cell death and decreased the tumor | [35] |
| CD44+/CD117+ | Recapitulate the original tumor in vivo | [33, 36] |
| CD44+/MyD88+ | Presented stem-like characteristics, including constitutive NF-κB activity, high capacity for tumor reconstitution, resistance to chemotherapeutics ability to recapitulate the tumor in vivo and | [37] |
| CD44+/E-cadherin−/CD34− |
Participate in neovascularization shorter tumor-free period in vivo and increased |
[38] |
|
CD44+/CD24+/EpCAM+ CD44+/CD24− |
migration and invasion characteristics in vitro differentiation potential and drug resistance accompanied by higher invasion ability |
[39] [40] |
Cancer stem cells and chemo resistance
There are many different biological aspects of the clinical development of ovarian cancer that support the role of cancer stem cells during tumor progression and disease survival. Ovarian cancer is often associated with peritoneal ascites, in which spheroids reside in tumor cells and survive and proliferate even in a non-adherent status. The anoikis resistance is a key feature of these stem cells [53]. Different aspects of stem cell biology, including quiescence, differentiation, EMT, and plasticity are regulated by different cellular niche components including non-stem cells, host cells, extracellular matrix, and soluble factors [54]. CSCs are a sub population of tumor cells with self-renewal properties which preserve the growth and heterogeneity of tumor during tumor relapse [55, 56]. During primary chemotherapeutic treatment, drug resistance in CSCs leads to the tumor relapse [57]. There are various percentages of CSCs in different tumor tissues. Ovarian CSCs were firstly observed through a multilayer spheroid culture. These spheroids have the ability to form new tumors in mice [58]. These cells have been suggested as key tumor-initiating factors which have an important role in tumor recurrence after chemotherapy through several chemotherapeutic resistance mechanisms. It has been shown that the CSCs have specific metabolic features such as higher glycolytic functions in comparison with differentiated tumor cells [59–62]. Such specific metabolic behaviors can be resulted in drug resistance. The rodent ovarian CSCs have higher glycolysis compared with parental cells, which can be associated with chemo resistance [63]. The CD44 + CD117 + ovarian CSCs also showed high levels of mitochondrial ROS, which suggested that the mitochondrial electron respiratory chain is mainly used to preserve the cells during nutrient starvation and stress conditions [64]. There are various drug resistance mechanisms in CSCs such as ABC transporters, Aldehyde dehydrogenase, DNA repair, and signaling pathways [65]. Hoechst 3342 is a method for CSCs detection, which is associated with the function of ABC transporters. P-glycoprotein (MDR1) and breast cancer resistance protein (ABCG2) are involved in dye exclusion and chemo resistance [66–68]. Although, Doxorubicin is excluded by both ABCB1 and ABCG2 [69], Paclitaxel is only pumped out by MDR1 [66, 69]. Therefore, higher expression of these transporters can be observed in different CSCs. The high levels of ABCG2 and ABCB1 have been observed in breast and ovarian CSCs, respectively [70, 71]. Moreover, it has been reported that there were high levels of ABCA1, ABCB5, and ABCC3/MRP3 expressions in ovarian tumor tissues [72] and high levels of ABCA1, ABCB1/MDR1/P-GP, and ABCG2/BCRP expression in ovarian CSCs [71, 73–75]. The correlation between ABC transporter type and chemo resistance mechanism is a critical issue to select a specific suppressor [76]. Aldehyde dehydrogenase (ALDH) is the other important mechanism of drug resistance among CSCs. There are different isoforms of human ALDH which are mainly expressed in kidney and liver [77]. ALDH over activity is considered as a prognostic marker for various cancers such as lung [78], breast [79], pancreas [80], intestine [81], and ovarian cancer [49]. The cyclophosphamide-resistance role of ALDH was identified in cyclophosphamide-resistant L1210 leukemic cell line which was retrievable by Disulfiram as an ALDH inhibitor [82]. The ALDH-mediated cyclophosphamide-resistance has been also reported in medullaoblastoma [83]. Moreover, ALDH is associated with CSCs phenotype, colony formation, self-renewal marker expression, tumor formation, and EMT process in ovarian cancer [84]. Therefore, ALDH inhibition may play an important role to sensitize the CSCs toward drugs. It has been reported that the ESA + CD44+ colon CSCs with high expression of ALDH were sensitized toward cyclophosphamide via ALDH1A1- siRNA [85]. The third mechanism which leads to chemo-resistance in CSCs is the role of B-cell lymphoma-2 (BCL-2) protein family. This protein family plays a significant role in balancing between survival, apoptosis, embryogenesis, neurogenesis, and hematopoiesis [86]. These proteins inhibit the BAX and BAK pro-apoptotic proteins to release Cytochrome c [87]. As a potential oncogene, BCL-2 protein is expressed in various neoplastic and hematopoietic lineage cells [88, 89]. The roles of BCL-2 family members have been widely studied in tumor cells survival and CSC biology. It has been reported that there were high levels of BCL-XL and BCL-2 expressions in quiescent leukemic CD34+ cells [90] and CD44+/CD24−/low breast CSCs [91]. Therefore, high levels of BCL-2 protein expression through signaling pathways are required for the CSCs survival and chemo-resistance. Decreased expression of BCL-2 is accompanied by increased sensitivity to FU-5 and Oxaliplatin [65]. Bcl-xl over expression has been observed in most of recurrent chemo-resistant ovarian cancers which were correlated with a shorter disease-free interval [92, 93]. Preclinical studies showed that the Bcl-xL inhibition increased chemo-sensitivity of ovarian cancer cells, which highlighted the inhibition of anti-apoptotic proteins as a promising therapeutic method for recurrent ovarian cancer [93, 94]. Signaling pathways such as WNT/β-catenin and NOTCH are also the other chemo-resistance processes in CSCs [95–99]. It has been observed that there was a correlation between WNT pathway and Cisplatin resistance OV6 + hepatic CSCs [100]. NOTCH signaling pathway has key functions in tumor progression, angiogenesis, epithelial-mesenchymal transition (EMT), and self-renewal [101–104]. It has been shown that the Notch 1 receptor knockdown or using γ-secretase inhibitors resulted in Oxaliplatin sensitization in intestine cancer cells [105]. Increased expression of Notch3 also plays a significant role in the biology of CSCs and Platinum resistance. The γ-secretase inhibitor (GSI) eliminates the CSCs via increasing the Platinum sensitivity. Altogether, the combination treatments including tumor resection and CSCs targeted therapy can be much more effective than routine treatments [106].
Targeted therapy of ovarian cancer stem cells
Despite recent findings, ovarian cancer has still a high ratio of mortality. Regarding the importance of ovarian CSCs in drug resistance and tumor relapse, their elimination could be considered as an efficient treatment method to decrease chemo-resistance and relapse in ovarian cancer [107]. Therefore, three different strategies are applicable; signaling pathways could be a good choice, surface markers could be used as precise target and finally we will briefly discuss about some other methods to eradicate the CSC.
Signaling pathways and targeted therapy
Targeting the signaling pathways is one of the best therapeutic options in CSCs. There are several key signaling pathways such as WNT, SONIC Hedgehog (SHH), NOTCH, PI3K/PTEN, and NF-kB which are associated with stem cell properties. Therefore, deregulation of these signaling pathways can be associated with CSCs survival [108]. We have summarized some recent studies in Table 2.
Table 2.
Signaling pathways and targeted therapy substance
| Targeted Pathway/s | Substance | Cancer/s type | Result/s | Clinical state |
|---|---|---|---|---|
| WNT | PRI-724 | colon cancer | apoptosis induction | Experimental |
| WNT | LGK974 | breast cancer, melanoma, pancreas cancer | determine the maximum tolerated dose and/or recommended dose for expansion, characterize the safety and tolerability, and assess preliminary antitumor activity | Phase 1 |
| WNT | Ipafricept | Pancreas, ovarian cancers | determination of dose-limiting toxicities (DLTs) | Phase 1a/1b |
| SHH | Cyclopamine | ovarian cell lines, EX2, TOV112D, OV90, SKOV3 | decreased spheroid formation | Experimental |
| SHH | Vismodegib | Basal tumor | Prevent metastatic cells | phase 1 |
| SHH | Sonidegib | Basal Cell Carcinoma | Prevent metastasis | FDA Approved |
| SHH, PTCH | 5E1 antibody | motor neuron | SMO inhibitors | Experimental |
| SHH | GDC-0449 | ovarian cancer | SMO inhibitors | phase 2 |
| NOTCH | Ƴ-secretase inhibitor, Cisplatin | ovarian cancer | increased chemo-sensitivity and decreased CSCs numbers | Experimental |
| NOTCH | Anti Jagged1 | Taxane-resistant cell line | Docetaxel sensitivity and decreased tumor weight | Experimental |
| NOTCH | cediranib maleate | breast cancer, malignant melanoma, colorectal cancer, pancreatic cancer, kidney cancer, high grade glioma, non-small-cell lung cancer, and ovarian cancer | determine the tolerability, maximum tolerated dose and safety profile of RO4929097 | phase 1 |
| NOTCH | Ƴ-secretase inhibitor RO4929097 | metastatic melanoma | Increased progression-free survival and 1-year overall survival rate | phase 1 |
| NOTCH | Ƴ-secretase inhibitor of LY900009 | ovarian cancer | inhibited plasma levels of amyloid-β peptide and inhibition of progression | phase 1 |
| NOTCH | monoclonal antibodies against DLL4 | ovarian tumors | increased apoptosis in tumor cells and reduced tumor weights | Experimental |
| NOTCH | Enoticumab | ovarian tumors | determine the safety, dose-limiting toxicities (DLT), pharmacokinetics (PK), and recommended phase II dose (RP2D) of enoticumab | Experimental |
| NOTCH | Demcizumab | ovarian tumors | increased apoptosis in tumor cells | Experimental |
| MAPK | Salinomycin | Ovarian cancer | decreased chemo-resistance | Experimental |
| MAPK | Salinomycin | OVCAR-3 | decrease the CSCs | Experimental |
| EpCAM | Catumaxomab | ovarian malignant ascites patients | Decreases malignancy | phase III |
WNT signaling pathway
The canonical WNT signaling pathway is considered to be an important and protected pathway during embryogenesis and tissue homeostasis (Fig. 1) [109]. Deregulation of WNT pathway disrupts the natural growth and differentiation of colonic crypt stem cells, and increases the expression of target genes such as c-myc and cyclin D which results in a CSC phenotype [110]. Moreover, It has been observed that there was a significant correlation between WNT pathway and CSC properties in CD44+/CD133+ colon CSCs [111]. This pathway is also associated with chemoresistance in ovarian cancer [112]. WNT pathway plays an important role in the maintenance of stem cells in ovarian epithelium, while R-spondins activate this pathway through LGR receptors. The presence of LGR5 and LGR6 in regulation of epithelial stem cells and the chemoresistance of these cells plays an important role in ovarian cancer [113]. Elimination of CSCs by inhibition of WNT signaling can be considered as an efficient approach in tumor treatment [114]. PRI-724 inhibits the WNT pathway through CREB-binding protein which results in apoptosis induction in colon cancer cells [115]. LGK974 is a WNT inhibitor which is in phase 1 of the clinical trial and functioning in breast cancer, melanoma, and pancreatic cancer (NCT Number: NCT01351103). Ipafricept (OMP-54F28) as an Fc-Frizzled 8 receptor is also on 1a/1b phase pancreatic and ovarian cancers (NCT02092363, NCT02050178).
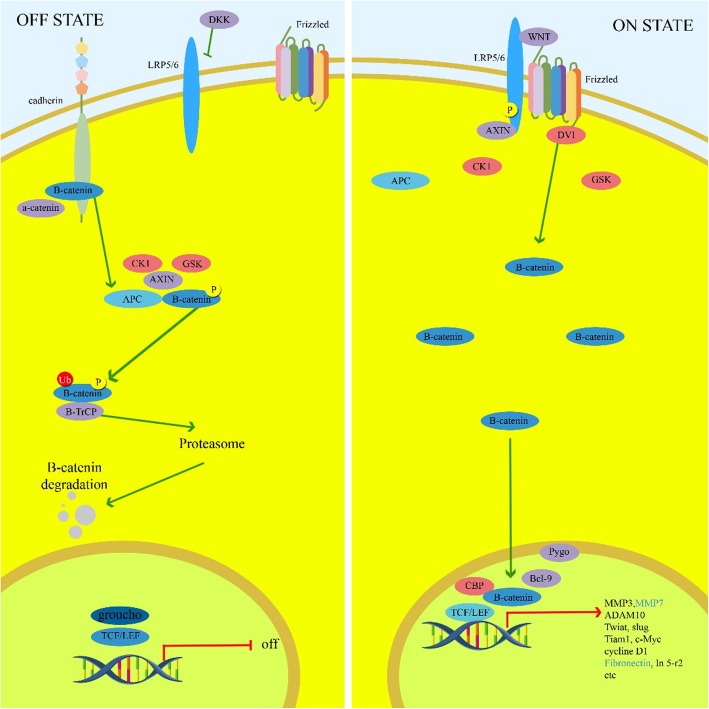
Fig. 1.
Schematic overview of the WNT signaling pathway. Wnt binds to (triggers) the receptor. Axin is removed from the “destruction complex.” β-catenin transfers into the nucleus, binds to a transcription factor on DNA, and stimulates transcription of a protein. Binding of Wnt to the receptors Frizzled (Fz) and LRP6 primes to inhibition of β-catenin degradation. β-catenin in turn interrelates with members of the TCF/Lef-1 family of transcription factors to co-activate target gene transcription
Sonic hedgehog signaling pathway
The SHH pathway has a critical role in a wide range of molecular and cellular processes such as embryogenesis, development, and adult tissue homeostasis (Fig. 2) [111, 116]. Deregulation of the SHH pathway has been reported in CSCs maintenance in several cancers such as breast cancer, pancreatic cancer, myeloma, lung cancer, glioblastoma, and CML [117–122]. SMO and Gli1 over expressions have been observed in myeloma CSCs [123]. Since the SHH pathway plays an important role in self-implantation of CSC and other characteristics of these cells, its inhibition may disrupt CSCs stemness through differentiation of these cells [124, 125]. Cyclopamine as a Hedgehog inhibitor is reported to decrease spheroid-formation (up to 10-folds) in several ovarian cell lines, such as EX2, TOV112D, OV90, and SKOV3 [126]. Vismodegib is a SHH inhibitor in phase 1 of the clinical trial, which targets SMO and is used against metastatic basal tumor cells [124, 125]. Sonidegib is also another SMO inhibitor approved by FDA for advanced BCC patients [127]. The 5E1 antibody inhibits conjunction of all three ligands of HH and PTCH [128, 129]. The GDC-0449 (Vismodegib derivative) and Sonidegic (LDE225) are also SMO inhibitors in phase 2 of the clinical trial (NCT00739661) and (NCT02195973) respectively in ovarian cancer.
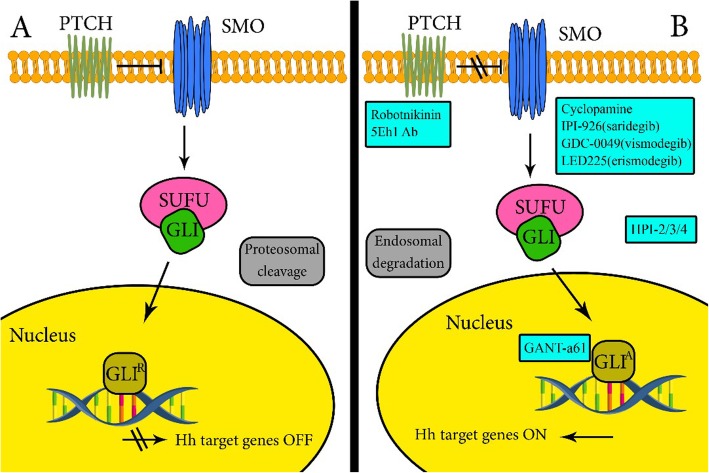
Fig. 2.
Schematic overview of the hedgehog signaling pathway and some inhibitors of the pathway in preclinical and clinical revisions. a In the absence of HH ligands, PTCH inhibits the role of SMO, and GLI proteins are changed by proteosomes to the transcriptional repressor form (GLIR). b Interaction of HH ligands with PTCH unrepresses SMO and creates activated GLI factors (GLIA) which encourage transcription of downstream HH genes. The bound of HH/PTHC complex develops adopted in the endosome and degraded
Notch signaling pathway
Canonical NOTCH signaling pathway is one of the most important evolutionarily conserved pathways during development and adult tissue homeostasis (Fig. 3) [130, 131]. Deregulation of NOTCH signaling has an important role in the maintenance and survival of the CSCs in breast cancer, pancreatic cancer, and glioblastoma. Fascin is an Actin binding protein that is involved in the regulation of breast CSCs through NOTCH signaling pathway [132]. Therefore, Fascin knockdown decreases the expression of pluripotent genes and sphere formation in breast stem cell-like cells [132]. It has been reported that there were increased expression of NOTCH signaling components such as NOTCH 1, NOTCH3, JAG1, JAG2, and HES1 in pancreatic CSCs and Ƴ-secretase inhibitor decreased the CSC population and tumorsphere formation [133]. Activation of Notch signaling pathway by Delta/Serrate/Lag-2 peptide also is increased in the pancreatic CSCs tumorsphere, whereas NOTCH inhibition through HES1 knockdown decreased tumorsphere formation of pancreatic CSCs [133]. A combination of Ƴ-secretase inhibitor (GSI) and Cisplatin to target the NOTCH signaling pathway increased chemo-sensitivity and decreased CSCs numbers [106]. Another group targeted Jagged1 in the Taxane-resistant cells that caused Docetaxel sensitivity and decreased tumor weight [134]. A phase 1 clinical trial was done about a combination of Ƴ-secretase inhibitor RO4929097 and cediranib maleate (NCT01131234). The Ƴ-secretase inhibitor of LY900009 was also used in a phase 1 clinical trial for advanced ovarian cancer patients [135]. The other NOTCH inhibition method is using monoclonal antibodies against DLL4 (Delta-like lignad4) which prevents the ligand binding. Enoticumab (REGN421) is an anti-DLL4 antibody which is used in DLL4 over expressed ovarian tumors. Moreover, Demcizumab as an anti-DLL4 antibody has been also used in advanced ovarian tumors [136].
Fig. 3.
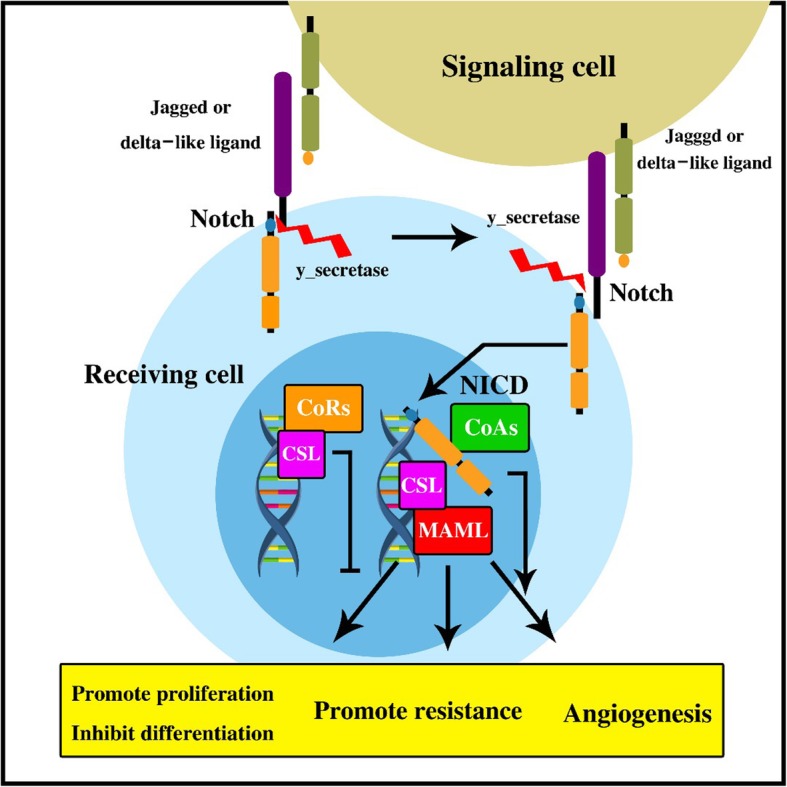
Schematic overview of the Notch signaling pathway. Ligands of the Jagged and Delta-like families interrelate with Notch family receptors on an adjacent cell. The Notch receptor exists at the cell surface as a proteolytically cleaved heterodimer containing of a large ectodomain and a membrane-tethered intracellular domain. The receptor-ligand interaction makes two additional proteolytic cleavages that free the Notch intracellular domain (NICD) from the cell membrane. The NICD moves to the nucleus, where it procedures a complex with the RBPJ protein, dislocating a histone deacetylase (HDAc)-co-repressor (CoR) complex from the RBPJ protein. Components of an activation complex, such as MAML1 and histone acetyltransferases (HAc), are engaged to the NICD-RBPJ complex, leading to the transcriptional activation of Notch target genes
Other signaling pathways
Salinomycin is an ionophore antibiotic which suppresses the ovarian CSCs through different mechanisms such as ABC transporters and MAPK pathway which leads to a decreased chemo-resistance [137, 138]. Also these antibiotics decrease the CSCs numbers in OVCAR-3 cell line through down regulation of Bcl-2 [139]. Catumaxomab is also a monoclonal antibody used for epithelial cell adhesion molecule (EpCAM), which is in the clinical trial phase III among ovarian malignant ascites patients [140, 141].
Eliminating CSCs by targeting their surface markers
Surface markers of the CSCs, like CD24, CD44, CD117 and CD133 can be targeted by several strategies [142]. The CD44+ SKOV3 cell lines were targeted by hyaluronic acid-paclitaxel (HA-TXL) which resulted in decreased tumor weight and nodules [143]. In another report, CD133+ OVCAR5-luc cells were targeted that resulted in a considerable decrease in tumor progression [144]. The CD24 inhibition decreased cell viability through apoptosis induction in SKOV3 cell line, and restricted the tumor growth in nude mice [145]. There is a correlation between CD117 surface marker and drug resistance in ovarian cancer [146]. Activation of Wnt/β –catenin-ABCG2 pathway for Cisplatin/Paclitaxel resistance is occurred by CD117 in ovarian CSC. The Imatinib Mesylate as a CD117 inhibitor has been used to treat various tumor types and chemo-resistant ovarian tumors [147, 148]. The growth of CD44+ and CD117+ chemo resistant ovarian CSCs were also inhibited by Paclitaxel and Salinomycin treatments [149]. Metformin is another drug associated with increased 5-year survival rate of ovarian cancer patients. It has been observed that the Metformin inhibited CD44+ and CD117+ CSCs and EMT process in SKOV3 and A2780 cell lines [150]. Another group showed that the Metformin decreased ALDH+ CSC population and angiogenesis [151]. Clostridium perfringens Enteroxin (CPE) can also be used to eliminate the chemo-resistant CD44+ ovarian CSCs in Xenograft mouse model [152].
Other potential strategies to eliminate CSCs (differentiation therapy, niches and miRNAs)
Differentiation therapy is one other method to eradicate the CSC [153]. Retinoic acids are the only factors that have been used in clinical trials of differentiation therapy [154]. It has been shown that the Carboplatin in combination with Novel Retinoid Compounds 3 efficiently reduced the growth of ovarian CSCs [155].
The tumorigenic ability of ovarian tumor cells is associated with niches derived from human embryonic stem cells [156]. Hypoxic Niches also provide suitable conditions to obtain the properties of ovarian cancerous stemness [157]. Therefore, these Niches can be considered as appropriate treatment targets.
MiRNAs are a group of noncoding RNAs, which are involved in tumor progression [158]. There are different miRNA expression profiles between normal and cancer stem cells [159, 160]. It has been reported that there was increased levels of miR-214 expression in ovarian CSCs which was correlated with self-renewal and chemo resistance [161]. MiR-199a also prevents the tumor growth and increases the sensitivity toward Cisplatin, Paclitaxel, and Adriamycin through down regulation of CD44 in ovarian CSCs [162]. It has been also shown that the miR-200a decreased the migration of ovarian CD133 + CSCs [163].
Conclusions
Regarding the importance of CSCs in ovarian cancer progression and metastasis, it is required to clarify the molecular biology of CSCs to introduce novel markers for the elimination of such cells in ovarian tumors. Indeed, molecular targeted therapy against the CSCs will improve patient’s survival and decrease the tumor relapse among ovarian cancer patients. According to the recent studies, it was concluded that a combination therapy including tumor resection and CSC targeted therapy can be one of the most efficient anti-cancer therapeutic methods against ovarian tumors.
Acknowledgements
Not applicable.
Abbreviations
- ABCG2
ATP-binding cassette sub-family G member 2
- ALDH
Adlehyde dehydrogenase
- BCL-2
B-cell lymphoma-2
- CPE
Clostridium perfringens Enteroxin
- CSCs
Cancer stem cells
- CT
Computed tomography
- FACS
Fluorescent-activated cell storing method
- GSCs
glioma stem cells
- GSI
γ-secretase inhibitor
- IDS
Interval debulking surgery
- MACS
Magnetic-activated cell storing method
- OSE
Surface epithelial cells
- SP
Side population
- STIC
Intra epithelial carcinomas
- VEGF
Vascular Endothelial Growth Factor
Authors’ contributions
VK, SAE and HY were involved in drafting. MF and MM edited and revised the draft. SRK and MRA supervised the project. All authors read and approved the final manuscript.
Funding
Not applicable.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Vahideh Keyvani, Email: vahidekeyvani@gmail.com.
Moein Farshchian, Email: moeinfarshchy@yahoo.com.
Seyed-Alireza Esmaeili, Email: imunoman2009@gmail.com.
Hadi Yari, Email: yari.hadi@gmail.com.
Meysam Moghbeli, Email: moghbelim@mums.ac.ir.
Seyed-Reza Kazemi Nezhad, Email: kazemi_reza@yahoo.de.
Mohammad Reza Abbaszadegan, Email: Abbaszadeganmr@mums.ac.ir.
References
- 1.Ottevanger, P.B. Ovarian cancer stem cells more questions than answers. In Seminars in cancer biology. 2017. Elsevier. [DOI] [PubMed]
- 2.Fathalla M. Incessant ovulation—a factor in ovarian neoplasia. Lancet. 1971;2(7716):163. doi: 10.1016/S0140-6736(71)92335-X. [DOI] [PubMed] [Google Scholar]
- 3.Dubeau L, Drapkin R. Coming into focus: the nonovarian origins of ovarian cancer. Ann Oncol. 2013;24(suppl_8):viii28–viii35. doi: 10.1093/annonc/mdt308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Matulonis UA, et al. Ovarian cancer. Nature Reviews Disease Primers. 2016;2:16061. doi: 10.1038/nrdp.2016.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meinhold-Heerlein I, Hauptmann S. The heterogeneity of ovarian cancer. Arch Gynecol Obstet. 2014;289(2):237–239. doi: 10.1007/s00404-013-3114-3. [DOI] [PubMed] [Google Scholar]
- 6.Zhan Q, Wang C, Ngai S. Ovarian cancer stem cells: a new target for cancer therapy. Biomed Res Int. 2013;2013. [DOI] [PMC free article] [PubMed]
- 7.Kurman RJ, et al. Early detection and treatment of ovarian cancer: shifting from early stage to minimal volume of disease based on a new model of carcinogenesis. Am J Obstet Gynecol. 2008;198(4):351–356. doi: 10.1016/j.ajog.2008.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferlay, J., et al., GLOBOCAN 2012 v1. 0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11. Lyon, France: International Agency for Research on Cancer; 2013. 2015.
- 9.Malvezzi M, et al. Global trends and predictions in ovarian cancer mortality. Ann Oncol. 2016;27(11):2017–2025. doi: 10.1093/annonc/mdw306. [DOI] [PubMed] [Google Scholar]
- 10.Rossing MA, et al. Ovarian tumors in a cohort of infertile women. N Engl J Med. 1994;331(12):771–776. doi: 10.1056/NEJM199409223311204. [DOI] [PubMed] [Google Scholar]
- 11.Reigstad, M.M., et al., Cancer risk in women treated with fertility drugs according to parity status—a registry-based cohort study. Cancer Epidemiology and Prevention Biomarkers, 2017. [DOI] [PMC free article] [PubMed]
- 12.Kurta, M.L., et al., Use of fertility drugs and risk of ovarian cancer: results from a US-based case–control study. Cancer Epidemiology and Prevention Biomarkers, 2012. [DOI] [PMC free article] [PubMed]
- 13.Landen CN, Jr, Birrer MJ, Sood AK. Early events in the pathogenesis of epithelial ovarian cancer. J Clin Oncol. 2008;26(6):995–1005. doi: 10.1200/JCO.2006.07.9970. [DOI] [PubMed] [Google Scholar]
- 14.Mayr D, et al. KRAS and BRAF mutations in ovarian tumors: a comprehensive study of invasive carcinomas, borderline tumors and extraovarian implants. Gynecol Oncol. 2006;103(3):883–887. doi: 10.1016/j.ygyno.2006.05.029. [DOI] [PubMed] [Google Scholar]
- 15.Gui T, et al. Tumor heterogeneity has important consequences for personalized medicine in ovarian cancer. Histol Histopathol. 2015;30(2):173–181. doi: 10.14670/HH-30.173. [DOI] [PubMed] [Google Scholar]
- 16.Kindelberger DW, et al. Intraepithelial carcinoma of the fimbria and pelvic serous carcinoma: evidence for a causal relationship. Am J Surg Pathol. 2007;31(2):161–169. doi: 10.1097/01.pas.0000213335.40358.47. [DOI] [PubMed] [Google Scholar]
- 17.Kuhn E, et al. TP53 mutations in serous tubal intraepithelial carcinoma and concurrent pelvic high-grade serous carcinoma—evidence supporting the clonal relationship of the two lesions. J Pathol. 2012;226(3):421–426. doi: 10.1002/path.3023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Network CGAR. Integrated genomic analyses of ovarian carcinoma. Nature. 2011;474(7353):609. doi: 10.1038/nature10166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Forstner R. Radiological staging of ovarian cancer: imaging findings and contribution of CT and MRI. Eur Radiol. 2007;17(12):3223–3235. doi: 10.1007/s00330-007-0736-5. [DOI] [PubMed] [Google Scholar]
- 20.Shaaban A, Rezvani M. Ovarian cancer: detection and radiologic staging. Top Magn Reson Imaging. 2010;21(4):247–259. doi: 10.1097/RMR.0b013e31823d8063. [DOI] [PubMed] [Google Scholar]
- 21.Forstner R, et al. ESUR guidelines: ovarian cancer staging and follow-up. Eur Radiol. 2010;20(12):2773–2780. doi: 10.1007/s00330-010-1886-4. [DOI] [PubMed] [Google Scholar]
- 22.Petricoin EF, III, et al. Use of proteomic patterns in serum to identify ovarian cancer. Lancet. 2002;359(9306):572–577. doi: 10.1016/S0140-6736(02)07746-2. [DOI] [PubMed] [Google Scholar]
- 23.Michielsen K, et al. Whole-body MRI with diffusion-weighted sequence for staging of patients with suspected ovarian cancer: a clinical feasibility study in comparison to CT and FDG-PET/CT. Eur Radiol. 2014;24(4):889–901. doi: 10.1007/s00330-013-3083-8. [DOI] [PubMed] [Google Scholar]
- 24.Cortez AJ, et al. Advances in ovarian cancer therapy. Cancer Chemother Pharmacol. 2018;81(1):17–38. doi: 10.1007/s00280-017-3501-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Basta A, et al. Recommendation of the polish Society of Oncological Gynaecology on the diagnosis and treatment of epithelial ovarian cancer. Oncology in Clinical Practice. 2015;11(5):233–243. [Google Scholar]
- 26.Urbański K. Consolidation therapy of ovarian cancer. Oncology in Clinical Practice. 2007;3(6):298–305. [Google Scholar]
- 27.López-Guerrero JA, Romero I, Poveda A. Trabectedin therapy as an emerging treatment strategy for recurrent platinum-sensitive ovarian cancer. Chinese journal of cancer. 2015;34(1):41. doi: 10.5732/cjc.014.10278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jain RK. Antiangiogenesis strategies revisited: from starving tumors to alleviating hypoxia. Cancer Cell. 2014;26(5):605–622. doi: 10.1016/j.ccell.2014.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yap TA, et al. Poly (ADP-ribose) polymerase (PARP) inhibitors: exploiting a synthetic lethal strategy in the clinic. CA Cancer J Clin. 2011;61(1):31–49. doi: 10.3322/caac.20095. [DOI] [PubMed] [Google Scholar]
- 30.Duan J-J, et al. Strategies for isolating and enriching cancer stem cells: well begun is half done. Stem Cells Dev. 2013;22(16):2221–2239. doi: 10.1089/scd.2012.0613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moghbeli M, et al. Cancer stem cell detection and isolation. Med Oncol. 2014;31(9):69. doi: 10.1007/s12032-014-0069-6. [DOI] [PubMed] [Google Scholar]
- 32.Mehrazma M, et al. Expression of stem cell markers, CD133 and CD44, in pediatric solid tumors: a study using tissue microarray. Fetal and pediatric pathology. 2013;32(3):192–204. doi: 10.3109/15513815.2012.701266. [DOI] [PubMed] [Google Scholar]
- 33.Meng X, et al. Both CD133+ and CD133− subpopulations of A549 and H446 cells contain cancer-initiating cells. Cancer Sci. 2009;100(6):1040–1046. doi: 10.1111/j.1349-7006.2009.01144.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zheng X, et al. Most C6 cells are cancer stem cells: evidence from clonal and population analyses. Cancer Res. 2007;67(8):3691–3697. doi: 10.1158/0008-5472.CAN-06-3912. [DOI] [PubMed] [Google Scholar]
- 35.Khan MI, et al. Current approaches in identification and isolation of human renal cell carcinoma cancer stem cells. Stem Cell Res Ther. 2015;6(1):178. doi: 10.1186/s13287-015-0177-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gassenmaier M, et al. CXC chemokine receptor 4 is essential for maintenance of renal cell carcinoma-initiating cells and predicts metastasis. Stem Cells. 2013;31(8):1467–1476. doi: 10.1002/stem.1407. [DOI] [PubMed] [Google Scholar]
- 37.Galleggiante V, et al. CTR2 identifies a population of cancer cells with stem cell-like features in patients with clear cell renal cell carcinoma. J Urol. 2014;192(6):1831–1841. doi: 10.1016/j.juro.2014.06.070. [DOI] [PubMed] [Google Scholar]
- 38.Foster R, Buckanovich RJ, Rueda BR. Ovarian cancer stem cells: working towards the root of stemness. Cancer Lett. 2013;338(1):147–157. doi: 10.1016/j.canlet.2012.10.023. [DOI] [PubMed] [Google Scholar]
- 39.Garson K, Vanderhyden BC. Epithelial ovarian cancer stem cells: underlying complexity of a simple paradigm. Reproduction. 2015;149(2):R59–R70. doi: 10.1530/REP-14-0234. [DOI] [PubMed] [Google Scholar]
- 40.Zhang S, et al. Identification and characterization of ovarian cancer-initiating cells from primary human tumors. Cancer Res. 2008;68(11):4311–4320. doi: 10.1158/0008-5472.CAN-08-0364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Curley MD, et al. CD133 expression defines a tumor initiating cell population in primary human ovarian cancer. Stem Cells. 2009;27(12):2875–2883. doi: 10.1002/stem.236. [DOI] [PubMed] [Google Scholar]
- 42.Shah V, et al. Targeted nanomedicine for suppression of CD44 and simultaneous cell death induction in ovarian cancer: an optimal delivery of siRNA and anticancer drug. Clin Cancer Res. 2013;19(22):6193–6204. doi: 10.1158/1078-0432.CCR-13-1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cao L, et al. Tissue transglutaminase links TGF-β, epithelial to mesenchymal transition and a stem cell phenotype in ovarian cancer. Oncogene. 2012;31(20):2521. doi: 10.1038/onc.2011.429. [DOI] [PubMed] [Google Scholar]
- 44.Alvero AB, et al. Molecular phenotyping of human ovarian cancer stem cells unravels the mechanisms for repair and chemoresistance. Cell Cycle. 2009;8(1):158–166. doi: 10.4161/cc.8.1.7533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Alvero AB, et al. Stem-like ovarian cancer cells can serve as tumor vascular progenitors. Stem Cells. 2009;27(10):2405–2413. doi: 10.1002/stem.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wei X, et al. Müllerian inhibiting substance preferentially inhibits stem/progenitors in human ovarian cancer cell lines compared with chemotherapeutics. Proc Natl Acad Sci. 2010;107(44):18874–18879. doi: 10.1073/pnas.1012667107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Meng E, et al. CD44+/CD24− ovarian cancer cells demonstrate cancer stem cell properties and correlate to survival. Clin Exp Metastasis. 2012;29(8):939–948. doi: 10.1007/s10585-012-9482-4. [DOI] [PubMed] [Google Scholar]
- 48.Almanaa TN, Geusz ME, Jamasbi RJ. A new method for identifying stem-like cells in esophageal cancer cell lines. J Cancer. 2013;4(7):536. doi: 10.7150/jca.6477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kuroda T, et al. ALDH1-high ovarian cancer stem-like cells can be isolated from serous and clear cell adenocarcinoma cells, and ALDH1 high expression is associated with poor prognosis. PLoS One. 2013;8(6):e65158. doi: 10.1371/journal.pone.0065158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kryczek I, et al. Expression of aldehyde dehydrogenase and CD133 defines ovarian cancer stem cells. Int J Cancer. 2012;130(1):29–39. doi: 10.1002/ijc.25967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Song J, et al. Characterization of side populations in HNSCC: highly invasive, chemoresistant and abnormal Wnt signaling. PLoS One. 2010;5(7):e11456. doi: 10.1371/journal.pone.0011456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ruan Z, Liu J, Kuang Y. Isolation and characterization of side population cells from the human ovarian cancer cell line SK-OV-3. Experimental and therapeutic medicine. 2015;10(6):2071–2078. doi: 10.3892/etm.2015.2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dontu G, et al. In vitro propagation and transcriptional profiling of human mammary stem/progenitor cells. Genes Dev. 2003;17(10):1253–1270. doi: 10.1101/gad.1061803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Boesch M, et al. Heterogeneity of cancer stem cells: rationale for targeting the stem cell niche. Biochimica et Biophysica Acta (BBA)-reviews on Cancer. 2016;1866(2):276–289. doi: 10.1016/j.bbcan.2016.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jordan CT, Guzman ML, Noble M. Cancer stem cells. N Engl J Med. 2006;355(12):1253–1261. doi: 10.1056/NEJMra061808. [DOI] [PubMed] [Google Scholar]
- 56.Kitamura H, et al. Cancer stem cell: implications in cancer biology and therapy with special reference to lung cancer. Lung Cancer. 2009;66(3):275–281. doi: 10.1016/j.lungcan.2009.07.019. [DOI] [PubMed] [Google Scholar]
- 57.Valent P, et al. Cancer stem cell definitions and terminology: the devil is in the details. Nat Rev Cancer. 2012;12(11):767. doi: 10.1038/nrc3368. [DOI] [PubMed] [Google Scholar]
- 58.Bapat SA, et al. Stem and progenitor-like cells contribute to the aggressive behavior of human epithelial ovarian cancer. Cancer Res. 2005;65(8):3025–3029. doi: 10.1158/0008-5472.CAN-04-3931. [DOI] [PubMed] [Google Scholar]
- 59.Liu P, et al. Metabolic regulation of cancer cell side population by glucose through activation of the Akt pathway. Cell Death Differ. 2014;21(1):124. doi: 10.1038/cdd.2013.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Palorini R, et al. Energy metabolism characterization of a novel Cancer stem cell-L ike line 3 AB-OS. J Cell Biochem. 2014;115(2):368–379. doi: 10.1002/jcb.24671. [DOI] [PubMed] [Google Scholar]
- 61.Zhou, Y., et al., Metabolic alterations in highly tumorigenic glioblastoma cells: preference for hypoxia and high dependency on glycolysis. Journal of Biological Chemistry, 2011: p. jbc. M111. 260935. [DOI] [PMC free article] [PubMed]
- 62.Deshmukh A, et al. Cancer stem cell metabolism: a potential target for cancer therapy. Mol Cancer. 2016;15(1):69. doi: 10.1186/s12943-016-0555-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Liao J, et al. Ovarian cancer spheroid cells with stem cell-like properties contribute to tumor generation, metastasis and chemotherapy resistance through hypoxia-resistant metabolism. PLoS One. 2014;9(1):e84941. doi: 10.1371/journal.pone.0084941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pastò A, et al. Cancer stem cells from epithelial ovarian cancer patients privilege oxidative phosphorylation, and resist glucose deprivation. Oncotarget. 2014;5(12):4305. doi: 10.18632/oncotarget.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Abdullah LN, Chow EK-H. Mechanisms of chemoresistance in cancer stem cells. Clinical and translational medicine. 2013;2(1):3. doi: 10.1186/2001-1326-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chow EKH, et al. Oncogene-specific formation of chemoresistant murine hepatic cancer stem cells. Hepatology. 2012;56(4):1331–1341. doi: 10.1002/hep.25776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shapiro AB, Corder AB, Ling V. P-glycoprotein-mediated Hoechst 33342 transport out of the lipid bilayer. Eur J Biochem. 1997;250(1):115–121. doi: 10.1111/j.1432-1033.1997.00115.x. [DOI] [PubMed] [Google Scholar]
- 68.Scharenberg CW, Harkey MA, Torok-Storb B. The ABCG2 transporter is an efficient Hoechst 33342 efflux pump and is preferentially expressed by immature human hematopoietic progenitors. Blood. 2002;99(2):507–512. doi: 10.1182/blood.V99.2.507. [DOI] [PubMed] [Google Scholar]
- 69.Litman T, et al. The multidrug-resistant phenotype associated with overexpression of the new ABC half-transporter, MXR (ABCG2) J Cell Sci. 2000;113(11):2011–2021. doi: 10.1242/jcs.113.11.2011. [DOI] [PubMed] [Google Scholar]
- 70.Chuthapisith S, et al. Breast cancer chemoresistance: emerging importance of cancer stem cells. Surg Oncol. 2010;19(1):27–32. doi: 10.1016/j.suronc.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 71.Eyre R, et al. Reversing paclitaxel resistance in ovarian cancer cells via inhibition of the ABCB1 expressing side population. Tumor Biol. 2014;35(10):9879–9892. doi: 10.1007/s13277-014-2277-2. [DOI] [PubMed] [Google Scholar]
- 72.Uhlén M, et al. Tissue-based map of the human proteome. Science. 2015;347(6220):1260419. doi: 10.1126/science.1260419. [DOI] [PubMed] [Google Scholar]
- 73.Chou, J.-L., et al., Hypomethylation of TGF-beta target gene, ABCA1 in ovarian cancer and cancer initialing cell and is associated with poor prognosis in cancer patients. 2011, AACR.
- 74.Hu L, McArthur C, Jaffe R. Ovarian cancer stem-like side-population cells are tumourigenic and chemoresistant. Br J Cancer. 2010;102(8):1276. doi: 10.1038/sj.bjc.6605626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kim DK, et al. Crucial role of HMGA1 in the self-renewal and drug resistance of ovarian cancer stem cells. Exp Mol Med. 2016;48(8):e255. doi: 10.1038/emm.2016.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dalton WS, et al. A phase III randomized study of oral verapamil as a chemosensitizer to reverse drug resistance in patients with refractory myeloma. A Southwest Oncology Group study. Cancer. 1995;75(3):815–820. doi: 10.1002/1097-0142(19950201)75:3<815::AID-CNCR2820750311>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 77.Sládek NE. Human aldehyde dehydrogenases: potential pathological, pharmacological, and toxicological impact. J Biochem Mol Toxicol. 2003;17(1):7–23. doi: 10.1002/jbt.10057. [DOI] [PubMed] [Google Scholar]
- 78.Liu J, et al. Lung cancer tumorigenicity and drug resistance are maintained through ALDHhiCD44hi tumor initiating cells. Oncotarget. 2013;4(10):1698. doi: 10.18632/oncotarget.1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Li X, et al. Intrinsic resistance of tumorigenic breast cancer cells to chemotherapy. J Natl Cancer Inst. 2008;100(9):672–679. doi: 10.1093/jnci/djn123. [DOI] [PubMed] [Google Scholar]
- 80.Rasheed ZA, et al. Prognostic significance of tumorigenic cells with mesenchymal features in pancreatic adenocarcinoma. J Natl Cancer Inst. 2010;102(5):340–351. doi: 10.1093/jnci/djp535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lugli A, et al. Prognostic impact of the expression of putative cancer stem cell markers CD133, CD166, CD44s, EpCAM, and ALDH1 in colorectal cancer. Br J Cancer. 2010;103(3):382. doi: 10.1038/sj.bjc.6605762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hilton J. Role of aldehyde dehydrogenase in cyclophosphamide-resistant L1210 leukemia. Cancer Res. 1984;44(11):5156–5160. [PubMed] [Google Scholar]
- 83.Friedman HS, et al. Cyclophosphamide resistance in medulloblastoma. Cancer Res. 1992;52(19):5373–5378. [PubMed] [Google Scholar]
- 84.Li Y, et al. High ALDH activity defines ovarian cancer stem-like cells with enhanced invasiveness and EMT progress which are responsible for tumor invasion. Biochem Biophys Res Commun. 2018;495(1):1081–1088. doi: 10.1016/j.bbrc.2017.11.117. [DOI] [PubMed] [Google Scholar]
- 85.Dylla SJ, et al. Colorectal cancer stem cells are enriched in xenogeneic tumors following chemotherapy. PLoS One. 2008;3(6):e2428. doi: 10.1371/journal.pone.0002428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Opferman JT, Kothari A. Anti-apoptotic BCL-2 family members in development. Cell Death Differ. 2018;25(1):37. doi: 10.1038/cdd.2017.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kim R, Emi M, Tanabe K. Role of mitochondria as the gardens of cell death. Cancer Chemother Pharmacol. 2006;57(5):545–553. doi: 10.1007/s00280-005-0111-7. [DOI] [PubMed] [Google Scholar]
- 88.Pegoraro L, et al. A 14; 18 and an 8; 14 chromosome translocation in a cell line derived from an acute B-cell leukemia. Proc Natl Acad Sci. 1984;81(22):7166–7170. doi: 10.1073/pnas.81.22.7166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Graninger WB, et al. Expression of Bcl-2 and Bcl-2-Ig fusion transcripts in normal and neoplastic cells. J Clin Invest. 1987;80(5):1512–1515. doi: 10.1172/JCI113235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Konopleva M, et al. The anti-apoptotic genes Bcl-XL and Bcl-2 are over-expressed and contribute to chemoresistance of non-proliferating leukaemic CD34+ cells. Br J Haematol. 2002;118(2):521–534. doi: 10.1046/j.1365-2141.2002.03637.x. [DOI] [PubMed] [Google Scholar]
- 91.Madjd Z, et al. CD44+ cancer cells express higher levels of the anti-apoptotic protein Bcl-2 in breast tumours. Cancer Immunity Archive. 2009;9(1):4. [PMC free article] [PubMed] [Google Scholar]
- 92.Williams J, et al. Expression of Bcl-xL in ovarian carcinoma is associated with chemoresistance and recurrent disease. Gynecol Oncol. 2005;96(2):287–295. doi: 10.1016/j.ygyno.2004.10.026. [DOI] [PubMed] [Google Scholar]
- 93.Wong, M., et al., Navitoclax (ABT-263) reduces Bcl-xL mediated chemo-resistance in ovarian cancer models. Molecular cancer therapeutics, 2012: p. molcanther. 0693.2011. [DOI] [PubMed]
- 94.Witham J, et al. The Bcl-2/Bcl-XL family inhibitor ABT-737 sensitizes ovarian cancer cells to carboplatin. Clin Cancer Res. 2007;13(23):7191–7198. doi: 10.1158/1078-0432.CCR-07-0362. [DOI] [PubMed] [Google Scholar]
- 95.Reya T, et al. A role for Wnt signalling in self-renewal of haematopoietic stem cells. Nature. 2003;423(6938):409. doi: 10.1038/nature01593. [DOI] [PubMed] [Google Scholar]
- 96.Zhao C, et al. Loss of β-catenin impairs the renewal of normal and CML stem cells in vivo. Cancer Cell. 2007;12(6):528–541. doi: 10.1016/j.ccr.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bisson I, Prowse DM. WNT signaling regulates self-renewal and differentiation of prostate cancer cells with stem cell characteristics. Cell Res. 2009;19(6):683. doi: 10.1038/cr.2009.43. [DOI] [PubMed] [Google Scholar]
- 98.Capodanno Y, et al. Notch pathway inhibition targets chemoresistant insulinoma cancer stem cells. Endocr Relat Cancer. 2018;25(2):131–144. doi: 10.1530/ERC-17-0415. [DOI] [PubMed] [Google Scholar]
- 99.Abbaszadegan MR, et al. WNT and NOTCH signaling pathways as activators for epidermal growth factor receptor in esophageal squamous cell carcinoma. Cell Mol Biol Lett. 2018;23:42. doi: 10.1186/s11658-018-0109-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Yang W, et al. Wnt/β-catenin signaling contributes to activation of normal and tumorigenic liver progenitor cells. Cancer Res. 2008;68(11):4287–4295. doi: 10.1158/0008-5472.CAN-07-6691. [DOI] [PubMed] [Google Scholar]
- 101.Ranganathan P, Weaver KL, Capobianco AJ. Notch signalling in solid tumours: a little bit of everything but not all the time. Nat Rev Cancer. 2011;11(5):338. doi: 10.1038/nrc3035. [DOI] [PubMed] [Google Scholar]
- 102.Moghbeli M, et al. Role of MAML1 in targeted therapy against the esophageal cancer stem cells. J Transl Med. 2019;17(1):126. doi: 10.1186/s12967-019-1876-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Moghbeli M, et al. Role of Msi1 and PYGO2 in esophageal squamous cell carcinoma depth of invasion. J Cell Commun Signal. 2016;10(1):49–53. doi: 10.1007/s12079-015-0314-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Abbaszadegan MR, Moghbeli M. Role of MAML1 and MEIS1 in esophageal squamous cell carcinoma depth of invasion. Pathol Oncol Res. 2018;24(2):245–250. doi: 10.1007/s12253-017-0243-1. [DOI] [PubMed] [Google Scholar]
- 105.Meng RD, et al. γ-Secretase inhibitors abrogate oxaliplatin-induced activation of the Notch-1 signaling pathway in colon cancer cells resulting in enhanced chemosensitivity. Cancer Res. 2009;69(2):573–582. doi: 10.1158/0008-5472.CAN-08-2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.McAuliffe SM, et al. Targeting notch, a key pathway for ovarian cancer stem cells, sensitizes tumors to platinum therapy. Proc Natl Acad Sci. 2012;109(43):E2939–E2948. doi: 10.1073/pnas.1206400109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Haygood CLW, et al. Ovarian cancer stem cells: can targeted therapy lead to improved progression-free survival? World journal of stem cells. 2014;6(4):441. doi: 10.4252/wjsc.v6.i4.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Reya T, et al. Stem cells, cancer, and cancer stem cells. Nature. 2001;414(6859):105. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 109.Komiya Y, Habas R. Wnt signal transduction pathways. Organogenesis. 2008;4(2):68–75. doi: 10.4161/org.4.2.5851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.MacDonald BT, Tamai K, He X. Wnt/β-catenin signaling: components, mechanisms, and diseases. Dev Cell. 2009;17(1):9–26. doi: 10.1016/j.devcel.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zhang S-S, et al. Identification of CD200+ colorectal cancer stem cells and their gene expression profile. Oncol Rep. 2016;36(4):2252–2260. doi: 10.3892/or.2016.5039. [DOI] [PubMed] [Google Scholar]
- 112.Arend RC, et al. The Wnt/β-catenin pathway in ovarian cancer: a review. Gynecol Oncol. 2013;131(3):772–779. doi: 10.1016/j.ygyno.2013.09.034. [DOI] [PubMed] [Google Scholar]
- 113.Schindler AJ, Watanabe A, Howell SB. LGR5 and LGR6 in stem cell biology and ovarian cancer. Oncotarget. 2018;9(1):1346. doi: 10.18632/oncotarget.20178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zhang X, Hao J. Development of anticancer agents targeting the Wnt/β-catenin signaling. Am J Cancer Res. 2015;5(8):2344. [PMC free article] [PubMed] [Google Scholar]
- 115.Emami, K., et al., A small molecule inhibitor of beta-catenin/CREB-binding protein transcription [corrected] Proc Natl Acad Sci U S A. 2004; 101: 12682–12687. [DOI] [PMC free article] [PubMed]
- 116.Varjosalo M, Taipale J. Hedgehog: functions and mechanisms. Genes Dev. 2008;22(18):2454–2472. doi: 10.1101/gad.1693608. [DOI] [PubMed] [Google Scholar]
- 117.Zhao C, et al. Hedgehog signalling is essential for maintenance of cancer stem cells in myeloid leukaemia. Nature. 2009;458(7239):776. doi: 10.1038/nature07737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Merchant AA, Matsui W. Targeting hedgehog—a cancer stem cell pathway. Clin Cancer Res. 2010;16(12):3130–3140. doi: 10.1158/1078-0432.CCR-09-2846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Clement V, et al. HEDGEHOG-GLI1 signaling regulates human glioma growth, cancer stem cell self-renewal, and tumorigenicity. Curr Biol. 2007;17(2):165–172. doi: 10.1016/j.cub.2006.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Bar EE, et al. Cyclopamine-mediated hedgehog pathway inhibition depletes stem-like cancer cells in glioblastoma. Stem Cells. 2007;25(10):2524–2533. doi: 10.1634/stemcells.2007-0166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Dierks C, et al. Expansion of Bcr-Abl-positive leukemic stem cells is dependent on hedgehog pathway activation. Cancer Cell. 2008;14(3):238–249. doi: 10.1016/j.ccr.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 122.Justilien V, et al. The PRKCI and SOX2 oncogenes are coamplified and cooperate to activate hedgehog signaling in lung squamous cell carcinoma. Cancer Cell. 2014;25(2):139–151. doi: 10.1016/j.ccr.2014.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Peacock CD, et al. Hedgehog signaling maintains a tumor stem cell compartment in multiple myeloma. Proc Natl Acad Sci. 2007;104(10):4048–4053. doi: 10.1073/pnas.0611682104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Von Hoff DD, et al. Inhibition of the hedgehog pathway in advanced basal-cell carcinoma. N Engl J Med. 2009;361(12):1164–1172. doi: 10.1056/NEJMoa0905360. [DOI] [PubMed] [Google Scholar]
- 125.Sekulic A, et al. Efficacy and safety of vismodegib in advanced basal-cell carcinoma. N Engl J Med. 2012;366(23):2171–2179. doi: 10.1056/NEJMoa1113713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ray A, et al. Hedgehog signaling pathway regulates the growth of ovarian cancer spheroid forming cells. Int J Oncol. 2011;39(4):797–804. doi: 10.3892/ijo.2011.1093. [DOI] [PubMed] [Google Scholar]
- 127.Doan HQ, Silapunt S, Migden MR. Sonidegib, a novel smoothened inhibitor for the treatment of advanced basal cell carcinoma. OncoTargets and therapy. 2016;9:5671. doi: 10.2147/OTT.S108171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ericson J, et al. Two critical periods of Sonic hedgehog signaling required for the specification of motor neuron identity. Cell. 1996;87(4):661–673. doi: 10.1016/S0092-8674(00)81386-0. [DOI] [PubMed] [Google Scholar]
- 129.Bosanac I, et al. The structure of SHH in complex with HHIP reveals a recognition role for the Shh pseudo active site in signaling. Nat Struct Mol Biol. 2009;16(7):691. doi: 10.1038/nsmb.1632. [DOI] [PubMed] [Google Scholar]
- 130.Artavanis-Tsakonas S, Rand MD, Lake RJ. Notch signaling: cell fate control and signal integration in development. Science. 1999;284(5415):770–776. doi: 10.1126/science.284.5415.770. [DOI] [PubMed] [Google Scholar]
- 131.Moghbeli M, et al. Correlation of Wnt and NOTCH pathways in esophageal squamous cell carcinoma. J Cell Commun Signal. 2016;10(2):129–135. doi: 10.1007/s12079-016-0320-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Barnawi R, et al. Fascin is critical for the maintenance of breast cancer stem cell pool predominantly via the activation of the notch self-renewal pathway. Stem Cells. 2016;34(12):2799–2813. doi: 10.1002/stem.2473. [DOI] [PubMed] [Google Scholar]
- 133.Abel EV, et al. The notch pathway is important in maintaining the cancer stem cell population in pancreatic cancer. PLoS One. 2014;9(3):e91983. doi: 10.1371/journal.pone.0091983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Steg, A.D., et al., Targeting the notch ligand JAGGED1 in both tumor cells and stroma in ovarian cancer. Clinical cancer research, 2011: p. clincanres. 0432.2011. [DOI] [PMC free article] [PubMed]
- 135.Pant S, et al. A first-in-human phase I study of the oral notch inhibitor, LY900009, in patients with advanced cancer. Eur J Cancer. 2016;56:1–9. doi: 10.1016/j.ejca.2015.11.021. [DOI] [PubMed] [Google Scholar]
- 136.Huang J, et al. Dll4 inhibition plus aflibercept markedly reduces ovarian tumor growth. Mol Cancer Ther. 2016;15(6):1344–1352. doi: 10.1158/1535-7163.MCT-15-0144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Naujokat C, Steinhart R. Salinomycin as a drug for targeting human cancer stem cells. Biomed Res Int. 2012;2012. [DOI] [PMC free article] [PubMed]
- 138.Zhang B, et al. Antitumor properties of salinomycin on cisplatin-resistant human ovarian cancer cells in vitro and in vivo: involvement of p38 MAPK activation. Oncol Rep. 2013;29(4):1371–1378. doi: 10.3892/or.2013.2241. [DOI] [PubMed] [Google Scholar]
- 139.Kaplan F, Teksen F. Apoptotic effects of salinomycin on human ovarian cancer cell line (OVCAR-3) Tumor Biol. 2016;37(3):3897–3903. doi: 10.1007/s13277-015-4212-6. [DOI] [PubMed] [Google Scholar]
- 140.Sebastian, M., et al., Catumaxomab: a bispecific trifunctional antibody. Drugs of today (Barcelona, Spain: 1998), 2009. 45(8): p. 589–597. [DOI] [PubMed]
- 141.Seimetz D, Lindhofer H, Bokemeyer C. Development and approval of the trifunctional antibody catumaxomab (anti-EpCAM× anti-CD3) as a targeted cancer immunotherapy. Cancer Treat Rev. 2010;36(6):458–467. doi: 10.1016/j.ctrv.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 142.Li S-D, Howell SB. CD44-targeted microparticles for delivery of cisplatin to peritoneal metastases. Mol Pharm. 2009;7(1):280–290. doi: 10.1021/mp900242f. [DOI] [PubMed] [Google Scholar]
- 143.Lee SJ, et al. Metronomic activity of CD44-targeted hyaluronic acid-paclitaxel in ovarian carcinoma. Clin Cancer Res. 2012. [DOI] [PMC free article] [PubMed]
- 144.Skubitz AP, et al. Targeting CD133 in an in vivo ovarian cancer model reduces ovarian cancer progression. Gynecol Oncol. 2013;130(3):579–587. doi: 10.1016/j.ygyno.2013.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Su D, et al. Targeting CD24 for treatment of ovarian cancer by short hairpin RNA. Cytotherapy. 2009;11(5):642–652. doi: 10.1080/14653240902878308. [DOI] [PubMed] [Google Scholar]
- 146.Raspollini M, et al. C-KIT expression and correlation with chemotherapy resistance in ovarian carcinoma: an immunocytochemical study. Ann Oncol. 2004;15(4):594–597. doi: 10.1093/annonc/mdh139. [DOI] [PubMed] [Google Scholar]
- 147.Chau W, et al. C-kit mediates chemoresistance and tumor-initiating capacity of ovarian cancer cells through activation of Wnt/β-catenin–ATP-binding cassette G2 signaling. Oncogene. 2013;32(22):2767. doi: 10.1038/onc.2012.290. [DOI] [PubMed] [Google Scholar]
- 148.Schilder RJ, et al. Phase II evaluation of imatinib mesylate in the treatment of recurrent or persistent epithelial ovarian or primary peritoneal carcinoma: a gynecologic oncology group study. J Clin Oncol. 2008;26(20):3418–3425. doi: 10.1200/JCO.2007.14.3420. [DOI] [PubMed] [Google Scholar]
- 149.Chung H, et al. The effect of salinomycin on ovarian cancer stem-like cells. Obstetrics & gynecology science. 2016;59(4):261–268. doi: 10.5468/ogs.2016.59.4.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Zhang R, et al. Inhibitory effects of metformin at low concentration on epithelial–mesenchymal transition of CD44+ CD117+ ovarian cancer stem cells. Stem Cell Res Ther. 2015;6(1):262. doi: 10.1186/s13287-015-0249-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Shank JJ, et al. Metformin targets ovarian cancer stem cells in vitro and in vivo. Gynecol Oncol. 2012;127(2):390–397. doi: 10.1016/j.ygyno.2012.07.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Casagrande F, et al. Eradication of chemotherapy-resistant CD44+ human ovarian cancer stem cells in mice by intraperitoneal administration of clostridium perfringens enterotoxin. Cancer. 2011;117(24):5519–5528. doi: 10.1002/cncr.26215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Sell S. Stem cell origin of cancer and differentiation therapy. Crit Rev Oncol Hematol. 2004;51(1):1–28. doi: 10.1016/j.critrevonc.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 154.Gudas LJ, Wagner JA. Retinoids regulate stem cell differentiation. J Cell Physiol. 2011;226(2):322–330. doi: 10.1002/jcp.22417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Whitworth JM, et al. The impact of novel retinoids in combination with platinum chemotherapy on ovarian cancer stem cells. Gynecol Oncol. 2012;125(1):226–230. doi: 10.1016/j.ygyno.2011.12.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Katz E, Skorecki K, Tzukerman M. Niche-dependent tumorigenic capacity of malignant ovarian ascites-derived cancer cell subpopulations. Clin Cancer Res. 2009;15(1):70–80. doi: 10.1158/1078-0432.CCR-08-1233. [DOI] [PubMed] [Google Scholar]
- 157.Liang D, et al. The hypoxic microenvironment upgrades stem-like properties of ovarian cancer cells. BMC Cancer. 2012;12(1):201. doi: 10.1186/1471-2407-12-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136(2):215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Lavon I, et al. Gliomas display a microRNA expression profile reminiscent of neural precursor cells. Neuro-oncology. 2010;12(5):422–433. doi: 10.1093/neuonc/nop061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.van Jaarsveld MT, et al. MicroRNAs in ovarian cancer biology and therapy resistance. Int J Biochem Cell Biol. 2010;42(8):1282–1290. doi: 10.1016/j.biocel.2010.01.014. [DOI] [PubMed] [Google Scholar]
- 161.Xu, C.-X., et al., MiR-214 regulates ovarian cancer cell stemness by targeting p53/nanog. Journal of Biological Chemistry, 2012: p. jbc. M112. 374611.
- 162.Cheng W, et al. MicroRNA-199a targets CD44 to suppress the tumorigenicity and multidrug resistance of ovarian cancer-initiating cells. FEBS J. 2012;279(11):2047–2059. doi: 10.1111/j.1742-4658.2012.08589.x. [DOI] [PubMed] [Google Scholar]
- 163.Wu Q, et al. MicroRNA-200a inhibits CD133/1+ ovarian cancer stem cells migration and invasion by targeting E-cadherin repressor ZEB2. Gynecol Oncol. 2011;122(1):149–154. doi: 10.1016/j.ygyno.2011.03.026. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.