Abstract
BACKGROUND
Increased expression of nerve growth factor in injured or inflamed tissue is associated with increased pain. This proof-of-concept study was designed to investigate the safety and analgesic efficacy of tanezumab, a humanized monoclonal antibody that binds and inhibits nerve growth factor.
METHODS
We randomly assigned 450 patients with osteoarthritis of the knee to receive tanezumab (administered at a dose of 10, 25, 50, 100, or 200 μg per kilogram of body weight) or placebo on days 1 and 56. The primary efficacy measures were knee pain while walking and the patient’s global assessment of response to therapy. We also assessed pain, stiffness, and physical function using the Western Ontario and Mc-Master Universities Osteoarthritis Index (WOMAC); the rate of response using the criteria of the Outcome Measures for Rheumatology Committee and Osteoarthritis Research Society International Standing Committee for Clinical Trials Response Criteria Initiative (OMERACT-OARSI); and safety.
RESULTS
When averaged over weeks 1 through 16, the mean reductions from baseline in knee pain while walking ranged from 45 to 62% with various doses of tanezumab, as compared with 22% with placebo (P<0.001). Tanezumab, as compared with placebo, was also associated with significantly greater improvements in the response to therapy as assessed with the use of the patients’ global assessment measure (mean increases in score of 29 to 47% with various doses of tanezumab, as compared with 19% with placebo; P≤0.001). The rate of response according to the OMERACT-OARSI criteria ranged from 74 to 93% with tanezumab treatment, as compared with 44% with placebo (P<0.001). The rates of adverse events were 68% and 55% in the tanezumab and placebo groups, respectively. The most common adverse events among tanezumab-treated patients were headache (9% of the patients), upper respiratory tract infection (7%), and paresthesia (7%).
CONCLUSIONS
In this proof-of-concept study, treatment with tanezumab was associated with a reduction in joint pain and improvement in function, with mild and moderate adverse events, among patients with moderate-to-severe osteoarthritis of the knee. (Funded by Rinat Neuroscience; ClinicalTrials.gov number, .)
NERVE GROWTH FACTOR IS A NEUROTRO-phin that regulates the structure and function of responsive sensory neurons, including small-diameter nociceptive afferents. There has been increasing recognition of the potential role of nerve growth factor in pain modulation through nociceptor sensitization.1–7 In animals and humans, exogenous nerve growth factor increases pain either locally or systemically, depending on the dose and the route of administration.8,9 Increased expression of nerve growth factor is found in inflamed tissues from patients with conditions such as arthritis, pancreatitis, and prostatitis.10–12 Levels of nerve growth factor are also elevated in animal models of inflammatory pain, and pharmacologic inhibition of the activity of nerve growth factor in these models reduces or blocks signs of pain. Therefore, nerve growth factor appears to have a role in causing and augmenting pain in these models.1,13–15 The development of therapeutic interventions that are based on antagonism of nerve growth factor is of interest.16,17
The treatment options for patients with painful osteoarthritis of the knee are inadequate. Nonsteroidal antiinflammatory drugs and narcotic analgesics are commonly used18,19; however, these medications have well-described gastrointestinal and cardiorenal side effects,20,21 and the response to them is unsatisfactory in some patients.22–23 Potent analgesic medications with acceptable side-effect profiles may help to avoid or delay surgical intervention.24
Tanezumab is a humanized IgG2 monoclonal antibody directed against nerve growth factor that blocks the interaction of nerve growth factor with its receptors, TrkA and p75.25 A small phase 1 clinical trial showed that a single intravenous injection of tanezumab substantially reduced pain in patients with osteoarthritis of the knee.26–27 We report the results of a proof-of-concept study of tanezumab in patients with advanced osteoarthritis of the knee who did not have a satisfactory response to nonopiate pain medications or who were considered to be candidates for invasive intervention. We compared the safety, side-effect profile, and efficacy of repeat doses of tanezumab as compared with placebo.
METHODS
STUDY POPULATION
We enrolled patients, 40 to 75 years of age, who had osteoarthritis of the knee as diagnosed on the basis of American College of Rheumatology criteria,28 with radiographic confirmation (Kellgren-Lawrence grade 2 or higher, on a scale of 0 to 4, with higher numbers indicating more severe signs of osteoarthritis). Patients were eligible only if they were unwilling to take nonopiate pain medications or had had an unsatisfactory response to them or if they were candidates for or seeking invasive interventions such as intraarticular injections or total knee replacement. All pain medications except the “rescue” medications, acetaminophen and tramadol, were discontinued at the screening visit. At the time of randomization, patients had to have pain while walking on a flat surface (the walking-pain measure of the Western Ontario and McMaster Universities Osteoarthritis Index [WOMAC]) that they rated between 50 and 90 on a visual-analogue scale that ranged from 0 to 100, with 100 indicating maximal pain. In addition, among patients who discontinued pain medication during the screening period, an increase in the walking-pain score of 10 or more was required between screening and randomization.
The exclusion criteria were pregnancy, a history of or current symptoms of an autoimmune disorder, cancer within the previous 5 years except for cutaneous basal-cell or squamous-cell cancer resolved by excision, allergic reaction to monoclonal antibodies or IgG-fusion proteins, infection with hepatitis B or hepatitis C virus or the human immunodeficiency virus, drug abuse, fibromyalgia, clinically significant cardiac disease, diabetes mellitus requiring oral treatment or insulin, clinically significant neurologic disease, or a clinically significant psychiatric disorder. All participants provided written informed consent.
STUDY DESIGN AND OVERSIGHT
Patients were recruited between March 30, 2006, and May 3, 2007, at 46 study centers in the United States and were screened within 30 days before randomization. Eligible patients who were taking pain medication other than acetaminophen and tramadol underwent a washout period (of at least 5 half-lives of the medication). Patients rated their knee pain and recorded the score in an electronic diary every day for 3 days before randomization to establish their baseline pain score. Eligible patients were randomly assigned on day 1, with the use of an interactive voice-response system, to placebo or to tanezumab at a dose of 10, 25, 50, 100, or 200 μg per kilogram of body weight, such that there were equal numbers in each study group. A pharmacist at each study site received each patient’s randomization number and prepared each patient’s dosing solution. Other than the pharmacist at each site and one statistician at the contract research organization, all the staff members and patients involved in the study were unaware of the group assignments.
The study medication was administered intravenously on days 1 and 56. Study visits were scheduled for days 14, 28, 70, 84, 112, 136, and 182, during which safety and efficacy assessments were performed and serum samples for routine laboratory tests and for pharmacokinetic analyses were obtained. In addition, patients were contacted by telephone on day 42 to ask about adverse events. Patients recorded their knee pain and their use of rescue medication daily in an electronic diary. The rescue medications that were permitted were acetaminophen at a dose of 3000 mg or less per day, tramadol at a dose of 400 mg or less per day, or both, during the washout period and days 1 through 28 (weeks 1 through 4) and acetaminophen at a dose of 3000 mg per day for the remainder of the study. Patients could enter an open-label extension of the trial (ClinicalTrials.gov number, ) at week 16 (day 112) if they had received two doses of the study drug and had been followed for at least 8 weeks after the last dose.
The study was designed and coordinated by Rinat Neuroscience, a subsidiary of Pfizer. An external data and safety monitoring board monitored safety, and day-to-day study operations, including data management, were overseen by PPDI (a contract research organization contracted by Pfizer). The data were analyzed by Pfizer. The first author wrote the first draft of the manuscript. All the authors were involved in the design of the study and interpretation of the data, contributed to the writing of the manuscript, made the decision to submit the manuscript for publication, attest that the study was performed in accordance with the protocol and the statistical analysis plan, and vouch for the accuracy and completeness of the reported results. Editorial support was provided by UBC Scientific Solutions and was funded by Pfizer. The study protocol was approved by the local ethics committee at each study center before patient enrollment began. The protocol, including the statistical analysis plan, is available with the full text of this article at NEJM.org.
EFFICACY ASSESSMENTS
The primary efficacy outcomes were the change from baseline in the pain the patient felt in the index knee while walking on a flat surface and in the patient’s global assessment of response to therapy, averaged over weeks 1 through 16. Secondary efficacy outcomes included the change from baseline in overall knee pain and in scores on the WOMAC subscales for pain, stiffness, and physical function. Pain while walking and overall knee pain were recorded daily in an electronic diary, whereas the patient’s global assessment of response to therapy and scores on the WOMAC subscales were recorded on study-visit days. Pain, the patient’s global assessment, and scores on the WOMAC subscales were assessed with the use of a visual-analogue scale that ranged from 0 to 100. In the case of pain and WOMAC scores, a lower score indicated improvement (i.e., less pain, less stiffness, and less limitation of physical function), whereas in the case of the patient’s global assessment, a higher score indicated improvement (i.e., a better response to therapy). Another secondary outcome was the response to therapy on the basis of the criteria of the Outcome Measures for Rheumatology Committee and Osteoarthritis Research Society International Standing Committee for Clinical Trials Response Criteria Initiative (OMERACT-OARSI).29 Patients were classified as having had a response if the WOMAC pain or physical-function score decreased by 50% or more and by 20 or more points on the visual-analogue scale or if two of the following three findings were recorded: a decrease in the WOMAC pain score by 20% or more and by 10 or more points on the visual-analogue scale, a decrease in the WOMAC physical-function score by 20% or more and by 10 or more points on the scale, or an increase in the score on the patient’s global assessment by 20% or more and by 10 or more points on the scale. Rescue medication use, also a prespecified secondary outcome, was recorded daily in the patients’ diaries.
SAFETY ASSESSMENTS
The nature, onset, duration, severity, and outcome of all adverse events, as well as any relationship of an adverse event to the study drug were ascertained and documented at each visit. Safety assessments included physical and neurologic examinations (e.g., evaluation of mental status, strength, reflexes, sensation, and coordination), cognitive testing with the use of the Hopkins Verbal Learning Test-Revised,30 assessment of postural vital signs, and electrocardiography. Clinically significant abnormalities on a neurologic examination performed by the investigator or adverse events suggestive of peripheral neuropathy were further evaluated by an independent neurologist.
STATISTICAL ANALYSIS
We estimated that we would need to enroll 75 patients in each group for the study to have 80% power to detect a difference between the tanezumab groups and the placebo group of 15 points or more on the visual-analogue scale for the primary outcomes (the average change from baseline through week 16 in knee pain while walking and in the patient’s global assessment of response to therapy), with an effect size of 0.5 (indicating a moderate difference).31 With respect to the intensity of pain, decreases of 10 or more points on a visual-analogue scale that ranges from 1 to 100 are considered to be minimally important improvements, and decreases of 20 or more points are considered to be moderately important improvements.32
Changes from baseline in all the measures that were assessed with the use of a visual-analogue scale were determined with a mixed-model, repeated-measures analysis, with model terms for study site, study group, study week, and the interaction between study medication and study week, and with the baseline score on the visual-analogue scale as a covariate, with no imputation for missing data. A repeated-measures analysis was also used to assess the number of rescue medication pills taken. For the analysis of rates of response according to OMERACT-OARSI criteria (calculated on the basis of the average change from baseline to week 16), we used the Cochran- Mantel-Haenszel test, stratified according to study site, to compare the proportions of patients in the tanezumab groups who had a response with the proportion of those in the placebo group who had a response.
RESULTS
BASELINE CHARACTERISTICS OF THE PATIENTS
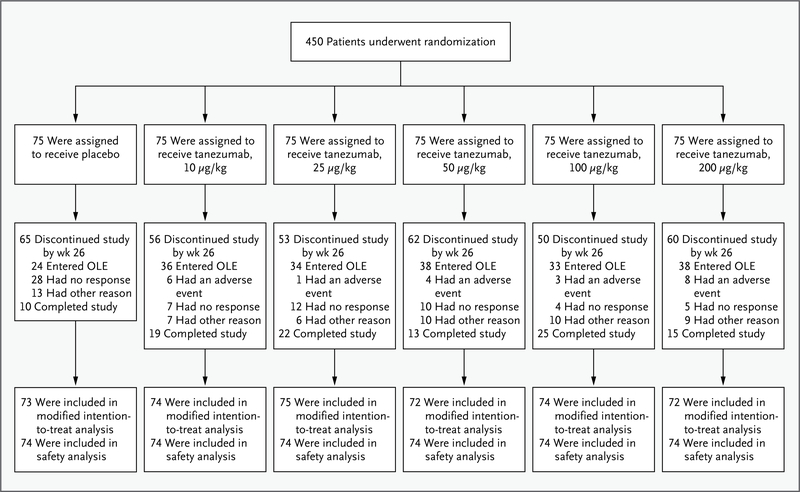
Of the 450 patients who underwent randomization, 440 received at least one dose of the study medication and underwent at least one efficacy assessment (the modified intention-to-treat population) (Fig. 1). Most patients in the modified intention-to-treat population had a Kellgren-Lawrence grade of 3 (52%) or 4 (17%) and severe pain33 both at the time of screening (mean [±SD] score on the visual-analogue scale, 58±12) and at the time of randomization, after washout of previous medications (score on the visual-analogue scale, 71±11). A total of 87% of the patients reported taking pain medication for knee pain before they enrolled in the study. The baseline characteristics of the patients were similar across study groups (Table 1).
Figure 1. Randomization and Follow-up.
Eligible patients could enter the open-label extension (OLE) of the study at week 16.
Table 1.
Baseline Characteristics of the Study Patients.*
| Characteristic | Placebo (N = 74) | Tanezumab, 10 μg/kg (N = 74) | Tanezumab, 25 μg/kg (N = 74) | Tanezumab, 50 μg/kg (N = 74) | Tanezumab, 100 μg/kg (N = 74) | Tanezumab, 200 μg/kg (N = 74) |
|---|---|---|---|---|---|---|
| Age — yr | 58.1±7.7 | 58.3±8.3 | 59.9±8.1 | 60.4±7.7 | 57.1±8.2 | 58.4±7.6 |
| Female sex — no. (%) | 42 (57) | 49 (66) | 50 (68) | 37 (50) | 44 (59) | 40 (54) |
| White race — no. (%)† | 66 (89) | 62 (84) | 67 (91) | 66 (89) | 67 (91) | 64 (86) |
| Kellgren–Lawrence grade — no./total no. (%)‡ | ||||||
| 2 | 18/73 (25) | 21/73 (29) | 23/74 (31) | 29/74 (39) | 22/74 (30) | 19/73 (26) |
| 3 or 4 | 55/73 (75) | 52/73 (71) | 51/74 (69) | 45/74 (61) | 52/74 (70) | 54/73 (74) |
| Knee pain while walking§ | 71.6±10.0 | 70.6±10.9 | 71.7±10.5 | 68.1±10.2 | 71.1±11.0 | 72.4±11.5 |
| Patient’s global assessment of response¶ | 48.8±20.8 | 55.7±20.3 | 51.0±20.6 | 51.6±16.9 | 49.9±19.9 | 54.4±22.4 |
| WOMAC score∥ | ||||||
| Pain | 69.0±11.9 | 65.8±13.9 | 69.2±12.5 | 62.1±12.3 | 68.3±13.2 | 68.4±12.0 |
| Stiffness | 74.4±13.5 | 69.7±13.1 | 75.0±12.4 | 66.7±17.5 | 71.2±17.9 | 73.3±13.1 |
| Physical function | 69.0±12.5 | 63.8±13.6 | 69.2±14.6 | 62.6±12.3 | 67.4±14.8 | 67.8±14.0 |
Plus–minus values are means ±SD.
Race was self-reported.
A Kellgren–Lawrence score of 2 (minimal signs of osteoarthritis) indicates definite osteophytes without reduction of the joint space; a score of 3 (moderate signs of osteoarthritis) indicates diminished joint space; and a score of 4 (severe signs of osteoarthritis) indicates greatly reduced joint space. Data are from the intention-to-treat population, and missing data are excluded.
Knee pain while walking was assessed with the use of a visual-analogue scale that ranged from 0 to 100, with higher scores indicating more pain. Data are from the modified intention-to-treat population.
Patients’ global assessment of response to therapy was assessed with the use of a visual-analogue scale that ranged from 0 to 100, with higher scores indicating a better response to therapy. Data are from the modified intention-to-treat population.
Scores on the pain, stiffness, and physical-function subscales of the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) were assessed with the use of a visual-analogue scale that ranged from 0 to 100, with higher scores indicating more pain, more stiffness, and more limitation of physical function, respectively. Data are from the modified intention-to-treat population.
EFFICACY
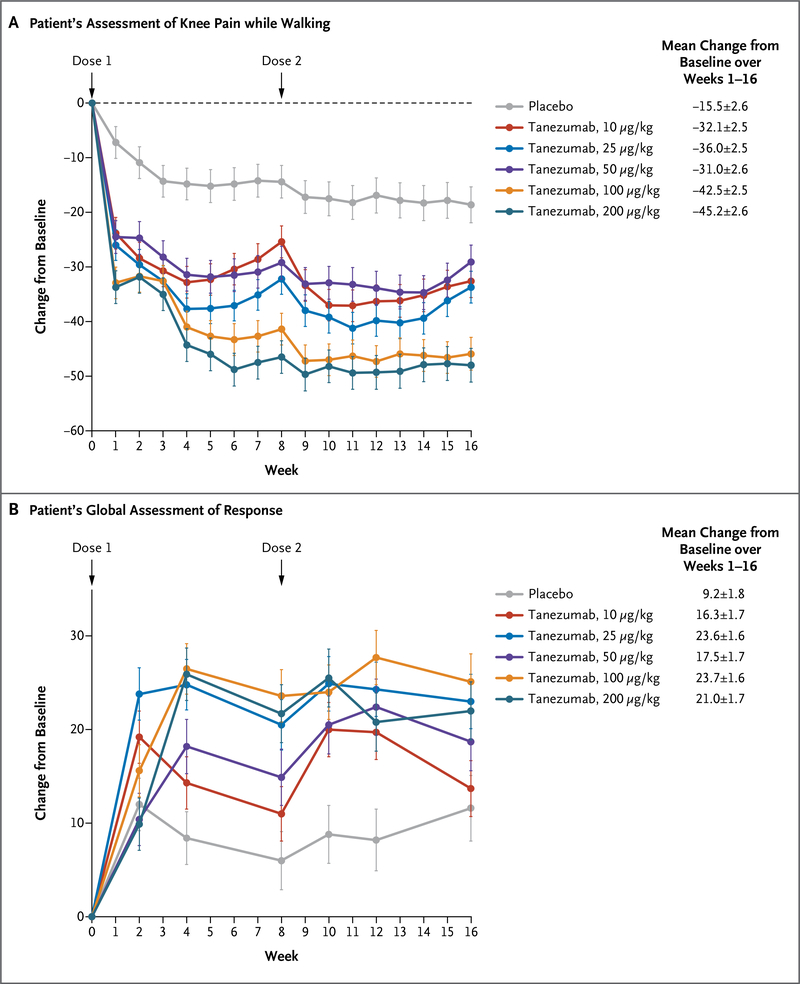
As compared with placebo, tanezumab, at all the doses studied, was associated with an improvement in the primary efficacy measures. The mean reduction from baseline in the score on the visual-analogue scale for knee pain while walking, averaged over weeks 1 through 16, ranged from 31.0 to 45.2 points with various doses of tanezumab, as compared with 15.5 points with placebo (a reduction of 45 to 62% with tanezumab vs. 22% with placebo, P<0.001 for the comparison of all doses of tanezumab with placebo) (Fig. 2A). We observed significant improvements among patients receiving tanezumab as compared with those receiving placebo by the end of the first week, and significant improvements continued to be seen throughout the remainder of the treatment period. The mean increase from baseline in the score on the patient’s global assessment of response to therapy, averaged over weeks 1 through 16, ranged from 16.3 to 23.7 points with various doses of tanezumab, as compared with 9.2 points with placebo (an increase of 29 to 47% with tanezumab vs. 19% with placebo, P≤0.001 for the comparison of all doses of tanezumab with placebo) (Fig. 2B). By week 2, the scores on the patient’s global assessment had improved in the group receiving 25 μg of tanezumab per kilogram, as compared with placebo (P=0.002); by week 4, the scores had improved in the groups receiving 50 μg, 100 μg, and 200 μg of tanezumab per kilogram, as compared with placebo (P = 0.01, P<0.001, and P<0.001 for the three comparisons, respectively); and during weeks 10 and 12, the scores improved in the group receiving tanezumab at a dose of 10 μg per kilogram, as compared with placebo (P=0.008). The improvements were maintained through week 16 in the groups receiving 25 μg, 100 μg, and 200 μg of tanezumab per kilogram.
Figure 2. Change from Baseline in Patients’ Assessment of Knee Pain while Walking and in Patients’ Global Assessment of Response to Therapy.
The patient’s assessment of knee pain while walking and the patient’s global assessment of response to therapy were obtained at baseline and at the indicated times with the use of a visual-analogue scale that ranged from 0 to 100. In the case of knee pain, a decrease in the score indicates improvement (i.e., less pain); in the case of the patient’s global assessment, an increase in the score indicates improvement (i.e., a better response to therapy). Changes are reported as least-squares means ±SE. P<0.001 for the comparisons of all doses of tanezumab with placebo in the assessment of knee pain and global assessment of response, except for the comparison of 10 μg of tanezumab per kilogram of body weight with placebo in the patient’s global assessment, for which P = 0.001.
The mean reductions from baseline in overall knee pain over the course of weeks 1 through 16 were similar in magnitude to those reported for knee pain while walking (reductions of 43 to 62% with tanezumab vs. 23% with placebo, P<0.001 for the comparison of all doses of tanezumab with placebo). Treatment with tanezumab, as compared with placebo, was also associated with reductions in the mean WOMAC scores for pain (reductions of 46 to 64% vs. 23%), stiffness (48 to 65% vs. 22%), and physical function (47 to 65% vs. 22%) over the same period (P<0.001 for all comparisons) (Table 2). The percentage of patients who had a response to therapy according to OMERACT-OARSI criteria, averaged over weeks 1 through 16, was significantly higher with tanezumab treatment than with placebo (74 to 93% vs. 44%, P<0.001 for the comparison of all doses of tanezumab with placebo) (Table 2). Rescue medications that were allowed per protocol were used less frequently by tanezumab-treated patients than by placebo-treated patients during weeks 1 through 16 (odds ratio with tanezumab, 0.50; 95% confidence interval [CI], 0.24 to 1.02; P=0.05) and was significantly lower during weeks 1 through 4 (odds ratio, 0.49; 95% CI, 0.24 to 0.99; P = 0.04).
Table 2.
Secondary Efficacy Outcomes.*
| Outcome | Placebo (N = 73) | Tanezumab, 10 μg/kg (N = 74) | Tanezumab, 25 μg/kg (N = 75)† | Tanezumab, 50 μg/kg (N = 72) | Tanezumab, 100 μg/kg (N = 74) | Tanezumab, 200 μg/kg (N = 72) |
|---|---|---|---|---|---|---|
| Change in WOMAC score from baseline through week 16 | ||||||
| Pain subscale | −16.2±2.4 | −30.1±2.3 | −36.0±2.2 | −29.0±2.4 | −39.6±2.2 | −43.5±2.3 |
| Stiffness subscale | −16.3±2.4 | −33.5±2.3 | −37.7±2.2 | −34.5±2.4 | −42.7±2.2 | −47.8±2.4 |
| Physical-function subscale | −15.2±2.3 | −30.1±2.3 | −34.9±2.2 | −30.8±2.4 | −40.5±2.2 | −43.8±2.3 |
| Response to therapy according to OMERACT–OARSI criteria by week 16 (% of patients)‡ | 43.8 | 74.3 | 84.0 | 75.0 | 93.2 | 93.1 |
Plus–minus values are means ±SE. P<0.001 for all comparisons of the five doses of tanezumab with placebo. These analyses were performed on data from the modified intention-to-treat population.
One patient who was randomly assigned to receive 25 μg of tanezumab per kilogram of body weight instead received 50 μg per kilogram.
According to the criteria of the Outcome Measures for Rheumatology Committee and Osteoarthritis Research Society International Standing Committee for Clinical Trials Response Criteria Initiative (OMERACT–OARSI), patients were classified as having had a response if the WOMAC pain or physical-function score decreased by 50% or more and by 20 or more points on the visual-analogue scale or if two of the following three findings were observed: a decrease in the WOMAC pain score by 20% or more and by 10 or more points on the scale, a decrease in the WOMAC physical-function score by 20% or more and by 10 or more points on the scale, or an increase in the score on the patient’s global assessment by 20% or more and by 10 or more points on the scale.
SAFETY
Among patients in the tanezumab groups, the three most common adverse events were headache, upper respiratory tract infection, and paresthesia (Table 3). The incidence of treatment-related adverse events was higher among patients treated with 100 μg or 200 μg of tanezumab per kilogram than among patients who received lower doses (28% and 35% in the groups receiving 100 μg and 200 μg per kilogram, respectively, vs. 11 to 18% in the groups receiving other doses).
Table 3.
Frequency of Adverse Events.
| Variable | Placebo (N = 74) | Tanezumab, 10 μg/kg (N = 74) | Tanezumab, 25 μg/kg (N = 74) | Tanezumab, 50 μg/kg (N = 74) | Tanezumab, 100 μg/kg (N = 74) | Tanezumab, 200 μg/kg (N = 74) |
|---|---|---|---|---|---|---|
| number of patients (percent) | ||||||
| Any adverse event | 41 (55) | 51 (69) | 49 (66) | 44 (59) | 51 (69) | 58 (78) |
| Treatment-related adverse event | 6 (8) | 11 (15) | 13 (18) | 8 (11) | 21 (28) | 26 (35) |
| Severe adverse event* | 2 (3) | 6 (8) | 3 (4) | 3 (4) | 3 (4) | 3 (4) |
| Treatment-related severe adverse event | 0 | 0 | 1 (1) | 0 | 1 (1) | 0 |
| Serious adverse event† | 1 (1) | 2 (3) | 0 | 2 (3) | 0 | 2 (3) |
| Adverse event occurring in ≥5% of tanezumab-treated patients‡ | ||||||
| Headache | 2 (3) | 8 (11) | 5 (7) | 8 (11) | 6 (8) | 6 (8) |
| Upper respiratory tract infection | 4 (5) | 2 (3) | 6 (8) | 5 (7) | 7 (9) | 7 (9) |
| Arthralgia | 0 | 1 (1) | 2 (3) | 5 (7) | 4 (5) | 7 (9) |
| Pain in extremity | 0 | 3 (4) | 1 (1) | 2 (3) | 6 (8) | 9 (12) |
| Peripheral edema | 2 (3) | 0 | 2 (3) | 5 (7) | 6 (8) | 8 (11) |
| Adverse event involving abnormal peripheral sensation | ||||||
| Allodynia | 0 | 0 | 0 | 0 | 1 (1) | 1 (1) |
| Burning sensation | 1 (1) | 0 | 0 | 0 | 1 (1) | 0 |
| Dysesthesia | 0 | 0 | 0 | 0 | 1 (1) | 1 (1) |
| Hyperesthesia | 0 | 0 | 0 | 3 (4) | 4 (5) | 4 (5) |
| Hypoesthesia | 0 | 1 (1) | 6 (8) | 2 (3) | 5 (7) | 5 (7) |
| Neuralgia | 0 | 0 | 0 | 0 | 1 (1) | 0 |
| Neuritis | 0 | 0 | 0 | 0 | 1 (1) | 0 |
| Pallanesthesia | 0 | 0 | 1 (1) | 0 | 0 | 1 (1) |
| Paresthesia | 2 (3) | 4 (5) | 4 (5) | 1 (1) | 8 (11) | 8 (11) |
| Sensory disturbance | 0 | 0 | 0 | 1 (1) | 1 (1) | 2 (3) |
| Sensory loss | 0 | 0 | 1 (1) | 0 | 0 | 1 (1) |
The severity of an adverse event refers to the maximum intensity of the event. An event was considered to be severe (as compared with mild or moderate) if it interfered substantially with the patient’s usual functioning.
An adverse event was classified as serious if it was fatal or life-threatening, required or prolonged inpatient hospitalization, was disabling, resulted in a congenital anomaly or birth defect, or required medical or surgical intervention to prevent permanent impairment or damage. No serious adverse event was considered to be treatment-related.
The determination of a rate of 5% or higher was made on the basis of all tanezumab groups combined.
Peripheral sensory symptoms, including paresthesia, were reported in 14% of the patients receiving tanezumab and in 4% of those receiving placebo (Table 3); the severity of these adverse events was mild in 56 of the 70 patients with these symptoms in the tanezumab groups and in all 3 patients with these symptoms in the placebo group and was moderate in the remaining 14 patients in the tanezumab groups. The results of neurologic examinations of these patients were predominantly normal; in patients with clinically significant changes, abnormalities were minor and consisted mainly of changes in sensation in the extremities and diminished deep-tendon reflexes. A total of 15 patients had abnormal peripheral sensation that was unresolved at the final visit. Of these 15 patients, 6 had clinically significant findings on neurologic examination: decreased ankle reflexes in 1 patient who was receiving 100 μg of tanezumab per kilogram; decreased temperature and a sensation of sharp pain, vibration, or both, in the toes or feet in 4 patients (1 each in the groups receiving 25 and 100 μg of tanezumab per kilogram and 2 in the group receiving 200 μg per kilogram); and bilateral decreased sensation in pain, fine touch, and temperature in a radial-nerve distribution in 1 patient who was receiving 100 μg of tanezumab per kilogram; all other aspects of the neurologic examination were normal.
Additional follow-up data were available for 7 patients with abnormal peripheral sensation who subsequently participated in the open-label extension study, and in each case, the adverse event resolved before the patient’s entry into the extension study. Similarly, follow-up data were available for 1 of the 6 patients with neurologic findings, and in the case of this patient, the neurologic findings had normalized by the time of the baseline visit in the open-label extension study.
The mean time to the onset of abnormal sensory symptoms was 33 days (median, 14) after the first dose of tanezumab, and the mean duration of symptoms was 18 days (median, 4) across tanezumab groups. Some differences in the onset and duration of these symptoms were noted: allodynia, dysesthesia, and hyperesthesia tended to develop primarily after the first dose of tanezumab had been administered and were relatively short-lived, whereas the onset and duration of paresthesia were more variable.
Serious adverse events were reported in 6 patients (2%) receiving tanezumab (appendicitis, bacterial arthritis, cellulitis, spinal stenosis, breast cancer, and syncope) and in 1 patient (1%) receiving placebo (noncardiac chest pain). A total of 6% of tanezumab-treated patients withdrew from the study because of adverse events; no placebo-treated patients withdrew because of adverse events (Fig. 1). We observed no clinically important changes in electrocardiographic findings, postural vital signs, or mental status or cognition in any of the study participants, and we did not detect the presence of antitanezumab antibodies in any of the patients assigned to a tanezumab group.
One site in the current study also participated in a subsequent phase 3 trial and was closed by the sponsor owing to substantial noncompliance with Good Clinical Practice guidelines and with the protocol in that phase 3 study. Therefore, all analyses from the current study were repeated, with the 23 patients from that site excluded; only very small changes in the efficacy and safety results and slight increases in P values were seen when the patients from that site were not included in the analyses (see the Supplementary Appendix, available at NEJM.org).
DISCUSSION
Two injections of tanezumab — a monoclonal antibody that inhibits nerve growth factor — 8 weeks apart at doses ranging from 10 to 200 μg per kilogram resulted in clinically significant reductions in knee pain, stiffness, and limitations of physical function in patients with moderate-to-severe knee osteoarthritis. Entries in daily pain diaries indicated that differences between tanezumab therapy and placebo were apparent within days after the first injection, and the efficacy persisted throughout the 4-month treatment period. Although the study was not powered to assess dose response, and no formal dose-response analysis was performed, the reductions in pain appeared to be greater among patients taking higher doses of tanezumab (100 or 200 μg per kilogram) than among those taking lower doses, with no clear benefit of the 200-μg dose over the 100-μg dose. Clinically meaningful pain relief is often described as a reduction in pain intensity of approximately 30% from the baseline level,34,35 and in this study, reductions ranged from 45 to 62% with tanezumab. Furthermore, reductions in pain with tanezumab therapy resulted in pain scores that were equal to or lower than those reported by patients at the time of screening, when they were taking their previously prescribed pain medications.
The majority of adverse events that were reported by patients taking tanezumab, including abnormal peripheral sensations, were mild to moderate in severity. The occurrence of adverse events appeared to be dose-dependent. Since nerve growth factor is thought to act on small-diameter sensory afferents, the occurrence of paresthesia and other signs associated with large-fiber sensory function is interesting. The more frequent occurrence of these events within a short time after administration of the first dose suggests that there may be transient changes in sensitivity or “tone” of different afferent fiber populations, leading to altered sensations. Owing to their largely transient nature, it is unlikely that these adverse events are indicative of neurodegenerative changes. The transient nature of these events is consistent with findings in long-term studies of the safety of highdose tanezumab in nonhuman primates.36 Elucidation of the mechanisms underlying these effects and any potential long-term consequences require further investigation. The assessed measures of mental status and cognition were unchanged, suggesting that the effects of tanezumab were limited to the peripheral nervous system.
The limitations of this study include the lack of a comparison group receiving a different active treatment, a study population that was too small for a statistical comparison of efficacy according to dose, and short-term exposure to tanezumab (since knee pain from osteoarthritis usually requires long-term treatment). However, our study shows efficacy in patients with more severe osteoarthritis than those in other trials. For example, two studies37,38 of glucosamine and chondroitin in patients with osteoarthritis of the knee excluded patients who had the highest degree of severity on radiography (a Kellgren-Lawrence grade of 4), whereas 17% of our study population had this degree of severity. Baseline scores for knee pain on the visual-analogue scale in these two other studies were 54 and 57, respectively, whereas our population had a mean score for knee pain on the day of randomization of 71±11.
Since the completion of this study and through May 24, 2010, progressively worsening osteoarthritis associated with radiographic evidence of bone necrosis developed in 16 subjects participating in 1 of 13 phase 3 studies of tanezumab for osteoarthritis of the hip and knee; all 16 subjects required total joint replacements. The affected joints were the knee, hip, or shoulder (predominantly unilateral involvement), with more than half the cases occurring in a joint other than the index joint under evaluation in the study. These 16 events led the Food and Drug Administration (FDA) on June 22, 2010, to put the osteoarthritis clinical program for tanezumab on clinical hold until more information can be obtained to determine the true incidence and the causality of these events. More recently, the FDA requested the suspension of two additional trials of tanezumab, one involving patients with low back pain and the other involving patients with diabetic neuropathy.
Our proof-of-concept study showed that tanezumab had a favorable efficacy profile for the treatment of moderate-to-severe knee pain associated with osteoarthritis. Longer trials involving larger samples are needed to better understand safety and tolerability issues and explore the clinical potential of tanezumab as an alternative to current pharmacologic treatments.
Supplementary Material
Acknowledgments
Supported by Rinat Neuroscience, now a subsidiary of Pfizer. Editorial support was provided by Papia Das and Elizabeth Young of UBC Scientific Solutions and was funded by Pfizer. Dr. Lane was also supported by a grant (K24-AR04884) from the National Institutes of Health.
We thank Leslie Tive, Susan Simpson, and Carol Zhao for assistance in the preparation of the manuscript.
Footnotes
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
Contributor Information
Nancy E. Lane, University of California at Davis Medical School, Sacramento
Thomas J. Schnitzer, Northwestern University Feinberg School of Medicine, Chicago
Charles A. Birbara, University of Massachusetts School of Medicine, Worcester
Masoud Mokhtarani, Rinat Neuroscience, South San Francisco, CA
David L. Shelton, Rinat Neuroscience, South San Francisco, CA
Mike D. Smith, Pfizer, New London, CT
Mark T. Brown, Pfizer, New London, CT
references
- 1.Woolf CJ, Safieh-Garabedian B, Ma QP, Crilly P, Winter J. Nerve growth factor contributes to the generation of inflammatory sensory hypersensitivity. Neuroscience 1994;62:327–31. [DOI] [PubMed] [Google Scholar]
- 2.McMahon SB. NGF as a mediator of inflammatory pain. Philos Trans R Soc Lond B Biol Sci 1996;351:431–40. [DOI] [PubMed] [Google Scholar]
- 3.Skaper SD. The biology of neurotrophins, signalling pathways, and functional peptide mimetics of neurotrophins and their receptors. CNS Neurol Disord Drug Targets 2008;7:46–62. [DOI] [PubMed] [Google Scholar]
- 4.Petruska JC, Mendell LM. The many functions of nerve growth factor: multiple actions on nociceptors. Neurosci Lett 2004; 361:168–71. [DOI] [PubMed] [Google Scholar]
- 5.Barker PA, Murphy RA. The nerve growth factor receptor: a multicomponent system that mediates the actions of the neurotrophin family of proteins. Mol Cell Biochem 1992;110:1–15. [DOI] [PubMed] [Google Scholar]
- 6.Frade JM, Barde YA. Nerve growth factor: two receptors, multiple functions. Bioessays 1998;20:137–45. [DOI] [PubMed] [Google Scholar]
- 7.Nicol GD, Vasko MR. Unraveling the story of NGF-mediated sensitization of nociceptive sensory neurons: ON or OFF the Trks? Mol Interv 2007;7:26–41. [DOI] [PubMed] [Google Scholar]
- 8.Hao J, Ebendal T, Xu X, Wiesenfeld-Hallin Z, Eriksdotter Jönhagen M. Intracerebroventricular infusion of nerve growth factor induces pain-like response in rats. Neurosci Lett 2000;286:208–12. [DOI] [PubMed] [Google Scholar]
- 9.Ruiz G, Ceballos D, Baños JE. Behavioral and histological effects of endoneurial administration of nerve growth factor: possible implications in neuropathic pain. Brain Res 2004;1011:1–6. [DOI] [PubMed] [Google Scholar]
- 10.Halliday DA, Zettler C, Rush RA, Scicchitano R, McNeil JD. Elevated nerve growth factor levels in the synovial fluid of patients with inflammatory joint disease. Neurochem Res 1998;23:919–22. [DOI] [PubMed] [Google Scholar]
- 11.Friess H, Zhu ZW, di Mola FF, et al. Nerve growth factor and its high-affinity receptor in chronic pancreatitis. Ann Surg 1999;230:615–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miller LJ, Fischer KA, Goralnick SJ, et al. Nerve growth factor and chronic prostatitis/chronic pelvic pain syndrome. Urology 2002;59:603–8. [DOI] [PubMed] [Google Scholar]
- 13.McMahon SB, Bennett DL, Priestley JV, Shelton DL. The biological effects of endogenous nerve growth factor on adult sensory neurons revealed by a trkA-IgG fusion molecule. Nat Med 1995;1:774–80. [DOI] [PubMed] [Google Scholar]
- 14.Ramer MS, Bisby MA. Adrenergic innervation of rat sensory ganglia following proximal or distal painful sciatic neuropathy: distinct mechanisms revealed by anti- NGF treatment. Eur J Neurosci 1999;11: 837–46. [DOI] [PubMed] [Google Scholar]
- 15.Zahn PK, Subieta A, Park SS, Brennan TJ. Effect of blockade of nerve growth factor and tumor necrosis factor on pain behaviors after plantar incision. J Pain 2004;5:157–63. [DOI] [PubMed] [Google Scholar]
- 16.Hefti FF, Rosenthal A, Walicke PA, et al. Novel class of pain drugs based on antagonism of NGF. Trends Pharmacol Sci 2006; 27:85–91. [DOI] [PubMed] [Google Scholar]
- 17.Dray A, Read SJ. Arthritis and pain: future targets to control osteoarthritis pain. Arthritis Res Ther 2007;9:212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dillon CF, Rasch EK, Gu Q, Hirsch R. Prevalence of knee osteoarthritis in the United States: arthritis data from the Third National Health and Nutrition Examination Survey 1991–94. J Rheumatol 2006;33:2271–9. [PubMed] [Google Scholar]
- 19.Zhang W, Moskowitz RW, Nuki G, et al. OARSI recommendations for the management of hip and knee osteoarthritis. II. OARSI evidence-based, expert consensus guidelines. Osteoarthritis Cartilage 2008; 16:137–62. [DOI] [PubMed] [Google Scholar]
- 20.Johnsen SP, Larsson H, Tarone RE, et al. Risk of hospitalization for myocardial infarction among users of rofecoxib, celecoxib, and other NSAIDs: a population-based case-control study. Arch Intern Med 2005;165:978–84. [DOI] [PubMed] [Google Scholar]
- 21.Whelton A Renal and related cardiovascular effects of conventional and COX-2-specific NSAIDs and non-NSAID analgesics. Am J Ther 2000;7:63–74. [DOI] [PubMed] [Google Scholar]
- 22.Bjordal JM, Ljunggren AE, Klovning A, Slørdal L. Non-steroidal anti-inflammatory drugs, including cyclo-oxygenase-2 inhibitors, in osteoarthritic knee pain: metaanalysis of randomised placebo controlled trials. BMJ 2004;329:1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bjordal JM, Klovning A, Ljunggren AE, Slørdal L. Short-term efficacy of pharmacotherapeutic interventions in osteo-arthritic knee pain: a meta-analysis of randomised placebo-controlled trials. Eur J Pain 2007;11:125–38. [DOI] [PubMed] [Google Scholar]
- 24.Hawker GA. Who, when, and why total joint replacement surgery? The patient’s perspective. Curr Opin Rheumatol 2006; 18:526–30. [DOI] [PubMed] [Google Scholar]
- 25.Abdiche YN, Malashock DS, Pons J. Probing the binding mechanism and affinity of tanezumab, a recombinant humanized anti-NGF monoclonal antibody, using a repertoire of biosensors. Protein Sci 2008;17:1326–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hefti F, Mokhtarani M, Gray M, Zhao C, Chan C. RN624 (anti-NGF) reduces pain and improves function in subjects with moderate to severe pain from osteoarthritis of the knee. J Pain 2006;7:Suppl:S45 abstract. [Google Scholar]
- 27.Lane N, Webster L, Lu S, Gray M, Hefti F, Walicke P. RN624 (anti-NGF) improves pain and function in subjects with moderate knee osteoarthritis: a phase I study. Arthritis Rheum 2005;52:Suppl: S461 abstract. [Google Scholar]
- 28.Altman R, Asch E, Bloch D, et al. Development of criteria for the classification and reporting of osteoarthritis: classification of osteoarthritis of the knee. Arthritis Rheum 1986;29:1039–49. [DOI] [PubMed] [Google Scholar]
- 29.Pham T, van der Heijde D, Altman RD, et al. OMERACT-OARSI initiative: Osteoarthritis Research Society International set of responder criteria for osteoarthritis clinical trials revisited. Osteoarthritis Cartilage 2004;12:389–99. [DOI] [PubMed] [Google Scholar]
- 30.Shapiro AM, Benedict RH, Schretlen D, Brandt J. Construct and concurrent validity of the Hopkins Verbal Learning Test-revised. Clin Neuropsychol 1999;13:348–58. [DOI] [PubMed] [Google Scholar]
- 31.Cohen J Statistical power analysis for the behavioral sciences. 2nd ed. Hillsdale, NJ: L. Erlbaum, 1988. [Google Scholar]
- 32.Dworkin RH, Turk DC, Wyrwich KW, et al. Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. J Pain 2008;9:105–21. [DOI] [PubMed] [Google Scholar]
- 33.Collins SL, Moore RA, McQuay HJ. The visual analogue pain intensity scale: what is moderate pain in millimetres? Pain 1997;72:95–7. [DOI] [PubMed] [Google Scholar]
- 34.Rowbotham MC. What is a “clinically meaningful” reduction in pain? Pain 2001; 94:131–2. [DOI] [PubMed] [Google Scholar]
- 35.Salaffi F, Stancati A, Silvestri CA, Ciapetti A, Grassi W. Minimal clinically important changes in chronic musculoskeletal pain intensity measured on a numerical rating scale. Eur J Pain 2004;8:283–91. [DOI] [PubMed] [Google Scholar]
- 36.Shelton DL, Zorbas M, Bales R, Rogers B, Pons J. Preclinical safety evaluation of PF04383119 after single and multiple-dose administration in monkeys. Anesthesiology 2008;109:A597 (Web only). abstract. (Accessed July 30, 2010, at http://www.asaabstracts.com/strands/asaabstracts/searchArticle.htm;jsessionid=618E87D0E0ABC77F4B2A108910544EA7?index=1&highlight=false&highlightcolor=1&bold=true&italic=false.) [Google Scholar]
- 37.Clegg DO, Reda DJ, Harris CL, et al. Glucosamine, chondroitin sulfate, and the two in combination for painful knee osteoarthritis. N Engl J Med 2006;354:795–808. [DOI] [PubMed] [Google Scholar]
- 38.Kahan A, Uebelhart D, De Vathaire F, Delmas PD, Reginster JY. Long-term effects of chondroitins 4 and 6 sulfate on knee osteoarthritis: the study on osteoarthritis progression prevention, a two-year, randomized, double-blind, placebo-controlled trial. Arthritis Rheum 2009;60: 524–33. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.