Abstract.
Congenital infection with Trypanosoma cruzi remains a major route for Chagas disease transmission in endemic and non-endemic regions. We evaluated an intervention strategy aimed to detect congenital Chagas disease cases at a major hospital in the Ecuadorian Amazon via cord blood analysis at the time of delivery. All women giving birth at the hospital during the study period (191) were invited to participate. Among them, two (1.0%) did not adjust to the inclusion criteria and four (2.1%) declined to participate in the study, showing the intervention had good acceptability among the mothers. It was possible to obtain cord blood samples during 146 of the deliveries, and only one woman was found to be seropositive, without evidence of transmission to the newborn at delivery or 8 months later. In addition, sociodemographic and economic characterization of the study population revealed that few women had previous knowledge about Chagas disease (16.1%) whereas more than half (62.5%) recognized the vector. Recognizing the vector and having seen it indoors were associated with women from rural families, involved in agriculture, and hunting in the forest. Interestingly, most women (87.3%) reported having easy access to Ecuador’s national health system, suggesting serological screening during prenatal visits would be of value in this province. With a proper prenatal screening system in place, cord blood screening would allow for timely detection of T. cruzi infection in newborns from both seropositive women and the minority (2.1%) of women who do not comply with prenatal care visits.
INTRODUCTION
Chagas disease, a neglected tropical disease, is endemic to Latin America. Six to seven million people worldwide are infected with Trypanosoma cruzi, the etiological agent, and more than 100 million people live under the risk of infection.1 Trypanosoma cruzi is mainly transmitted through contact with infected triatomine bug feces. Other less frequent transmission routes include ingestion of contaminated food or beverages, transfusion of contaminated blood products, and transplant of infected organs.1 In addition, mothers acute and chronically infected by T. cruzi can transmit the parasite vertically during pregnancy or birth (congenital Chagas disease)2; furthermore, in the absence of proper treatment, an infected woman may potentially transmit the infection in each successive pregnancy. Notably, congenital transmission constitutes an important means of T. cruzi spread both in endemic and non-endemic regions. In 2006, more than 15,000 new congenital cases per year were estimated in Latin America3; although this number may have declined since 2006, several recent publications have reported new congenital transmission.4–6 Congenital T. cruzi infection is mostly asymptomatic in newborns; however, more severe manifestations may occur7,8 and mortality can reach up to 15% for untreated severe cases.8 Many congenital cases remain undiagnosed and will eventually give rise to disabling cardiac or digestive pathology. In contrast to chronically infected adults, treatment of congenitally infected newborns and infants less than a year of age is well tolerated and highly effective (up to 100%).9–11 Therefore, timely diagnosis and treatment of congenital infections is crucial.
Several diagnostic methods for T. cruzi infection in newborns are available. Although parasitemia at birth may be very low, and thus undetectable, several parasitological methods are used. These include direct identification of trypomastigotes via microscopic examination of cord blood collected at birth or in newborn venous blood collected during the first months of life.2 Hemoculture can also be used; however, parasite growth in culture may not be detectable for up to a month or more.12 Although serological methods are more sensitive, maternal anti–T. cruzi IgG is passed on to the fetus transplacentally, making early serological tests unreliable during the first 6–8 months of life.2 Maternal IgG gradually disappears, whereas the anti–T. cruzi humoral immune response is activated in infected newborns, and detection of anti–T. cruzi antibodies after 6 months of age constitutes the most reliable means of diagnosis.2 Nonetheless, in the absence of good postnatal monitoring, the opportunity to provide timely diagnosis and treatment to these patients is frequently lost.
Forty-eight percent of the Ecuadorian territory is located in the Amazon, and the risk of Chagas disease transmission within this region has been previously recognized.13–15 The seroprevalence rate in 2003 in a sample of 6,866 people living in 162 communities of the northern Ecuadorian Amazon was estimated to be 2.4%.13 More recent studies have reported similar seroprevalences in the southern Ecuadorian Amazon.16,17
In the Amazon region in general, Chagas disease transmission is believed to depend on human contact with non-domiciliated vectors, which sporadically invade human dwellings.18 This epidemiological scenario results from complex interactions between social and biological determinants that remain to be clarified. Furthermore, it has been proposed that transdisciplinary approaches to study the living conditions of the inhabitants and improve their understanding of the transmission process could help reduce Chagas disease incidence.19,20
In this context, the main objectives of this study were 1) to screen women and their newborns for T. cruzi infection at the time of delivery at a major Amazonian hospital; 2) to characterize the study population at sociodemographic and economic levels and to examine knowledge, habits, and practices related to Chagas disease, to identify risk factors for infection; and 3) to evaluate the feasibility, acceptability, and relevance of the proposed intervention.
Our results highlight the need for measures to detect and manage cases of congenital Chagas within the population of women infected with T. cruzi at the national level in Ecuador.
MATERIALS AND METHODS
Study design.
From June 22 to August 15, 2016, a cross-sectional study was conducted among pregnant women at the Francisco de Orellana Hospital (El Coca) located in the Amazon region in Ecuador (Figure 1). A serological screening for infection with T. cruzi and a questionnaire to describe the study population and its knowledge about vectors and Chagas disease as well as to examine risk factor associations were implemented. The study was approved by the Committee of Ethics in Research with Human Subjects from Pontificia Universidad Católica del Ecuador (approval number CEISH-358-2017) and by the Ecuadorian Ministry of Health (MSP-DIS-2016-0095-0, June 10, 2016).
Figure 1.
Map of Ecuador showing relevant features. The H symbol marks the location of Francisco de Orellana Hospital, within Orellana Province. Also shown are the cities of Quito (Ecuador’s capital) in the highlands, and Guayaquil, in the coast, where the national reference laboratory for Chagas disease is located. Manabí Province, a well-known endemic region within Ecuador, is also shown.
Recruitment and inclusion/exclusion criteria.
All pregnant women (minors and adults) giving birth at the Francisco de Orellana Hospital during the study period were invited to participate. The inclusion criteria were as follows: giving birth during the study period, agreeing to provide a cord blood sample, and providing signed informed consent. In the case of minors, both the young woman and her parent or legal guardian provided signed informed consent. Women not eligible for participation were those not mentally able to provide informed consent, those rejecting the study, or those who did not provide written informed consent.
Interview.
A questionnaire consisting of ∼40 items was applied to all study participants. The questions covered the following aspects: 1) demographic and socioeconomic characteristics of the population, 2) behaviors and environmental traits that could constitute risk factors for house infestation by triatomines and Chagas disease transmission, and 3) knowledge of the women about triatomines and Chagas disease. Questions regarding triatomine recognition were asked after the participants observed a box containing individuals from different species of triatomines in the various developmental stages of their life cycle. Also, a few questions addressed the feasibility of setting up the Chagas disease diagnosis protocol within the maternity ward. Tables 1–3 show the questions and answers obtained.
Table 1.
Demographic and socioeconomic profile of the pregnant women study population at the Francisco de Orellana Hospital (El Coca), and results of bivariate analyses of associations with pregnant women 1) reporting to recognize the vector and 2) having seen triatomines indoors
| Variables | Recognizing the triatomine | Having seen triatomines indoors | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | Yes | No | Yes | No | ||||||||
| N | % | N | % | N | % | P* | N | % | N | % | P* | |
| Age (years) | 0.86 | 0.065 | ||||||||||
| ≤ 18 | 22 | 15.07 | 14 | 14.74 | 8 | 15.69 | 2 | 4.76 | 20 | 19.61 | ||
| 18–35 | 113 | 77.40 | 73 | 76.84 | 40 | 78.43 | 36 | 85.71 | 75 | 73.53 | ||
| > 35 | 11 | 7.53 | 8 | 8.42 | 3 | 5.88 | 4 | 9.52 | 7 | 6.86 | ||
| Geographical region in which the woman was born | 0.13 | 0.35 | ||||||||||
| Coastal region | 27 | 18.49 | 15 | 15.79 | 12 | 24.49 | 6 | 14.29 | 20 | 20.00 | ||
| Interandean region | 16 | 10.96 | 10 | 10.53 | 6 | 12.24 | 5 | 11.90 | 11 | 11.00 | ||
| Amazonian region | 95 | 65.07 | 68 | 71.58 | 27 | 55.10 | 31 | 73.81 | 63 | 63.00 | ||
| Foreign | 6 | 4.11 | 2 | 2.11 | 4 | 8.16 | 0 | 0.00 | 6 | 6.00 | ||
| Speaking a local language | 0.00 | 0.37 | 0.64 | |||||||||
| Yes | 27 | 18.49 | 20 | 21.05 | 7 | 14.29 | 9 | 21.43 | 18 | 18.00 | ||
| No | 119 | 81.51 | 75 | 78.95 | 44 | 89.80 | 33 | 78.57 | 84 | 84.00 | ||
| Educational level | 0.42 | 0.94 | ||||||||||
| Primary | 20 | 13.70 | 13 | 13.68 | 7 | 14.29 | 6 | 14.29 | 13 | 12.74 | ||
| Secondary | 117 | 80.14 | 78 | 82.11 | 39 | 79.59 | 34 | 80.95 | 82 | 80.40 | ||
| University | 9 | 6.16 | 4 | 4.21 | 5 | 10.20 | 2 | 4.76 | 7 | 6.86 | ||
| Main occupation | 1.00 | 0.43 | ||||||||||
| Housewife or without remunerated activity | 99 | 67.81 | 64 | 67.37 | 35 | 68.63 | 32 | 76.19 | 66 | 64.70 | ||
| Student | 20 | 13.70 | 13 | 13.68 | 7 | 13.72 | 4 | 9.52 | 16 | 15.69 | ||
| Paid work | 27 | 18.49 | 18 | 18.95 | 9 | 17.65 | 6 | 14.29 | 20 | 19.61 | ||
| Monthly income (US$) | 0.71 | 0.18 | ||||||||||
| < 250 | 64 | 48.48 | 40 | 47.06 | 23 | 51.11 | 15 | 38.46 | 49 | 52.69 | ||
| ≥ 250 | 68 | 51.51 | 45 | 52.94 | 22 | 48.89 | 24 | 61.54 | 44 | 47.31 | ||
| Distance of current domicile to El Coca city (km) | 0.052 | 0.001 | ||||||||||
| 0 (living in El Coca city) | 51 | 36.43 | 27 | 29.35 | 24 | 50.00 | 6 | 15.00 | 45 | 45.92 | ||
| < 60 | 40 | 28.57 | 28 | 30.43 | 12 | 25.00 | 17 | 42.50 | 22 | 22.45 | ||
| ≥ 60 | 49 | 35.00 | 37 | 40.22 | 12 | 25.00 | 17 | 42.50 | 31 | 31.63 | ||
| Environment in which current domicile is located | 0.043 | 0.00 | 0.00 | 0.12 | ||||||||
| City and village | 79 | 58.52 | 46 | 51.11 | 33 | 73.33 | 18 | 45.00 | 59 | 63.44 | ||
| Rural community in close proximity to the forest | 48 | 35.55 | 38 | 42.22 | 10 | 22.22 | 19 | 47.50 | 29 | 31.18 | ||
| Rural community located far away from the forest | 8 | 5.92 | 6 | 6.67 | 2 | 4.44 | 3 | 7.50 | 5 | 5.38 | ||
| Number of house elements which increase the risk of triatomine presence (i.e., thatched roof and cane walls) | 0.38 | 0.23 | ||||||||||
| 0 | 45 | 31.91 | 32 | 34.78 | 13 | 26.53 | 11 | 27.50 | 33 | 33.00 | ||
| 1 | 13 | 9.22 | 6 | 6.52 | 7 | 14.28 | 1 | 2.50 | 12 | 12.00 | ||
| 2 | 75 | 53.19 | 48 | 52.17 | 27 | 55.10 | 25 | 62.50 | 50 | 50.00 | ||
| 3 | 8 | 5.67 | 6 | 6.52 | 2 | 4.08 | 3 | 7.50 | 5 | 5.00 | ||
| Drinking fresh, unboiled, fruit juice | 0.52 | 0.36 | ||||||||||
| Yes | 116 | 79.45 | 77 | 81.05 | 39 | 76.47 | 36 | 85.71 | 79 | 77.45 | ||
| No | 30 | 20.55 | 18 | 18.94 | 12 | 23.53 | 6 | 14.29 | 23 | 22.55 | ||
| Cooking with firewood | 0.076 | 0.71 | ||||||||||
| Yes | 58 | 39.73 | 52 | 54.74 | 36 | 70.59 | 24 | 57.14 | 63 | 61.76 | ||
| No | 88 | 60.27 | 43 | 45.26 | 15 | 29.41 | 18 | 42.86 | 39 | 38.23 | ||
| Sleeping sometimes outdoors in field near the forest or in the countryside | 0.38 | 0.85 | ||||||||||
| Yes | 62 | 42.46 | 43 | 45.26 | 19 | 37.25 | 17 | 40.48 | 44 | 43.14 | ||
| No | 84 | 57.53 | 52 | 54.74 | 32 | 62.74 | 25 | 59.52 | 58 | 56.86 | ||
| At least one member of the household works in the field | 0.038 | 0.27 | ||||||||||
| Yes | 66 | 45.2 | 49 | 51.58 | 17 | 33.33 | 22 | 52.38 | 43 | 42.16 | ||
| No | 80 | 54.79 | 46 | 48.42 | 34 | 66.67 | 20 | 47.62 | 59 | 57.84 | ||
| At least one member of the household hunts | 0.046 | 0.35 | ||||||||||
| Yes | 28 | 19.18 | 23 | 24.21 | 5 | 9.80 | 10 | 23.80 | 17 | 16.67 | ||
| No | 118 | 80.82 | 72 | 75.79 | 46 | 90.20 | 32 | 76.19 | 85 | 83.33 | ||
| Reporting seing wild animals in or near the dwelling | 0.042 | 0.28 | ||||||||||
| Yes | 35 | 23.97 | 28 | 29.47 | 7 | 13.72 | 13 | 30.95 | 22 | 21.57 | ||
| No | 111 | 76.03 | 67 | 70.53 | 44 | 86.27 | 29 | 69.05 | 80 | 78.43 | ||
| Presence of animals as pets, farm animals, and/or wild animals in dwelling (indoors and/or outdoors) | 0.29 | 0.38 | ||||||||||
| Yes | 114 | 78.08 | 77 | 81.05 | 37 | 72.55 | 35 | 83.33 | 77 | 75.49 | ||
| No | 32 | 21.92 | 18 | 18.95 | 14 | 27.45 | 7 | 16.67 | 25 | 24.51 | ||
| Owning chickens | 0.08 | 0.11 | ||||||||||
| Yes | 42 | 28.77 | 32 | 33.68 | 10 | 19.61 | 16 | 38.09 | 25 | 24.51 | ||
| No | 104 | 71.23 | 63 | 66.31 | 41 | 80.39 | 26 | 61.90 | 77 | 75.49 | ||
* P-values ≥ 0.25 that were selected in the down-step regression multivariate analysis are shown in bold.
Table 3.
Knowledge about Chagas disease and its vectors among the study population at Franscico de Orellana Hospital (El Coca)
| Variables | N | % |
|---|---|---|
| Knowledge of the vector | ||
| Recognizing the triatomine* | 95/146 | 65.1 |
| Knowing the triatomine name (chinche or chinchorro) | 54/146 | 37 |
| Reporting having seen triatomines | ||
| Indoors | 42/144 | 29.2 |
| Outdoors | 53/144 | 36.8 |
| Indoors and/or outdoors | 93/144 | 64.6 |
| Knowledge of the disease | ||
| Having heard about Chagas disease | 23/146 | 16.1 |
| Information source† | ||
| Primary health center | 11/23 | 47.8 |
| Television and internet | 5/23 | 21.7 |
| School | 2/23 | 8.7 |
| Physician | 2/23 | 8.7 |
| Family | 3/23 | 13 |
| Formal training (nursing school) | 2/23 | 8.7 |
| Identifying the triatomine as a vector of disease | 11/135 | 7.5 |
* Response obtained after observation of a box containing triatomine species presently found in the Amazon, on their different developmental stages.
† optional multiple response.
Collection of cord blood samples.
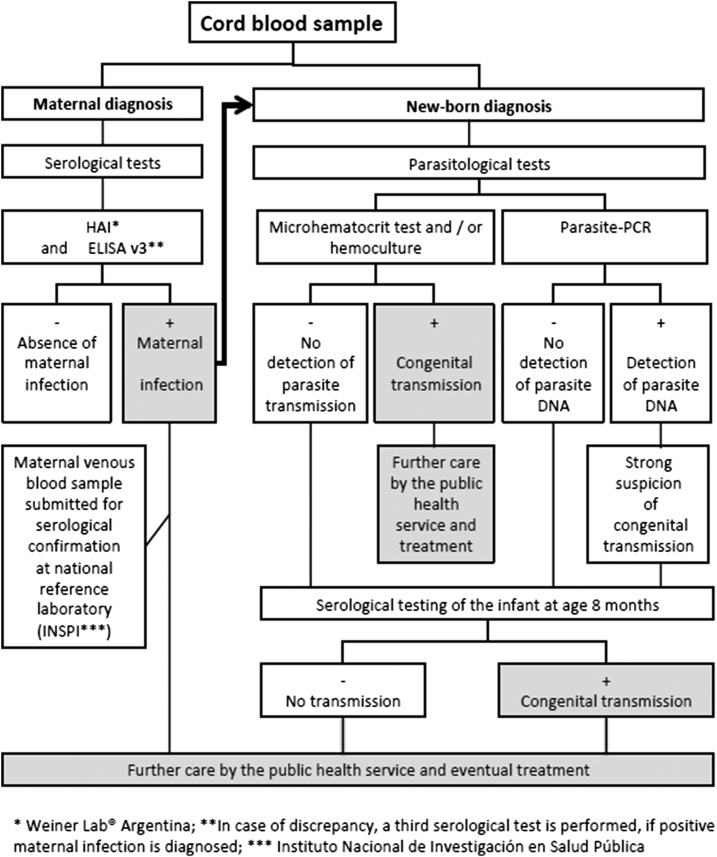
A cord blood sample of ∼20 mL was collected by the midwife or gynecologist at the time of delivery from study participants. The blood was drawn after the newborn was delivered and before the placenta was expelled (Figure 2), and it was immediately divided into three subsamples: 1) a subsample for serum separation and subsequent anti–T. cruzi serological analysis, 2) a heparinized subsample (15.96 IU/mL of heparine) for microscopic trypomastigote search by the microhematocrit method and hemoculture, and 3) an ethylenediaminetetraacetic acid (EDTA) subsample (1.8 mg/mL) for parasite DNA-Polymerase chain reaction (PCR). The flowchart in Figure 3 shows the laboratory processes involving the cord blood samples, which are described in the following section.
Figure 2.
Pictures taken during the study, illustrating the collection of umbilical cord blood samples. (A) Sampling 20 mL of cord blood with syringe. (B) Filling three vacutainer tubes with ∼ 6 mL each. This figure appears in color at www.ajtmh.org.
Figure 3.
Diagnostic strategy followed in the study. Flowchart for the detection of Trypanosoma cruzi infection among mothers and their newborns from cord blood samples taken at the delivery in Francisco de Orellana Hospital (El Coca).
Serological diagnosis.
Two conventional serological tests, hemagglutination and ELISA, were applied to each cord blood sample. Within 24 hours of being drawn, each sample was analyzed by indirect hemagglutination (Chagastest HAI, Weiner® laboratories, Rosario, Argentina), following the manufacturer’s instructions, and the results were provided to each study participant before release from the hospital. Samples were then stored at −20°C and, within the following week, tested by Chagas test ELISA Recombinante V3 (Weiner laboratory), following the manufacturer’s instructions. According to the WHO guidelines,21 an individual was considered seropositive when these two different tests yielded positive results. If positive results were obtained from both serological tests, both tests were also performed with serum derived from peripheral blood of the woman.
Parasitological testing.
When the indirect haemagglutination (IHA) test yielded positive results, a microscopic search for trypomastigotes was immediately conducted as previously described,22 with slight modifications. Briefly, six heparinized microhematocrit tubes were filled with the heparinized cord blood sample and centrifuged at 12,000 rpm for 7 minutes. The microhematocrit tubes were then cut at the buffy coat level, and an ∼10 µL buffy coat drop was deposited on each of the two microscope slides. Coverslips were applied, and the samples were examined at ×400 magnification for the presence of trypomastigotes. In addition, the remaining heparinized cord blood was centrifuged and the buffy coat was aseptically distributed into two culture tubes containing biphasic Novy-MacNeal-Nicolle medium, which were incubated at 25°C and microscopically examined at ×400 in a weekly basis for 4 months.
Polymerase chain reaction.
For seropositive cases and a subset of seronegative cases, PCR amplification of parasite DNA sequences was performed at CISeAL, Quito. DNA was extracted from blood sample collected in EDTA, and PCRs were performed as previously described23; the chosen oligonucleotide primers amplified the hypervariable region of the kDNA minicircles.24 Polymerase chain reaction products were analyzed by electrophoresis on 1% agarose gels and visualized using SYBR® Green fluorescent dye (Sigma-Aldrich, St. Louis, MO).
Data analyses.
The seroprevalence of T. cruzi infection among the pregnant women was estimated as the ratio of cases seropositive for both serological diagnostic tests over the total number of participants. The 95% CI was calculated under the assumption of a Poisson distribution of the data, using the online “Free Statistics Calculators, version 4.0” calculator website (Free Statistics Calculator, version 4.0. Daniel Soper, Fullerton, CA).25 Two dependent variables (“Recognizing the triatomine” and “Having seen triatomines indoors”) obtained from the knowledge questionnaire were tested for their association with 17 independent variables (Table 1). The Fisher’s exact test was used to evaluate the association of each independent variable with the two dependent variables under study (Fisher’s exact test is more accurate than the chi-square test of independence when the expected numbers are small). Variables with a P-value ≥ 0.25 in the univariate analysis were retained for multiple linear regression model analysis (gml).26 The descending method was applied, starting with all the selected variables in the model. Subsequently, the less significant variables (i.e., the highest P-value ≥ 0.05) were removed stepwise until all remaining variables were significant. To find out which of the explanatory variables in the model are significant, the Wald test and the likelihood-ratio test were applied. The analysis was performed using R software27,28 (R Foundation for Statistical Computing, Vienna, Austria) with the Epidisplay package.29
Acceptability of the current screening pipeline by the participants was expressed as the ratio between the number of women accepting the survey and those refusing; acceptability by the health-care personal was expressed as the ratio between the number of total deliveries and the number of blood samples taken.
RESULTS
Study participants.
Of 191 pregnant women giving birth during the study period, 146 agreed to participate and provided written informed consent. For 41 women, it was not possible to obtain the cord blood sample, four women refused to participate, and two did not adjust to the inclusion criteria.
Demographic, socioeconomic characteristics and habits of the population under study.
Table 1 summarizes the sociodemographic characteristics of the study population, the type of dwellings the women reported to inhabit, and the risk factors related for Chagas disease transmission. The age of the women ranged from 14 to 42 years and the average age was 24 ± 6.7 years. The patients were mostly multiparous, with an average of three children per woman. Most of the women (65.1%) were born in the Amazon, whereas 15.1% of those not natives to the Amazon were from other areas endemic for Chagas disease in Ecuador. All interviewed women understood and spoke Spanish, and some of them reported to additionally speak one of the local languages (14.5% Kichwa and 4.1% Shuar). Most of the women (80.1%) reported having studied until completion of the secondary education level (high school), and being housewives (67.8%). About half (48.5%) of them reported having a monthly household income less than US$250, an amount lower than the Ecuadorian minimum wage in 2016 (US$366). Most women reported living outside the city of El Coca, and a third (35.0%) reported living more than 60 km away from the hospital. Furthermore, 35.5% stated that their community is near the forest. Several practices considered as risk factors for Chagas transmission are shared by a majority of women: drinking natural homemade juice (∼80%) (unpasteurized juice, made from fresh fruit contaminated with triatomine feces, has been associated with oral Chagas transmission) and owning domestic and/or wild animals (which can be parasite reservoirs) and keeping them within the dwellings (78.1%).
Infection by T. cruzi and estimation of seroprevalence.
Of the 146 participants in the study, only one cord blood sample yielded positive results in both serological tests used (HIA and ELISA). In addition, both serological tests were also positive for serum-derived peripheral venous blood of the corresponding woman. Subsequently, in accordance with the Ecuadorian National protocol, the hospital submitted the venous blood sample of the woman to the reference laboratory at the National Institute of Public Health and Research, which confirmed the positive result 1 month later. The woman was 26 years old and had migrated to the Amazon region from the province of Manabí (a well-known endemic area for Chagas disease, Figure 1) over 10 years ago. She was asymptomatic, and no clinical manifestations of Chagas disease were evident on clinical examination. In addition, the clinical examination of the newborn was normal. All parasitological tests, including cord blood microhematocrit test and hemoculture, failed to yield evidence of T. cruzi presence. In addition, PCR for amplification of T. cruzi DNA, from both cord blood and maternal venous blood, was unsuccessful. Furthermore, all other children of the infected woman were summoned, and serological tests could be carried out for five of six of them (the oldest child does not any longer live with the mother). All of them were found to be seronegative by IHA and ELISA. The newborn was found to be seronegative after 8 months of age, ruling out congenital transmission. The estimated seroprevalence of T. cruzi infection in pregnant women was 0.68%, with a 95% CI between 0.02% and 3.80%.
Access to health care.
A short interview allowed assessing the conditions of access to health care by pregnant women (Table 2). Although most women (89.7%) reported not being affiliated to the Ecuadorian Social Security system, almost all of them (97.9%) had access to prenatal care. Most women (87.3%) report having easy access to Ecuador’s national health-care system and, among those reporting otherwise, the main reasons given were the distance from their homes to the health-care facilities and the waiting time.
Table 2.
Access to health care among pregnant women in the study population at the Francisco de Orellana Hospital (El Coca)
| Variables | N | % |
|---|---|---|
| Type of health-care coverage | ||
| Public | 15/146 | 10.3 |
| None | 131/146 | 89.8 |
| Prenatal visit | ||
| Yes | 143/146 | 97.9 |
| No | 3/146 | 2.1 |
| First health-care option used by women in the event of a health problem* | ||
| Primary health-care center | 125/152 | 82.2 |
| Other (doctor, drugstore, and medicine-man) | 27/152 | 17.8 |
| Reporting difficulties in access to health care | ||
| No | 128/146 | 87.7 |
| Yes | 18/146 | 12.3 |
| Reason for reported difficulties* | ||
| Distance | 9/19 | 47.4 |
| Waiting time | 9/19 | 47.4 |
| Inadequate service perceived | 1/19 | 5.3 |
* Optional multiple response.
Knowledge of vectors and Chagas disease.
Table 3 summarizes the results concerning the knowledge by study participants about Chagas disease and its vectors. A box containing triatomine specimens in their different developmental stages was presented to the study participants and 65.1% of them recognized the insect; however, only 37% of them named it correctly (chinche or chinchorro). Around 30% of the participants reported indoor presence of triatomines, whereas other 30% reported seeing them outdoors. Only two participants reported having observed triatomines both indoors and outdoors. Importantly, our results highlight the lack of knowledge about Chagas disease: only 16.1% of the women report having heard about Chagas. Health centers were reported to be their main source of information about this topic, followed by the media (internet and television). However, very few women knew whether the triatomine bite or contact with its feces is implicated in transmission of the disease.
Factors associated with vector recognition.
In the univariate analysis, four factors were identified to be significantly associated with the recognition of the vector (Table 1): “a domicile located in rural community in close proximity to the forest,” “at least one member of the household works in the field,” “at least one member of the household hunts,” and “reporting seeing wild animals in or near the dwelling”; in the final model obtained via step-down multivariate analysis, two of these factors were significant, “at least one member of the household hunts” and “reporting seeing wild animals in or near the dwelling” (Table 4). Furthermore, through the univariate analysis, only one factor was detected to be significantly associated with the report of having observed triatomines indoors: living outside the city of El Coca; this factor was also significant in the multivariate analysis along with two others, “age” (range, 18–35 years) and “monthly income” (≥ 250 $US).
Table 4.
Multivariate analysis models of demographic and socioeconomic traits, practices, and environmental traits among pregnant women in the study population at the Francisco de Orellana Hospital (El Coca) for two dependent variables: “Recognizing the vector” and “having seen triatomines indoors”
| Recognizing the vector | ||||
|---|---|---|---|---|
| Variables | Adjusted OR | P-value for Wald’s test | P-value for LR test | |
| Geographical region in which the woman was born | 0.07 | |||
| Coastal region | 1 | – | – | |
| Interandean region | 1.04 | [0.27; 4] | 0.954 | |
| Amazonian region | 2.05 | [0.82; 5.14] | 0.125 | |
| Foreign | 0.23 | [0.03; 2.01] | 0.185 | |
| At least one member of the household hunts | 0.031 | |||
| No | 1 | – | – | |
| Yes | 3.3 | [1.01; 10.85] | 0.049 | |
| Reports seing wild animals in or near the dwelling | 0.026 | |||
| No | 1 | – | – | |
| Yes | 3.01 | [1.07; 8.41] | 0.036 | |
| Having seen triatomines indoors | ||||
| Age (years) | 0.028 | |||
| ≤ 18 | 1 | – | – | |
| 18–35 | 6.93 | [1.33; 36.08] | 0.021 | |
| > 35 | 3.97 | [0.46; 34.32] | 0.21 | |
| Monthly income (US$) | 0.012 | |||
| < 250 | 1 | – | – | |
| ≥ 250 | 3.32 | [1.25; 8.84] | 0.016 | |
| Distance of current domicile to El Coca city (km) | < 0.001 | |||
| 0 (living in El Coca city) | 1 | – | – | |
| < 60 | 12.26 | [3.44; 43.72] | < 0.001 | |
| ≥ 60 | 8.51 | [2.54; 28.53] | < 0.001 | |
OR = odds ratio; LR = likelihood ratio.
DISCUSSION
Sociodemographic characteristics of the study population.
The study population is young (24 ± 6.7 years) and multiparous (three children per woman in average). Our data are consistent with previous estimates for age30 and fertility rate16 of women in Orellana Province. In 2014, 29% of the newborns in Orellana Province were delivered by women living in rural areas (Instituto Nacional de Estadísticas y Censos [INEC] data obtained at www.ecuadorencifras.gov.ec, Anuario de Estadisticas vitales–nacimientos y defunciones 2014). In this study 41, 4% of women reported living in rural areas, suggesting that the Francisco de Orellana Hospital provides good coverage of the rural population. In addition, 48.5% of study participants reported a monthly income less than US$250. In 2010, the INEC considered the average income in Orellana Province to be lower than the national average (www.ecuadorencifras.gov.ec, Informe de resultados Encuesta de condiciones de vida 2013–2014), with 85.0% of its population considered poor. Although the questionnaire used in the study does not allow for poverty-level estimation, at least half of the study population can be considered poor because their monthly income did not reach the 2016 minimum wage. Most women in the study reported secondary or higher education levels (86.3%); nevertheless, 67.8% of them report being a housewife or having no paid activity. Most of the women (89.8%) reported not being affiliated to the social security system. This rate is higher than the percentage recorded for the province in 2010 (69.5%). The overrepresentation of women without social security may occur because the attention in public hospital is free of cost. On the other hand, an excellent score was obtained for prenatal care because the great majority of women (97.9%) reported having attended at least one prenatal care consultation.
Estimation of the seroprevalence rate.
The estimated seroprevalence rate for T. cruzi infection in this study (0.68%, [0.02; 3.80]) lacks precision because of the small sample size. However, this figure is compatible with previous studies.16 With a seroprevalence of 3.8%, a sample of > 500 women would be required to reduce the CI between 1.5% and 4.5%. The T. cruzi infection case detected in the study is a woman born in Manabí Province (Figure 1), who migrated a few years later to the Amazon. It is not possible to determine whether she was infected in the Amazon because Manabí Province is also endemic for Chagas disease. However, this case highlights the presence of chagasic women living in Orellana Province, who are not presently diagnosed and do not receive proper medical attention for their condition. Therefore, the risk of congenital transmission persists.
Public health and congenital Chagas disease.
In the absence of vectorial transmission, congenital T. cruzi transmission could still maintain Chagas disease indefinitely31 and congenital Chagas control relies on active case identification. Recent studies show the cost-effectiveness of maternal screening in the United States5,32,33 and in the Latin American population living in Europe.33 However, control measures must be tailored to the local conditions; therefore, we explored congenital Chagas disease in the Ecuadorian Amazon. Francisco de Orellana Hospital was selected for the study because it is the only hospital in Orellana Province and, thus, hosts more deliveries than other health-care facilities in the region. In addition, the serological tests used in the study could be performed on its laboratory facilities. Prenatal Chagas screening is highly recommended; however, women who do not attend prenatal care visits cannot benefit from it. The application of a noninvasive screening method, that is, testing umbilical cord blood, is useful for both maternal and neonatal diagnosis,34 was very well accepted among women in the Orellana hospital (97.9% acceptance rate), and was also well received by the hospital personnel (78.5%.) We recommend the IHA test as the first-line test because it is easy, rapid, and does not require complex infrastructure or equipment. Because almost all women (97.9%) reported attending to at least one prenatal visit, the primary health centers where prenatal care takes place could use IHA tests and the hospital could perform ELISAs on a weekly basis. The combination of these two independent tests would allow for early detection of infected women and their specific follow-up. The two tests for serological diagnosis should be available in the hospital for women who arrive for delivery without any previous prenatal screening. Cord blood screening would allow for early detection of T. cruzi infection in newborns from seropositive women and the minority (2.1%) of women who do not comply with prenatal care visits. When parasites are not detected in the cord blood, newborns from seropositive women should be serologically tested at 8 months of age.
Anthropological perspective of Chagas disease.
A small proportion of enrolled women have heard of Chagas disease (16.1%) and report having been informed mostly in primary health-care centers, highlighting the role of the Ministry of Health in community education, which should be supported and strengthened. Our analyses show that vector recognition is associated with a rural population of women living in families involved in agriculture and hunting in the forest. In Amazonia, many triatomine species are part of the zoonotic cycle of T. cruzi. Humans disrupt this cycle with settlements, subsoil exploitation, or forest clearing for agriculture, displacing mammals and triatomines, the latter being frequently attracted into houses by light. In this context, human populations inhabiting in areas near residual forest are more likely to come into contact with the vectors. In our study, the identification of these risk profiles shows that the implementation of screening and prevention campaigns in the Amazonian rural areas of Ecuador should be a priority.
CONCLUSION
Our data confirm the occurrence of Chagas disease among pregnant women in the Orellana Province, indicating a latent risk of congenital transmission in the Ecuadorian Amazon region. Many of the women in the study population recognize triatomines; however, they do not necessarily associate them with disease transmission, highlighting the importance of implementing educational programs about Chagas in this region. The women recognizing the vector tend to live in rural areas near the forest, as would be expected in the Amazonian scenario.
We have shown the feasibility of implementing an intervention strategy aimed to detect congenital Chagas disease cases at a major hospital in the Ecuadorian Amazon at the time of delivery, using the presently available infrastructure and resources. Serological screening of the mothers should be implemented during prenatal care visits, which most pregnant women attend. In this context, parasitological tests performed in umbilical cord blood would allow for immediate detection of congenital transmission cases among newborns from seropositive women. This is of paramount importance because early treatment of newborns is highly effective. If parasitological tests are negative, serological test for the newborn at age > 6 months is warranted. Moreover, conditions are met for serological and parasitological diagnosis when women reach the hospital for delivery without previous screening.
Acknowledgments:
We would like to acknowledge the support received from Dirección de Estrategia y Control from Ministerio de Salud Pública del Ecuador; Nelson Dueñas Basurto, director of Hospital General Francisco de Orellana; the medical personnel at Hospital General Francisco de Orellana who assisted with the cord blood collection; and César Yumiseva, for his assistance generating the map shown in Figure 1. In addition, we would like to thank Mario Grijalva, CISeAL´s director, for his support to the project.
REFERENCES
- 1.World Health Organization , 2018. Chagas Disease (American Trypanosomiasis). Fact Sheet. Available at: http://www.who.int/news-room/fact-sheets/detail/chagas-disease-(american-trypanosomiasis). Accessed April 1, 2019. [Google Scholar]
- 2.Carlier Y, Sosa-Estani S, Luquetti AO, Buekens P, 2015. Congenital Chagas disease: an update. Mem Inst Oswaldo Cruz 110: 363–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization , 2006. Estimación Euantitativa de la Enfermedad de Chagas en las Américas. Available at: http://ops-uruguay.bvsalud.org/pdf/chagas19.pdf. Accessed April 1, 2019. [Google Scholar]
- 4.Rodari P, Angheben A, Gennati G, Trezzi L, Bargiggia G, Maino M, Ruggeri M, Rampello S, Soavi L, Rizzi M, 2018. Congenital Chagas disease in a non-endemic area: results from a control programme in Bergamo province, northern Italy. Travel Med Infect Dis 25: 31–34. [DOI] [PubMed] [Google Scholar]
- 5.Stillwaggon E, Perez-Zetune V, Bialek SR, Montgomery SP, 2018. Congenital Chagas disease in the United States: cost savings through maternal screening. Am J Trop Med Hyg 98: 1733–1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gulin JEN, Bisio M, Rocco DM, Altcheh J, Solana ME, Garcia-Bournissen F, 2018. Molecular and biological characterization of a highly pathogenic Trypanosoma cruzi strain isolated from a patient with congenital infection. Exp Parasitol 186: 50–58. [DOI] [PubMed] [Google Scholar]
- 7.Torrico F, Alonso-Vega C, Suarez E, Rodriguez P, Torrico MC, Dramaix M, Truyens C, Carlier Y, 2004. Maternal Trypanosoma cruzi infection, pregnancy outcome, morbidity, and mortality of congenitally infected and non-infected newborns in Bolivia. Am J Trop Med Hyg 70: 201–209. [PubMed] [Google Scholar]
- 8.Salas NA, Cot M, Schneider D, Mendoza B, Santalla JA, Postigo J, Chippaux JP, Brutus L, 2007. Risk factors and consequences of congenital Chagas disease in Yacuiba, south Bolivia. Trop Med Int Health 12: 1498–1505. [DOI] [PubMed] [Google Scholar]
- 9.Altcheh J, Moscatelli G, Moroni S, Garcia-Bournissen F, Freilij H, 2011. Adverse events after the use of benznidazole in infants and children with Chagas disease. Pediatrics 127: e212–e218. [DOI] [PubMed] [Google Scholar]
- 10.Carlier Y, Torrico F, Sosa-Estani S, Russomando G, Luquetti A, Freilij H, Albajar Vinas P, 2011. Congenital Chagas disease: recommendations for diagnosis, treatment and control of newborns, siblings and pregnant women. PLoS Negl Trop Dis 5: e1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Altcheh J, Moscatelli G, Mastrantonio G, Moroni S, Giglio N, Marson ME, Ballering G, Bisio M, Koren G, Garcia-Bournissen F, 2014. Population pharmacokinetic study of benznidazole in pediatric Chagas disease suggests efficacy despite lower plasma concentrations than in adults. PLoS Negl Trop Dis 8: e2907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mora MC, Sanchez Negrette O, Marco D, Barrio A, Ciaccio M, Segura MA, Basombrio MA, 2005. Early diagnosis of congenital Trypanosoma cruzi infection using PCR, hemoculture, and capillary concentration, as compared with delayed serology. J Parasitol 91: 1468–1473. [DOI] [PubMed] [Google Scholar]
- 13.Grijalva MJ, Escalante L, Paredes RA, Costales JA, Padilla A, Rowland EC, Aguilar HM, Racines J, 2003. Seroprevalence and risk factors for Trypanosoma cruzi infection in the Amazon region of Ecuador. Am J Trop Med Hyg 69: 380–385. [PubMed] [Google Scholar]
- 14.Chico M, Sandoval C, Guevara A, Calvopina M, Cooper PJ, Reed SG, Guderian RH, 1997. Chagas disease in Ecuador: evidence for disease transmission in an Indigenous population in the Amazon region. Mem Inst Oswaldo Cruz 92: 317–320. [DOI] [PubMed] [Google Scholar]
- 15.Amunarriz M, Chico ME, Guderian RH, 1991. Chagas disease in Ecuador: a sylvatic focus in the Amazon region. J Trop Med Hyg 94: 145–149. [PubMed] [Google Scholar]
- 16.Carrera Vargas C, Narvaez AO, Muzzio Aroca J, Shiguango G, Robles LM, Herrera C, Dumonteil E, 2015. Seroprevalence of Trypanosoma cruzi infection in schoolchildren and in pregnant women from an Amazonian region in Orellana Province, Ecuador. Am J Trop Med Hyg 93: 774–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guevara AG, Atherton RD, Wauters MA, Vicuna Y, Nelson M, Prado J, Kato H, Calvopina MH, Hashiguchi Y, 2013. Seroepidemiological study of Chagas disease in the southern Amazon region of Ecuador. Trop Med Health 41: 21–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dumonteil E, Herrera C, Martini L, Grijalva MJ, Guevara AG, Costales JA, Aguilar HM, Brenière SF, Waleckx E, 2016. Chagas disease has not been controlled in Ecuador. PLoS One 11: e0158145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rosecrans K, Cruz-Martin G, King A, Dumonteil E, 2014. Opportunities for improved Chagas disease vector control based on knowledge, attitudes and practices of communities in the Yucatan Peninsula, Mexico. PLoS Negl Trop Dis 8: e2763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sanmartino M, Crocco L, 2000. Conocimientos sobre la enfermedad de Chagas y factores de riesgo en comunidades epidemiológicamente diferentes de Argentina. Rev Panam Salud Publica 7: 173–178. [DOI] [PubMed] [Google Scholar]
- 21.World Health Organization , 2002. Control of Chagas Disease: Second Report of the WHO Expert Committee. WHO Technical Report Series. Geneva, Switzerland: WHO, 1–109. [PubMed] [Google Scholar]
- 22.La Fuente C, Saucedo E, Urjel R, 1984. The use of microhaematocrit tubes for the rapid diagnosis of Chagas disease and malaria. Trans R Soc Trop Med Hyg 78: 278–279. [DOI] [PubMed] [Google Scholar]
- 23.Wincker P, Bosseno MF, Britto C, Yaksic N, Cardoso MA, Morel CM, Brenière SF, 1994. High correlation between Chagas’ disease serology and PCR-based detection of Trypanosoma cruzi kinetoplast DNA in Bolivian children living in an endemic area. FEMS Microbiol Lett 124: 419–423. [DOI] [PubMed] [Google Scholar]
- 24.Britto C, Cardoso MA, Ravel C, Santoro A, Pereira JB, Coura JR, Morel CM, Wincker P, 1995. Trypanosoma cruzi: parasite detection and strain discrimination in chronic Chagasic patients from northeastern Brazil using PCR amplification of kinetoplast DNA and nonradioactive hybridization. Exp Parasitol 81: 462–471. [DOI] [PubMed] [Google Scholar]
- 25.Soper DS, 2018. Poisson Confidence Interval Calculator [Software]. Available at: http://www.danielsoper.com/statcalc. Accessed October 9, 2016. [Google Scholar]
- 26.Gillaizeau F, Grabar S, 2011. Modèles de régression multiple. Sang Thromb Vaiss 23: 360–370. [Google Scholar]
- 27.Mantel N, 1967. The detection of disease clustering and a generalized regression approach. Cancer Res 27: 209–220. [PubMed] [Google Scholar]
- 28.R Development Core Team , 2008. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; Available at: http://www.R-project.org. Accessed January 24, 2019. [Google Scholar]
- 29.Chongsuvivatwong V, 2018. EpiDisplay V 3.5.0.1: Epidemiological Data Display Package. Available at: https://rdrr.io/cran/epiDisplay/. Accessed January 24, 2019. [Google Scholar]
- 30.Costales JA, Sanchez-Gomez A, Silva-Aycaguer LC, Cevallos W, Tamayo S, Yumiseva CA, Jacobson JO, Martini L, Carrera CA, Grijalva MJ, 2015. A national survey to determine prevalence of Trypanosoma cruzi infection among pregnant women in Ecuador. Am J Trop Med Hyg 92: 807–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Monge-Maillo B, Lopez-Velez R, 2017. Challenges in the management of Chagas disease in Latin-American migrants in Europe. Clin Microbiol Infect 23: 290–295. [DOI] [PubMed] [Google Scholar]
- 32.Sicuri E, Munoz J, Pinazo MJ, Posada E, Sanchez J, Alonso PL, Gascon J, 2011. Economic evaluation of Chagas disease screening of pregnant Latin American women and of their infants in a non endemic area. Acta Trop 118: 110–117. [DOI] [PubMed] [Google Scholar]
- 33.Requena-Mendez A, Bussion S, Aldasoro E, Jackson Y, Angheben A, Moore D, Pinazo MJ, Gascon J, Munoz J, Sicuri E, 2017. Cost-effectiveness of Chagas disease screening in Latin American migrants at primary health-care centres in Europe: a Markov model analysis. Lancet Glob Health 5: e439–e447. [DOI] [PubMed] [Google Scholar]
- 34.Sosa-Estani S, et al. 2008. Use of a rapid test on umbilical cord blood to screen for Trypanosoma cruzi infection in pregnant women in Argentina, Bolivia, Honduras, and Mexico. Am J Trop Med Hyg 79: 755–759. [PubMed] [Google Scholar]