Abstract.
Mannose-binding lectin (MBL) and MBL-associated serine protease-2 (MASP-2) are important proteins in the lectin pathway of the immune system. Mannose-binding lectin and MASP-2 deficiencies have been reported to be responsible for various fungal infections. We investigated the association of MBL and MASP-2 variants with sporotrichosis in a Chinese population and revealed one rare heterozygous mutation in a disseminated cutaneous patient without immunosuppressive conditions (MASP2, p.156_159dupCHNH). We also found that sporotrichosis patients had decreased levels of MBL and MASP-2 in their serum samples compared with controls. Our findings linked, for the first time, MASP-2 deficiencies with susceptibility to Sporothrix sp.
Sporotrichosis is a chronic granulomatous mycotic infection caused by species of the Sporothrix schenckii complex.1 Such infections may progress into chronic cutaneous, subcutaneous, and/or even deeper infections involving the lymphatics, fascia, muscles, cartilage, and bones. The prognosis of the disease is related to the immune status of the host, and it is believed that genetic variation plays an important role in host susceptibility.2
The complement system is one of the major effectors of the innate immune system and an important bridge between innate and adaptive immunity. The MBL-associated serine protease-2 (MASP-2) is a key enzyme involved in the initiation of innate immune responses by binding to mannose-binding lectin (MBL) and activating the complement lectin pathway. The complex formed by MBL and MASP-2 cleaves C4 and C2 to form the C3 convertase (C4b2a). Subsequent complement activation leads to opsonization and phagocytosis of the target microbes, as well as formation of membrane attack complexes.3,4 Previous studies suggest that variants of MBL and MASP-2 are associated with fungal infectious diseases such as invasive aspergillosis and recurrent vulvovaginal candidiasis.5–7 It remains unknown whether MBL and MASP-2 deficiencies are associated with sporotrichosis. This study explores the association between MBL and MASP-2 variants and their corresponding serum levels in sporotrichosis patients.
The study was approved by the human medical and ethics committee of Shandong Provincial Institute of Dermatology and Venereology. We performed the mutation analysis of MBL and MASP-2 gene in 61 cases with cutaneous sporotrichosis and 300 controls by Sanger sequencing (Table 1). After informed consent, genomic DNA was extracted from the peripheral blood of all participants. All the exons of MBL and MASP-2 gene were amplified by polymerase chain reaction. After amplification, the products were purified and directly sequenced on ABI 3130xl Genetic Analyser (Applied Biosystems ABI, Carlsbad, CA). Mannose-binding lectin and MASP-2 concentrations were measured in the sera of 35 patients and 47 controls using commercial ELISA kits (Hycult Biotech, Uden, The Netherlands/HK326; Hycult Biotechnology). Minimum and maximum concentration which can be measured is 0.16 ng/mL and 3.13 ng/mL, respectively. Color intensity was evaluated at 450 nm in an ELISA reader.
Table 1.
Clinical information
| Characteristics | Patients (n = 61) | Controls (n = 300) |
|---|---|---|
| Female | 22 (36%) | 133 (44%) |
| Male | 39 (64%) | 167 (56%) |
| Mean age at diagnosis | 62.81 | 54.56 |
| Clinical presentations | ||
| Fixed cutaneous | 35 (57%) | – |
| Lymphocutaneous | 21 (35%) | – |
| Disseminated cutaneous | 5 (8%) | – |
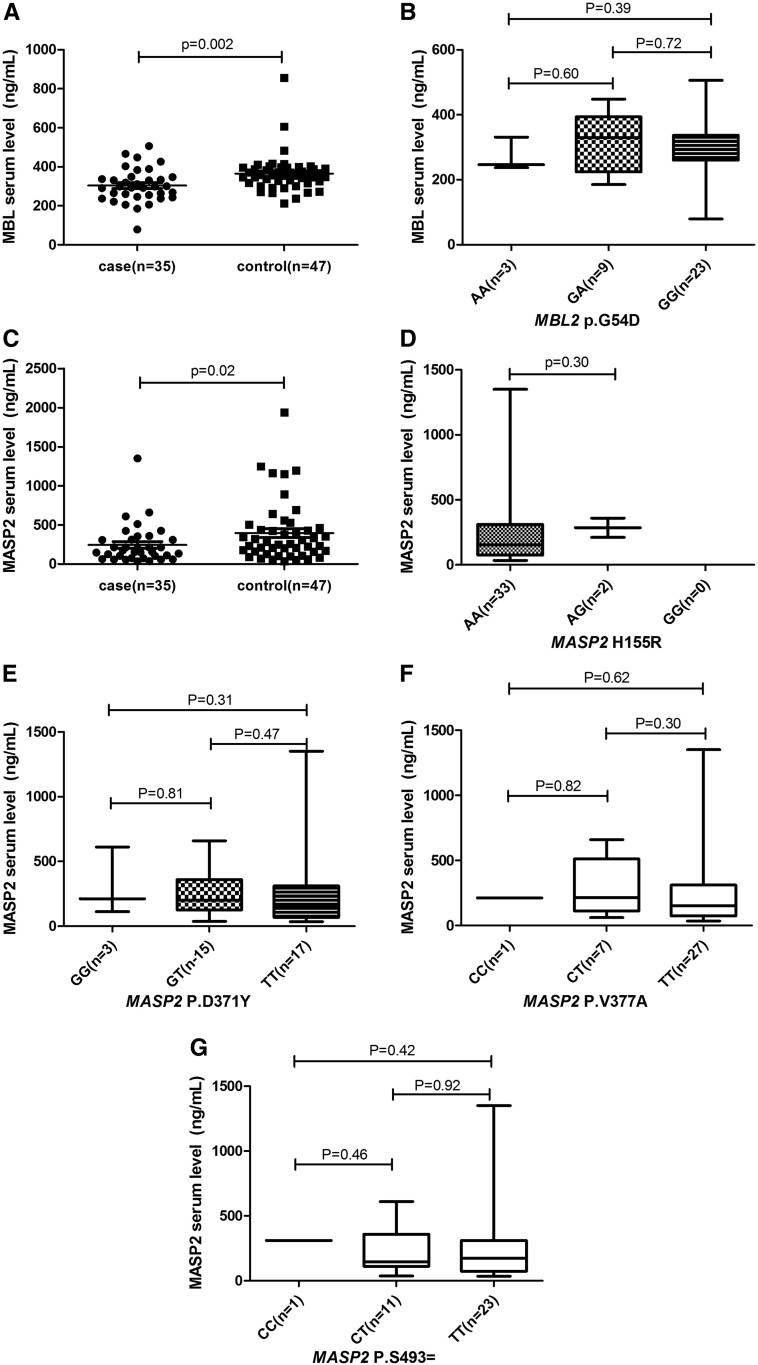
We identified one variant in exon 1 of the MBL2 gene in patient group: p.G54D (c.161G>A). The control data are derived from Han Chinese (Beijing, China) in 1000 Genomes Project Phase 3 allele frequencies. No statistical significance of the allele frequencies and genotype frequencies was observed between the patient and control groups. The mean serum level of MBL in patients and controls were 304 ng/mL and 365 ng/mL, respectively (P < 0.05 t- test) (Figure 1A). There were no significant differences between the patients with and without p.G54D variant (Figure 1B).
Figure 1.
Mannose-binding lectin (MBL) and MBL-associated serine protease-2 (MASP-2) levels. (A) MBL levels in patients and controls. (B) Mannose-binding lectin levels according to the genotypes of MBL-2 (p.G54D) in patients. (C) MBL-associated serine protease-2 levels in patients and controls. (D–G) MBL-associated serine protease-2 levels according to the genotypes of MASP-2 (p.H155R, p.D371Y, p.V377A, and p.S493) in patients.
Mannose-binding lectin-associated serine protease-2 sequencing revealed one rare heterozygous mutation in a disseminated cutaneous patient without immunosuppressive conditions: p.156_159dupCHNH (c.477_478insTGCCACAACCAC). This mutation was not detected in the other matched normal controls (0/300, P = 0.169, Fisher’s exact test) and in the East Asian population (0/4290, Exome Aggregation Consortium database, P = 0.014, Fisher’s exact test). The duplication of four amino acids within the epidermal growth factor domain (p.156_159dupCHNH) was predicted to cause damage to the MASP-2 protein and probably results in MASP-2 misfolding.8,9 In addition, four variants of MASP-2 (p.H155R [c.464 A>G], p.D371Y [c.1111 G>T], p.V377A [c.1130 T>C], and p.S493 [c.1479 C>T]) were identified in patient group, and there were no significant differences in the allele frequencies and genotype frequencies between the patients and controls (P > 0.05 Fisher’s Test) (Table 2). We found that sporotrichosis patients had decreased levels of MASP-2 in their serum samples compared with controls (median 244 versus 396 ng/mL, P < 0.05 t- test) (Figure 1C). There were no significant differences between patients with and without the aforementioned variant (Figure 1D–G). Our studies showed that the heterozygous mutation p.156_159dupCHNH did not affect the protein expression (212 ng/mL), but whether the mutation affected the protein function needs further experimental exploration.
Table 2.
MBL and MASP-2 gene polymorphism
| Gene region | SNP ID | Allele | mRNA | Genotype | Case | Control | Allele frequency (in case) | Allele frequency (in control) | P-value (Fisher’s test) |
|---|---|---|---|---|---|---|---|---|---|
| MASP-2 exon 4 | rs2273343 | p.H155R | c.464 A>G | AA | 59 (96.7%) | 282 (94.0%) | A: 98.4% | A: 97.0% | 0.216 |
| AG | 2 (3.3%) | 18 (6.0%) | G: 1.6% | G: 3.0% | |||||
| GG | 0 | 0 | – | – | |||||
| GG+AG | 2 (3.3%) | 18 (6.0%) | – | – | |||||
| MASP-2 exon 10 | rs12711521 | p.D371Y | c.1111 G>T | GG | 5 (8.0%) | 49 (16.3%) | G: 31.1% | G: 36.8% | 0.311 |
| GT | 28 (46.0%) | 123 (41.0%) | T: 68.9% | T: 63.2% | |||||
| TT | 28 (46.0%) | 128 (42.7%) | – | – | |||||
| TT+GT | 56 (92%) | 251 (83.7%) | – | – | |||||
| MASP-2 exon 10 | rs2273346 | p.V377A | c.1130 T>C | TT | 46 (75.4%) | 204 (68.0%) | T: 86.1% | T: 80.8% | 0.398 |
| CT | 13 (21.3%) | 77 (25.7%) | C: 13.9% | C: 19.2% | |||||
| CC | 2 (3.3%) | 19 (6.3%) | – | – | |||||
| CC+TC | 15 (24.6%) | 96 (32.0%) | – | – | |||||
| MASP-2 exon 12 | rs1782455 | p.S493= | c.1479 C>T | CC | 2 (3.3%) | 3 (1.0%) | C: 18.0% | C: 14.3% | 0.701 |
| CT | 18 (29.5%) | 80 (26.7%) | T: 82.0% | T: 85.7% | |||||
| TT | 41 (67.2%) | 217 (72.3%) | – | – | |||||
| TT+CT | 59 (96.7%) | 297 (99.0%) | |||||||
| MBL-2 exon 1 | rs1800450 | p.G54D | c.161G>A | GG | 42 (68.9%) | 217 (72.3%) | G: 81.1% | G: 84.8% | 0.312 |
| AG | 15 (24.6%) | 75 (25.0%) | A: 18.9% | A: 15.2% | |||||
| AA | 4 (6.5%) | 8 (2.7%) | – | – | |||||
| AA+AG | 19 (31.1%) | 83 (27.7%) | – | – |
MASP-2 = MBL-associated serine protease-2; MBL-2 = mannose-binding lectin-2; SNP = single nucleotide polymorphism.
To summarize, MASP-2 sequencing in sporotrichosis patients revealed one rare mutation. Various inborn errors of MASP-2 gene have been reported to be responsible for various infections. Our findings linked, for the first time, MASP-2 deficiencies with susceptibility to Sporothrix sp. However, more ethnic lines and many studies are needed to affirm the consideration in future.
Acknowledgment:
We thank the Natural Science Foundation of Shandong Province (ZR2019PH069).
REFERENCES
- 1.López-Romero E, Reyes-Montes Mdel R, Pérez-Torres A, Ruiz-Baca E, Villagómez-Castro JC, Mora-Montes HM, Flores-Carreón A, Toriello C, 2011. Sporothrix schenckii complex and sporotrichosis, an emerging health problem. Future Microbiol 6: 85–102. [DOI] [PubMed] [Google Scholar]
- 2.Carvalho A, Cunha C, Pasqualotto AC, Pitzurra L, Denning DW, Romani L, 2010. Genetic variability of innate immunity impacts human susceptibility to fungal diseases. Int J Infect Dis 14: 460–468. [DOI] [PubMed] [Google Scholar]
- 3.Holmberg V, Onkamo P, Lahtela E, Lahermo P, Bedu-Addo G, 2012. Mutations of complement lectin pathway genes MBL2 and MASP2 associated with placental malaria. Malar J 11: 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wallis R, Dodds AW, Mitchell DA, Sim RB, Reid KB, Schwaeble WJ, 2007. Molecular interactions between MASP-2, C4, and C2 and their activation fragments leading to complement activation via the lectin pathway. J Biol Chem 282: 7844–7851. [DOI] [PubMed] [Google Scholar]
- 5.Fernández-Ruiz M, Giménez E, Lora D, Aguado JM, Pascual M, Manuel O, 2019. Impact of MBL2 gene polymorphisms on the risk of infection in solid organ transplant recipients: a systematic review and meta-analysis. Am J Transplant 19: 1072–1085. [DOI] [PubMed] [Google Scholar]
- 6.Kalia N, Singh J, Sharma S, Kaur M, 2019. SNPs in 3′-UTR region of MBL2 increases susceptibility to recurrent vulvovaginal infections by altering sMBL levels. Immunobiology 224: 42–49. [DOI] [PubMed] [Google Scholar]
- 7.Beltrame MH, Boldt AB, Catarino SJ, Mendes HC, Boschmann SE, Goeldner I, Messias-Reason I, 2015. MBL-associated serine proteases (MASPs) and infectious diseases. Mol Immunol 67: 85–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thiel S, Kolev M, Degn S, Steffensen R, Hansen AG, Ruseva M, Jensenius JC, 2009. Polymorphisms in mannan-binding lectin (MBL)-associated serine protease 2 affect stability, binding to MBL, and enzymatic activity. J Immunol 182: 2939–2947. [DOI] [PubMed] [Google Scholar]
- 9.Thiel S, Steffensen R, Christensen I, Ip WK, Lau Y, Reason I, Eiberg H, Gadjeva M, Ruseva M, Jensenius JC, 2007. Deficiency of mannan-binding lectin associated serine protease-2 due to missense polymorphisms. Genes Immun 8: 154–163. [DOI] [PubMed] [Google Scholar]