Abstract.
Influenza A and B outbreaks occur each year with different activity and molecular patterns. To date, knowledge of seasonal epidemiology remains a prerequisite not only to put in place the most effective immunization strategy against influenza but also to identify population groups at higher risk of developing serious complications. A retrospective analysis of influenza surveillance data from 2010 to 2018 aimed to explore the epidemiology of influenza in Sicily, at the primary care and hospital level. Overall, 6,740 patients with acute respiratory infection were tested, of which 3,032 (45.0%) were positive for influenza. The relative proportion of type A and B viruses markedly varied across seasons. Type A similarly spreads among children and adults, whereas type B was more commonly identified among pediatric population aged 5–9 years. The median age of confirmed influenza cases differed by health-care setting, increasing according to disease severity (range: 8–54 years). Among influenza-confirmed cases, more than 80% of hospitalized patients had an underlying medical condition. Cardiovascular disease, lung disease, diabetes, and obesity were some of the most frequent. Overall, patients admitted to an intensive care unit were more likely to have multiple comorbidities and being infected with influenza infection strongly increased the risk of severe clinical outcomes. Understanding of the epidemiology of influenza and the molecular features of circulating viruses is of paramount importance to optimize prevention and control strategies. Knowledge of predictors for the occurrence of severe forms of the disease may help to address adequate preventive measures to high-risk population groups.
INTRODUCTION
Influenza A and B outbreaks occur annually in both Northern and Southern Hemispheres, displaying seasonal patterns that vary in distribution and severity.
Despite preventive measures adopted, influenza epidemics are responsible for considerable morbidity and mortality, and impose a significant economic burden through health-care direct costs and lost productivity.1 Illnesses range from mild to severe infections and even death, globally resulting in about three to five million severe infections and up to 500,000 deaths each year.2,3
During epidemics, influenza infection occurs in subjects of all ages and a higher incidence is regularly observed in pediatric population.4,5 Elderly population, defined as those aged 65 years and older, and, more specifically, elderly people with underlying conditions, are at increased risk for hospitalization and death, also in industrialized countries.6–8
The best strategy to prevent influenza is vaccination, the effectiveness of which strongly depends on the variability of circulating viruses, representing a major challenge for public health systems worldwide.9 Therefore, it is of paramount importance a continuous monitoring of genetic and antigenic features of influenza strains.
In this regard, a broad range of countries collect and share epidemiological and virological data of laboratory-confirmed influenza cases, through their public health services and National Influenza Centers under the coordination of the WHO in the Global Influenza Surveillance and Response System.10
During seasonal epidemics, on a weekly basis, both regional and national laboratories contribute to the knowledge of the burden of influenza disease, collecting the genetic information of spreading viruses to support diagnostics and the annual update of vaccine antigenic composition at a global level.
Since the 2009 HIN1 influenza pandemic, the reference laboratory (RRL) of Sicily (Italy) takes part in the national influenza surveillance network (InfluNet) and contributes to the surveillance of the fourth most populous administrative region of the country, which accounts for more than five million inhabitants.
This retrospective study aimed to explore the epidemiology of influenza disease and the heterogeneity of circulating strains in Sicily, at the primary care and hospital level, over eight influenza seasons after the 2009 pandemic.
MATERIALS AND METHODS
Case definition.
The standard case definition was in accordance with the operative protocol of the Italian Influenza Epidemiological and Virological Surveillance Network (InfluNet).11
A case of influenza-like illness (ILI)/acute respiratory infection (ARI) was defined as one individual with a sudden onset of at least one of the following systemic symptoms: fever (≥ 37.5°C), general discomfort or asthenia, headache, and muscle pain, and at least one of the respiratory symptoms between cough, sore throat, and shortness of breath.
In addition, a case of ILI/ARI requiring hospitalization was defined as severe acute respiratory infection.12
Study population and retrospective data collection.
In Sicily, a regional RRL for influenza surveillance has been identified with decree n°4812/09 of the Sicilian Health Department dated April 30, 2009. The RRL yearly participates in the national surveillance as part of the InfluNet network.13
In this article, all available clinical and virological data of eight consecutive influenza surveillance seasons between 2010 and 2018, usually collected from week 42 to week 17 of the following year, were analyzed. All relevant data collected during the influenza pandemic occurred in 2009 were previously reported14 and were not included in the present study.
All surveillance data are aggregated at a regional level, shared at a national level on a weekly basis, and ultimately flow into the WHO’s global influenza program.
A number of pediatricians and general practitioners belonging to the regional health system are invited to contribute to the community-based ILI/ARI surveillance, whereas hospital wards throughout Sicily allow the monitoring of patients admitted with severe respiratory complications, potentially correlated to an influenza infection.
Data on birthdate, gender, date of onset, outcomes, and underlying illnesses as major risk factors for developing influenza-related complications were gathered from each case. All personal data were anonymized and stored into a digital dataset.
Routine testing and influenza virus genotyping.
Viral nucleic acids were obtained from respiratory samples, which were collected and transported to the RRL by using Virocult swabs (MWE, Medical Wire & Equipment, Corsham, England) or bronchoalveolar lavages.
Viral RNA was extracted using QIAamp Viral RNA extraction kit (QIAGEN, Hilden, Germany) according to the manufacturer’s suggested protocol, and the RNA was eluted from the spin column in 60 μL of elution buffer. Eluted RNA was divided into aliquots and stored immediately at −80°C until further use. Each sample was tested by one-step real-time reverse transcription-polymerase chain reaction for the presence of influenza virus RNA and genotyping (protocols available on request) using a QuantStudio 7 Flex Real-Time PCR system (Applied Biosystems, Carlsbad, CA).
Data managements and statistical analysis.
The study population was arbitrarily subdivided into 10 different age-groups: < 6 months, 6–23 months, 2–4, 5–9, 10–14, 15–19, 20–34, 35–49, 50–64, and ≥ 65 years. According to the Italian Health System, children were subjects ≤ 14 years of age.
Descriptive statistics were used to summarize each of the sociodemographic and clinical variables included in the dataset. Medians and interquartile ranges (IQRs) were used to describe continuous variables, whereas frequency analyses for categorical variables were described with percentages.
Comparisons of continuous variables were conducted using the Student’s t-test or the Mann–Whitney U-test, according to data distribution, and a P-value < 0.05 was considered to indicate statistical significance (two tailed).
Univariate logistic regression was used to examine the relation between variables of interest and severity of disease. For this purpose, patients were grouped into ordered categories including community-acquired and treated influenza cases and, for hospitalized cases, into two further groups according to their admission in a medical intensive care unit (ICU) or not. Results were expressed as odds ratios (ORs) and/or adjusted odds ratio (aORs) with 95% CIs.
The analyses were also conducted within both the groups of influenza-negative and influenza-positive subjects. The first group allowed to assess the baseline risk for admission to ICU due to respiratory complications, whereas the excess risk of disease severity due to influenza was evaluated by relating the risk values obtained for individuals who tested negative for influenza and for those who had a laboratory-confirmed infection.
More specifically, an “aOR ratio” was calculated by dividing the aOR obtained for influenza-positive subjects by the corresponding aOR obtained for those testing negative. Data were processed with the STATA MP statistical software package v14.2 for Apple™ (StataCorp LLC, College Station, TX).
RESULTS
Epidemiology of influenza viruses between 2010 and 2018.
The surveillance of ILI/ARI showed year-by-year different patterns in terms of level and timing of influenza activity, although the number of documented cases progressively increased also according to a significant improvement of the surveillance system in Sicily (Figure 1).
Figure 1.
Distribution of influenza-like illness/acute respiratory infection and confirmed influenza cases documented in Sicily between 2010 and 2018, according to annual epidemics. This figure appears in color at www.ajtmh.org.
On average, the duration of annual epidemic peak was in the range of 8–12 weeks, usually between January and March, with the exception of the 2016–2017 and 2017–2018 influenza epidemics which grew in December, nearly 1 month faster than expected.
Over the whole study period, 6,740 subjects with ILI/ARI are monitored (Table 1), they had a median age of 12 years (IQR = 43), and, among those with available data, about half (54.2%; n = 3,647/6,726) were children younger than 14 years. In general, all age-groups were represented in our study population and a male:female ratio of 1.12 was found.
Table 1.
Number of ILI/ARI subjects, confirmed influenza cases, and relative proportions attributable to influenza A and B virus
| ILI/ARI subjects | Influenza cases | Influenza type A | Influenza type B | |
|---|---|---|---|---|
| Study population (n [%], % by row) | 6,740 | 3,032 (45.0) | 1,924 (63.5) | 1,108 (36.5) |
| Influenza season (n [%], % by row) | ||||
| 2010–2011 | 335 | 106 (31.6) | 79 (74.5) | 27 (25.5) |
| 2011–2012 | 246 | 90 (36.6) | 90 (100.0) | 0 |
| 2012–2013 | 329 | 159 (48.3) | 45 (28.3) | 114 (71.7) |
| 2013–2014 | 253 | 77 (30.4) | 77 (100.0) | 0 |
| 2014–2015 | 512 | 220 (43.0) | 167 (75.9) | 53 (24.1) |
| 2015–2016 | 1,408 | 646 (45.9) | 387 (59.9) | 259 (40.1) |
| 2016–2017 | 1,593 | 608 (38.2) | 595 (97.9) | 13 (2.1) |
| 2017–2018 | 2,064 | 1,126 (54.6) | 484 (43.0) | 642 (57.0) |
| Age (years; median [IQR]) | 12 (43) | 10 (35) | 11 (40) | 9 (26) |
| Age-group (years; n [%], % by column) | n = 6,726 | n = 3,028 | n = 1,922 | n = 1,106 |
| Children (≤ 14 years) | 3,647 (54.2) | 1,831 (60.5) | 1,083 (56.3) | 748 (67.6) |
| Adults (> 14 years) | 3,079 (45.8) | 1,137 (39.5) | 839 (43.7) | 358 (32.4) |
| < 6 months | 53 (0.8) | 13 (0.4) | 11 (0.6) | 2 (0.2) |
| 6–23 months | 472 (7.0) | 175 (5.8) | 116 (6.0) | 59 (5.3) |
| 2–4 | 1,041 (15.5) | 436 (14.4) | 311 (16.2) | 125 (11.3) |
| 5–9 | 1,405 (20.9) | 812 (26.8) | 421 (21.9) | 391 (35.3) |
| 10–14 | 676 (10.0) | 395 (13.0) | 224 (11.6) | 171 (15.5) |
| 15–19 | 211 (3.1) | 111 (3.7) | 63 (3.3) | 48 (4.3) |
| 20–34 | 537 (8.0) | 202 (6.7) | 152 (7.9) | 50 (4.5) |
| 35–49 | 765 (11.4) | 307 (10.1) | 222 (11.6) | 85 (7.7) |
| 50–64 | 863 (12.8) | 336 (11.1) | 247 (12.8) | 89 (8.1) |
| ≥ 65 | 703 (10.5) | 241 (8.0) | 155 (8.1) | 86 (7.8) |
| Gender (n [%], % by column) | ||||
| Female | 3,182 (47.2) | 1,460 (48.2) | 915 (47.6) | 545 (49.2) |
| Male | 3,558 (52.8) | 1,572 (51.8) | 1,009 (52.4) | 563 (50.8) |
| Health-care setting (n [%], % by column)* | ||||
| Community-based (sentinel physicians) | 5,290 (78.5) | 2,635 (49.8) | 1,610 (61.1) | 1,025 (38.9) |
| Hospital-based | 1,450 (21.5) | 397 (27.4) | 314 (79.1) | 83 (20.9) |
| Non-ICU admission | 909 (62.7) | 225 (24.7) | 161 (71.6) | 64 (28.4) |
| ICU admission | 541 (37.3) | 172 (31.8) | 153 (89.0) | 19 (11.0) |
| Managed with ECMO | 58 (10.7) | 38 (22.1) | 36 (94.7) | 2 (5.3) |
ARI = acute respiratory infection; ECMO = extracorporeal membrane oxygenation; ICU = intensive care unit; ILI = influenza-like Illness; IQR = interquartile range. Period: 2010–2018.
* Intra-stratum percentages are calculated by rows.
In this study, community-based surveillance was prevalent (78.5%; n = 5,290/6,740). Among subjects who required hospitalization (n = 1,450), 62.7% (n = 909/1,450) were non-critically ill patients, whereas 37.3% (n = 541/1,450) were admitted to an ICU. About 10% of these latter patients (n = 58/541) were assisted with extracorporeal membrane oxygenation support.
In total, 5,532 of 6,740 subjects provided information about their vaccination status and a very low proportion (7.4%; n = 411/5,532) was immunized against influenza (data not shown).
All respiratory specimens were tested for influenza viruses. The overall proportion of positive samples was 45.0% (n = 3,032/6,740), ranging between 30.4% in 2013–2014 and 54.6% in 2017–2018.
Overall, influenza type A virus was prevalent over the whole period (63.5%; n = 1,924/3,032), although the relative proportion of viruses belonging to influenza type A or B markedly varied across seasons.
Influenza type A was more frequently detected than type B in six seasons, whereas influenza B accounted for at least 20% of all confirmed cases in five different seasons, greatly predominating in 2012–2013 (71.7%).
Type A similarly spread among children and adults (56.3% versus 43.7%, respectively), following a bimodal pattern with a first peak in the age-group 5–9 years and a second peak in the age-group 50–64 years. Conversely, influenza B infections were more frequently identified among pediatric population and the 5–9 years age-group exhibited the highest proportion.
As observed in the whole study population, no gender-related differences were found among confirmed influenza cases caused by type A or type B strains.
Prevalence of influenza infection varied in different health settings; this was consistently lower in the hospital than in the community (27.4% versus 49.8%), regardless of whether the patients were admitted to critical or noncritical hospital wards (31.8% versus 24.7%, respectively).
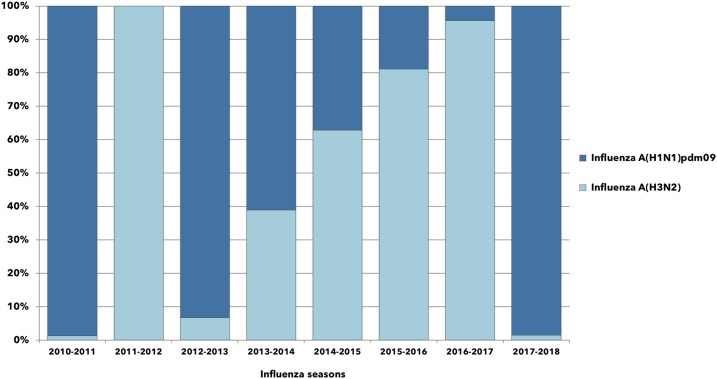
All influenza A cases were further characterized to quantify the relative proportion of subtypes documented during each studied season (Figure 2).
Figure 2.
Relative proportions of influenza A cases documented in Sicily between 2010 and 2018, according to annual epidemics. This figure appears in color at www.ajtmh.org.
Influenza A(H1N1)pdm09 circulated in all but one surveilled seasons and this was the dominant subtype in 2010–2011, 2012–2013, 2013–2014, and 2017–2018.
Influenza A(H3N2) co-circulated in 2010–2011, although poorly represented, whereas during the season 2011–2012, it was the unique influenza subtype identified in Sicily. After that, the proportion of A(H3N2) increased progressively until 2016–2017, when it markedly prevailed over A(H1N1)pdm09 (95.8% versus 4.2%).
In our study population, the impact of influenza on hospitalization was also evaluated together with any relevant chronic medical conditions or respiratory complications. Table 2 reports the demographic and clinical characteristics of patients with laboratory-confirmed infection stratified by health-care setting.
Table 2.
Demographic and clinical characteristics of patients with laboratory-confirmed influenza infection (% by column).
| Health-care setting | P-value* | |||
|---|---|---|---|---|
| Community (n = 2,635) | Hospital (other than ICU) (n = 225) | ICU (n = 172) | ||
| Age (years; median [IQR]) | 8 (21) | 53 (28) | 54 (24) | < 0.001 |
| Age-group (years; n (%)] | ||||
| Children (≤ 14 years) | 1,806 (68.6) | 21 (9.3) | 5 (2.9) | |
| Adults (> 14 years) | 829 (31.4) | 204 (90.7) | 167 (97.1) | < 0.001 |
| Gender (n [%]) | ||||
| Female | 1,297 (49.2) | 93 (41.3) | 70 (40.7) | |
| Male | 1,338 (50.8) | 132 (58.7) | 102 (59.3) | 0.004 |
| Underlying medical conditions (n [%]) | n = 2,603 | n = 204 | n = 157 | |
| Any comorbidity† | 374 (14.4) | 165 (80.9) | 137 (87.3) | < 0.001 |
| Cardiovascular disease | 155 (41.4) | 65 (39.4) | 73 (53.3) | 0.043 |
| Lung disease | 93 (24.9) | 51 (30.9) | 53 (38.7) | 0.002 |
| Diabetes | 62 (16.6) | 37 (22.4) | 40 (29.2) | 0.001 |
| Obesity | 50 (13.4) | 31 (18.8) | 53 (38.7) | < 0.001 |
| BMI 30–40 | 44 (11.8) | 21 (12.7) | 34 (24.8) | 0.001 |
| BMI > 40 | 6 (1.6) | 10 (6.1) | 19 (13.9) | < 0.001 |
| Metabolic disorders | 43 (11.5) | 15 (9.1) | 19 (13.9) | 0.649 |
| Cancer | 20 (5.3) | 16 (9.7) | 7 (5.1) | 0.681 |
| Immunological disorders | 18 (4.8) | 18 (10.9) | 13 (9.5) | 0.023 |
| Genetic disorders | 11 (2.9) | 5 (3.0) | 7 (5.1) | 0.279 |
| Renal disease | 9 (2.4) | 34 (20.6) | 14 (10.2) | < 0.001 |
| Neurological disease | 4 (1.1) | 3 (1.8) | 6 (4.4) | 0.021 |
| Liver disease | 4 (1.1) | 17 (10.3) | 2 (1.5) | 0.106 |
| Pancreatic disease | 0 | 1 (0.6) | 0 | 0.659 |
| Respiratory complications (n [%]) | n = 2,603 | n = 206 | n = 167 | |
| Any complication† | 18 (0,7) | 117 (56.8) | 161 (96.4) | < 0.001 |
| Pneumonia | 15 (0.6) | 93 (79.5) | 56 (34.8) | < 0.001 |
| Respiratory failure | 2 (0.1) | 8 (6.8) | 23 (14.3) | 0.126 |
| ARDS | 0 | 16 (13.7) | 110 (68.3) | < 0.001 |
| Influenza type (n [%]) | n = 2,615 | n = 222 | n = 167 | |
| Influenza type A | 1,589 (60.8) | 158 (71.2) | 148 (88.6) | < 0.001 |
| A(H1N1)pdm09 | 548 (34.5) | 109 (69.0) | 119 (80.4) | < 0.001 |
| A(H3N2) | 1,041 (65.5) | 49 (31.0) | 29 (19.6) | < 0.001 |
| Influenza type B | 1,026 (39.2) | 64 (28.2) | 19 (11.4) | < 0.001 |
ARDS = acute respiratory distress syndrome; BMI = body mass index; ICU = intensive care unit; IQR = interquartile range. Period: 2010–2018.
* Trend test across ordered group.
† Percentages are not mutually exclusive.
The median age of influenza cases significantly increased with the severity of disease (P < 0.001). It was 8 years (IQR = 21) for mild cases occurred in the community, 53 years (IQR = 28) for uncomplicated subjects admitted to hospital wards other than ICU, and 54 years (IQR = 24) for critically ill patients with ICU admission. Influenza-associated hospitalizations were more common in males than females (P = 0.004).
As expected, most hospitalized patients had an underlying medical condition. Cardiovascular disease, lung disease, diabetes, and obesity were more frequently reported with a significant upward trend in proportions by health-care setting.
Most individuals admitted to hospital had or developed a respiratory complication, pneumonia was commonly reported among non-ICU patients (79.5%; n = 93/206), whereas acute respiratory distress syndrome was documented in 68.3% (n = 110/167) of those admitted to an ICU.
On note, influenza subtypes differently spread in the community or in the hospital. More specifically, A(H1N1)pdm09 was overrepresented among severely ill patients (80.4%; n = 119/148), whereas A(H3N2) mostly circulated in the general population (65.5%; n = 1,041/1,589; P < 0.001), as also observed for influenza type B.
Table 3 reports a comparison between community subjects and those admitted to ICU, which aimed to identify predictors of disease severity, also in relation to confirmed or unconfirmed influenza infection.
Table 3.
Predictors of disease severity. Comparison between community patients and those admitted to ICU, according to influenza virus positivity
| Characteristic | aOR (95% CI) | aOR ratio (Influenza-positive: Influenza-negative) | |
|---|---|---|---|
| Influenza-negative subjects | Influenza-positive subjects | ||
| Age-group (years, OR), ≤ 14 vs. > 14 | 10.79 (7.52–15.51) | 17.03 (10.19–28.45) | 1.58 |
| Female vs. male | 1.95 (1.50–2.53) | 1.39 (0.99–1.95) | 0.71 |
| Any comorbidity* | 1.70 (1.54–1.87) | 1.51 (1.35–1.69) | 0.89 |
| 1–2 comorbidities | Reference | Reference | |
| ≥ 3 comorbidities | 4.20 (2.69–6.57) | 6.33 (3.60–11.16) | 1.51 |
| Cardiovascular disease | 1.75 (1.25–2.46) | 2.63 (1.70–4.06) | 1.50 |
| Obesity | 2.98 (2.01–4.42) | 9.16 (5.76–14.55) | 3.07 |
| BMI 30–40 | 2.00 (1.26–3.17) | 5.19 (3.09–8.72) | 2.60 |
| BMI > 40 | 6.02 (2.91–12.46) | 25.40 (9.62–67.06) | 4.22 |
| Diabetes | 3.50 (2.26–5.40) | 3.08 (1.89–5.00) | 0.88 |
| Lung disease | 5.34 (3.87–7.37) | 4.58 (2.94–7.14) | 0.86 |
| Lung disease + diabetes | 6.29 (2.92–13.56) | 12.13 (4.24–34.71) | 1.93 |
| Lung disease + obesity | 7.89 (4.08–15.27) | 17.86 (7.59–41.99) | 2.26 |
| Lung disease + cardiovascular disease | 5.20 (3.15–8.58) | 5.55 (2.95–10.42) | 1.07 |
aOR = age-adjusted odds ratio; BMI = body mass index; OR = odds ratio.
* Cancer, cardiovascular disease, diabetes, genetic disorders, immunologic disorders, renal disease, liver disease, lung disease, metabolic disorders, neurological disease, obesity, pancreatic disease.
In general, subjects older than 14 years had a higher probability of being admitted to ICU than those in the pediatric population (OR = 10.79; 95% CI: 7.52–15.51) and the odds increased by 1.58 times among influenza-positive cases (OR = 17.03; 95% CI: 10.19–28.45).
Males showed an overall higher propensity to severe outcomes than females, as well as the group of individuals having any underlying medical condition. In this latter case, the odds were even more pronounced in the presence of multiple chronic comorbidities, especially among patients infected with influenza (OR = 6.33; 95% CI: 3.60–11.16; aOR ratio = 1.51).
The age-adjusted OR for ICU admission associated with cardiovascular disease was 1.75 (95% CI: 1.25–2.46) among influenza-negative and 2.63 (95% CI: 1.70–4.06) among influenza-positive subjects (aOR ratio = 1.50). Much more higher odds were calculated in obese influenza cases (OR = 9.16; 95% CI: 5.76–14.55; aOR ratio = 3.07) and the excess risk due to an influenza infection greatly increased according to the body mass index (BMI) value (BMI 30–40, aOR ratio = 2.60; BMI > 40, aOR ratio = 4.22).
Finally, taken alone, diabetes and lung disease were predictors of severe outcomes, even though the odds were similar between the two groups (aOR ratio = 0.88 and 0.86, respectively). Nevertheless, among patients with influenza, an interaction effect emerged considering the two factors combined (OR = 12.13; 95% CI: 4.24–34.71; aOR ratio = 1.93), as well as for lung disease + obesity (OR = 17.86; 95% CI: 7.59–41.99; aOR ratio = 2.26), and for lung disease + cardiovascular disease (OR = 5.55; 95% CI: 2.95–10.42; aOR ratio = 1.07).
DISCUSSION
This study presented data on the circulation of influenza viruses in Sicily, from the epidemic seasons 2010–2011 to 2017–2018.
In line with surveillance studies conducted in other Italian regions15–17 or in Europe,18–20 each surveillance season showed a specific virological profile and depicted epidemiological patterns with concurrent viral types and subtypes.
In general, the relative proportions of influenza cases attributable to type A and B viruses were quite similar to those observed at the national level.21 However, some variations in the regional prevalences were reported during the seasons 2015–2016 and 2017–2018, which respectively exceeded by 18.9% and 20.6% those documented in Italy, thus reflecting some peculiarities in the local epidemiology of influenza epidemics.
Overall, the frequency of influenza cases did not differ by gender, also in terms of infecting viral types. Conversely, a different distribution was clearly observed by age, with children sustaining most influenza infections.
Our findings highlighted that influenza type A circulated at higher frequency among adults and the elderly, whereas influenza type B was the most prominent in children. Similar considerations were made by Caini and others22 in a large case-based global surveillance study including several countries from all continents, as also claimed by Panatto et al.23 in a recent meta-regression analysis covering about 20 different countries in the Northern Hemisphere.
A detailed picture concerning trends of influenza B infection, molecular dynamics, and the impact of vaccine mismatch have been recently reported,24,25 evidencing the co-circulation of the two major B lineages, Yamagata and Victoria, in our geographic area.
Notably, severely ill subjects admitted to the ICU, as well as those who experienced the need of an extracorporeal membrane oxygenation, were mostly affected by an influenza type A infection, regardless of the seasonal molecular dynamics of circulating influenza viruses.
Torner et al.,8 in Catalonia, displayed a similar picture among severe hospitalized cases during five post-pandemic seasons (2010–2015), although the authors hypothesized a bias explained by a greater prevalence of influenza A viruses spread in the population during the observed period.
In France, a cross-sectional study analyzing the influenza seasons from 2009–2010 to 2012–2013 underlined the predominance of influenza virus A among ICU-admitted cases, and this virus type was always overrepresented when compared with that observed in the general population.26 Also, Shah and others27 in the United States found a significant higher frequency of type A viruses among patients who had a severe influenza infection.
Besides the description of the molecular epidemiology of seasonal influenza outbreaks in Sicily, the present article aimed to investigate demographic variables and/or clinical features of surveyed patients, according to the location of care, to analyze their potential association with increased severity of disease.
On this point, the age of patients has been reported to positively correlate with a more severe outcome and death, either during 2009 influenza pandemic28,29 or subsequent seasonal epidemics.19,27,30–32 In our setting, adults older than 14 years had a baseline higher risk of being admitted to an ICU than pediatric population, and the age-adjusted OR further increased among influenza-positive cases. In general, the median age of patients significantly grew up with disease severity and this was about 50 years and older among hospitalized patients.
To date, the current European Center for Disease Prevention and Control guidance indicates two large priority risk groups to which might be routinely offered immunization against the influenza viruses. These include all individuals older than 6 months of age with chronic medical conditions and older adults. However, there is no sharp cutoff age for older adults, and many European countries still use the age of 65 years as a threshold, being at higher risk for complications.33
More recently, the newest update to the recommendations for the use of influenza vaccines in the United States34 clearly targeted a lower age for vaccination, identifying all persons of 50 years and older.
Male gender had a statistically significant tendency to a higher proportion across the health-care settings considered, even though the positivity to influenza did not increase the probability of being admitted to an ICU among this group of subjects.
Other authors have supported an association between male gender and more severe outcomes in influenza infection6,27 and, interestingly, it has been recently proposed in mice model the role of endogenous estrogen, such as estriol, in ameliorating the disease severity of influenza in females through a reduced induction of pulmonary cytokine and chemokine, which may lead to an early mitigation of pulmonary inflammation.35
Our results confirmed that, in a significant portion of severe influenza cases, patients had at least one chronic medical condition, and the tendency to a more severe outcome generally increased with most of the reported chronic diseases, further enhancing in case of multiple comorbidities.
This has been amply documented by studies based on data collected during the pandemic outbreak of 20097,28,36–38 and other post-pandemic seasonal influenza epidemics,39 also exhibiting heterogeneous viral molecular patterns.30
In this study, cardiovascular disease and obesity are independent predictors of disease severity, especially among confirmed influenza cases. Notably, our results highlighted that an influenza infection defined a 4-fold or greater increase in the odds of being admitted to an ICU, particularly in severe obese subjects with BMI > 40.
Following the 2009 influenza pandemic, most published studies focusing on risk factors for hospitalization and severe outcomes in infected subjects resulted in identifying the obesity, among others, as one of the most important predictors of major complications and death.7,40 Since that time, obesity has also been found as a risk factor along with seasonal and emerging influenza strains,41 playing an important role in influenza transmission,42 including the ability to interfere with protective immune response to vaccines,43,44 potentially through unbalanced levels of bioactive adipokines able to interfere with an immunosuppressive effect.45
Finally, patients with multiple underlying medical conditions were particularly prone to develop complications and severe clinical outcomes, especially when infected with influenza.46,47
Our findings reinforce the need for immunization against seasonal influenza of these high-risk populations.34,48
This study, like all observational studies, has strengths and limitations. The main strength is that, to date, few studies covering about a decade of seasonal influenza surveillance activity have been conducted in Italy. To our knowledge, this is the first report, referring to the recent timescale, carried out in one of the most populated areas in Southern Italy. Our findings rely on data that originate from a network of primary care physicians operating across the whole region and therefore are able to provide a solid picture of the epidemic spread of influenza viruses in general population.
Moreover, the heterogeneity of the study population, which included both cases occurred in the community and in the hospital settings, permitted to compare groups of subjects at different risk levels for developing influenza-related clinical complications.
However, there are also some limitations. Even though representative of our geographic area, the study was based on data collected only in one Italian region and, consequently, the results may have been biased by the structure of the local surveillance network, which substantially improved during the time period monitored.
In this setting, the predominance of the community over the hospital-based surveillance may have resulted in a limited representativeness of some chronic diseases, which have been suggested to increase the risk of serious complications in subjects with an influenza infection.31
Furthermore, in this context, we were unable to evaluate other important individual variables such as pregnancy, administration of antiviral treatments, and mortality, given the lack of robust data on these topics.
In conclusion, the epidemiology of influenza is an ever-changing world driven by natural dynamics and immune pressures, which may govern evolution and diversity of seasonal circulating viruses.
Therefore, this makes influenza surveillance of crucial importance for the collection of reliable and timely data on both the epidemiology and molecular characteristics of circulating virus types, to optimize current vaccine formulations, which remains the most effective strategy for the reduction of global burden of disease in all age-groups, as well as influenza-related hospitalizations, severe complications, and deaths.49
REFERENCES
- 1.Karve S, Meier G, Davis KL, Misurski DA, Wang CC, 2013. Influenza-related health care utilization and productivity losses during seasons with and without a match between the seasonal and vaccine virus B lineage. Vaccine 31: 3370–3388. [DOI] [PubMed] [Google Scholar]
- 2.GBD 2015 LRI Collaborators , 2017. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of lower respiratory tract infections in 195 countries: a systematic analysis for the global burden of disease study 2015. Lancet Infect Dis 17: 1133–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Iuliano AD, et al. 2018. Estimates of global seasonal influenza-associated respiratory mortality: a modelling study. Lancet 391: 1285–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nair H, et al. 2011. Global burden of respiratory infections due to seasonal influenza in young children: a systematic review and meta-analysis. Lancet 378: 1917–1930. [DOI] [PubMed] [Google Scholar]
- 5.Caini S, et al. 2018. Distribution of influenza virus types by age using case-based global surveillance data from twenty-nine countries, 1999–2014. BMC Infect Dis 18: 269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Snacken R, Quinten C, Devaux I, Plata F, Broberg E, Zucs P, Amato-Gauci A, 2012. Surveillance of hospitalised severe cases of influenza A(H1N1)pdm09 and related fatalities in nine EU countries in 2010–2011. Influenza Other Respir Viruses 6: e93–e96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Van Kerkhove MD, et al. 2011. Risk factors for severe outcomes following 2009 influenza A (H1N1) infection: a global pooled analysis. PLoS Med 8: e1001053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Torner N, et al. 2018. Descriptive study of severe hospitalized cases of laboratory-confirmed influenza during five epidemic seasons (2010–2015). BMC Res Notes 11: 244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Restivo V, Costantino C, Bono S, Maniglia M, Marchese V, Ventura G, Casuccio A, Tramuto F, Vitale F, 2018. Influenza vaccine effectiveness among high-risk groups: a systematic literature review and meta-analysis of case-control and cohort studies. Hum Vaccin Immunother 14: 724–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.World Health Organization (WHO) , 2019. Global Influenza Surveillance and Response System (GISRS), National Infuenza Center. Geneva, Switzerland: WHO. Available at: https://www.who.int/influenza/gisrs_laboratory/national_influenza_centres/en/. Accessed June 28, 2019.
- 11.Ministry of Health - National Institute of Health (ISS) , 2018. InfluNet: Sorveglianza Epidemiologica e Virologica dell’Influenza, Protocollo operativo 2018–2019. Rome, Italy: ISS. Available at: http://www.salute.gov.it/imgs/C_17_pubblicazioni_2786_allegato.pdf. Accessed June 28, 2019.
- 12.European Centre for Disease Prevention and Control (ECDC) , 2001. Surveillance Report: Influenza Surveillance in Europe 2010–2011. Stockholm, Sweden: ECDC; Available at: https://ecdc.europa.eu/sites/portal/files/media/en/publications/Publications/111209_SUR_Influenza_surveillance_Europe%20_2010_2012.pdf. Accessed June 28, 2019. [Google Scholar]
- 13.National Institute of Health , 2019. InfluNet: Influenza Virologic Surveillance. Rome, Italy: National Institute of Health; Available at: http://old.iss.it/fluv/. Accessed June 28, 2019. [Google Scholar]
- 14.Tramuto F, et al. 2011. Surveillance of hospitalised patients with influenza-like illness during pandemic influenza A(H1N1) season in Sicily. Euro Surveill 16: 19957. [PubMed] [Google Scholar]
- 15.Pariani E, et al. 2015. Ten years (2004–2014) of influenza surveillance in northern Italy. Hum Vaccin Immunother 11: 198–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Campa A, Quattrocchi M, Guido M, Gabutti G, Germinario C, De Donno A, Group TI, 2010. Ten-year (1999–2009) epidemiological and virological surveillance of influenza in south Italy (Apulia). Influenza Res Treat 2010: 642492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Trucchi C, et al. 2017. Fifteen years of epidemiologic, virologic and syndromic influenza surveillance: a focus on type B virus and the effects of vaccine mismatch in Liguria region, Italy. Hum Vaccin Immunother 13: 456–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mosnier A, Caini S, Daviaud I, Bensoussan JL, Stoll-Keller F, Bui TT, Lina B, Van der Werf S, Cohen JM; GROG network , 2015. Ten influenza seasons in France: distribution and timing of influenza A and B circulation, 2003–2013. BMC Infect Dis 15: 357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Snacken R, Broberg E, Beauté J, Lozano JE, Zucs P, Amato-Gauci AJ, 2014. Influenza season 2012–2013 in Europe: moderate intensity, mixed (sub)types. Epidemiol Infect 142: 1809–1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Adlhoch C, Snacken R, Melidou A, Ionescu S, Penttinen P; The European Influenza Survillance Network, 2018. Dominant influenza A(H3N2) and B/Yamagata virus circulation in EU/EEA, 2016/17 and 2017/18 seasons, respectively. Euro Surveill 23: pii=18-00146. Available at: 10.2807/1560-7917.ES.2018.23.13.18-00146. [DOI] [PMC free article] [PubMed]
- 21.National Institute of Health , 2019. InfluNet: Influenza Virologic Surveillance – Archive of Previous Epidemic Seasons. Rome, Italy: National Institute of Health; Available at: http://old.iss.it/fluv/index.php?lang=1&anno=2019&tipo=13. Accessed June 28, 2019. [Google Scholar]
- 22.Caini S, Kroneman M, Wiegers T, El Guerche-Séblain C, Paget J, 2018. Clinical characteristics and severity of influenza infections by virus type, subtype and lineage: a systematic literature review. Influenza Other Respir Viruses 12: 780–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Panatto D, Signori A, Lai PL, Gasparini R, Amicizia D, 2018. Heterogeneous estimates of influenza virus types A and B in the elderly: results of a meta-regression analysis. Influenza Other Respir Viruses 12: 533–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Orsi A, Colomba GME, Pojero F, Calamusa G, Alicino C, Trucchi C, Canepa P, Ansaldi F, Vitale F, Tramuto F, 2018. Trends of influenza B during the 2010–2016 seasons in 2 regions of north and south Italy: the impact of the vaccine mismatch on influenza immunisation strategy. Hum Vaccin Immunother 14: 523–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tramuto F, Orsi A, Maida CM, Costantino C, Trucchi C, Alicino C, Vitale F, Ansaldi F, 2016. The molecular epidemiology and evolutionary dynamics of influenza B virus in two Italian regions during 2010–2015: the experience of Sicily and Liguria. Int J Mol Sci 17: 549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bonmarin I, Belchior E, Bergounioux J, Brun-Buisson C, Mégarbane B, Chappert JL, Hubert B, Le Strat Y, Lévy-Bruhl D, 2015. Intensive care unit surveillance of influenza infection in France: the 2009/10 pandemic and the three subsequent seasons. Euro Surveill 20: pii=30066. Available at: 10.2807/1560-7917.ES.2015.20.46.30066. [DOI] [PubMed]
- 27.Shah NS, et al. 2015. Severe influenza in 33 US hospitals, 2013–2014: complications and risk factors for death in 507 patients. Infect Control Hosp Epidemiol 36: 1251–1260. [DOI] [PubMed] [Google Scholar]
- 28.Zarychanski R, Stuart TL, Kumar A, Doucette S, Elliott L, Kettner J, Plummer F, 2010. Correlates of severe disease in patients with 2009 pandemic influenza (H1N1) virus infection. CMAJ 182: 257–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chudasama RK, Verma PB, Amin CD, Gohel B, Savariya D, Ninama R, 2010. Correlates of severe disease in patients admitted with 2009 pandemic influenza A (H1N1) infection in Saurashtra region, India. Indian J Crit Care Med 14: 113–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meerhoff TJ, et al. 2015. Surveillance for severe acute respiratory infections (SARI) in hospitals in the WHO European region–an exploratory analysis of risk factors for a severe outcome in influenza-positive SARI cases. BMC Infect Dis 15: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Domínguez A, Romero-Tamarit A, Soldevila N, Godoy P, Jané M, Martínez A, Torner N, Caylà JA, Rius C; Surveillance of Hospitalized Cases of Severe Influenza in Catalonia Working Group , 2018. Effectiveness of antiviral treatment in preventing death in severe hospitalised influenza cases over six seasons. Epidemiol Infect 146: 799–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dimitrijević D, Ilić D, Rakić Adrović S, Šuljagić V, Pelemiš M, Stevanović G, Milinković M, Šipetić Grujićić S, 2017. Predictors of hospitalization and admission to intensive care units of influenza patients in Serbia through four influenza seasons from 2010/2011 to 2013/2014. Jpn J Infect Dis 70: 275–283. [DOI] [PubMed] [Google Scholar]
- 33.European Centre for Disease Prevention and Control (ECDC) , 2008. Guidance Report: Priority Risk Groups for Influenza Vaccination. Stockholm, Sweden: ECDC; Available at: https://ecdc.europa.eu/sites/portal/files/media/en/publications/Publications/0808_GUI_Priority_Risk_Groups_for_Influenza_Vaccination.pdf. Accessed June 28, 2019. [Google Scholar]
- 34.Grohskopf LA, Sokolow LZ, Broder KR, Walter EB, Fry AM, Jernigan DB, 2018. Prevention and control of seasonal influenza with vaccines: recommendations of the advisory committee on immunization practices-United States, 2018–19 influenza season. MMWR Recomm Rep 67: 1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vermillion MS, Ursin RL, Attreed SE, Klein SL, 2018. Estriol reduces pulmonary immune cell recruitment and inflammation to protect female mice from severe influenza. Endocrinology 159: 3306–3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kusznierz G, et al. 2013. Clinical features of the hospitalized patients with 2009 pandemic influenza A (H1N1) in Santa Fe, Argentina. Influenza Other Respir Viruses 7: 410–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gilca R, De Serres G, Boulianne N, Ouhoummane N, Papenburg J, Douville-Fradet M, Fortin É, Dionne M, Boivin G, Skowronski DM, 2011. Risk factors for hospitalization and severe outcomes of 2009 pandemic H1N1 influenza in Quebec, Canada. Influenza Other Respir Viruses 5: 247–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cardeñosa N, et al. 2011. Epidemiological analysis of severe hospitalized 2009 pandemic influenza A (H1N1) cases in Catalonia, Spain. Hum Vaccin 7 (Suppl): 226–229. [DOI] [PubMed] [Google Scholar]
- 39.Poulakou G, Souto J, Balcells J, Pérez M, Laborda C, Roca O, Tórtola T, Pujol M, Palomar M, Rello J, 2012. First influenza season after the 2009 pandemic influenza: characteristics of intensive care unit admissions in adults and children in Vall d’Hebron Hospital. Clin Microbiol Infect 18: 374–380. [DOI] [PubMed] [Google Scholar]
- 40.Rodríguez-Baño J, Paño-Pardo JR, Múñez Rubio E, Segura Porta F, 2012. Pregnancy, obesity and other risk factors for complications in influenza A(H1N1) pdm09 infection. Enferm Infecc Microbiol Clin 30 (Suppl 4): 32–37. [DOI] [PubMed] [Google Scholar]
- 41.Segaloff HE, Evans R, Arshad S, Zervos MJ, Archer C, Kaye KS, Martin ET, 2018. The impact of obesity and timely antiviral administration on severe influenza outcomes among hospitalized adults. J Med Virol 90: 212–218. [DOI] [PubMed] [Google Scholar]
- 42.Maier HE, et al. 2018. Obesity increases the duration of influenza a virus shedding in adults. J Infect Dis 218: 1378–1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Neidich SD, Green WD, Rebeles J, Karlsson EA, Schultz-Cherry S, Noah TL, Chakladar S, Hudgens MG, Weir SS, Beck MA, 2017. Increased risk of influenza among vaccinated adults who are obese. Int J Obes (Lond) 41: 1324–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Green WD, Beck MA, 2017. Obesity impairs the adaptive immune response to influenza virus. Ann Am Thorac Soc 14 (Suppl 4): S406–S409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Grant RW, Dixit VD, 2015. Adipose tissue as an immunological organ. Obesity (Silver Spring) 23: 512–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mertz D, et al. 2013. Populations at risk for severe or complicated influenza illness: systematic review and meta-analysis. BMJ 347: f5061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Restivo V, Vizzini G, Mularoni A, Di Benedetto C, Gioè SM, Vitale F, 2017. Determinants of influenza vaccination among solid organ transplant recipients attending Sicilian reference center. Hum Vaccin Immunother 13: 346–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fukuta H, Goto T, Wakami K, Kamiya T, Ohte N, 2018. The effect of influenza vaccination on mortality and hospitalization in patients with heart failure: a systematic review and meta-analysis. Heart Fail Rev 24: 109–114. [DOI] [PubMed] [Google Scholar]
- 49.Costantino C, Vitale F, 2016. Influenza vaccination in high-risk groups: a revision of existing guidelines and rationale for an evidence-based preventive strategy. J Prev Med Hyg 57: E13–E18. [PMC free article] [PubMed] [Google Scholar]