Abstract.
Community-based active case detection of malaria parasites with conventional rapid diagnostic tests (cRDTs) is a strategy used most commonly in low-transmission settings. We estimated the sensitivity of this approach in a high-transmission setting in Western Kenya. We tested 3,547 members of 912 households identified in 2013–2014 by index children with (case) and without (control) cRDT-positive malaria. All were tested for Plasmodium falciparum with both a cRDT targeting histidine-rich protein 2 and with an ultrasensitive real-time polymerase chain reaction (PCR). We computed cRDT sensitivity against PCR as the referent, compared prevalence between participant types, and estimated cRDT detectability as a function of PCR-estimated parasite density. Parasite prevalence was 22.9% by cRDTs and 61.5% by PCR. Compared with children aged < 5 years or adults aged > 15 years, geometric mean parasite densities (95% CI) were highest in school-age children aged 5–15 years (8.4 p/uL; 6.6–10.6). The overall sensitivity of cRDT was 36%; among asymptomatic household members, cRDT sensitivity was 25.5% and lowest in adults aged > 15 years (15.8%). When modeled as a function of parasite density, relative to school-age children, the probability of cRDT positivity was reduced in both children aged < 5 years (odds ratio [OR] 0.48; 95% CI: 0.34–0.69) and in adults aged > 15 years (OR: 0.35; 95% CI: 0.27–0.47). An HRP2-detecting cRDT had poor sensitivity for active P. falciparum case detection in asymptomatic community members, and sensitivity was lowest in highly prevalent low-density infections and in adults. Future studies can model the incremental effects of high-sensitivity rapid diagnostic tests and the impacts on transmission.
INTRODUCTION
Between 2000 and 2015, malaria cases in Africa have declined by 40%1,2 and the proportion of Africans who live in hyper- or holoendemic transmission settings has declined by more than 70%.1 These successes have enabled fresh approaches to enhanced control measures, some of which rely on active case detection (ACD) in asymptomatic individuals in community settings.3 Active case detection is typically deployed either in response to an index case as reactive case detection (RACD) or in high-risk populations as proactive case detection (PACD).4 These ACD programs are common strategies of malaria control programs in low-transmission settings in Asia.5 In African studies, PACD and RACD identify large numbers of additional cases,6–12 but commonly cited barriers to ACD in African settings are operational barriers, limited spatial aggregation, high parasite prevalence, and uncertain suitability of common malaria rapid diagnostic tests (RDTs) to detect abundant low-density infections in community settings.13
Conventional rapid diagnostic tests (cRDTs) enhance clinical diagnosis and case management14 but are increasingly used for community-based detection in asymptomatic people.15 Because of their operability and availability, cRDTs have been repurposed for community-based studies for detection of “hot spots,”16 for RACD,12 and mass screen and treat programs.17 Despite these applications, the sensitivity of cRDTs in such settings and how this varies between groups remain unclear. Generally, cRDTs that detect the Plasmodium falciparum histidine-rich protein 2 (HRP2) are benchmarked to detect parasitemias at a density of 200 parasites/µL (p/µL) of whole blood18 but are believed to have a limit of detection of approximately 100 p/µL.15 Many studies have compared cRDT detection with molecular detection as a reference,12,19–21 but few19,22 have used PCR estimates of parasite density by which to explore the quantitative limit of detection of cRDT. Therefore, there exists a paucity of direct estimates of the probability of cRDT positivity as a function of parasite density among asymptomatic individuals of all ages in high-transmission settings.
In this study, we developed and applied a sensitive molecular P. falciparum detection assay that allows for infections to be quantified and then used this assay to directly estimate the sensitivity of a conventional HRP2-based RDT applied in a case–control study of malaria prevention in Western Kenya. Our field study enrolled children as cases (cRDT-positive) or controls (cRDT-negative), along with their household members; we hypothesized that, in this high-transmission setting, this HRP2-detecting cRDT would detect a large proportion of PCR-positive infections and that detectability would be highest in children.
MATERIALS AND METHODS
Ethical review.
The study was approved by the Ethical Review Boards of Moi University (000778) and Duke University (Pro00044098). All participants provided written informed consent; assent was obtained from children older than 8 years.
Specimen collection.
The field study was conducted in Webuye, Kenya, in 2013–2014, and described elsewhere.23 Webuye has perennial P. falciparum transmission with a seasonal peak in May–June, and the principal vectors are Anopheles arabiensis and Anopheles gambiae s.s. Briefly, index case and control children and their households were enrolled: case children aged 1–10 years were consecutively identified when admitted to Webuye Sub-County Hospital with cRDT-confirmed falciparum malaria; on discharge, these case children were age-, village-, and gender-matched to community-dwelling control children. Control children were cRDT negative and excluded if they were unwell or had taken antimalarials in the prior month. All household members of index children were sampled within 1 week of case child hospital discharge. All index and household participants were tested at enrollment with the SD Bioline Malaria Ag P.f. (HRPII) cRDT (Standard Diagnostics, Inc., Suwon city, Korea). Participants with positive cRDT results were treated: case children were treated with usual care as per local guidelines—typically parenteral quinine—and community participants with artemether–lumefantrine. At the time of cRDT testing, capillary blood was stored on a filter paper as a dried blood spot (DBS) in plastic bags with a desiccant.
Parasite detection assay development.
We designed a duplex TaqMan real-time PCR assay targeting the P. falciparum multicopy motif pfr36424 and the human gene β-tubulin.25 We aligned 41 separate sequences of pfr364 in the P. falciparum reference genome 3D7 in ClustalW26 and used Primer3 to design primers to amplify a 126-nt segment from 22 of these sequences and to design a TaqMan probe targeting a conserved segment of this amplicon with the reporter FAM and the quencher QSY; the β-tubulin TaqMan probe was synthesized with the VIC reporter and a QSY quencher (Supplemental Table 1).
We first amplified purified genomic DNA (gDNA) templates of reference P. falciparum lines. These individual 12 µL reactions consisted of 250 nM of each pfr364 primer, 300 nM of pfr364 probe, 300 nM of each β-tubulin primer and probe, 6 µL of TaqMan Environmental MasterMix (Applied Biosystems, Foster City, CA), 1 µL of template gDNA, and water, and were amplified on an ABI QuantStudio 6 platform. Reference parasite lines were 3D7, Dd2, K1, FCR3/FMG, and 7G8, each obtained from MR4, and were quantified using the Qubit dsDNA HS Assay Kit (ThermoFisher, Waltham, MA). We then prepared quantitative mocked DBSs of P. falciparum at known densities of 2,000, 1,000, 200, 100, 20, 10, 2, 1, 0.2, and 0.1 p/µL with which to test the lower limit of detection of P. falciparum. To do so, we cultivated P. falciparum strain 3D7 using standard methods and pelleted erythrocytes, and diluted these 1:20 into uninfected whole blood; we measured % parasitemia by Giemsa-stained thin smear and erythrocytes/µL using a hemocytometer. Serial dilutions of this source material were made into uninfected whole blood, and these were each preserved in duplicate as DBSs. We first tested the duplex pfr364/β-tubulin assay using these templates in six technical replicates; we then used these templates to directly compare in parallel the performance of this assay with that of reported real-time assays targeting varATS27 and pfldh28; for these tests, cycle threshold (Ct) lines were set manually for each assay at identical ΔRn values above background fluorescence and in the exponential phase of amplification. Reaction mixtures were as reported for these assays, excepting for the enzyme (TaqMan Environmental MasterMix), the platform (QuantStudio 6), and the number of cycles (45, as reported for the varATS assay).
Parasite detection procedures.
Field-collected DBSs were stored at room temperature for up to 2 years and then singly punched into individual wells of a 96-well deepwell plate; each plate also contained a punch from a mock, nonquantitative DBS containing 3D7 gDNA as an extraction control. In this study, gDNA was extracted from DBSs in a plate format using Chelex-100.29 Each gDNA specimen was tested with the duplex pfr364/β-tubulin real-time PCR assay in duplicate in 384-well reaction plates, with reaction conditions as described previously. Each reaction plate included 10 positive controls in duplicate as a standard curve, at parasite densities of 2,000, 1,000, 200, 100, 20, 10, 2, 1, 0.2, and 0.1 p/µL. Negative controls were included on each reaction plate, and all reaction plates were prepared in dedicated workspaces and with filtered tips. Cycle threshold lines were set manually above baseline fluorescence.
Statistical analyses.
We compared between assays test positivity with chi-squared tests and Ct values by computing pairwise correlation coefficients. For testing of clinical samples, the analytical population was defined as nonmissing specimens from participants with nonmissing individual clinical/demographic data who were from households where the index child (case or control) was non-missing. Specimens positive for P. falciparum met the following criteria: 1) positive amplification of human β-tubulin and 2) amplification of pfr364 in either both replicates or in a single replicate with a Ct value ≤ 38. We assigned parasite densities based on mean estimated quantities using a standard curve generated from standards from 2,000 to 1 p/µL. Prevalence ratios (PRs) were computed with Poisson regression. We summarized parasite densities by computing geometric means (geomean) and compared densities using analysis of variance (ANOVA) of log10-transformed densities. We modeled the probability of cRDT positivity as a function of log10-transformed parasite density with logistic regression. We repeated these analyses in adjusted models including indicator variables for age to estimate age-specific odds ratios (ORs) controlling for parasite density. Statistical analyses were computed in Stata/SE v14.2 (Stata Corp, College Station, TX).
RESULTS
Parasite detection assay performance.
When tested against purified gDNA from P. falciparum reference strains, the duplex pfr364 TaqMan real-time PCR assay reported lower limits of detection, ranging from 2.2 × 10−5 ng/µL to 1 × 10−2 ng/µL of gDNA. When tested initially on gDNA extracted from mock quantitative DBSs prepared with known densities of parasites, the assay was positive down to 0.1 p/µL (Supplemental Table 2). A standard curve fit to Ct measurements from densities of 1–2,000 p/µL returned an R2 of 0.9624 with an efficiency of 90%.
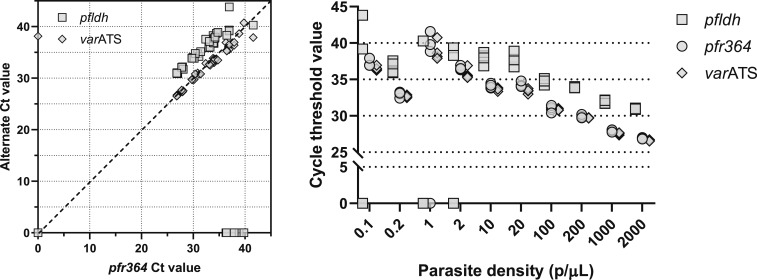
These templates were then used to directly compare the performance of this pfr364 assay with that of real-time PCR assays targeting pfldh and varATS (Figure 1). In 12 replicates at densities of 1 p/µL or less, detection was successful (defined as Ct ≤ 40) less often for pfldh (5/12) than for varATS (11/12) or pfr364 (10/12) (P < 0.05 for each comparison with pfldh). When compared with varATS and pfldh parasite detection assays, Ct values among successful tests for pfr364 were highly correlated with those for both pfldh (correlation efficient 0.9392, P < 0.001) and varATS (0.9833, P < 0.001).
Figure 1.
Comparison of cycle threshold (Ct) values of real-time PCR Plasmodium falciparum detection assays genomic DNA (gDNA) extracted from dried blood spots (DBSs) prepared by spiking into whole blood P. falciparum 3D7 parasites at densities of 0.1 to 2,000 parasites/µL (p/µL) were tested in parallel in real-time PCR assays targeting pfr364, pfldh, and varATS. Each template was tested in quadruplicate in each assay, and Ct lines were manually set for each assay at identical ΔRn values above background fluorescence and in the exponential phase of amplification. A Ct value of 0 indicates failed amplification. Left: scatterplot of Ct values of pfr364 (x axis) and corresponding Ct values of pfldh or varATS (y axis) during amplification of gDNA of mocked DBSs across a range of parasite densities. Dotted line indicates equality. Right: Ct values for pfldh (squares), pfr364 (circles), and varATS (diamonds) as a function of parasite density.
Parasite prevalence.
The parent field study enrolled 4,377 participants (2,203 in case households and 2,154 in control households). From these, DBS samples were available for 4,116 participants; of these, 4,075 were able to be matched to clinical data and tested for malaria parasites with real-time PCR. We further restricted analyses only to those households for which clinical and molecular data were available for the index child, resulting in 3,547 participants in 912 households.
The overall prevalence of parasites was 22.9% (813/3,547) by cRDT and 61.5% (2,180/3,547) by real-time PCR (Table 1); overall, the prevalence of subpatent infections, defined as cRDT-negative and PCR-positive, was 39.3% (1,395/3,547). The prevalence of parasites in members of case households was higher than that in members of control households by both cRDT (PR: 2.21; 95% CI: 1.82–2.70) and PCR (PR: 1.74; 95% CI: 1.58–1.92). Compared with children aged < 5 years, the prevalence of parasites in adults aged > 15 years was lower by cRDT (PR: 0.65; 95% CI: 0.49–0.87) but not by PCR (PR: 1.0; 95% CI: 0.88–1.15). We observed similar patterns in both case and control households, in that adults had lower prevalence by cRDT but similar prevalence to children by PCR (Supplemental Table 3).
Table 1.
Parasite prevalence by detection method and subgroup
| Conventional malaria rapid diagnostic test–positive, % (n/N) | Prevalence ratio (95% CI) | Real-time PCR positive, % (n/N) | Prevalence ratio (95% CI) | Subpatent infections,* % (n/N) | Prevalence ratio (95% CI) | |
|---|---|---|---|---|---|---|
| Overall | 22.9 (813/3,547) | NA | 61.5 (2,180/3,547) | NA | 39.3 (1,395/3,547) | NA |
| Index children | ||||||
| Controls | 0 (0/419) | REF | 33.7 (141/419) | REF | 33.7 (141/419) | REF |
| Cases | 100 (360/360) | NA | 98.9 (356/360) | 2.94 (2.42–3.57) | 0 | NA |
| Household members† | 16.4 (453/2,768) | – | 60.8 (1,683/2,768) | – | 45.3 (1,254/2,768) | – |
| Controls | 10.3 (144/1,405) | REF | 44.6 (626/1,405) | REF | 35.1 (493/1,405) | REF |
| Cases | 22.7 (309/1,363) | 2.21 (1.82–2.70) | 77.6 (1,057/1,363) | 1.74 (1.58–1.92) | 55.8 (761/1,363) | 1.59 (1.42–1.78) |
| Age (years) | ||||||
| < 5 | 14.6 (75/513) | REF | 58.1 (298/513) | REF | 44.6 (229/513) | REF |
| 5–15 | 26.6 (254/955) | 1.82 (1.41–2.35) | 65.3 (624/955) | 1.12 (0.98–1.29) | 40.2 (384/955) | 0.90 (0.76–1.06) |
| ≥ 15 | 9.5 (124/1,300) | 0.65 (0.49–0.87) | 58.5 (761/1,300) | 1.0 (0.88–1.15) | 49.3 (641/1,300) | 1.10 (0.95–1.28) |
NA = not applicable; REF = referent category.
* Defined as the proportion of real-time PCR-positive infections that were negative by cRDT.
† Excluding index children.
Parasite density distribution.
The geomean (95% CI) parasite density was 12.1 p/µL (10.4–14.0) (Table 2). Predictably, geomean (95% CI) densities were highest among case children (1,717 p/µL; 1,172–2,514) and lowest among control children, who were defined as cRDT-negative (1.3 p/µL; 1.0–1.8); surprisingly, among asymptomatic household members, geomean (95% CI) densities were higher in case (5.9 p/µL; 5.0–6.9) than in control households (4.0 p/uL; 3.2–4.9) (P = 0.005 by ANOVA). Excluding index children, geomean (95% CI) densities were highest in school-age children aged 5–15 years (8.4 p/µL; 6.6–10.6) compared with younger (5.2 p/uL; 3.7–7.2) and older (3.4 p/uL; 2.9–4.0) participants (P < 0.001 by ANOVA).
Table 2.
Parasite density by participant type and age
| No. of PCR-positive infections | Plasmodium falciparum density, p/µL, geometric mean (95% CI) | P-value* | |
|---|---|---|---|
| Overall | 2,180 | 12.1 (10.4–14.0) | Not applicable |
| Type of participant | |||
| Control child | 141 | 1.3 (1.0–1.8) | < 0.001 |
| Case child | 356 | 1,717 (1,172–2,514) | |
| Control household member | 626 | 4.0 (3.2–4.9) | |
| Case household member | 1,057 | 5.9 (5.0–6.9) | |
| Age (years)† | |||
| < 5 | 298 | 5.2 (3.7–7.2) | < 0.001 |
| 5–15 | 624 | 8.4 (6.6–10.6) | |
| ≥ 15 | 761 | 3.4 (2.9–4.0) | |
* Computed with analysis of variance comparing log10-transformed densities.
† Excluding case children.
Sensitivity of parasite detection by cRDT.
Among the 2,180 PCR-positive infections, we computed cRDT sensitivity against PCR as the reference standard after binning PCR-estimated densities (Table 3). The overall sensitivity of cRDT was 36% (785/2,180); this declined from 98.2% (54/55) at densities in excess of 100,000 p/µL to 8.5% (55/650) at densities < 1 p/µL; above the conventional limit of detection of 100 p/µL, the aggregate sensitivity was 84% (436/517); less than 100 p/µL, the sensitivity was 21% (349/1,663). By age, the overall sensitivity was highest in children aged < 5 years (49.3%; 322/653) and lowest in adults aged > 15 years (15.9%; 121/762). Among only 1,683 asymptomatic household members, the overall sensitivity of cRDT was 25.5% (429/1,683); this was lower in adults aged > 15 years (15.8%; 120/761) than in children aged < 5 years (23.2%; 69/298) and with school-age children (38.5%; 240/624) (P < 0.01 for each).
Table 3.
Sensitivity of conventional malaria rapid diagnostic test by parasite density and age
| PCR-estimated parasite density, p/uL | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Any density | ≥ 100,000 | 100,000–10,000 | 10,000–5,000 | 5,000–1,000 | 1,000–500 | 500–200 | 200–100 | 100–10 | 10–1 | < 1 | |
| Age of all participants (years) | 36.0 (785/2,180) | 98.2 (54/55) | 96.8 (119/123) | 79.3 (23/29) | 86.7 (65/75) | 75.6 (31/41) | 76.5 (65/85) | 72.5 (79/109) | 41.6 (159/382) | 21.4 (135/631) | 8.5 (55/650) |
| < 5 | 49.3 (322/653) | 100 (37/37) | 98.6 (72/73) | 83.3 (10/12) | 85.2 (23/27) | 76.9 (10/13) | 88.6 (31/35) | 84.1 (37/44) | 44.2 (38/86) | 31.3 (51/163) | 8.0 (13/163) |
| 5–15 | 44.7 (342/765) | 100 (17/17) | 95.8 (46/48) | 92.3 (12/13) | 94.4 (34/36) | 85.0 (17/20) | 71.4 (25/35) | 82.1 (32/39) | 49.0 (72/147) | 27.3 (56/205) | 15.1 (31/205) |
| ≥ 15 | 15.9 (121/762) | 0 (0/1) | 50 (1/2) | 25 (1/4) | 66.7 (8/12) | 50 (4/8) | 60 (9/15) | 38.5 (10/26) | 32.9 (49/149) | 10.7 (28/263) | 3.9 (11/282) |
| P-value | < 0.001 | < 0.001 | 0.001 | 0.013 | 0.048 | 0.149 | 0.061 | < 0.001 | 0.017 | < 0.001 | < 0.001 |
| Age of asymptomatic household members (years) | 25.5 429/1,683) | 50.0 (1/2) | 76.5 (13/17) | 64.7 (11/17) | 82.4 (42/51) | 71.0 (22/31) | 66.7 (38/57) | 58.2 (39/67) | 35.9 (117/326) | 17.1 (93/545) | 9.3 (53/570) |
| < 5 | 23.2 (69/298) | NA | 75.0 (3/4) | 66.7 (4/6) | 70.0 (7/10) | 50.0 (2/4) | 63.6 (7/11) | 45.5 (5/11) | 19.2 (9/47) | 20.2 (20/99) | 11.3 (12/106) |
| 5–15 | 38.5 (240/624) | 1 (1/1) | 83.3 (10/12) | 85.7 (6/7) | 93.1 (27/29) | 84.2 (16/19) | 71.0 (22/31) | 80.0 (24/30) | 45.4 (59/130) | 24.6 (45/183) | 16.5 (30/182) |
| ≥ 15 | 15.8 (120/761) | 0 (0/1) | 0 (0/1) | 25.0 (1/4) | 66.7 (8/12) | 50.0 (4/8) | 60.0 (9/15) | 38.5 (10/26) | 32.9 (49/149) | 10.7 (28/263) | 3.9 (11/282) |
| P-value | < 0.001 | 0.157 | 0.168 | 0.127 | 0.068 | 0.124 | 0.740 | 0.005 | 0.003 | < 0.001 | < 0.001 |
Influence of parasite density on cRDT detection.
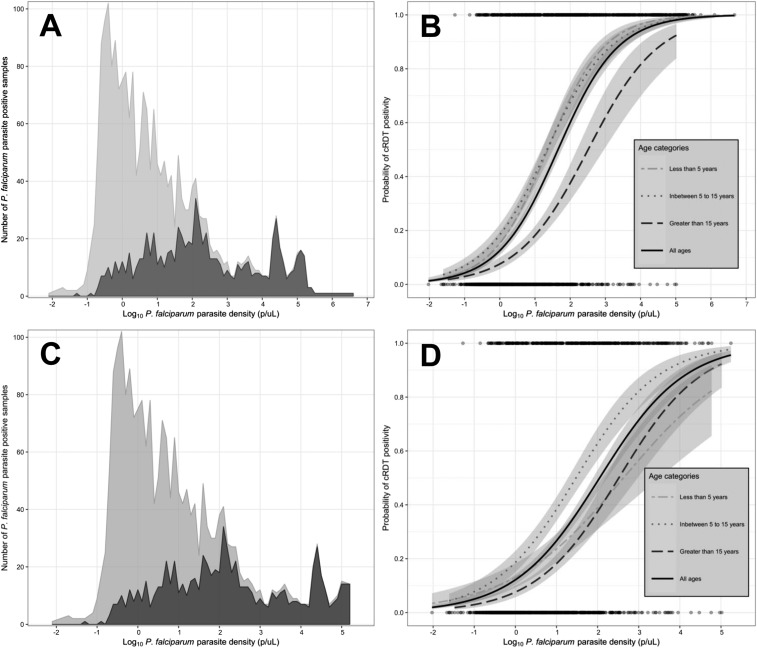
We used logistic regression to estimate the probability of cRDT positivity as a function of PCR-estimated density. The overall distribution of densities remained right-skewed, and this was largely driven by cRDT-negative infections; the distribution of cRDT-positive infections was nearly normal (Figure 2A). The probability of cRDT detection was directly related to the log10-transformed density. Using this model, the probability of cRDT positivity at the conventional density threshold of 100 p/µL was 60.1% (95% CI: 56.6–63.6). The lowest parasite density below which the 95% CI of the probability of cRDT detection no longer crossed 0.95 was 7,377 p/µL (probability 93.1%; 95% CI: 90.9–94.8). We conditioned this model on age-groups (Figure 2B); controlling for parasite density, relative to school-age children, the probability of cRDT positivity was unchanged in children aged < 5 years (OR: 0.93; 95% CI: 0.71–1.23) but significantly lower in adults aged > 15 years (OR: 0.32; 95% CI: 0.24–0.43).
Figure 2.
Parasite density distribution and probability of detection by conventional rapid diagnostic test (cRDT). (A) Distribution of parasite densities estimated by PCR among cRDT-positive (dark gray) and cRDT-negative (light gray) participants for all participants (case, control, and asymptomatic household members). (B) Probability of cRDT positivity as a function of PCR-estimated parasite density for all participants (case, control, and asymptomatic household members), modeled by logistic regression and stratified by age categories. Dots at top and bottom indicate positive and negative cRDT results, and gray shading indicates 95% CI of modeled probability. (C) Distribution of parasite densities estimated by PCR among cRDT-positive (dark gray) and cRDT-negative (light gray) participants for only asymptomatic household members. (D) Probability of cRDT positivity as a function of PCR-estimated parasite density for only asymptomatic household members, modeled by logistic regression and stratified by age categories. Dots at top and bottom indicate positive and negative cRDT results, and gray shading indicates 95% CI of modeled probability.
Finally, we used logistic regression to estimate the probability of cRDT positivity as a function of PCR-estimated density in models conditioned on age (Figure 2C and D) only among asymptomatic household members. Among asymptomatic household members, the lowest parasite density for which the sensitivity CI included 0.95 was 36,790 p/uL (probability of cRDT positive 91.9%; 95% CI: 88.2–94.5). When conditioned on age-group, relative to school-age children, the probability of cRDT detection was reduced in both children younger than 5 years (OR: 0.48; 95% CI: 0.34–0.69) and adults aged > 15 years (OR: 0.35; 95% CI: 0.27–0.47).
DISCUSSION
In this molecular epidemiologic study of malaria parasites in Kenya, we developed and used an ultrasensitive real-time PCR assay for P. falciparum parasites to describe the distribution of infections and directly measure the sensitivity of a common cRDT. We report that, among asymptomatic household members of index children who were admitted to hospital with cRDT-positive malaria, most infections were undetectable by cRDT and the overall sensitivity of the HRP2-detecting cRDT was very poor. This poor sensitivity was partially owing to a large abundance of low-density infections that was unexpected in this high-transmission setting. Finally, cRDT sensitivity was consistently lower in adults aged > 15 years, irrespective of parasite density. Collectively, these findings suggest that cRDTs are not suitable tools for effective malaria parasite case detection in asymptomatic residents of high-transmission communities.
The sensitivity of a widely used cRDT was very low. The cRDT we used detects HRP2, achieved a 95% detection rate against samples at 200 p/µL in rigorous testing,18 and was a WHO-prequalified product. Despite this, the overall sensitivity in asymptomatic household members was 25.5%, similar to that of recent studies in Ethiopia30 and Eswatini.12 Quantitatively, in our study, only at densities greater than 36,790 p/µL did the probability of cRDT positivity exceed 95% in asymptomatic participants. We observed this poor performance despite cRDT testing in a high-transmission setting in which HRP2 persistence would be expected to enhance the detectability of parasites. It is unlikely that parasites with HRP2 deletions contributed to this poor performance because these parasites are rare in Western Kenya,31 and, given that most infections in our study area are polyclonal,32 this would require the co-occurrence of more than one HRP2-deleted (and HRP3-deleted) parasite to remain undetected. The low sensitivity and the resulting inability to identify a large proportion of low-density infections in asymptomatic, community members suggest cRDTs in high-transmission settings would be an ineffective approach to case detection.
Conventional rapid diagnostic test sensitivity was lowest in adults, in whom only 15.9% of PCR-positive infections were detected. Although this partially resulted from a more skewed density distribution in adults toward low densities, it is notable that sensitivity was poorer in adults even at similar densities. Specifically, among asymptomatic household members in models controlling for parasite density, compared with school-age children, the odds of detection with cRDT in adults was reduced by 65% (OR: 0.35; 95% CI: 0.27–0.47). Epidemiologically, enhanced detectability in children could result from HRP2 antigen accumulation in school-age children resulting from preceding infections, but this is not supported by the similar parasite point prevalence by PCR we measured in each age-group. The influence of preceding infections on cRDT sensitivity could be mediated by differential clearance rates of HRP2 by age, specifically here if HRP2 is cleared more rapidly in adults; this idea is supported by models indicating that HRP2 persistence after treatment is more prolonged in children than in adults,33 but it is unclear if this observation is the result of higher starting densities in children. Alternatively, HRP2 may be rendered less detectable in adults by anti-HRP2 IgG, which can be present in measurable quantities in clinical samples34 but are of uncertain diagnostic impact.35 Because the PCR-estimated parasite prevalence was similar in adults to other age-groups and because these represent a large proportion of prevalent, likely transmissible infections, the low sensitivity in adults further renders cRDTs unsuitable as a tool by which to identify and treat infections, and thereby efficiently reduce transmission.
To a greater degree than other studies in sub-Saharan Africa, we report a large number of infections that are below the limits of detection of conventional diagnostics. We found that 74.5% (1,254/1,683) of infections in asymptomatic household members were cRDT-negative and 33.7% of these (570/1,683) were below the putative lower limit of detection of 1 p/µL for the high-sensitivity RDT (HS-RDT).36 Similarly skewed distributions of parasite densities are more typically reported from low-transmission sites,37–39 which are typically defined on the basis of a far lower PCR-estimated parasite prevalence (typically less than 5%). By contrast, our PCR prevalence was 60.8%, and reports in other settings with this degree of transmission have typically reported less right-skewed distributions of parasite densities with a much larger proportion of parasites at densities greater than 100 p/µL.19,40,41 Even though our study differed by enrolling two parallel groups of household members with different levels of risk, the prevalence was high enough in both control (44.6%) and case (77.6%) households to expect that larger proportions would have been detectable by cRDTs.42 The reasons for this unexpectedly high prevalence of very low-density infections may include the high levels of ownership and use of insecticide-treated nets.23 In addition, the proportion of participants who were adults and therefore most likely to harbor low-density infections was higher in our study (47%) than in Tanzania (35% > 20 years)41 or Burkina Faso (25% > 15 years), potentially skewing density profiles.40 These directly measured density distributions support the use in high-transmission settings of newly available HS-RDT, which has demonstrated enhanced analytical sensitivity for low-density parasitemias in Uganda,19 Papua New Guinea,22 and Myanmar.43 This enhanced detectability of HRP2 should enable the identification of a substantially higher proportion of low-density infections in asymptomatic community members.
The ability to capture these low-density infections in a range of transmission settings will be enhanced by the expanding use of ultrasensitive, high-throughput, real-time PCR detection assays like our duplex pfr364 assay, which offers a new option for robust and scalable parasite detection in molecular epidemiologic studies. Like the parasite detection assay targeting varATS,27 this assay targets a multicopy motif in the P. falciparum genome, specifically annealing to 22 identical sequences in the parasite genome. In direct comparisons, Ct values obtained by pfr364 and varATS assays on identical templates were highly correlated and nearly identical (Figure 1). The assay amplified a wide range of reference parasite genomes from diverse settings, and, when applied to quantitative standards during the production phase of testing, returned positive results in 51/52 replicate tests of a standard at 0.1 p/µL. In addition, the assay includes an internal human control β-tubulin to confirm gDNA extraction, and, similar to the varATS assay but unlike other ultrasensitive PCR detection assays, does not require a large blood volume from venipuncture37 or RNA extraction with reverse transcription,44 two steps which limit application to large field studies owing to both cost and specialized sampling requirements. Given the demonstrated ability of the similarly ultrasensitive varATS assay to identify gametocytemic infections22 and therefore the likely contribution of these low-density infections to onward transmission,42 future studies can adapt and improve these assays to better sample these cryptic infections in diverse settings.
As we previously reported with cRDT detection,23 we observed with PCR detection the clustering of P. falciparum infections within the households of children with malaria. Notably, this household clustering has typically been reported from low-transmission areas, including in Southeast Asia,45 Zambia,46 Namibia,47 Zanzibar,48 Rwanda,49 and coastal Kenya,50 and less often investigated in high-transmission settings like ours. Potential reasons for the spatial aggregation of infections typically include shared risks of vector exposure, similar accessibility to health care, or participation in household-level chains of parasite transmission. Understanding these factors, and how they may be interrupted in high-transmission settings, could serve as a rationale for household-level interventions.
This study has some limitations. Because enrollment was triggered by hospitalized malaria cases, we sampled few households during the low-transmission season, when parasite distributions may be quite different. Therefore, we could not compare detectability and parasite epidemiology between seasons. In addition, insofar as our observations may influence the design of RACD interventions, this approach may limit the generalizability of our findings to interventions which may use alternate triggers for screening, such as uncomplicated malaria. Our parasite density estimations could have been biased, owing to differential efficiency of gDNA extraction between samples or to stochastic variability between technical replicates at low densities. In mitigation, we observed consistent human β-tubulin amplification between samples within a very narrow range of Ct values, and we enforced additional criteria on parasite amplification output to reduce the risk of false-positives. In addition, the assay targets P. falciparum only and therefore would not be a suitable tool where non-falciparum species predominate. Finally, we did not have direct measurements of HRP2, the antigen detected by the cRDT, to better understand if HRP2 differences account for differences in parasite detectability.
We describe a large proportion of cRDT-negative infections in this high-transmission setting. Additional observations are that parasite densities in all participant subgroups were skewed toward very low densities, and therefore that the sensitivity was poor of a cRDT detecting HRP2. In addition, age mediated the probability of detecting an infection with cRDT, in that adults aged > 15 years had the lowest detectability irrespective of parasite density. These findings suggest that cRDT is not likely to be effective at detecting a large proportion of asymptomatic infections. Further steps include credible modeling of the impact on transmission of the detection and treatment of cRDT-positive infections, as well as of the incremental benefits on detectability and downstream impact of new HS-RDTs. These data and tools will enable us to test the efficiency and effectiveness of community-based detection strategies on malaria transmission in diverse settings.
Supplemental tables
Acknowledgments:
We thank Assumpta Nantume and Verona Liao for their assistance in the laboratory, and Ibrahim Khaoya, Isaac Kunusia, Eznah Mukeli, Eric Nalianya, Lilian Nukewa, Edith Wamalwa, and Aggrey Wekesa for their assistance with the field study. The following reagents obtained through BEI Resources, NIAID, NIH: P. falciparum, Strain 3D7 (MRA-102, contributed by Daniel J. Carucci), and genomic DNA from P. falciparum strains 3D7 (MRA-102G, contributed by Daniel J. Carucci), Dd2 (MRA-150G, contributed by David Walliker), FCR-3/FMG (Gambia) (MRA-736G, contributed by ATCC), 7G8 (MRA-926G, contributed by Thomas E. Wellems), and K1 (MRA 159G, contributed by Dennis E. Kyle). We are indebted to the individuals who participated in the field study.
Note: Supplemental tables appear at www.ajtmh.org.
REFERENCES
- 1.Bhatt S, et al. 2015. The effect of malaria control on Plasmodium falciparum in Africa between 2000 and 2015. Nature 526: 207–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gething PW, et al. 2016. Mapping Plasmodium falciparum mortality in Africa between 1990 and 2015. N Engl J Med 375: 2435–2445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sturrock HJ, Hsiang MS, Cohen JM, Smith DL, Greenhouse B, Bousema T, Gosling RD, 2013. Targeting asymptomatic malaria infections: active surveillance in control and elimination. PLoS Med 10: e1001467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.WHO , 2017. A Framework for Malaria Elimination. Available at: http://www.who.int/malaria/publications/atoz/9789241511988/en/. Accessed October 8, 2018. [Google Scholar]
- 5.Smith Gueye C, et al. 2013. Active case detection for malaria elimination: a survey among Asia Pacific countries. Malar J 12: 358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Larsen DA, Winters A, Cheelo S, Hamainza B, Kamuliwo M, Miller JM, Bridges DJ, 2017. Shifting the burden or expanding access to care? Assessing malaria trends following scale-up of community health worker malaria case management and reactive case detection. Malar J 16: 441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pinchoff J, Henostroza G, Carter BS, Roberts ST, Hatwiinda S, Hamainza B, Hawela M, Curriero FC, 2015. Spatial patterns of incident malaria cases and their household contacts in a single clinic catchment area of Chongwe district, Zambia. Malar J 14: 305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chihanga S, Haque U, Chanda E, Mosweunyane T, Moakofhi K, Jibril HB, Motlaleng M, Zhang W, Glass GE, 2016. Malaria elimination in Botswana, 2012–2014: achievements and challenges. Parasit Vectors 9: 99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deutsch-Feldman M, et al. 2018. Efficiency of a malaria reactive test-and-treat program in southern Zambia: a prospective, observational study. Am J Trop Med Hyg 98: 1382–1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Littrell M, Sow GD, Ngom A, Ba M, Mboup BM, Dieye Y, Mutombo B, Earle D, Steketee RW, 2013. Case investigation and reactive case detection for malaria elimination in northern Senegal. Malar J 12: 331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sturrock HJ, Novotny JM, Kunene S, Dlamini S, Zulu Z, Cohen JM, Hsiang MS, Greenhouse B, Gosling RD, 2013. Reactive case detection for malaria elimination: real-life experience from an ongoing program in Swaziland. PLoS One 8: e63830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hsiang MS, et al. 2019. Active case-finding for malaria: a three-year national evaluation of optimal approaches to detect infections and hotspots through reactive case detection in the low transmission setting of Eswatini. Clin Infect Dis. Available at: 10.1093/cid/ciz403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Campillo A, Daily J, Gonzalez IJ, 2017. International survey to identify diagnostic needs to support malaria elimination: guiding the development of combination highly sensitive rapid diagnostic tests. Malar J 16: 385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abba K, Deeks JJ, Olliaro P, Naing CM, Jackson SM, Takwoingi Y, Donegan S, Garner P, 2011. Rapid diagnostic tests for diagnosing uncomplicated P. falciparum malaria in endemic countries. Cochrane Database Syst Rev 7: CD008122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu L, van den Hoogen LL, Slater H, Walker PG, Ghani AC, Drakeley CJ, Okell LC, 2015. Comparison of diagnostics for the detection of asymptomatic Plasmodium falciparum infections to inform control and elimination strategies. Nature 528: S86–S93. [DOI] [PubMed] [Google Scholar]
- 16.Mogeni P, et al. 2017. Detecting malaria hotspots: a comparison of rapid diagnostic test, microscopy, and polymerase chain reaction. J Infect Dis 216: 1091–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cook J, et al. 2015. Mass screening and treatment on the basis of results of a Plasmodium falciparum-specific rapid diagnostic test did not reduce malaria incidence in Zanzibar. J Infect Dis 211: 1476–1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.WHO , 2017. Malaria Rapid Diagnostic Test Performance. Results of WHO Product Testing of Malaria RDTs: Round 7 (2015–2016). Available at: http://www.who.int/malaria/publications/atoz/978924151268/en/. Accessed October 10, 2018. [Google Scholar]
- 19.Das S, et al. 2017. Performance of a high-sensitivity rapid diagnostic test for Plasmodium falciparum malaria in asymptomatic individuals from Uganda and Myanmar and naive human challenge infections. Am J Trop Med Hyg 97: 1540–1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aydin-Schmidt B, Xu W, Gonzalez IJ, Polley SD, Bell D, Shakely D, Msellem MI, Björkman A, Mårtensson A, 2014. Loop mediated isothermal amplification (LAMP) accurately detects malaria DNA from filter paper blood samples of low density parasitaemias. PLoS One 9: e103905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cook J, et al. 2015. Loop-mediated isothermal amplification (LAMP) for point-of-care detection of asymptomatic low-density malaria parasite carriers in Zanzibar. Malar J 14: 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hofmann NE, et al. 2018. Assessment of ultra-sensitive malaria diagnosis versus standard molecular diagnostics for malaria elimination: an in-depth molecular community cross-sectional study. Lancet Infect Dis 18: 1108–1116. [DOI] [PubMed] [Google Scholar]
- 23.Obala AA, Mangeni JN, Platt A, Aswa D, Abel L, Namae J, Prudhomme O’Meara W, 2015. What is threatening the effectiveness of insecticide-treated bednets? A case-control study of environmental, behavioral, and physical factors associated with prevention failure. PLoS One 10: e0132778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Demas A, et al. 2011. Applied genomics: data mining reveals species-specific malaria diagnostic targets more sensitive than 18S rRNA. J Clin Microbiol 49: 2411–2418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beshir KB, Hallett RL, Eziefula AC, Bailey R, Watson J, Wright SG, Chiodini PL, Polley SD, Sutherland CJ, 2010. Measuring the efficacy of anti-malarial drugs in vivo: quantitative PCR measurement of parasite clearance. Malar J 9: 312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thompson JD, Higgins DG, Gibson TJ, 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 22: 4673–4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hofmann N, Mwingira F, Shekalaghe S, Robinson LJ, Mueller I, Felger I, 2015. Ultra-sensitive detection of Plasmodium falciparum by amplification of multi-copy subtelomeric targets. PLoS Med 12: e1001788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rantala AM, Taylor SM, Trottman PA, Luntamo M, Mbewe B, Maleta K, Kulmala T, Ashorn P, Meshnick SR, 2010. Comparison of real-time PCR and microscopy for malaria parasite detection in Malawian pregnant women. Malar J 9: 269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Plowe CV, Djimde A, Bouare M, Doumbo O, Wellems TE, 1995. Pyrimethamine and proguanil resistance-conferring mutations in Plasmodium falciparum dihydrofolate reductase: polymerase chain reaction methods for surveillance in Africa. Am J Trop Med Hyg 52: 565–568. [DOI] [PubMed] [Google Scholar]
- 30.Girma S, Cheaveau J, Mohon AN, Marasinghe D, Legese R, Balasingam N, Abera A, Feleke SM, Golassa L, Pillai DR, 2019. Prevalence and epidemiological characteristics of asymptomatic malaria based on ultrasensitive diagnostics: a cross-sectional study. Clin Infect Dis 69: 1003. [DOI] [PubMed] [Google Scholar]
- 31.Beshir KB, Sepulveda N, Bharmal J, Robinson A, Mwanguzi J, Busula AO, de Boer JG, Sutherland C, Cunningham J, Hopkins H, 2017. Plasmodium falciparum parasites with histidine-rich protein 2 (pfhrp2) and pfhrp3 gene deletions in two endemic regions of Kenya. Sci Rep 7: 14718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Levitt B, Obala A, Langdon S, Corcoran D, O’Meara WP, Taylor SM, 2017. Overlap extension barcoding for the next generation sequencing and genotyping of Plasmodium falciparum in individual patients in western Kenya. Sci Rep 7: 41108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dalrymple U, Arambepola R, Gething PW, Cameron E, 2018. How long do rapid diagnostic tests remain positive after anti-malarial treatment? Malar J 17: 228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Markwalter CF, Mudenda L, Leelawong M, Kimmel DW, Nourani A, Mbambara S, Thuma PE, Wright DW, 2018. Evidence for histidine-rich protein 2 immune complex formation in symptomatic patients in southern Zambia. Malar J 17: 256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Taylor DW, Bobbili N, Khadka VS, Quakyi IA, Leke RG, 2017. Individuals living in a malaria-endemic area of Cameroon do not have an acquired antibody response to Plasmodium falciparum histidine-rich protein 2. Malar J 16: 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Das S, Peck RB, Barney R, Jang IK, Kahn M, Zhu M, Domingo GJ, 2018. Performance of an ultra-sensitive Plasmodium falciparum HRP2-based rapid diagnostic test with recombinant HRP2, culture parasites, and archived whole blood samples. Malar J 17: 118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Imwong M, Hanchana S, Malleret B, Rénia L, Day NP, Dondorp A, Nosten F, Snounou G, White NJ, 2014. High-throughput ultrasensitive molecular techniques for quantifying low-density malaria parasitemias. J Clin Microbiol 52: 3303–3309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cheng Z, Wang D, Tian X, Sun Y, Sun X, Xiao N, Zheng Z, 2015. Capture and ligation probe-PCR (CLIP-PCR) for molecular screening, with application to active malaria surveillance for elimination. Clin Chem 61: 821–828. [DOI] [PubMed] [Google Scholar]
- 39.Lucchi NW, Narayanan J, Karell MA, Xayavong M, Kariuki S, DaSilva AJ, Hill V, Udhayakumar V, 2013. Molecular diagnosis of malaria by photo-induced electron transfer fluorogenic primers: PET-PCR. PLoS One 8: e56677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Goncalves BP, et al. 2017. Examining the human infectious reservoir for Plasmodium falciparum malaria in areas of differing transmission intensity. Nat Commun 8: 1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mwingira F, Genton B, Kabanywanyi AN, Felger I, 2014. Comparison of detection methods to estimate asexual Plasmodium falciparum parasite prevalence and gametocyte carriage in a community survey in Tanzania. Malar J 13: 433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Slater HC, et al. 2019. The temporal dynamics and infectiousness of subpatent Plasmodium falciparum infections in relation to parasite density. Nat Commun 10: 1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Landier J, et al. 2018. Operational performance of a Plasmodium falciparum ultrasensitive rapid diagnostic test for detection of asymptomatic infections in eastern Myanmar. J Clin Microbiol 56: e00565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Murphy SC, et al. 2012. Real-time quantitative reverse transcription PCR for monitoring of blood-stage Plasmodium falciparum infections in malaria human challenge trials. Am J Trop Med Hyg 86: 383–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lawpoolsri S, Chavez IF, Yimsamran S, Puangsa-Art S, Thanyavanich N, Maneeboonyang W, Chaimungkun W, Singhasivanon P, Maguire JH, Hungerford LL, 2010. The impact of human reservoir of malaria at a community-level on individual malaria occurrence in a low malaria transmission setting along the Thai-Myanmar border. Malar J 9: 143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stresman GH, Kamanga A, Moono P, Hamapumbu H, Mharakurwa S, Kobayashi T, Moss WJ, Shiff C, 2010. A method of active case detection to target reservoirs of asymptomatic malaria and gametocyte carriers in a rural area in southern province, Zambia. Malar J 9: 265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Smith JL, Auala J, Tambo M, Haindongo E, Katokele S, Uusiku P, Gosling R, Kleinschmidt I, Mumbengegwi D, Sturrock HJW, 2017. Spatial clustering of patent and sub-patent malaria infections in northern Namibia: implications for surveillance and response strategies for elimination. PLoS One 12: e0180845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bjorkman A, Cook J, Sturrock H, Msellem M, Ali A, Xu W, Molteni F, Gosling R, Drakeley C, Mårtensson A, 2017. Spatial distribution of falciparum malaria infections in zanzibar: implications for focal drug administration strategies targeting asymptomatic parasite carriers. Clin Infect Dis 64: 1236–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rulisa S, et al. 2013. Malaria prevalence, spatial clustering and risk factors in a low endemic area of eastern Rwanda: a cross sectional study. PLoS One 8: e69443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bejon P, et al. 2010. Stable and unstable malaria hotspots in longitudinal cohort studies in Kenya. PLoS Med 7: e1000304. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.