Abstract.
Leptospirosis is a zoonotic bacterial disease caused by pathogenic species of the genus Leptospira. Disease incidence is known to be attributed to environmental and social conditions which promote the spread of reservoir hosts, primarily rodents. A well-being program was conducted to determine the seroprevalence and risk factors associated with leptospirosis in urban poor communities occupying low-cost flat accommodation and squatter settlements in the vicinity of Wilayah Persekutuan, Kuala Lumpur. Blood samples from a total of 532 volunteers were screened for the detection of IgG and IgM antibodies against leptospirosis using ELISA. Demographic data were collected for each participant through a questionnaire survey before blood collection. The overall seroprevalence was low (12.6%, n = 67/532; 95% CI: 9.9–15.7%), with 8.1% (n = 43/532) being seropositive for anti-Leptospira IgG, indicating previous infection, and 4.9% (n = 26/532) for anti-Leptospira IgM, indicating current infection. Two significant factors such as host age (P ≤ 0.01) and knowledge of disease transmission (P = 0.017) significantly influenced the presence of anti-Leptospira IgM, whereas the detection of anti-IgG indicated the presence of clean drinking water sources (P = 0.043). Despite the low prevalence, the transmission of leptospirosis does occur among urban poor communities, suggesting the need for undertaking public awareness programs.
INTRODUCTION
The cumulative growth of urbanization in Malaysia from 43.2% (1989) to 71% (2010) has resulted in a population increase from 27% in 1970 to 71% in 2010.1 Many members of the population flock into the city to seek better lives, and such an increase in population density in the urban environment can impact on public health risks through the creation of dense infrastructures and inadequate sanitation, thereby facilitating the spread of communicable diseases. Urban poor is described as a condition whereby individuals or communities live in a city with the inability to cover costs for basic living needs. Urbanization, local and global migration from rural settlements to urban cities, together with increasing costs of living has contributed to a rise in urban poverty in Malaysia.2 Urban health risks are also distributed unequally and largely among marginalized social groups, particularly those living in slum areas where up to 40% of urban population growth has occurred. Air-borne diseases, such as tuberculosis, are associated with overcrowding and inadequate ventilation, whereas water- and vector-borne diseases, such as leptospirosis, are linked to unsafe water storage and poor waste management.
Leptospirosis has been recognized globally as a zoonotic disease and a major cause of illness both in humans and animals and is caused by spirochete bacteria belonging to the genus Leptospira.3 A total of 22 species has been described and classified according to DNA–DNA hybridization and phylogenetic analysis,4,5 with more than 300 serovars based on agglutinating lipopolysaccharide antigens.6 Human transmission is via direct contact with infected blood, tissues, organs, or urine of infected hosts or through indirect contact with contaminated fomites, soil, mud, fresh water, and rodent-infested habitats.7,8 Transmission can also occur via direct penetration of Leptospira through the conjunctiva or surface epithelium.9 The role of rats as a source of human infection was discovered in 1917 and recognized as a most important reservoir for infection as these rodents are abundant in most environments. Many clinical manifestations are observed with leptospirosis, ranging from asymptomatic and mild symptoms to a self-limited febrile and fulminant life-threatening one.7
Leptospirosis is highly prevalent and considered to be a reemerging disease in the Asia Pacific region. Malaysia is ranked in the top 20 countries, relative to high incidences of leptospirosis,10 which increased sharply from 2,268 cases in 2011 to 8,291 in 2015.11 The state of Wilayah Persekutuan in Kuala Lumpur is ranked with the highest number of outbreaks in the country, especially due to overcrowding, poverty, and poor sanitation in urban slum areas.12,13 The present study targeted communities residing in the People’s Housing Program (Program Perumahan Rakyat [PPR]) developed by the Ministry of Housing and Local Government Malaysia. Such a housing program was established following the demolition of squatter dwellings previously occupied by individuals within lower income groups and to fulfill their need for low-cost housing. Generally, urban housing comprises high-density flats equipped with basic facilities including clean water and sanitation. However, the situation is a far cry from reality as most PPR developments in Kuala Lumpur are vastly overcrowded with poor waste management.14
In view of the wide range of clinical manifestations shown by leptospirosis, severe cases are only detected when hospitalized. Therefore, a seroprevalence study was conducted for the first time in the urban poor community of Wilayah Persekutuan to identify risk factors associated with leptospirosis infection.
METHODS
Study population.
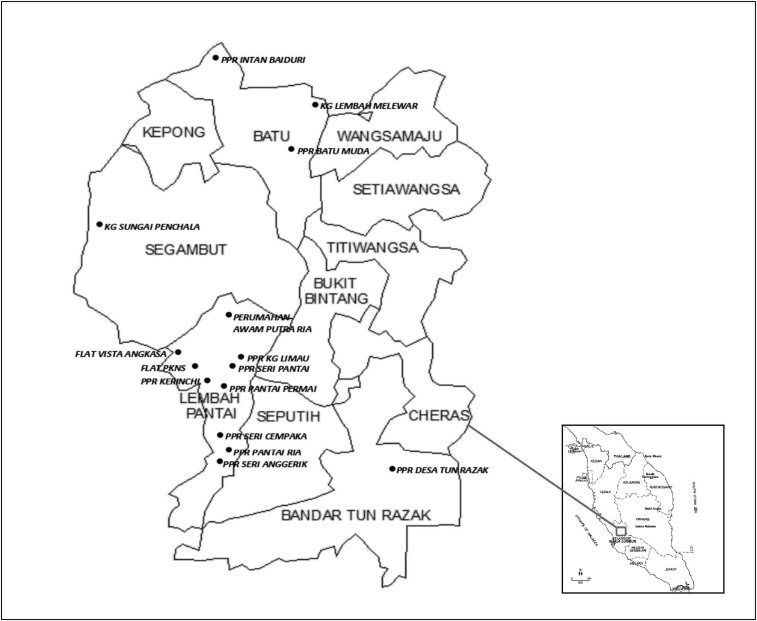
Using a well-being community program, an investigation of a targeted community within the state of Wilayah Persekutuan, Kuala Lumpur, was undertaken between October 2017 and March 2018 (Table 1, Figure 1). A minimum sample size of the population was calculated using a formula by Leedy and Ormrod15 and based on earlier estimates of prevalence (12.6%) in Malaysia.16 A total of 532 volunteers were successfully recruited, and each individual was given a set of questionnaires relating to sociodemographic factors, health status, environmental health, and awareness of leptospirosis. Consent forms were collected from each individual before collection of blood samples. Ethical clearance was obtained before commencing the study (reference number: BK-MIS-1117-E01).
Table 1.
Locations of urban poor communities with global positioning system (GPS) coordinates
| Locations | Coordinates | Locations | Coordinates |
|---|---|---|---|
| PPR Kerinchi | 3.107149, 101.668984 | Flat PKNS | 3.112573, 101.663235 |
| PPR Seri Anggerik | 3.092198, 101.673858 | PPR Kampung Limau | 3.109022, 101.674798 |
| Kampung Lembah Melewar | 3.218245, 101.698487 | Flat Vista Angkasa | 3.113418, 101.661690 |
| PPR Desa Tun Razak | 3.078596, 101.717112 | PPR Pantai Permai | 3.108372, 101.668760 |
| Perumahan Awam Putra Ria | 3.123049, 101.676385 | Kampung Sungai Penchala | 3.162505, 101.625649 |
| PPR Intan Baiduri | 3.235292, 101.655946 | PPR Batu Muda | 3.208055, 101.682405 |
| PPR Seri Cempaka | 3.100525, 101.674443 | PPR Pantai Ria | 3.097437, 101.671294 |
| PPR Sri Pantai | 3.106026, 101.674388 | – | – |
PPR = Program Perumahan Rakyat.
Figure 1.
Locations of urban poor communities from Kuala Lumpur in Peninsular Malaysia.
Sample collection.
A 5-mL sample of venous blood was drawn from each volunteer by medically trained personnel, and the samples were placed into BD Vacutainer SST™ II Advance Plus (Fisher Scientific, Loughborough, Leicestershire, UK) blood collection tubes (each with a yellow top cap). Each tube was kept in an ice box and immediately transported to the Parasitology Laboratory, Institute of Biological Sciences, University of Malaya. Tubes were centrifuged using a benchtop centrifuge (Universal 320) (Hettich, Tuttlingen, Germany) at 1,500 × g for 10 minutes and the serum samples maintained at −20°C until use.
Detection of immunoglobulin G and M antibodies to Leptospira spp.
Seropositivity for Leptospira spp. infection was demonstrated by anti-Leptospira IgG and IgM antibodies using standard ELISA commercial kits (SERION ELISA classic, Institut Virion/Serion GmbH, Warburg, Germany). All reagents were maintained at room temperature for testing and sera allowed to thaw at the same temperature.
First, washing solutions were prepared by diluting the buffer concentrate (V1) 1:30 with distilled water to reach a final volume of 1,000 mL (V2). Before running the test, a rheumatoid factor (Rf) absorbent was used to dilute the buffer at a ratio of 1:4. A total of 200 µL of Rf absorbent was added to 800 µL of dilution buffer, and then in the case of IgM Leptospira kits, 10 µL of each volunteer’s sample was diluted with 1,000 µL of Rf dilution buffer. For IgG Leptospira kits, 10 μL of volunteer samples was diluted with 1,000 μL of Rf dilution buffer (1:100). Following dilution and before pipetting into microtiter plates, samples were mixed thoroughly to ensure the solutions were homogenous.
Rheumatoid factors are autoantibodies mainly of the IgM class, which preferably bind to IgG immune complexes. The presence of nonspecific IgM antibodies (Rfs) can lead to false-positive results, and in addition, weak-binding pathogen-specific IgM antibodies may be displaced by stronger binding ones such as IgG, leading to false-negatives. Samples must, therefore, be treated with Rf absorbents before antibody detection. Sera must also be diluted with buffer during the assay because heterophilic antibodies in human serum/plasma are capable of binding to both capture and detection antibodies and cross-linking can result in false-positives.
Apart from the blank substrate, each of 100 µL of diluted sample or ready-to-use controls were added to appropriate wells of the microtiter test strips and incubated at 37°C for 60 minutes in a moist chamber. Following incubation, wells were washed four times with 300 µL washing solution and 100 µL of the ready-to-use antihuman IgM conjugate (for Leptospira IgM) and antihuman IgG conjugate (for Leptospira IgG). Apart from the blank substrate, alkaline phosphatase conjugate was added to the wells and incubated again for 30 minutes. Following the second incubation, all wells were rinsed with the washing solution and 100 µL of ready-to-use p-nitrophenyl phosphate (pNPP) substrate was added, followed by incubation at 37°C for 30 minutes in a moist chamber. Finally, 100 µL of sodium hydroxide (stopping solution) was added to each well, and following gentle shaking of the microtiter plate, the enzyme–substrate reaction was stopped. Within 60 minutes, optical densities of samples against blank substrates were read at a wavelength of 405 nm (yellow water-soluble reaction products absorb light at this wavelength) against a background of 650 nm.
Data analysis.
Seroprevalences of infection (%) are shown with 95% CIs as described by Rohlf and Sokal.17 Seroprevalences were analyzed using maximum likelihood techniques based on log linear analysis of contingency tables using the software package SPSS (version 22) (IBM, Armonk, NY).
RESULTS
Seroprevalences of anti-Leptospira IgG and IgM within the poor communities.
A total of 532 participants from 15 locations were involved in this study, and these locations included PPR Seri Anggerik (n = 53; 9.96%), Kampung Lembah Melewar (n = 7; 1.32%), PPR Desa Tun Razak (n = 53; 9.96%), Perumahan Awam Putra Ria (n = 31; 5.83%), PPR Intan Baiduri (n = 34; 6.39%), PPR Seri Cempaka (n = 66; 12.41%), PPR Sri Pantai (n = 46; 8.65%), Flat Perbadanan Kemajuan Negeri Selangor (PKNS) (n = 12; 2.26%), PPR Kampung Limau (n = 41; 7.71%), Flat Vista Angkasa (n = 24; 4.51%), PPR Pantai Permai (n = 37; 6.95%), Kampung Sungai Penchala (n = 76; 14.29%), PPR Batu Muda (n = 8; 1.50%), PPR Kerinchi (n = 6; 1.13%), and PPR Pantai Ria (n = 38; 7.1%).
The overall seroprevalence of Leptospira infections among 532 participants was 12.6% (n = 67; 95% CI: 9.9–15.7%), with 8.1% (n = 43; 95% CI: 5.9–10.7%) seropositive to anti-Leptospira IgG and 4.9% (n = 26; 95% CI: 3.2–7.1%) seropositive to anti-Leptospira IgM. Only a total of 0.4% (n = 2; 95% CI: 0–1.4%) were positive to both anti-Leptospira IgG and IgM. According to SERION ELISA classic, Germany, samples positive for both IgG and IgM antibodies suggest acute infections, although in the present study positive participants did not present any clinical disease symptoms.
Seroprevalences of antibodies to Leptospira infections relative to sociodemographic and environmental factors.
The sociodemographic profile mainly comprised women (n = 326; 61.3%) and participants of age groups more than 55 years (n = 179, 33.6%). A large proportion was Muslims (n = 480; 90.2%), with more than half unemployed (n = 345; 64.8%). Of the four factors considered (gender, age, religion, and occupation), only age was associated with seropositivity of anti-Leptospira IgM ( = 34.145, P < 0.01), with seroprevalence being significantly associated with the 13–17 years age group (Table 2).
Table 2.
Sociodemographic factors and seroprevalences of IgG+ and IgM+ antibodies to Leptospira infections among urban poor communities in Wilayah Persekutuan, Kuala Lumpur
| Factors | IgG+ | IgM+ | IgG+ IgM+ | ||||
|---|---|---|---|---|---|---|---|
| % (95% CI) | P-value | % (95% CI) | P-value | % (95% CI) | P-value | ||
| Gender | Male (n = 206) | 9.7 [6.0–14.6] | 0.274 | 2.9 [1.1–6.2] | 0.093 | 0.0 [0.0–0.0] | 0.161 |
| Female (n = 326) | 7.1 [4.5–10.4] | 6.1 [3.8–9.3] | 0.6 [0.1–2.2] | ||||
| Age (years) | < 12 (n = 13) | 0.0 [0.0–24.7] | 0.737 | 15.4 [1.9–45.4] | < 0.01* | 0.0 [0.0–0.0] | 0.317 |
| 13–17 (n = 15) | 13.3 [1.7–40.5] | 40.0 [16.3–67.7] | 6.7 [0.2–31.9] | ||||
| 18–24 (n = 23) | 4.3 [0.1–21.9] | 13.0 [2.8–33.6] | 0.0 [0.0–0.0] | ||||
| 25–34 (n = 51) | 7.8 [2.2–18.9] | 0.0 [0.0–7.0] | 0.0 [0.0–0.0] | ||||
| 35–44 (n = 107) | 6.5 [2.7–13.0] | 7.5 [3.3–14.2] | 0.0 [0.0–0.0] | ||||
| 45–54 (n = 144) | 10.4 [5.9–16.6] | 2.1 [0.4–6.0] | 0.7 [0.0–3.8] | ||||
| > 55 (n = 179) | 7.8 [4.3–12.8] | 2.2 [0.6–5.6] | 0.0 [0.0–0.0] | ||||
| Religion | Islam (n = 480) | 8.1 [5.8–10.9] | 0.836 | 4.8 [3.1–7.1] | 0.912 | 0.2 [0.0–1.2] | 0.639 |
| Buddhist (n = 5) | 20.0 [0.5–71.6] | 0.0 [0.0–52.2] | 0.0 [0.0–0.0] | ||||
| Hindu (n = 42) | 7.1 [1.5–19.5] | 7.1 [1.5–19.5] | 2.4 [0.1–12.6] | ||||
| Christian (n = 3) | 0.0 [0.0–70.8] | 0.0 [0.0–70.8] | 0.0 [0.0–0.0] | ||||
| Others (n = 2) | 0.0 [0.0–84.2] | 0.0 [0.0–84.2] | 0.0 [0.0–0.0] | ||||
| Occupation | Employed (n = 187) | 7.5 [4.2–12.2] | 0.710 | 3.7 [1.5–7.6] | 0.368 | 0.5 [0.0–2.9] | 0.667 |
| Not employed (n = 345) | 8.4 [5.7–11.8] | 5.5 [3.3–8.5] | 0.3 [0.0–1.6] | ||||
* Significant at P = 0.05.
Environmental health factors considered in the community included the standard of accommodation, sources of drinking water, waste disposal methods, and pet ownership. A significant association was recorded between the seropositivity of anti-Leptospira IgG and sources of drinking water ( = 4.087, P = 0.043), and no risk factors were linked with the seroprevalence of anti-Leptospira IgM (Table 3).
Table 3.
Environmental health factors and seroprevalences of IgG+ and IgM+ antibodies to Leptospira infections among urban poor communities in Wilayah Persekutuan, Kuala Lumpur
| Factors | IgG+ | IgM+ | IgG+ IgM+ | ||||
|---|---|---|---|---|---|---|---|
| % (95% CI) | P-value | % (95% CI) | P-value | % (95% CI) | P-value | ||
| Accommodation | PPR or Flat (n = 448) | 7.6 [5.3–10.4] | 0.335 | 5.1 [3.3–7.6] | 0.542 | 0.4 [0.1–1.6] | 0.407 |
| Squatter home (n = 84) | 10.7 [5.0–19.4] | 3.6 [0.7–10.1] | 0.0 [0.0–0.0] | ||||
| Drinking water sources | Piped and boiled (n = 363) | 9.6 [6.8–13.2] | 0.043* | 5.2 [3.2–8.1] | 0.586 | 0.6 [0.1–2.0] | 0.216 |
| Mineral water (n = 169) | 4.7 [2.1–9.1] | 4.1 [1.7–8.3] | 0.0 [0.0–0.0] | ||||
| Waste disposal near housing area | Yes (n = 345) | 8.7 [5.9–12.2] | 0.481 | 4.6 [2.7–7.4] | 0.717 | 0.3 [0.0–1.6] | 0.667 |
| No (n = 187) | 7.0 [3.8–11.6] | 5.3 [2.6–9.6] | 0.5 [0.0–2.9] | ||||
| Domestic animals at residences | Yes (n = 182) | 6.6 [3.5–11.2] | 0.363 | 2.7 [0.9–6.3] | 0.099 | 0.0 [0.0–0.0] | 0.195 |
| No (n = 350) | 8.9 [6.1–12.3] | 6.0 [3.8–9.0] | 0.6 [0.1–2.0] | ||||
* Significant at P = 0.05.
Prior knowledge, etiology, and clinical symptoms of leptospirosis.
Up to 64.7% of volunteers were knowledgeable in the fundamental aspects of the disease, with 58.6% being aware that leptospirosis was fatal, and 75% appreciated that rats were major carriers of the disease. Those volunteers who lacked knowledge of disease transmission showed significant correlation with seropositivity of anti-Leptospira IgM ( = 5.666, P = 0.017) (Table 4).
Table 4.
Seroprevalences of IgG+ and IgM+ antibodies to leptospirosis relative to prior knowledge of the disease among urban poor communities in Wilayah Persekutuan, Kuala Lumpur
| Prior knowledge | IgG+ | IgM+ | IgG+ IgM+ | ||||
|---|---|---|---|---|---|---|---|
| % (95% CI) | P-value | % (95% CI) | P-value | % (95% CI) | P-value | ||
| Basic knowledge | Yes (n = 344) | 6.4 [4.1–9.5] | 0.053 | 3.2 [1.6–5.6] | 0.017* | 0.3 [0.0–1.6] | 0.671 |
| No (n = 188) | 11.2 [7.0–16.6] | 8.0 [4.5–12.8] | 0.5 [0.0–2.9] | ||||
| Fatal disease | Yes (n = 312) | 7.4 [4.7–10.9] | 0.474 | 3.5 [1.8–6.2] | 0.083 | 0.3 [0.0–1.8] | 0.805 |
| No (n = 220) | 9.1 [5.6–13.7] | 6.8 [3.9–11.0] | 0.5 [0.0–2.5] | ||||
| Transmission by rats | Yes (n = 399) | 7.8 [5.3–10.8] | 0.646 | 4.3 [2.5–6.7] | 0.246 | 0.3 [0.0–1.4] | 0.447 |
| No (n = 133) | 9.0 [4.7–15.2] | 6.8 [3.1–12.5] | 0.8 [0.0–4.1] | ||||
* Significant at P = 0.05.
With reference to etiology, majority of the participants had no contact with the urine of rats (n = 523; 98.3%) and never walked with bare feet (n = 494; 92.9%). A high proportion demonstrated frequent occurrences not only of hand washing (n = 424; 79.7%) but also of hand washing before food consumption (n = 521; 97.9%). A small proportion had neither engaged in aquatic recreational activities recently (n = 24; 4.5%) nor involved in previous flood disasters (n = 55; 10.34%). Overall, none of the issues analyzed were found to be associated with seropositivity of anti-Leptospira IgG and IgM (Table 5).
Table 5.
Seroprevalences of IgG+ and IgM+ antibodies to leptospirosis relative to etiological factors among urban poor communities in Wilayah Persekutuan, Kuala Lumpur
| Etiological factors | IgG+ | IgM+ | IgG+ IgM+ | ||||
|---|---|---|---|---|---|---|---|
| % (95% CI) | P-value | % (95% CI) | P-value | % (95% CI) | P-value | ||
| Rat’s urine contact | Yes (n = 9) | 0.0 [0.0–33.6] | 0.216 | 0.0 [0.0–33.6] | 0.340 | 0.0 [0.0–0.0] | 0.794 |
| No (n = 523) | 8.2 [6.0–10.9] | 5.0 [3.3–7.2] | 0.4 [0.0–1.4] | ||||
| Walking without shoes | Yes (n = 38) | 5.3 [0.6–17.7] | 0.484 | 7.9 [1.7–21.4] | 0.408 | 0.0 [0.0–0.0] | 0.586 |
| No (n = 494) | 8.3 [0.6–11.1] | 4.7 [3.0–6.9] | 0.4 [0.1–1.5] | ||||
| Involved in flood | Yes (n = 24) | 16.7 [4.7–37.4] | 0.159 | 0.0 [0.0–14.2] | 0.117 | 0.0 [0.0–0.0] | 0.667 |
| No (n = 508) | 7.7 [5.5–10.3] | 5.1 [3.4–7.4] | 0.4 [0.0–1.4] | ||||
| Waterfall/river visit | Yes (n = 55) | 5.5 [1.1–15.1] | 0.427 | 7.3 [2.0–17.6] | 0.414 | 0.0 [0.0–0.0] | 0.508 |
| No (n = 477) | 8.4 [6.1–11.2] | 4.6 [2.9–6.9] | 0.4 [0.1–1.5] | ||||
| Hand wash | < 3 times (n = 20) | 5.0 [0.1–24.9] | 0.814 | 5.0 [0.1–24.9] | 0.932 | 0.0 [0.0–0.0] | 0.635 |
| 3–5 times (n = 88) | 9.1 [4.0–17.1] | 5.7 [1.9–12.8] | 0.0 [0.0–0.0] | ||||
| > 5 times (n = 424) | 8.0 [5.6–11.0] | 4.7 [2.9–7.2] | 0.5 [0.1–1.7] | ||||
| Eating with hand | Yes (n = 521) | 8.1 [5.9–10.7] | 0.903 | 5.0 [3.3–7.2] | 0.291 | 0.4 [0.0–1.4] | 0.772 |
| No (n = 11) | 9.1 [0.2–41.3] | 0.0 [0.0–28.5] | 0.0 [0.0–0.0] | ||||
Symptoms of illness among participants, including headaches, jaundice, myalgia, diarrhea, abdominal discomfort, chills, and fevers, appeared to be unrelated to leptospirosis (Table 6).
Table 6.
Seroprevalences of IgG+ and IgM+ antibodies to leptospirosis relative to clinical symptoms among urban poor communities in Wilayah Persekutuan, Kuala Lumpur
| Clinical symptoms | IgG+ | IgM+ | IgG+ IgM+ | ||||
|---|---|---|---|---|---|---|---|
| % (95% CI) | P-value | % (95% CI) | P-value | % (95% CI) | P-value | ||
| Headaches | Yes (n = 237) | 10.1 [6.6–14.7] | 0.122 | 6.8 [3.9–10.7] | 0.075 | 0.4 [0.0–2.3] | 0.877 |
| No (n = 295) | 6.4 [3.9–9.9] | 3.4 [1.6–6.1] | 0.3 [0.0–1.9] | ||||
| Jaundice | Yes (n = 1) | 0.0 [0.0–97.5] | 0.681 | 0.0 [0.0–97.5] | 0.751 | 0.0 [0.0–0.0] | 0.931 |
| No (n = 531) | 8.1 [5.9–10.8] | 4.9 [3.2–7.1] | 0.4 [0.0–1.4] | ||||
| Myalgia | Yes (n = 191) | 6.3 [3.3–10.7] | 0.246 | 4.2 [1.8–8.1] | 0.572 | 0.0 [0.0–0.0] | 0.182 |
| No (n = 341) | 9.1 [6.3–12.7] | 5.3 [3.2–8.2] | 0.6 [0.1–2.1] | ||||
| Chills | Yes (n = 42) | 2.4 [0.1–12.6] | 0.104 | 9.5 [2.7–22.6] | 0.191 | 0.0 [0.0–0.0] | 0.566 |
| No (n = 490) | 8.6 [6.2–11.4] | 4.5 [2.8–6.7] | 0.4 [0.0–1.5] | ||||
| Diarrhea | Yes (n = 117) | 8.5 [4.2–15.2] | 0.836 | 6.0 [2.4–11.9] | 0.543 | 0.0 [0.0–0.0] | 0.318 |
| No (n = 415) | 8.0 [5.5–11.0] | 4.6 [2.8–7.1] | 0.5 [0.1–1.7] | ||||
| Abdominal discomfort | Yes (n = 140) | 9.3 [5.0–15.4] | 0.548 | 7.1 [3.5–12.7] | 0.165 | 0.7 [0.0–3.9] | 0.475 |
| No (n = 392) | 7.7 [5.2–10.7] | 4.1 [2.4–6.5] | 0.3 [0.0–1.4] | ||||
| Recent fever | Yes (n = 76) | 7.9 [3.0–16.4] | 0.948 | 5.3 [1.5–12.9] | 0.871 | 0.0 [0.0–0.0] | 0.432 |
| No (n = 456) | 8.1 [5.8–11.0] | 4.8 [3.0–7.2] | 0.4 [0.1–1.6] | ||||
DISCUSSION
Leptospirosis has been recognized as a reemerging infectious disease with global importance in urban and rural settings both in tropical and subtropical countries.18 Leptospirosis remains a major environmental and social disease in the Asia Pacific region with sporadic outbreaks in Southeast Asia, especially in Thailand, India, Malaysia, and Indonesia.6 The number of human cases worldwide is either underestimated or not well-documented, largely because of inadequate surveillance and similarities in clinical manifestation with other febrile diseases.19 Malaysia appears to have one of the highest number of disease incidences worldwide,10 especially in the state of Wilayah Persekutuan, Kuala Lumpur.11
The present work represents the first large-scale attempt to determine the seroprevalence of this disease in a targeted community. The discovery of relatively low seroprevalences among urban poor communities in Wilayah Persekutuan, Kuala Lumpur, is an indication of a successful attempt by the Malaysian government to provide basic housing and amenities to these marginalized communities. Nevertheless, there is concern that these communities are at risk of being infected from contaminated water, and although water samples were not examined, the present study has shown a significant association between the seroprevalence of anti-Leptospira IgG with piped water sources. Evidence of pathogenic Leptospira in recreational lakes and sewer effluents has previously been recorded in Kuala Lumpur,20 and many leptospirosis outbreaks have been reported worldwide because of poor sanitation and contamination of urban water supplies.21–24 In addition, 69 residents from a nurses’ hostel in Chennai, South India, were infected with leptospirosis by drinking contaminated water from an underground storage tank.25 In developing countries, water is treated with chlorine, although recontamination is a major problem often due to the design, construction, and inadequate maintenance of storage facilities and also poor quality control.26
Substantial improvements are also needed to improve waste disposal management systems in these residential locations because uncollected garbage provides ideal conditions for rodent reservoir hosts to breed and thrive. A recent study recorded two predominant pathogenic Leptospira serovars from two dominant rat species: Leptospira borgpetersenii serovar Javanica and Leptospira interrogans serovar Bataviae in Kuala Lumpur.27,28 The presence of pathogenic Leptospira thriving in urban rat populations, aided with an abundance of food, poor garbage management, and rapid urbanization, can ultimately result in rodents living in close proximity to human populations, thereby facilitating Leptospira transmission.
In the present study, SERION ELISA classic IgG and IgM tests demonstrated a reliable sensitivity of 94.7% and specificity of > 99% compared with the microscopic agglutination test (MAT) (sensitivity of 90%; specificity > 90%) previously shown by Russell et al.29 and Da Silva et al.30 This type of serology test, apart from exhibiting a high specificity and sensitivity, typically provides results within 4 hours31 and also distinguishes acute and previous infections.32 By contrast, the MAT technique takes several hours to complete18 and is also unable to differentiate between current, recent, and past infections. Polymerase chain reaction (PCR) methods, on the other hand, offer greater sensitivity and specificity when used in clinical cases because infections can be diagnosed rapidly and completed within the first week of illness.33 But PCR is more costly to run, requiring expensive equipment and skilled laboratory personnel.30
However, positive IgM antibody is a strong indicator of acute Leptospira infections.34 Normally, IgM antibodies can be detected not only 2 days following the onset of symptoms but also up to a further 5 months in infected patients. Yet, not all patients demonstrate IgG production following a Leptospira infection. Silva et al.34 showed that a maximum of 87.5% patients had IgG antibody titers up to 2–3 months postinfection and the remaining patients demonstrated no responses. Therefore, in the present study, participants who were positive for both anti-Leptospira IgG and IgM (n = 2) were considered to harbor acute infections.
Young participants, especially within the 13- to 17-year age group, showed a relatively high prevalence of 40% of anti-Leptospira IgM compared with 17.1% in the 20- to 29-year age group in a previous study in Malaysia.35 In China, a high proportion of students in the younger age group were also diagnosed with leptospirosis likely to be related to the frequency of outdoor recreational activities, including jungle trekking, swimming, kayaking, climbing, and mountain biking.36,37 On the other hand, in Malaysia from 2004 to 2012, a higher number of leptospirosis cases occurred in the middle-aged 30- to 39-year age group,35 and these findings are similar to those reported in the Netherlands and Germany, where greater levels of mobility of this age group may increase the risk of exposure.38,39 Furthermore, increases in the incidences of leptospirosis in more active groups in the community are likely to be associated with recreational activities, such as water sports.13,35,40
In the present investigation, a lack of knowledge on the transmission of leptospirosis was identified as a risk factor, particularly the presence of anti-Leptospira IgM and IgG in blood samples examined from a range of participants. Poor awareness of this disease, including inadequate sanitation and hygiene practices, together with the presence of rodent populations contributed to the risk of infection as in other communities.41–43 Effective cleaning and disinfection, to alleviate infrequent or improper hand washing, are necessary for disease prevention because leptospirosis is primarily spread through direct or indirect contact with contaminated urine. Therefore, those urban poor communities who are at risk of infection in Malaysia must be made aware of the mode of transmission of leptospirosis so that systematic surveillance, prevention, treatment, and control of the disease can be implemented.
In conclusion, leptospirosis is among one of the leading zoonotic causes of morbidity worldwide and also accounts for much mortality in vulnerable human populations in both urban and rural settings. Low seroprevalences of leptospirosis infection were found among urban poor communities in Wilayah Persekutuan, Kuala Lumpur, and these findings suggest the importance of introducing public awareness programs on the epidemiology of leptospirosis and particularly the role of rodents as reservoirs of infection. Rodent control management should, therefore, be implemented, especially in urban slum areas where poor sanitation and infrastructure, together with inadequate waste management, can contribute to disease outbreaks.
Acknowledgments:
We express our sincere and grateful thanks to all participants, participating companies and agencies, and Ministry of Health, Malaysia, and to the medical staff and nurses from University Malaya Medical Centre (UMMC) and Hospital Universiti Kebangsaan Malaysia (HUKM) for their invaluable technical assistance.
REFERENCES
- 1.Siwar C, Ahmed F, Bashawir A, Mia MS, 2016. Urbanization and urban poverty in Malaysia: consequences and vulnerability. J Appl Sci 16: 154–160. [Google Scholar]
- 2.Zainal NR, Kaur G, Ahmad NA, Khalili JM, 2012. Housing conditions and quality of life of the urban poor in Malaysia. Procedia Soc Behav Sci 50: 827–838. [Google Scholar]
- 3.Waitkins S, 1986. Leptospirosis as an occupational disease. Br J Ind Med 43: 721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bourhy P, Collet L, Brisse S, Picardeau M, 2014. Leptospira mayottensis sp. nov., a pathogenic species of the genus Leptospira isolated from humans. Int J Syst Evol Microbiol 64: 4061–4067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saito M, Villanueva SY, Chakraborty A, Miyahara S, Segawa T, Asoh T, Yoshida S, 2013. Comparative analysis of Leptospira strains isolated from environmental soil and water in the Philippines and Japan. Appl Environ Microbiol 79: 601–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Victoriano AF, et al. 2009. Leptospirosis in the Asia Pacific region. BMC Infect Dis 9: 147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Terpstra WJ, 2003. Human Leptospirosis: Guidance for Diagnosis, Surveillance and Control, World Health Organization, International Leptospirosis Society. Available at: http://apps.who.int/iris/handle/10665/42667. Accessed October 10, 2018. [Google Scholar]
- 8.Adler B, de la Peña Moctezuma A, 2010. Leptospira and leptospirosis. Vet Microbiol 140: 287–296. [DOI] [PubMed] [Google Scholar]
- 9.Russ A, Jali I, Bahaman A, Tuen A, Ismail G, 2003. Seroepidemiological Study of Leptospirosis among the Indigenous Communities Living in the Periphery of Crocker Range Park Sabah, Malaysia. Available at: http://www.arbec.com.my/pdf/art10janmar03.pdf. Accessed October 12, 2018. [Google Scholar]
- 10.Pappas G, Papadimitriou P, Siozopoulou V, Christou L, Akritidis N, 2008. The globalization of leptospirosis: worldwide incidence trends. Int J Infect Dis 12: 351–357. [DOI] [PubMed] [Google Scholar]
- 11.Ministry of Health Malaysia , 2015. Epidemiology and current situation of leptospirosis in Malaysia. Dr. Zainudin AW Persidangan Kesihatan Persekitaran Pihak Berkuasa Tempatan, 8–9 September, 2015, Wilayah Persekutuan Labuan. Available at: http://jkt.kpkt.gov.my/jkt/resources/PDF/Persidangan_2015/persidangan%20kesihatan/Leptospirosis_in_Malaysia.pdf. Accessed September 9, 2018. [Google Scholar]
- 12.Thayaparan S, Robertson I, Fairuz A, Suut L, Abdullah M, 2013. Leptospirosis, an emerging zoonotic disease in Malaysia. Malays J Pathol 35: 123–132. [PubMed] [Google Scholar]
- 13.Lau CL, Smythe LD, Craig SB, Weinstein P, 2010. Climate change, flooding, urbanisation and leptospirosis: fuelling the fire? Trans R Soc Trop Med Hyg 104: 631–638. [DOI] [PubMed] [Google Scholar]
- 14.Siti Salmiah AH, 2016. Jauh Cantik Dekat Jelik in My Metro. Available at: https://www.hmetro.com.my/node/186139. Accessed October 15, 2018. [Google Scholar]
- 15.Leedy PD, Ormrod JE, 2001. Practical Research: Planning and Design. Upper Saddle River, NJ: Merrill Prentice Hall. [Google Scholar]
- 16.El Jalii IM, Bahaman AR, Mohd-Azmi ML, Mutalib AR, 2002. Seroprevalence of human leptospirosis in representative population in Malaysia. Trop Biomed 19: 97–101. [Google Scholar]
- 17.Rohlf FJ, Sokal RR, 1995. Statistical Tables, 3rd edition San Francisco, CA: W.H. Freeman and Company. [Google Scholar]
- 18.Bharti AR, Nally JE, Ricaldi JN, Matthias MA, Diaz MM, Lovett MA, Gotuzzo E, 2003. Leptospirosis: a zoonotic disease of global importance. Lancet Infect Dis 3: 757–771. [DOI] [PubMed] [Google Scholar]
- 19.Socolovschi C, Angelakis E, Renvoisé A, Fournier PE, Marie JL, Davoust B, Raoult D, 2011. Strikes, flooding, rats, and leptospirosis in Marseille, France. Int J Infect Dis 15: e710–e715. [DOI] [PubMed] [Google Scholar]
- 20.Douadi B, Woh PY, Mohd Zain SN, Amran F, Thong KL, 2013. Pathogenic and saprophytic Leptospira species in water and soils from selected urban sites in Peninsular Malaysia. Microbes Environ 28: 135–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wynwood SJ, Graham GC, Weier SL, Collet TA, McKay DB, Craig SB, 2014. Leptospirosis from water sources. Pathog Glob Health 108: 334–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Padma V, Javid SM, 2014. Leptospirosis-a cross sectional study. WASJ 29: 879–883. [Google Scholar]
- 23.Johnson MA, Smith H, Joseph P, Gilman RH, Bautista CT, Campos KJ, Terry H, 2004. Environmental exposure and leptospirosis, Peru. Emerg Infect Dis 10: 1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ganoza CA, Matthias MA, Collins-Richards D, Brouwer KC, Cunningham CB, Segura ER, Vinetz JM, 2006. Determining risk for severe leptospirosis by molecular analysis of environmental surface waters for pathogenic Leptospira. PLoS Med 3: e308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ramakrishnan R, Patel M, Gupte M, Manickam P, Venkataraghavan S, 2003. An institutional outbreak of leptospirosis in Chennai, south India. J Comm Dis 35: 1–8. [PubMed] [Google Scholar]
- 26.Pitkanen T, Karinen P, Miettinen IT, Lettojarvi H, Heikkila A, Maunula R, 2011. Microbial contamination of groundwater of small community water supplies in Finland. Ambio 40: 377–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Douadi B, Mohd Zain SN, Amran F, Galloway RL, Kwai Lin T, 2013. Isolation and molecular characterization of Leptospira interrogans and Leptospira borgpetersenii isolates from the urban rat populations of Kuala Lumpur, Malaysia. Am J Trop Med Hyg 88: 704–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Douadi B, Mohd Zain SN, Ahmed AA, Mohd Khalid MKN, Hartskeerl R, Kwai Lin T, 2016. Predominance of the ST143 and ST50 Leptospira clones in the urban rat populations of Peninsular Malaysia. J Med Microbiol 65: 574–577. [DOI] [PubMed] [Google Scholar]
- 29.Russell KL, Montiel Gonzalez MA, Watts DM, Lagos-figueroa RC, Chauca G, Ore M, Vinetz JM, 2003. An outbreak of leptospirosis among Peruvian military recruits. Available at: https://www.ajtmh.org/content/journals/10.4269/ajtmh.2003.69.53. Accessed October 13, 2018. [PubMed]
- 30.Da Silva JB, Carvalho E, Hartskeerl RA, Ho PL, 2011. Evaluation of the use of selective PCR amplification of LPS biosynthesis genes for molecular typing of Leptospira at the serovar level. Curr Microbiol 62: 518–524. [DOI] [PubMed] [Google Scholar]
- 31.Musso D, La Scola B, 2013. Laboratory diagnosis of leptospirosis: a challenge. J Microbiol Immunol Infect 46: 245–252. [DOI] [PubMed] [Google Scholar]
- 32.Niloofa R, Fernando N, de Silva NL, Karunanayake L, Wickramasinghe H, Dikmadugoda N, Premawansa S, 2015. Diagnosis of leptospirosis: comparison between microscopic agglutination test, IgM-ELISA and IgM rapid immunochromatography test. PLoS One 10: e0129236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ahmed A, Engelberts MF, Boer KR, Ahmed N, Hartskeerl RA, 2009. Development and validation of a real-time PCR for detection of pathogenic Leptospira species in clinical materials. PLoS One 4: e7093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Silva M, Camargo E, Batista L, Vaz A, Brandao A, Nakamura P, Negrao J, 1995. Behaviour of specific IgM, IgG and IgA class antibodies in human leptospirosis during the acute phase of the disease and during convalescence. J Trop Med Hyg 98: 268–272. [PubMed] [Google Scholar]
- 35.El Jalii I, Bahaman A, Mohd-Azmi M, Mutalib A, 2000. Occurrence of human leptospirosis in Malaysia: a retrospective study. Trop Biomed 16: 1–5. [Google Scholar]
- 36.Zhang C, Wang H, Yan J, 2012. Leptospirosis prevalence in Chinese populations in the last two decades. Microbes Infect 14: 317–323. [DOI] [PubMed] [Google Scholar]
- 37.Tan WL, Soelar SA, Mohd Suan M, Hussin N, Cheah WK, Verasahib K, Goh PP, 2016. Leptospirosis incidence and mortality in Malaysia. Southeast Asian J Trop Med Public Health 47: 434–440. [PubMed] [Google Scholar]
- 38.Goris MGA, Boer KR, Duarte TATE, Kliffen SJ, Hartskeerl RA, 2013. Human leptospirosis trends The Netherlands, 1925–2008. Emerg Infect Dis 19: 371–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jansen A, Stark K, Schneider T, Schoeneberg I, 2007. Sex differences in clinical leptospirosis in Germany 1997–2005. Clin Infect Dis 44: 69–72. [DOI] [PubMed] [Google Scholar]
- 40.Shafei MN, Sulong MR, Yaacob NA, Hassan H, Wan Mohd Zahiruddin W, Daud A, Abdullah MR, 2012. Seroprevalence of leptospirosis among town service workers on northeastern state of Malaysia. Int J Collab Res Intern Med Public Health 4: 395–403. [Google Scholar]
- 41.Barcellos C, Sabroza PC, 2000. Socio-environmental determinants of the leptospirosis outbreak of 1996 in western Rio de Janeiro: a geographical approach. Int J Environ Health Res 10: 301–313. [DOI] [PubMed] [Google Scholar]
- 42.Felzemburgh RD, et al. 2014. Prospective study of leptospirosis transmission in an urban slum community: role of poor environment in repeated exposures to the Leptospira agent. PLoS Negl Trop Dis 8: e2927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Oliveira D, Guimarães M, Portugal J, Medeiros Z, 2009. The socio–demographic, environmental and reservoir factors associated with leptospirosis in an urban area of north–eastern Brazil. Ann Trop Med Parasitol 103: 149–157. [DOI] [PubMed] [Google Scholar]