Abstract.
Presently, it is difficult to accurately diagnose sepsis, a common cause of childhood death in sub-Saharan Africa, in malaria-endemic areas, given the clinical and pathophysiological overlap between malarial and non-malarial sepsis. Host biomarkers can distinguish sepsis from uncomplicated fever, but are often abnormal in malaria in the absence of sepsis. To identify biomarkers that predict sepsis in a malaria-endemic setting, we retrospectively analyzed data and sera from a case–control study of febrile Malawian children (aged 6–60 months) with and without malaria who presented to a community health center in Blantyre (January–August 2016). We characterized biomarkers for 29 children with uncomplicated malaria without sepsis, 25 without malaria or sepsis, 17 with malaria and sepsis, and 16 without malaria but with sepsis. Sepsis was defined using systemic inflammatory response criteria; biomarkers (interleukin-6 [IL-6], tumor necrosis factor receptor-1, interleukin-1 β [IL-1β], interleukin-10 [IL-10], von Willebrand factor antigen-2, intercellular adhesion molecule-1, and angiopoietin-2 [Ang-2]) were measured with multiplex magnetic bead assays. IL-6, IL-1β, and IL-10 were elevated, and Ang-2 was decreased in children with malaria compared with non-malarial fever. Children with non-malarial sepsis had greatly increased IL-1β compared with the other subgroups. IL-1β best predicted sepsis, with an area under the receiver operating characteristic (AUROC) of 0.71 (95% CI: 0.57–0.85); a combined biomarker–clinical characteristics model improved prediction (AUROC of 0.77, 95% CI: 0.67–0.85). We identified a distinct biomarker profile for non-malarial sepsis and developed a sepsis prediction model. Additional clinical and biological data are necessary to further explore sepsis pathophysiology in malaria-endemic regions.
INTRODUCTION
In 2016, 2.7 million children younger than 18 years died because of severe infections globally; children in sub-Saharan Africa (SSA) were disproportionately affected, representing two-thirds of these deaths.1 Pediatric sepsis, defined as a systemic inflammatory response syndrome (SIRS) in the presence of a suspected or proven infection, is the final common pathway for most infectious disease–related deaths (Box 1).2 Sepsis entails a complex interplay between the infectious pathogen and the host’s compensatory response and is a continuum of inflammation on a backdrop of a disrupted state of homeostasis.3,4 Sepsis is characterized by the host’s reaction to a pathogen, characterized by inflammation, microcirculatory disruption, endothelial activation and injury, direct end-organ damage from circulating cytokines, and dysregulation of the coagulation system.3,5,6 Inflammatory tumor necrosis factor, tumor necrosis factor receptor 1 (TNF-R1), interleukin-6 (IL-6), interleukin-1 β (IL-1β), and chemokines cause a systemic inflammatory response, whereas von Willebrand factor antigen-2 (vWFA2), intercellular adhesion molecule-1 (ICAM-1), and angiopoietin-2 (Ang-2) are released because of endothelial injury and activation.7–13 Increased anti-inflammatory mediators such as interleukin-10 (IL-10), transforming growth factor-1β, and interleukin-1ra characterize the compensatory anti-inflammatory response.3,4,6,7,10,14–16
Box 1.
Study definitions based on the presently accepted systemic inflammatory response syndrome (SIRS) and sepsis criteria for children developed by Goldstein et al.2,67
| Term | Definition |
|---|---|
| SIRS | The presence of > 2 of the following 4 criteria, one must be abnormal temperature or leukocyte count: |
| Axillary temperature of > 37.8°C or < 36°C | |
| Abnormal HR: tachycardia (HR > 2 SD above normal for age) or bradycardia for children aged < 1 year (HR < 10th% for age) | |
| Increased work of breathing (mean respiratory rate > 2 SD above normal for age or shortness of breath on examination) | |
| Leukocyte count elevated or depressed for age or > 10% immature neutrophils | |
| Infection | Suspected or proven, caused by any pathogen or a clinical syndrome associated with a high probability of infection. |
| Sepsis | SIRS in the presence/as a result of a suspected or proven infection. |
HR = heart rate; RR = respiratory rate.
In non–malaria-endemic regions, clinicians use pro-inflammatory host biomarkers to distinguish patients with severe disease from those with mild febrile disease.17–19 For example, there are data suggesting that the pro-inflammatory biomarker IL-6 may be useful for detecting the presence of a serious bacterial infection, a common cause of sepsis, in febrile infants20 and is used clinically to screen for neonatal sepsis.21,22 Elevations in IL-1β and IL-10 correlate with the degree of sepsis severity.14 Biomarkers of endothelial injury, such as Ang-2, are elevated in sepsis23 and also correlate with an increased risk of mortality and organ dysfunction.7
Although sepsis is a leading cause of death worldwide,3 appropriately identifying which febrile children have sepsis and, thus, are more likely to need urgent intervention and immediate hospital referral is challenging. Most pediatric case management guidelines for resource-limited settings combine history, symptoms, and vital signs to distinguish a healthy child from a sick child.24 However, using only the clinical examination has an unsatisfactory level of sensitivity and specificity for the detection of sepsis.25 Considering that an important aspect of improving the survival of septic patients is early and accurate diagnosis for timely initiation of appropriate treatment,26–29 there is still a need to develop a sepsis diagnostic method that is both sensitive and specific.
Identifying sepsis is further complicated in malaria-endemic regions of SSA for several reasons. First, malaria can be a cause of sepsis, but not every malaria infection results in sepsis. Second, there are often indistinguishable clinical features between malarial sepsis and sepsis caused by other organisms, making it difficult for clinicians to differentiate between these two common entities.30,31 In addition, there is overlapping pathophysiology between malaria infection and sepsis, making it difficult to diagnostically distinguish between the two clinical syndromes; for example, malaria infection is associated with endothelial injury, acute inflammation, and coagulation system activation even in the absence of SIRS and sepsis.32–41 Pro-inflammatory (TNF-R1) and anti-inflammatory (IL-10) biomarkers are elevated in young children with malaria,42 and, similar to sepsis, levels of IL-1β, IL-10, and Ang-2 correlate with the severity of malaria.35,43 Furthermore, higher parasite density has been associated with increased production of both anti-inflammatory biomarkers, such as IL-10,37,44 and pro-inflammatory cytokines, such as TNF-α, interferon-γ, and IL-1β.43
There are presently no highly sensitive, specific, and validated biomarkers that predict sepsis in malaria-endemic settings. This critical limitation impedes accurate risk stratification and development of therapeutic interventions and management algorithms for children with sepsis in SSA. This has direct clinical relevance, as a biomarker that is sensitive and specific for sepsis could aid in the early identification of children needing urgent interventions and hospital referral, and also help to distinguish septic children with and without malaria, which has immediate management implications. The objective of this study was, therefore, to describe serum biomarker profiles in Malawian children presenting with fever to a community clinic. We aimed to compare profiles between febrile children with and without sepsis and with and without malaria, and to identify a biomarker(s) that could distinguish children with and without sepsis.
MATERIALS AND METHODS
Study design and population.
We retrospectively analyzed the available serum collected during a case–control study of 156 children aged 6–60 months presenting with fever in Blantyre, Malawi, between January and August 2016. Immune responses in these children have previously been reported.45 The original study recruited three groups: 59 children with uncomplicated malaria (axillary temperature > 37.8°C at presentation, malaria positive, normal mental status by the Blantyre coma score, and absence of severe anemia [defined as a hemoglobin < 5g/dL]), 50 children with non-malarial fever (axillary temperature > 37.8°C at presentation and malaria negative), and 47 healthy controls (afebrile at the time of recruitment, malaria test negative, medically well, and no history of malaria in the past 3 months). Children with abnormal mental status by the Blantyre coma score, severe anemia, hypoglycemia (serum glucose < 45 mg/dL), HIV infection, or chronic illness were excluded.45 Malarial and non-malarial fever cases were recruited at the time of presentation to the community health center (a primary health-care facility) in Blantyre. Healthy controls were recruited from the vaccination clinic or surrounding community by study field-workers.
All subjects received a clinical assessment performed by an experienced research nurse at the time of enrollment. For all participants, venous blood samples (3 mL) were collected on enrollment, before vaccination for controls, and processed within 4 hours of collection. Blood analyses included malaria rapid diagnostic test (RDT) (1 drop); HIV RDT (1 drop); dried blood spot for polymerase chain reaction (2 drops); plasma (1 mL) and whole blood (1.5 mL) for immunologic, hematologic, and molecular investigations as previously described45; and remaining serum (50 μL) for biomarker analyses. Serum for biomarker experiments, as described in the following text, was stored at −70°C until the samples were analyzed. Malaria infection was tested with the SD Bioline Malaria Antigen Pf RDT for Plasmodium falciparum, and positive tests were confirmed by a field stain A and B thick blood smear, which was read and confirmed by two trained, independent, non-blinded microscopists. A Giemsa-stained thin smear was performed for species identification. A Beckman Coulter analyzer was used for full blood count analyses. Laboratory investigations were performed at Malawi Liverpool Wellcome Trust laboratories. There were no discrepant rapid test and smear results (malaria positive or negative). On thick smear, 500 parasites were counted against the number of white blood cells (WBCs) or 1,000 WBCs were counted against parasites, and results were expressed as parasites per microliter of blood, using the following formula: parasites/microliter blood = (parasites/WBCs) × known WBC count per microliter.
Past medical and immunization history were determined based on interviews with parents or guardians. We calculated three measures of malnutrition: weight-for-height Z-score or wasting, height-for-age Z-score or stunting, and weight-for-age Z-score (WAZ) or underweight. Written informed consent was obtained from parents or guardians of participating children, and participants were treated according to Malawi Government guidelines. Ethical approval for the study was obtained from the Malawi College of Medicine Research Ethics Committee (protocol number P.08/15/1785).
Biomarker measurements.
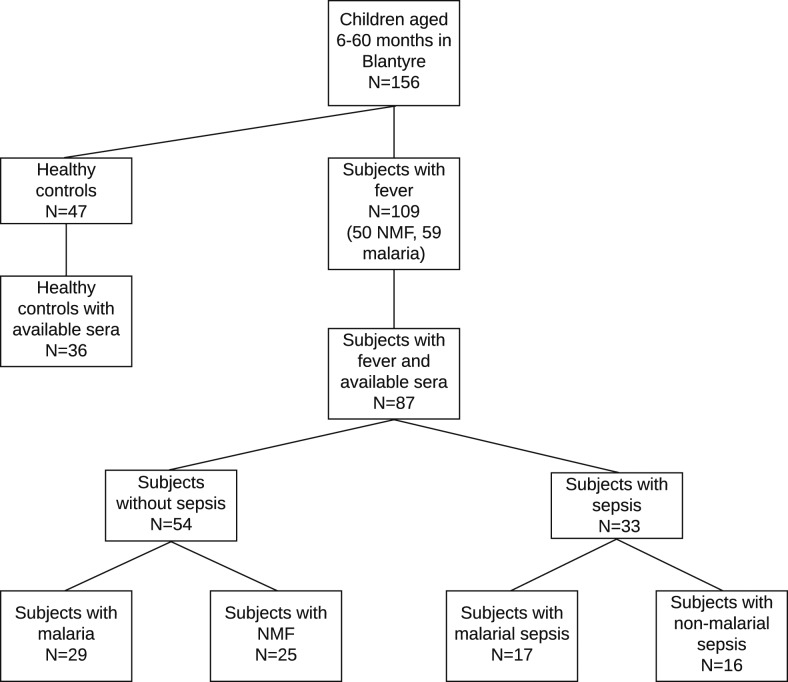
For this study, we obtained sera from 87 febrile children and determined their sepsis status by SIRS criteria (Box 1) using age-specific vital signs (Box 2), regardless of malaria status.2 Figure 1 shows the breakdown of patient subgroups, with and without sera; healthy controls were not included in the analysis. We compared baseline patient characteristics between the subset of 87 febrile children with sera and the 109 febrile children from the original study and found no significant differences (Supplemental Table 1). We also obtained and analyzed the sera of 36 of 47 healthy controls recruited for the original study, and we report their biomarker concentrations in the supplemental materials for reference. Malaria infection was determined as described earlier. Malarial fever included children with fever and malaria who did not meet the criteria for sepsis; non-malarial fever included children with fever, without malaria, and who did not meet the criteria for sepsis; malarial sepsis included children with malaria who met the criteria for sepsis; and non-malarial sepsis included children without malaria who met the criteria for sepsis.
Box 2.
Age-specific vital signs adapted from Goldstein et al.2
| Heart rate (beats/minutes) | ||||
|---|---|---|---|---|
| Age-group | Tachycardia | Bradycardia | Respiratory rate, (breaths/minutes) | Leukocyte count, (leukocytes × 103/millimeter) |
| 1–12 months | > 180 | < 90 | > 34 | > 17.5 or < 5 |
| 13–60 months | > 140 | Not applicable | > 22 | > 15.5 or < 6 |
Figure 1.
Patient flowchart showing the original and study sample sizes, as well as the number of children in each key subgroup. NMF = non-malarial fever.
We selected biomarkers known to be elevated in sepsis and/or malaria infection, specifically serum pro-inflammatory (IL-6, TNF-R1, and IL-1β), anti-inflammatory (IL-10), and endothelial injury (vWFA2, ICAM-1, and Ang-2) biomarkers. We measured biomarker concentrations in duplicate with blank and positive controls, as specified by the manufacturer (Luminex, Austin, TX), using the MAGPIX magnetic bead–based system (Luminex) and a Human Magnetic Assay (R&D Systems, Minneapolis, MN). Plate design was as specified by the manufacturer; the plate was provided in test kits. We excluded from analyses samples with a coefficient of variation between duplicates ≥ 10% or outside the level of quantification. Because distributions were highly skewed, we log-transformed mean duplicate values of each biomarker.
Data analysis.
We explored the distribution of values for each variable and summarized the data as means with SD, medians with interquartile ranges, or as group frequencies with percentages as appropriate. We used the chi-squared test to compare binary variables (e.g., patient gender or prevalence of symptoms), analysis of variance (ANOVA) to compare normally distributed continuous variables (e.g., anthropometric measurements and cell counts), and the Kruskal–Wallis test to compare the difference in distributions of non-normally distributed variables (patient age). We used a two-way ANOVA to compare log-transformed biomarker concentrations across and between subgroups—children with malarial fever, non-malarial fever, malarial sepsis, and non-malarial sepsis—and to identify any interactions, and present the results in the form of boxplots. Healthy controls were not included in the comparisons.
We determined the predictive ability of individual biomarkers and combinations of biomarkers to identify sepsis in febrile children (with and without malaria) by calculating the area under the receiver characteristic (AUROC) and the corresponding 95% CIs.46 We then tested a series of logistic regression models and their ability to predict sepsis, as determined by the AUROC. The logistic regression models included combinations of the highest performing biomarkers as well as risk factors for sepsis (age, malaria infection, and malnutrition determined by WAZ) selected a priori based on the literature.47–49 To test whether parasite density was correlated with biomarkers, we tested for correlations between log-transformed biomarker concentrations and log-transformed malaria parasite density using Spearman’s rank test. We used Stata MP 14.2 for data analysis. We considered a probability of < 0.05 to be statistically significant.
RESULTS
Baseline patient characteristics.
Among the 87 subjects providing serum, 33 (40%) met the criteria for sepsis (17 with malarial sepsis and 16 with non-malaria sepsis) and 54 (62%) had fever without sepsis (29 with malaria and 25 with non-malarial fever) (Figure 1). All malaria cases were infected with P. falciparum. Baseline patient characteristics (Table 1), including serum availability, were similar between subgroups, with a few notable exceptions. Children with non-malarial sepsis were younger than those in other subgroups. There were small but statistically significant differences in hemoglobin level, absolute lymphocyte, and platelet counts between the four subgroups: children with malaria had lower hemoglobin levels than children without malaria and children with sepsis had the lowest (malarial sepsis) and highest (non-malarial sepsis) mean absolute lymphocyte and platelet counts among the four subgroups. Presenting symptoms were similar between the four groups, with the exception of vomiting, which occurred most frequently in the non-malarial sepsis subgroup and least frequently in the malarial sepsis subgroup, and an enlarged spleen, which occurred more frequently in the malarial subgroups (Table 2). Patient characteristics and presenting symptoms between children with and without sepsis were also similar and are shown in Supplemental Tables 2 and 3. Regarding the SIRS criteria, all children met the criterion of fever, about half had an abnormal WBC count, and half had an abnormal respiratory rate; no children met > 2 criteria (Supplemental Table 4).
Table 1.
Patient characteristics for Malawian children younger than 5 years without sepsis (malarial and non-malarial fever) and with sepsis (malarial and non-malarial sepsis)
| No sepsis | Sepsis | P-value across groups | |||
|---|---|---|---|---|---|
| Malarial fever (N = 29) | Non-malarial fever (N = 25) | Malarial sepsis (N = 17) | Non-malarial sepsis (N = 16) | ||
| Demographics | |||||
| Age (months), median (interquartile range) | 20 (12–28) | 26 (20–35) | 30 (20–40) | 16 (10–23) | 0.01 |
| Female gender, n (%) | 18 (62) | 11 (44) | 11 (65) | 11(69) | 0.35 |
| Nutritional status | |||||
| Weight-for-height Z-score, mean (SD) | −1.1 (1.2) | −1.0 (1.7) | −1.1 (1.4) | −2.1 (1.4) | 0.1 |
| Height-for-age, mean (SD) | 0.4 (2.6) | −0.2 (2.5) | −0.9 (1.9) | 1.2 (2.5) | 0.09 |
| Weight-for-age Z-score, mean (SD) | −0.7 (1.4) | −0.9 (1.3) | −1.3 (1) | −1 (1.1) | 0.48 |
| Laboratory values | |||||
| Hemoglobin (g/dL), mean (SD) | 9.6 (1.8) | 11.4 (1.3) | 9.7 (1.9) | 11.3 (0.9) | < 0.0001 |
| White blood cell count, mean (SD) | 9.9 (2.7) | 10.1 (2.8) | 8.6 (4.9) | 12.6(6.3) | N/A |
| Absolute neutrophil count, mean (SD) | 4.1 (1.6) | 4.3 (2.7) | 3.9 (3.1) | 4.5 (3.3) | 0.9 |
| Absolute lymphocyte count, mean (SD) | 4.3 (2) | 4.6 (1.7) | 3.5 (2.3) | 6.3(4.2) | < 0.0001 |
| Platelet count × 103, mean (SD) | 252 (176) | 389 (150) | 198 (172) | 427 (148) | 0.0001 |
| Glucose (mg/dL), mean (SD) | 107 (23) | 96 (16) | 98 (24) | 93(23) | 0.13 |
| Serum available, n/total study sample (%) | 29/39 (74) | 25/30 (83) | 17/20 (85) | 16/20 (80) | 0.73 |
| Recent past medical history (within the last 3 months) | |||||
| Antibiotics, n (%) | 16(55) | 12 (48) | 7 (41) | 8 (50) | 0.8 |
| Antimalarial, n (%) | 6 (21) | 1(4) | 4 (24) | 0 (0) | 0.052 |
| Hospital admission, n (%) | 2 (7) | 1(4) | 3 (18) | 0 (0) | 0.21 |
| Immunization history | |||||
| Pneumococcal, n (%) | 29 (100) | 25 (100) | 17 (100) | 16 (100) | N/A |
| Rotavirus, n (%) | 29 (100) | 24 (96) | 16 (94) | 16 (100) | 0.5 |
N/A = not applicable. A P-value is provided for the comparison across all four groups. Tests of significance: Kruskal–Wallis (medians); chi-squared (percents); ANOVA (means). A P-value for WBC count is not included as this is a SIRS criterion used to define subgroups.
Table 2.
Presenting symptoms in Malawian children younger than 5 years without sepsis (malarial and non-malarial fever) and with sepsis (malarial and non-malarial sepsis)
| Symptom | No sepsis | Sepsis | P-value across groups | ||
|---|---|---|---|---|---|
| Malarial fever (N = 29) | Non-malarial fever (N = 25) | Malarial sepsis (N = 17) | Non-malarial sepsis (N = 16) | ||
| Fever duration (days), mean (range) | 2.1 (1–3) | 1.7 (1–3) | 2 (1–3) | 2.2 (1–4) | 0.48 |
| Vomiting, n (%) | 9 (31) | 10 (40) | 4 (24) | 11 (69) | 0.038 |
| Diarrhea, n (%) | 6 (21) | 7 (28) | 4 (24) | 3 (19) | 0.9 |
| Cough, n (%) | 14 (48) | 11 (44) | 5 (29) | 11 (69) | 0.15 |
| Increased work of breathing, n (%) | 1 (4) | 0 (0) | 7 (41) | 10 (63) | Not applicable |
| Spleen grade 1, n (%) | 7 (24) | 1 (4) | 6 (35) | 1 (6.2) | 0.029 |
A P-value is provided for the comparison across all four groups. Tests of significance: chi-squared (percents); ANOVA (means). A P-value for increased work of breathing is not included as it is a SIRS criterion used to define subgroups.
Biomarker profiles.
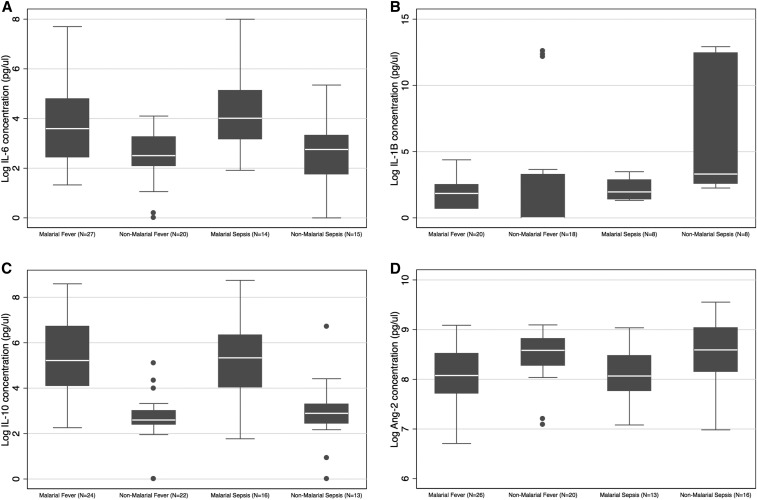
There were significant differences in IL-6 concentration across subgroups (P = 0.0007): levels were higher in children with malaria than in children without malaria (P = 0.0001), but there was no significant difference between children with and without sepsis (P = 0.43) and no interaction between malaria infection and sepsis (P = 0.58) (Figure 2A). Interleukin-1β concentration also varied across subgroups (P = 0.016): levels were increased in children with malaria compared with those without malaria (P = 0.011) and in those with sepsis compared with those without sepsis (P = 0.06), but there was not a statistically significant interaction between malaria infection and sepsis (P = 0.51) (Figure 2B). There were also significant differences in IL-10 concentration across subgroups (P < 0.0001): children with malaria had increased levels compared with those without (P = 0.01), but there was no significant difference between children with and without sepsis (P = 0.81) and no malaria–sepsis interaction (P = 0.56) (Figure 2C). In addition, there were significant differences in Ang-2 concentration across subgroups (P = 0.04): levels were decreased in malaria subgroups compared with non-malaria subgroups (P = 0.006), but there was no significant difference between sepsis subgroups and non-sepsis subgroups (P = 0.8), and there was no significant interaction between malaria infection and sepsis (P = 0.98) (Figure 2D). There were no statistically significant differences in TNFR-1, vWFA2, or ICAM-1 concentrations across or between subgroups (Supplemental Figure 1A–C). Log-transformed biomarker concentrations for each subgroup are shown in Supplemental Table 5. A summary of the biomarker profiles for subgroup is shown in Table 3.
Figure 2.
Boxplots of (A) IL-6, Boxplot represents median and interquartile range (IQR); whiskers adjacent values (extreme values within 1.5 IQR of nearest quartile); and points outside values. Test of significance: Two-way ANOVA. Model p=0.0007; malaria vs. no malaria p=0.0001; sepsis vs. no sepsis p=0.43; malaria-sepsis interaction p=0.58, (B) IL-1β, Boxplot represents median and interquartile range (IQR); whiskers adjacent values (extreme values within 1.5 IQR of nearest quartile); and points outside values. Test of significance: Two-way ANOVA. Model p=0.016; malaria vs. no malaria p=0.011; sepsis vs. no sepsis p=0.06; malaria-sepsis interaction p=0.11, (C) IL-10, Boxplot represents median and interquartile range (IQR); whiskers adjacent values (extreme values within 1.5 IQR of nearest quartile); and points outside values. Test of significance: Two-way ANOVA. Model p<0.0001; malaria vs. no malaria p<0.0001; sepsis vs. no sepsis p=0.81; malaria-sepsis interaction p=0.56, and (D) Ang-2 concentrations in Malawian children younger than 5 years with malarial fever, non-malarial fever, malarial sepsis, and non-malarial sepsis, Boxplot represents median and interquartile range (IQR); whiskers adjacent values (extreme values within 1.5 IQR of nearest quartile); and points outside values. Test of significance: Two-way ANOVA. Model p=0.04; malaria vs. no malaria p=0.0066; sepsis vs. no sepsis p=0.8; malaria-sepsis interaction p=0.98.
Table 3.
Summary of biomarker profiles for each subgroup
| Biomarker | No sepsis | Sepsis | ||
|---|---|---|---|---|
| Malarial fever | Non-malarial fever | Malarial sepsis | Non-malarial sepsis | |
| IL-6 | ↑ | – | ↑ | – |
| IL-1β | ↑ | – | ↑ | ↑↑ |
| IL-10 | ↑ | – | ↑ | – |
| Angiopoietin-2 | ↓ | – | ↓ | – |
IL: interleukin. Increases (↑) and decreases (↓) in biomarker concentration are shown and are with respect to the non-malarial fever subgroup (reference). Only biomarkers with statistically different concentrations across subgroups are shown.
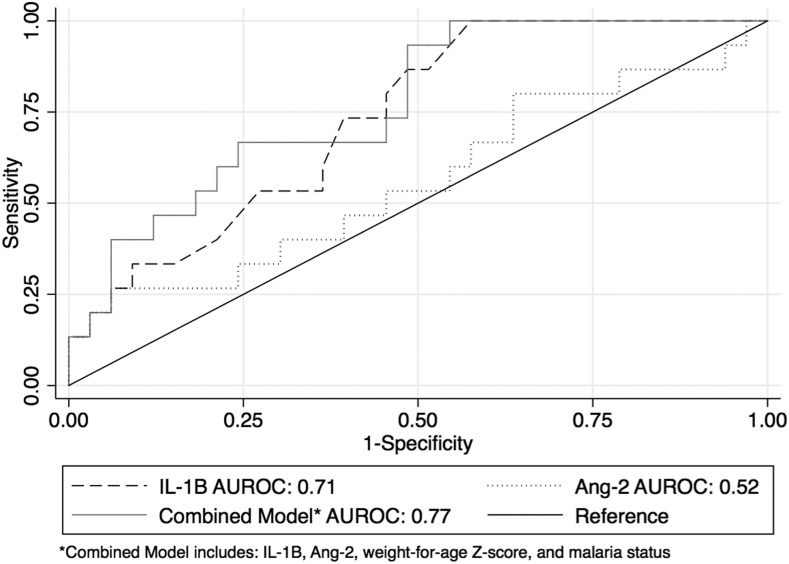
To determine the discriminatory ability of biomarkers to predict sepsis, we calculated the AUROC for each biomarker (Table 4). Biomarker IL-1β performed the best at predicting sepsis among children with fever, with an AUROC of 0.71 (95% CI: 0.57–0.85). A combined model incorporating IL-1β, Ang-2, WAZ, and malaria infection yielded a slightly higher AUROC (0.77, 95% CI: 0.67–0.85) (Figure 3). Other models were also tested and resulted in either equivalent or marginally lower AUROCs for predicting sepsis.
Table 4.
Area under the receiver operating characteristic (AUROC) and 95% CI for log-transformed biomarker concentrations for predicting sepsis in Malawian children younger than 5 years with fever
| Biomarker | AUROC | 95% CI |
|---|---|---|
| Pro-inflammatory | ||
| IL-6 (n = 76) | 0.54 | 0.4–0.68 |
| Tumor necrosis factor receptor-1 (n = 77) | 0.52 | 0.38–0.66 |
| IL-1β (n = 54) | 0.71 | 0.57–0.85 |
| Anti-inflammatory | ||
| IL-10 (n = 75) | 0.53 | 0.39–0.67 |
| Endothelial injury | ||
| von Willebrand factor antigen-2 (n = 98) | 0.46 | 0.33–0.6 |
| Intercellular adhesion molecule-1 (n = 85) | 0.5 | 0.37–0.63 |
| Angiopoietin-2 (n = 75) | 0.53 | 0.39–0.67 |
IL = interleukin.
Figure 3.
Area under the receiver operator characteristic curves for IL-1β, Ang-2, and a combined biomarker–clinical characteristics model for predicting sepsis in febrile Malawian children with and without malaria.
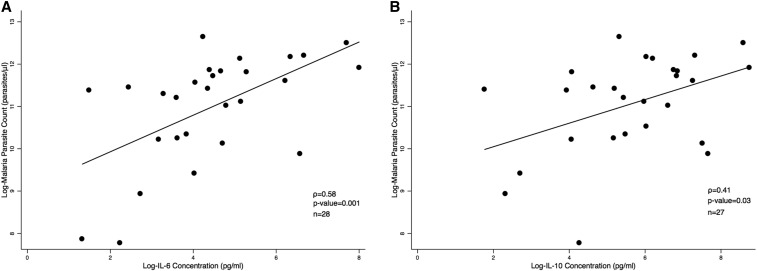
In a subgroup of malaria-positive children with and without sepsis (N = 46), we observed a positive correlation between IL-6 concentration and parasite density (ρ = 0.58, P = 0.001) and IL-10 concentration and parasite density (ρ = 0.41, P = 0.03) (Table 5, Figure 4A and B).
Table 5.
Spearman’s correlation (ρ) between log-transformed parasite density and pro- and anti-inflammatory biomarkers and endothelial injury biomarker concentrations in Malawian children younger than 5 years with malaria
| Biomarker | Parasitemia (number of parasites/µL) | |
|---|---|---|
| Correlation (ρ) | P-value | |
| Pro-inflammatory | ||
| IL-6 (n = 28) | 0.58 | 0.001 |
| Tumor necrosis factor receptor-1 (n = 30) | 0.32 | 0.08 |
| IL-1β (n = 23) | 0.11 | 0.62 |
| Anti-inflammatory | ||
| IL-10 (n = 27) | 0.41 | 0.03 |
| Endothelial injury | ||
| von Willebrand factor antigen-2 (n = 29) | 0.08 | 0.68 |
| Intercellular adhesion molecule-1 (n = 32) | −0.05 | 0.78 |
| Angiopoietin-2 (n = 26) | 0.17 | 0.4 |
IL = interleukin.
Figure 4.
Correlation between (A) IL-6 and (B) IL-10 concentrations and malaria parasite density in febrile Malawian children.
DISCUSSION
We identified distinct biomarker profiles that characterized malaria infection, non-malarial fever, and non-malarial sepsis. Malarial fever and malarial sepsis had significantly overlapping biomarker profiles, and we were unable to distinguish these two clinical entities by biomarker concentrations alone. Using non-malarial fever as the reference group, malaria infection was characterized by increased pro-inflammatory biomarker IL-6, increased anti-inflammatory biomarker IL-10, and decreased endothelial activation and injury biomarker Ang-2, signifying a simultaneous inflammatory and anti-inflammatory response in the setting of less endothelial injury. Malaria infection is known to cause endothelial injury, acute inflammation, and coagulation system activation.32–41 Similar to prior studies, we found that IL-10 was elevated in children with malaria, although we observed an increase in IL-6 and not the previously observed increase in TNFR-1 as a sign of acute inflammation.42 High levels of both pro- and anti-inflammatory cytokines characterize malaria, and higher levels are associated with increased disease severity35,43,50,51; this study only included children with uncomplicated (non-severe) malaria, which may explain the absence of an increase in IL-1β and the relative decrease in Ang-2 levels compared with non-malarial fever.
Non-malarial sepsis was characterized by increased pro-inflammatory biomarker IL-1β, without an increase in IL-6 or IL-10 or a decrease in Ang-2 as observed in malarial sepsis (Table 3). Interleukin-6 has previously been shown to be a sensitive and specific marker for the presence of a serious bacterial infection in febrile infants,20 implying that the etiology of non-malarial sepsis was not bacterial in study participants. This is further supported by the most common presenting symptom in the non-malarial sepsis subgroup, vomiting, which is a sign of gastrointestinal illness, most frequently caused by viral infections in this age-group and region.52 Unfortunately, we do not have supporting microbiological evidence to support the etiology of non-malarial sepsis in this study; future studies should focus on non-malarial pathogen identification. Interestingly, elevations in IL-1β have previously been correlated with the degree of sepsis severity14; we observed an increase in IL-1β in this study population, despite the limited severity of illness in the outpatient clinic setting.
Incorporation of inflammatory biomarker tests into an established clinical algorithm has been shown to increase the utility of clinical guidelines in resource-limited settings.53 Biomarkers such as C-reactive protein and procalcitonin have previously been studied in SSA, and predicted the presence of severe bacterial infections, a common cause of sepsis in SSA.54–56 A diagnostic assay capable of differentiating sepsis based on the causative pathogen would be ideal because it would allow focused therapy and also reduce the unnecessary use of antibiotics.57 Children with malarial sepsis had significantly elevated IL-6 and IL-10 compared with those with non-malarial sepsis. Thus, although there may be pathophysiologic similarities for all etiologies of sepsis, we distinguished malarial from non-malarial sepsis using biomarker analyses. Interleukin-6 has previously been shown to be one of the biomarkers capable of discriminating septic from non-septic patients,21,22,58 and findings of our study provide additional evidence that this biomarker could also be useful to differentiate between malarial and non-malarial sepsis. Follow-up studies should investigate if IL-6 and other biomarkers differentiate between other etiologies of sepsis.
We also aimed to identify biomarkers that distinguished children with sepsis from those without, regardless of malaria status. Although IL-1β performed the best at predicting sepsis (AUROC 0.71), this modest AUROC was driven by the increased level of IL-1β only in the non-malarial sepsis subgroup. Therefore, we were unsuccessful at identifying a single biomarker to identify all cases of sepsis.
Biomarkers have previously been targeted as a potential diagnostic tool for sepsis in a number of studies with varied outcomes.59 In recent years, the notion that a single biomarker can predict sepsis has fallen out of favor. Studies in high-income settings have shown that a combination of biomarkers in conjunction with clinical signs is superior to a single sepsis biomarker.60,61 When IL-1β was incorporated into a combined biomarker–clinical characteristics sepsis prediction model, performance improved (AUROC = 0.77), demonstrating that a combined model had reasonable predictive ability to discriminate children with and without sepsis. Thus, similar to sepsis biomarker studies in non–malaria-endemic settings,60,61 we found that a combination of biomarkers and clinical characteristics outperformed single biomarkers for predicting the presence of sepsis. However, the AUROC of this combined sepsis prediction model is rather low, which could be due to the relatively low illness severity of children presenting to the outpatient clinic resulting in minimal differences between cases (children with sepsis) and controls (children with fever only). The low AUROC may also reflect the poor sensitivity of the current definition of pediatric sepsis, which is dependent on the nonspecific SIRS criteria.
Pediatric sepsis in malaria-endemic regions of SSA may be affected by the child’s age as well as alterations in the host immune system due to repeated episodes of malaria. In Ugandan children presenting with acute malaria infection, younger children (aged 1–3 years) had significantly higher levels of pro-inflammatory (including IL-6 and TNF-R1) and anti-inflammatory (IL-10) biomarkers than older children (aged 7–10 years), suggesting that cytokine-dependent immune responses were dampened in older children, potentially by repeated exposure to malaria parasites.42 In addition, active and convalescent P. falciparum malaria infection has been shown to impair humoral and cellular immunity, placing children at risk for invasive bacterial infections and potentially increasing susceptibility to sepsis.45 The presence of a bacterial coinfection alters the biomarker profile further. In a cohort of Kenyan children with proven malaria infection, children coinfected with Gram-negative bacteremia had higher IL-1β and lower IL-10 expression than children who presented with only malaria.37 In a separate cohort of children with severe malaria from two hospitals in Kenya and Uganda, Gram-negative associated endotoxin levels in coinfected children were negatively correlated with IL-6 and IL-10 concentrations.62
In addition to the differences in biomarker profiles that we identified, we also observed that the malaria parasite burden correlated with increased IL-10 and IL-6 levels (Table 5, Figure 4A and B). In a cohort of Ugandan children with malaria and no coinfections, a higher parasite density was associated with increased production of the anti-inflammatory cytokine IL-10 by interferon gamma–positive CD4 T cells.44 This observation is in-line with the hypothesis that IL-10 and other anti-inflammatory cytokines provide a conducive environment for parasite replication. This hypothesis was supported by the association of increased inflammatory biomarkers and reduced parasite density in Kenyan children with bacterial coinfection.37 By contrast, a negative correlation between IL-10 levels and malaria parasitemia was seen in Indian adults and children, suggesting that in this setting, pro-inflammatory cytokines favored parasite replication.43
There were some differences in baseline characteristics and presenting symptoms between the subgroups. For example, children with non-malarial sepsis had the lowest median age (16 months) and the highest frequency of vomiting as a presenting symptom; however, IL-1β concentration was not significantly associated with age or vomiting in additional analyses (not shown). Children with malaria, unsurprisingly, had a lower mean hemoglobin value than children without malaria. Also not surprisingly, children with sepsis were more likely to have abnormally high or low white blood cell and absolute lymphocyte counts, and children with malarial sepsis had the lowest mean platelet count.
There were several limitations to our study. First, serum samples were not available for all subjects; however, baseline characteristics were similar between children with and without serum samples (Supplemental Table 1), the mean biomarker concentration was approximately the same between children with and without serum samples, and the same statistically significant associations are present in both the study population with available sera and the entire study population (Supplemental Table 6). Second, because of resource constraints, we tested this study population only for malaria and not for other pathogens, and in children classified as having malarial sepsis, we were unable to test for coinfections. This is an important limitation because other studies have previously shown differences in biomarker profiles between septic study participants with malaria only and those with malaria and bacteremia. Similarly, grouping all non-malarial sepsis cases without specifying the etiology is another limitation. Third, we did not collect data on patient clinical outcomes. Future studies should test for associations between biomarker profiles and clinical outcomes. Fourth, this study had a small sample size and it was not powered to detect a difference in biomarker concentrations; still, we did identify interesting associations. It should also be noted that reliable IL-1β measurements were obtained on fewer samples than for other biomarkers. The logistic regression model used to calculate the AUROC for the combined model in Figure 3 is, therefore, restricted to subjects with available IL-1β measurements, limiting generalizability. Fifth, the SIRS criteria are the current accepted pediatric definition of sepsis, but these criteria have been criticized for being too sensitive and nonspecific for pediatric sepsis and outcomes49,63; new criteria similar to the adult Sepsis-3 and Sequential Organ Failure Assessment (SOFA) score may be released shortly.64–66 Finally, generalizability of the study is uncertain; there may be important differences between our study population, which excluded children with severe malaria, HIV infection, and chronic illnesses, and the general SSA pediatric population. However, in SSA, most malaria cases treated at health centers are uncomplicated, and the pediatric prevalence of HIV infection is low, suggesting that our results are reasonably representative of other SSA pediatric populations. Future studies should characterize biomarker profiles across the illness severity spectrum of sepsis and malaria, focus on pathogen detection beyond malaria identification, and capture clinical outcomes.
In conclusion, our analyses demonstrate that a multiple biomarker assay can identify distinct biomarker profiles associated with malaria infection, non-malarial fever, and non-malarial sepsis in Malawian children. In addition, our results highlight the challenge of diagnosing sepsis in malaria-endemic regions. Children with malaria have a similar clinical presentation and biomarker profile, regardless of whether they meet the sepsis criteria. It appears that malaria, the infectious agent, drives the expression of host biomarkers, adding more evidence that sepsis is a heterogeneous clinical syndrome due to host–pathogen interactions. Furthermore, we did not identify a characteristic biomarker profile specific to all children with sepsis, although we did identify a combined biomarker–clinical characteristics model with modest sepsis predictive ability. Because malaria infection profoundly affects the host’s immune system and inflammatory response, sepsis biomarker data from high-income countries are unlikely to represent the pathophysiology of sepsis in malaria-endemic settings. Additional clinical and biological data from SSA are, therefore, necessary to better elucidate sepsis host–pathogen interactions and to develop improved algorithms to manage sepsis in malaria-endemic settings in SSA.
Supplemental tables and figures
Acknowledgments:
We thank study research nurse, Alice Lwanda, and field research assistant, Joseph Kumwenda, for their involvement in recruitment of the study participants, and the staff at Blantyre District Health Office, College of Medicine, and the Blantyre Malaria Project for their technical help. We would also like to thank Karl Seydel and his laboratory staff for access to and assistance with the MAGPIX system. We are also grateful to all study participants for their participation in the study.
Note: Supplemental tables and figures appear at www.ajtmh.org.
REFERENCES
- 1.Global Burden of Disease Child and Adolescent Health Collaboration et al. 2017. Child and adolescent health from 1990 to 2015: findings from the global burden of diseases, injuries, and risk factors 2015 study. JAMA Pediatr 171: 573–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goldstein B, Giroir B, Randolph A; International Consensus Conference on Pediatric Sepsis , 2005. International pediatric sepsis consensus conference: definitions for sepsis and organ dysfunction in pediatrics. Pediatr Crit Care Med 6: 2–8. [DOI] [PubMed] [Google Scholar]
- 3.Deutschman CS, Tracey KJ, 2014. Sepsis: current dogma and new perspectives. Immunity 40: 463–475. [DOI] [PubMed] [Google Scholar]
- 4.Schulte W, Bernhagen J, Bucala R, 2013. Cytokines in sepsis: potent immunoregulators and potential therapeutic targets–an updated view. Mediators Inflamm 2013: 165974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jaehne AK, Rivers EP, 2016. Early liberal fluid therapy for sepsis patients is not harmful: hydrophobia is unwarranted but drink responsibly. Crit Care Med 44: 2263–2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carcillo JA, Halstead ES, Hall MW, Nguyen TC, Reeder R, Aneja R, Shakoory B, Simon D; Eunice Kennedy Shriver National Institute of Child Health and Human Development Collaborative Pediatric Critical Care Research Network Investigators , 2017. Three hypothetical inflammation pathobiology phenotypes and pediatric sepsis-induced multiple organ failure outcome. Pediatric Critical Care Med 18: 513–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mikacenic C, Hahn WO, Price BL, Harju-Baker S, Katz R, Kain KC, Himmelfarb J, Liles WC, Wurfel MM, 2015. Biomarkers of endothelial activation are associated with poor outcome in critical illness. PLoS One 10: e0141251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang K, Bhandari V, Giuliano JS, Jr., O Hern CS, Shattuck MD, Kirby M, 2014. Angiopoietin-1, angiopoietin-2 and bicarbonate as diagnostic biomarkers in children with severe sepsis. PLoS One 9: e108461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hou PC; ProCESS Investigators et al. 2017. Endothelial permeability and hemostasis in septic shock: results from the ProCESS trial. Chest 152: 22–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Edgar JD, Gabriel V, Gallimore JR, McMillan SA, Grant J, 2010. A prospective study of the sensitivity, specificity and diagnostic performance of soluble intercellular adhesion molecule 1, highly sensitive C-reactive protein, soluble E-selectin and serum amyloid A in the diagnosis of neonatal infection. BMC Pediatr 10: 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Skibsted S, Jones AE, Puskarich MA, Arnold R, Sherwin R, Trzeciak S, Schuetz P, Aird WC, Shapiro NI, 2013. Biomarkers of endothelial cell activation in early sepsis. Shock 39: 427–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mihajlovic DM, Lendak DF, Draskovic BG, Mikic AS, Mitic GP, Cebovic TN, Brkic SV, 2015. Thrombomodulin is a strong predictor of multiorgan dysfunction syndrome in patients with sepsis. Clin Appl Thromb Hemost 21: 469–474. [DOI] [PubMed] [Google Scholar]
- 13.Krafte-Jacobs B, Brilli R, 1998. Increased circulating thrombomodulin in children with septic shock. Crit Care Med 26: 933–938. [DOI] [PubMed] [Google Scholar]
- 14.Bozza FA, Salluh JI, Japiassu AM, Soares M, Assis EF, Gomes RN, Bozza MT, Castro-Faria-Neto HC, Bozza PT, 2007. Cytokine profiles as markers of disease severity in sepsis: a multiplex analysis. Crit Care 11: R49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wong HR, et al. 2012. The pediatric sepsis biomarker risk model. Crit Care 16: R174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Erdman LK, et al. 2015. Biomarkers of host response predict primary end-point radiological pneumonia in Tanzanian children with clinical pneumonia: a prospective cohort study. PLoS One 10: e0137592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ivaska L, Niemela J, Leino P, Mertsola J, Peltola V, 2015. Accuracy and feasibility of point-of-care white blood cell count and C-reactive protein measurements at the pediatric emergency department. PLoS One 10: e0129920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Singer AJ, Taylor M, Domingo A, Ghazipura S, Khorasonchi A, Thode HC, Jr., Shapiro NI, 2014. Diagnostic characteristics of a clinical screening tool in combination with measuring bedside lactate level in emergency department patients with suspected sepsis. Acad Emerg Med 21: 853–857. [DOI] [PubMed] [Google Scholar]
- 19.Carcillo JA, Sward K, Halstead ES, Telford R, Jimenez-Bacardi A, Shakoory B, Simon D, Hall M; Eunice Kennedy Shriver National Institute of Child Health and Human Development Collaborative Pediatric Critical Care Research Network Investigators , 2017. A systemic inflammation mortality risk assessment contingency table for severe sepsis. Pediatr Crit Care Med 18: 143–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zarkesh M, Sedaghat F, Heidarzadeh A, Tabrizi M, Bolooki-Moghadam K, Ghesmati S, 2015. Diagnostic value of IL-6, CRP, WBC, and absolute neutrophil count to predict serious bacterial infection in febrile infants. Acta Med Iran 53: 408–411. [PubMed] [Google Scholar]
- 21.Batfalsky A, Lohr A, Heussen N, Neunhoeffer F, Orlikowsky TW, 2012. Diagnostic value of an interleukin-6 bedside test in term and preterm neonates at the time of clinical suspicion of early- and late-onset bacterial infection. Neonatology 102: 37–44. [DOI] [PubMed] [Google Scholar]
- 22.Pfafflin A, Schleicher E, 2009. Inflammation markers in point-of-care testing (POCT). Anal Bioanal Chem 393: 1473–1480. [DOI] [PubMed] [Google Scholar]
- 23.Davis JS, Yeo TW, Piera KA, Woodberry T, Celermajer DS, Stephens DP, Anstey NM, 2010. Angiopoietin-2 is increased in sepsis and inversely associated with nitric oxide-dependent microvascular reactivity. Crit Care 14: R89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.World Health Organization DoCaAHaD, UNICEF 2005. Handbook IMCI Integrated Management of Childhood Illness. Geneva, Switzerland: WHO Press. [Google Scholar]
- 25.Thompson M, et al. 2012. Systematic review and validation of prediction rules for identifying children with serious infections in emergency departments and urgent-access primary care. Health Technol Assess 16: 1–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carcillo JA, Davis AL, Zaritsky A, 1991. Role of early fluid resuscitation in pediatric septic shock. JAMA 266: 1242–1245. [PubMed] [Google Scholar]
- 27.de Oliveira CF, 2010. Early goal-directed therapy in treatment of pediatric septic shock. Shock 34 (Suppl 1): 44–47. [DOI] [PubMed] [Google Scholar]
- 28.Dellinger RP; Sepsis Campaign Guidelines Committee including The Pediatric Subgroup et al. 2013. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med 41: 580–637. [DOI] [PubMed] [Google Scholar]
- 29.Han YY, Carcillo JA, Dragotta MA, Bills DM, Watson RS, Westerman ME, Orr RA, 2003. Early reversal of pediatric-neonatal septic shock by community physicians is associated with improved outcome. Pediatrics 112: 793–799. [DOI] [PubMed] [Google Scholar]
- 30.Chipwaza B, Mhamphi GG, Ngatunga SD, Selemani M, Amuri M, Mugasa JP, Gwakisa PS, 2015. Prevalence of bacterial febrile illnesses in children in Kilosa district, Tanzania. PLoS Negl Trop Dis 9: e0003750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nielsen MV, Amemasor S, Agyekum A, Loag W, Marks F, Sarpong N, Dekker D, Adu-Sarkodie Y, May J, 2015. Clinical indicators for bacterial co-infection in Ghanaian children with P. falciparum infection. PLoS One 10: e0122139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Conroy AL, Lafferty EI, Lovegrove FE, Krudsood S, Tangpukdee N, Liles WC, Kain KC, 2009. Whole blood angiopoietin-1 and -2 levels discriminate cerebral and severe (non-cerebral) malaria from uncomplicated malaria. Malar J 8: 295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Conroy AL, Phiri H, Hawkes M, Glover S, Mallewa M, Seydel KB, Taylor TE, Molyneux ME, Kain KC, 2010. Endothelium-based biomarkers are associated with cerebral malaria in Malawian children: a retrospective case-control study. PLoS One 5: e15291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Erdman LK, et al. 2011. Combinations of host biomarkers predict mortality among Ugandan children with severe malaria: a retrospective case-control study. PLoS One 6: e17440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lovegrove FE, et al. 2009. Serum angiopoietin-1 and -2 levels discriminate cerebral malaria from uncomplicated malaria and predict clinical outcome in African children. PLoS One 4: e4912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mankhambo LA, et al. 2010, The role of angiogenic factors in predicting clinical outcome in severe bacterial infection in Malawian children. Crit Care 14: R91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Davenport GC, et al. 2016. Reduced parasite burden in children with falciparum malaria and bacteremia coinfections: role of mediators of inflammation. Mediators Inflamm 2016: 4286576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mita-Mendoza NK, et al. 2013. A potential role for plasma uric acid in the endothelial pathology of Plasmodium falciparum malaria. PLoS One 8: e54481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Phiri HT, et al. 2011. Elevated plasma von Willebrand factor and propeptide levels in Malawian children with malaria. PLoS One 6: e25626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tchinda VH, Tadem AD, Tako EA, Tene G, Fogako J, Nyonglema P, Sama G, Zhou A, Leke RG, 2007. Severe malaria in Cameroonian children: correlation between plasma levels of three soluble inducible adhesion molecules and TNF-alpha. Acta Trop 102: 20–28. [DOI] [PubMed] [Google Scholar]
- 41.O’Donnell A, Fowkes FJ, Allen SJ, Imrie H, Alpers MP, Weatherall DJ, Day KP, 2009. The acute phase response in children with mild and severe malaria in Papua New Guinea. Trans R Soc Trop Med Hyg 103: 679–686. [DOI] [PubMed] [Google Scholar]
- 42.Farrington L, Vance H, Rek J, Prahl M, Jagannathan P, Katureebe A, Arinaitwe E, Kamya MR, Dorsey G, Feeney ME, 2017. Both inflammatory and regulatory cytokine responses to malaria are blunted with increasing age in highly exposed children. Malar J 16: 499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mahanta A, Kar SK, Kakati S, Baruah S, 2015. Heightened inflammation in severe malaria is associated with decreased IL-10 expression levels and neutrophils. Innate Immunity 21: 546–552. [DOI] [PubMed] [Google Scholar]
- 44.Boyle MJ, et al. 2017. The development of Plasmodium falciparum-specific IL10 CD4 T cells and protection from malaria in children in an area of high malaria transmission. Front Immunol 8: 1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nyirenda TS, et al. 2017. Loss of humoral and cellular immunity to invasive nontyphoidal Salmonella during current or convalescent Plasmodium falciparum infection in Malawian children. Clin Vaccine Immunol 24: e00057–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hajian-Tilaki K, 2013. Receiver operating characteristic (ROC) curve analysis for medical diagnostic test evaluation. Caspian J Inter Med 4: 627–635. [PMC free article] [PubMed] [Google Scholar]
- 47.Kumar N, Thomas N, Singhal D, Puliyel JM, Sreenivas V, 2003. Triage score for severity of illness. Indian Pediatr 40: 204–210. [PubMed] [Google Scholar]
- 48.Berkley JA, Ross A, Mwangi I, Osier FH, Mohammed M, Shebbe M, Lowe BS, Marsh K, Newton CR, 2003. Prognostic indicators of early and late death in children admitted to district hospital in Kenya: cohort study. BMJ 326: 361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kortz TB, Sawe HR, Murray B, Enanoria W, Matthay MA, Reynolds T, 2017. Clinical presentation and outcomes among children with sepsis presenting to a public tertiary hospital in Tanzania. Front Pediatr 5: 278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lyke KE, et al. 2004. Serum levels of the proinflammatory cytokines interleukin-1 beta (IL-1beta), IL-6, IL-8, IL-10, tumor necrosis factor alpha, and IL-12(p70) in Malian children with severe Plasmodium falciparum malaria and matched uncomplicated malaria or healthy controls. Infect Immun 72: 5630–5637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mandala WL, Msefula CL, Gondwe EN, Drayson MT, Molyneux ME, MacLennan CA, 2017. Cytokine profiles in Malawian children presenting with uncomplicated malaria, severe malarial anemia, and cerebral malaria. Clin Vaccine Immunol 24: e00533–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Revelas A. 2012. Review: acute gastroenteritis among children in the developing world. South Afr J Epidemiol Infect 27: 156–162. [Google Scholar]
- 53.Keitel K, et al. 2017. A novel electronic algorithm using host biomarker point-of-care tests for the management of febrile illnesses in Tanzanian children (e-POCT): a randomized, controlled non-inferiority trial. PLoS Med 14: e1002411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Carrol ED, et al. 2009. The diagnostic and prognostic accuracy of five markers of serious bacterial infection in Malawian children with signs of severe infection. PLoS One 4: e6621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Elfving K, et al. 2016. Acute uncomplicated febrile illness in children aged 2–59 months in Zanzibar–aetiologies, antibiotic treatment and outcome. PLoS One 11: e0146054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Diez-Padrisa N, Bassat Q, Machevo S, Quintó L, Morais L, Nhampossa T, O’Callaghan-Gordo C, Torres A, Alonso PL, Roca A, 2010. Procalcitonin and C-reactive protein for invasive bacterial pneumonia diagnosis among children in Mozambique, a malaria-endemic area. PLoS One 5: e13226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pradipta IS, Sodik DC, Lestari K, Parwati I, Halimah E, Diantini A, Abdulah R, 2013. Antibiotic resistance in sepsis patients: evaluation and recommendation of antibiotic use. N Am J Med Sci 5: 344–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tsalik EL, Jaggers LB, Glickman SW, Langley RJ, van Velkinburgh JC, Park LP, Fowler VG, Cairns CB, Kingsmore SF, Woods CW, 2012. Discriminative value of inflammatory biomarkers for suspected sepsis. J Emer Med 43: 97–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liu Y, Hou JH, Li Q, Chen KJ, Wang SN, Wang JM, 2016. Biomarkers for diagnosis of sepsis in patients with systemic inflammatory response syndrome: a systematic review and meta-analysis. SpringerPlus 5: 2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gomez B, Mintegi S, Bressan S, Da Dalt L, Gervaix A, Lacroix L; European Group for Validation of the Step-by-Step Approach , 2016. Validation of the “step-by-step” approach in the management of young febrile infants. Pediatrics 138: e20154381. [DOI] [PubMed] [Google Scholar]
- 61.Leroy S, Bressan S, Lacroix L, Andreola B, Zamora S, Bailey B, Da Dalt L, Manzano S, Gervaix A, Galetto-Lacour A, 2018. Refined lab-score, a risk score predicting serious bacterial infection in febrile children less than 3 years of age. Pediatr Infect Dis J 37: 387–393. [DOI] [PubMed] [Google Scholar]
- 62.Olupot-Olupot P, et al. 2013. Endotoxaemia is common in children with Plasmodium falciparum malaria. BMC Infect Dis 13: 117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wiens MO, et al. 2016. Application of sepsis definitions to pediatric patients admitted with suspected infections in Uganda. Pediatr Crit Care Med 17: 400–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schlapbach LJ, Straney L, Bellomo R, MacLaren G, Pilcher D, 2018. Prognostic accuracy of age-adapted SOFA, SIRS, PELOD-2, and qSOFA for in-hospital mortality among children with suspected infection admitted to the intensive care unit. Intensive Care Med 44: 179–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Singer M, et al. 2016. The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA 315: 801–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.van Nassau SC, van Beek RH, Driessen GJ, Hazelzet JA, van Wering HM, Boeddha NP, 2018. Translating sepsis-3 criteria in children: prognostic accuracy of age-adjusted quick SOFA score in children visiting the emergency department with suspected bacterial infection. Front Pediatr 6: 266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dunser MW, Festic E, Dondorp A, Kissoon N, Ganbat T, Kwizera A, Haniffa R, Baker T, Schultz MJ; Global Intensive Care Working Group of European Society of Intensive Care Medicine , 2012. Recommendations for sepsis management in resource-limited settings. Intensive Care Med 38: 557–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.