Abstract.
Although human infections of Plasmodium knowlesi have been found throughout Southeast Asia, most cases originated from Malaysian Borneo. In Thailand, P. knowlesi malaria was considered extremely rare. However, during October 2017–September 2018, there was a surge in the number of reported P. knowlesi cases. Here, a series of six cases of P. knowlesi malaria found during this period in Songkhla and Narathiwat provinces of southern Thailand are presented. All cases were confirmed by polymerase chain reaction. The unprecedented case number in the affected area is a warning sign of an increasing P. knowlesi burden in the south of Thailand.
INTRODUCTION
Plasmodium knowlesi is increasingly recognized as a major cause of human malaria. The natural hosts of this parasite are the long-tailed and pig-tailed macaques of Southeast Asia.1,2 The transmission of P. knowlesi is generally considered to be from monkeys to humans through local anopheline vectors,3–5 with reported human-to-human transmissions confined to blood transfusion6,7 and experimental infection.8 The first documented natural infection of humans with P. knowlesi was in 1965 when a traveler acquired the parasite from a visit to Southeast Asia.9 More recently, in 2004 and 2008, a large number of naturally acquired P. knowlesi infections in humans were reported in Sarawak state of Malaysian Borneo. In addition to Malaysia, P. knowlesi infections have now been observed throughout Southeast Asia.10–17 A number of infections were also found in international travelers.18–24
In Thailand, a retrospective study of blood samples obtained from malaria patients in northwestern Tak Province during 1996 uncovered a case of mixed species infection of Plasmodium vivax and P. knowlesi.25 In 2000, a case of human P. knowlesi malaria infection was stated in Prachuap Khiri Khan Province.16 During October 2006 and September 2007, 10 P. knowlesi infections were identified by polymerase chain reaction (PCR) in 1,751 malaria patients.17 During October 2008 and September 2009, a survey identified 23 P. knowlesi infections in 3,446 patients from various parts of the country.25 A separate study provided evidence of P. knowlesi infection in two patients working near the Thai-Myanmar border in Ranong Province.26
Recently, P. knowlesi clinical cases were reported, for the first time, by the Thai National Malaria Control Program (NMCP), with the nationwide total of 23 cases during October 2017 –September 2018.27 Here, we present six of these cases from Songkhla and Narathiwat provinces of Southern Thailand.
METHODS
Sample collection and malarial DNA extraction.
Six blood samples were collected from malaria patients who sought treatment at the Vector-Borne Disease Control Center (VBDC) in Songkhla Province or at Naradhiwas Rajanagarindra Hospital in Narathiwat Province from November 2017 to April 2018. Thin and thick smears were prepared, stained with 10% Giemsa, and examined under a microscope. Genomic DNA was extracted from dried blood spots on filter paper or blood pellets and used in nested PCR and quantitative PCR (qPCR) to confirm the parasite species. This study received human use exemption (certificate no. MUTM-EXMPT 2018-009) by the Ethics Committee of the Faculty of Tropical Medicine, Mahidol University, Thailand.
Plasmodium species identification.
Nested PCR targeting the 18S rRNA genes was performed to detect the Plasmodium species.28 The first round of nested PCR was performed with Plasmodium genus–specific outer primers (ACGATCAGATACCGTCGTAATCTT and GAACCCAAAGACTTTGATTTCTCAT, 0.4 µM each). The reaction was set using GoTaq® Green Master Mix (Promega, Madison, WI) in a 25-µL reaction volume under thermocycling conditions 95°C for 20 seconds, 55°C for 30 seconds, and 72°C for 30 seconds. One microliter of 1:50 dilution of the first-round product was used as the template for the second round of PCR with the same forward primer (ACGATCAGATACCGTCGTAATCTT) and a reverse primer specific to each species (CAATCTAAAAGTCACCTCGAAAGATG for Plasmodium falciparum, CAATCTAAGAATAAACTCCGAGAGGAAA for P. vivax, ACTGAAGGAAGCAATCTAAGAAATTT for Plasmodium ovale, AAGGAAGCTATCTAAAAGAAACACTCAT for Plasmodium malariae, and CTGAAGGAAGCAATCTAAGAGTTC for P. knowlesi). The second PCR was set in a 25-µL reaction volume using GoTaq Green Master Mix with 0.4 µM concentration of each primer and thermocycling conditions 95°C for 20 seconds, 60°C for 30 seconds, and 72°C for 30 seconds. The final PCR products were analyzed on 2% agarose gel. Dye-terminator sequencing of the nested PCR products was performed in both directions using the nested PCR primers through commercial service (Macrogen, Seoul, Republic of Korea). Positive P. knowlesi detection was further confirmed by P. knowlesi–specific TagMan 18S qPCR which used primers (GTTAGCGAGAGCCACAAAAAAGCGAAT and ACTCAAAGTAACAAAATCTTCCATA, 0.6 µM each) and a probe (HEX–TGCTTTATGTGCGCATCCTCTACCTA-BFQ, 0.5 µM) with iTaq™ Universal Probes Supermix (Bio-Rad, Hercules, CA) and two-step thermocycling conditions 95°C for 15 seconds and 60°C for 60 seconds.28
CASE PRESENTATIONS
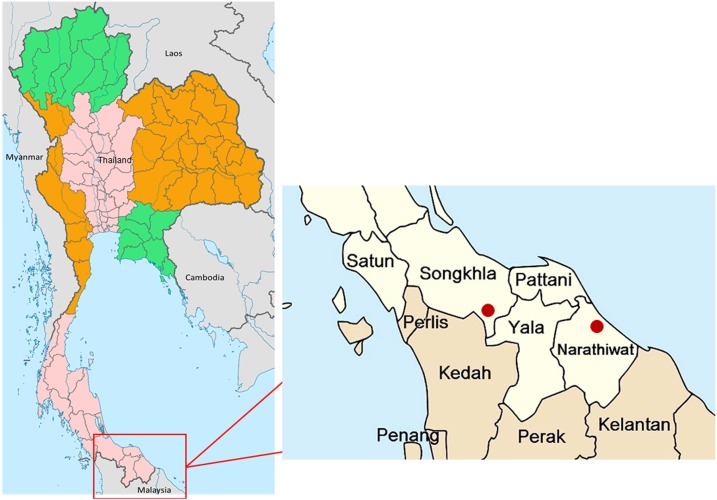
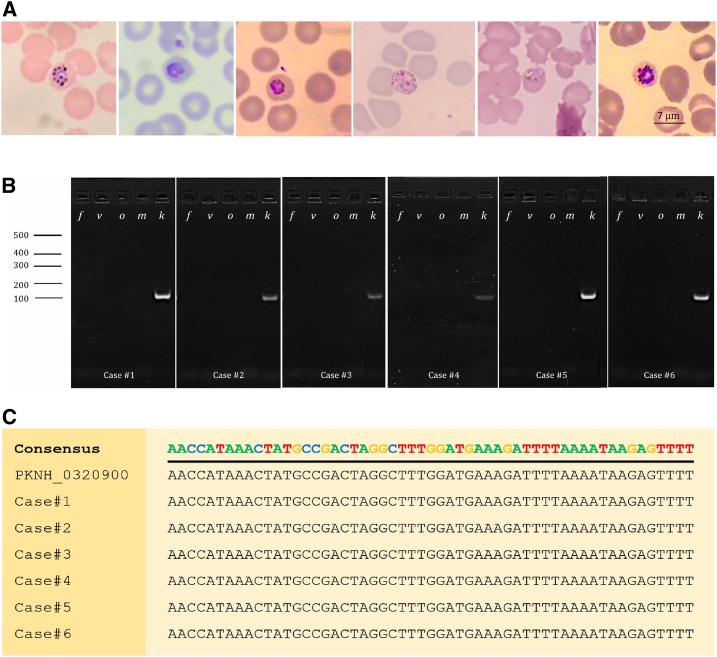
From November 2017 to April 2018, we identified six malaria patients with P. knowlesi infections, five from Songkhla Province and one from Narathiwat Province (Figure 1). These cases were suspected for P. knowlesi infection because of the unusual parasite morphology compared with more common P. vivax and P. falciparum. Blood films (Figure 2) of all cases showed trophozoites or schizonts without host cell enlargement, a feature consistent with P. knowlesi infection. Nested PCR, sequencing of the nested PCR products (Figure 2), and qPCR confirmed that infections were indeed due to P. knowlesi (Table 1).
Figure 1.
Map of Songkhla and Narathiwat provinces of Thailand and the Thai-Malaysian border. This figure appears in color at www.ajtmh.org.
Figure 2.
Diagnosis of Plasmodium knowlesi infection. (A) Light microscopic images of parasites with characteristics of P. knowlesi from Giemsa-stained thin blood smears. (B) Agarose gel images of nested polymerase chain reaction (PCR) demonstrating P. knowlesi infection using species-specific primers with the expected PCR product size of 110 base pairs. f, v, m, o, and k denote the species of parasites targeted by each PCR, from Plasmodium falciparum, Plasmodium vivax, Plasmodium malariae, Plasmodium ovale, and P. knowlesi. Numbers on the left indicate size in base pairs. (C) Sequences of the nested PCR products. The sequences of all six cases are identical to those of the reference P. knowlesi 18S rRNA gene PKNH_0320900 and differ from all known 18S rRNA genes of other human malaria parasites. This figure appears in color at www.ajtmh.org.
The first case, a 22-year-old male resident of Na Thawi district, Songkhla Province, presented himself at the malaria clinic of the VBDC in Na Thawi in November 2017 with six days of fever, chills, and headache. The initial diagnosis by light microscopy was P. vivax malaria, and the patient was treated with chloroquine (25 mg/kg over 3 days) and primaquine (0.25 mg/kg/day for 14 days) and recovered fully. The patient had worked in a rubber plantation and camped out in the forest at Ban Na Kha, Malaysia. The camping cottage was surrounded by a wooded area populated by wild macaques, the potential reservoir of the parasite.
The second case, a 45-year-old man, presented at the Naradhiwas Rajanagarindra Hospital in Narathiwat Province in January 2018. He displayed symptoms including a 6-day history of daily fever, shivering, headache, nausea, vomiting, jaundice, pallor, and dark urine. His temperature during admittance to the hospital was 39.3°C. He did not have a history of either malaria or blood transfusion. A malaria rapid diagnosis test (RDT: SD BIOLINE Malaria Ag P.f/Pan test) was negative for P. falciparum histidine rich protein 2 antigen but positive for Plasmodium lactate dehydrogenase. The patient was considered to have P. vivax infection and treated with chloroquine (25 mg/kg over 3 days) and primaquine (0.25 mg/kg/day for 14 days) according to the standard P. vivax treatment. The patient reported to have worked in palm plantations and traveled to work in Garuntun, Malaysia, before becoming sick. His work place was near a damp rainforest inhabited by monkeys.
The third case was a 50-year-old man in Na Thawi district, Songkhla Province. He had fever for 9 days with a mild headache. He was initially diagnosed as having influenza, but did not recover after treatment with antiflu medication. The patient returned to seek medical aid at the malaria clinic of VBDC in Na Thawi in February 2018. After being diagnosed as having P. vivax infection by light microscopy, the patient was treated with chloroquine (25 mg/kg over 3 days) and primaquine (0.25 mg/kg/day for 14 days) and recovered fully. The patient was a rubber plantation worker and reported to have had camped out for 4 days in the forest at Baan Keun Nam, Kedah state of Malaysia.
The fourth case was a 48-year-old woman in Sadao district, Songkhla Province. The patient presented at the Sadao Hospital in April 2018 after having experienced fever, chills, abdominal pain, and severe headache for 4 days. The patient was initially diagnosed as having P. vivax malaria and cured by the standard chloroquine (25 mg/kg over 3 days) and primaquine (0.25 mg/kg/day for 14 days). The patient lived in a rubber plantation and was a rubber tapper, herdsman, and nontimber forest product finder. She frequently visited the forest along the Thai-Malaysian border.
The fifth case was a 32-year-old male resident of Sadao district, Songkhla Province. The patient presented at the malaria clinic of VBDC in Sadao in April 2018 with 5-day fever, chills, and severe headache. Initial diagnosis was P. falciparum malaria due to the predominance of ring-stage parasites in blood smears. The patient was cured by standard 3-day dihydroartemisinin (2.5 mg/kg/day)–piperaquine (20 mg/kg/day) treatment with a single dose of primaquine (30 mg) according to Thailand’s national guideline. The patient was a rubber plantation worker and nontimber forest product finder. He reported to have had regularly visited the forest inhabited by wild monkeys near Satun Province of Thailand.
The sixth case, also detected in April 2018, was a 35-year-old man in Saba Yoi district, Songkhla Province. The patient came to the malaria clinic of VBDC in Saba Yoi with 2-day fever and a mild headache. The patient was initially diagnosed by light microscopy as having P. vivax malaria and treated with chloroquine (25 mg/kg over 3 days) and primaquine (0.25 mg/kg/day for 14 days). The patient was a rubber plantation worker and wild animal hunter. He reported to have spent 4 days in a hilly forest at Baan Keun Nam, Kedah state of Malaysia, before becoming sick.
DISCUSSION
We report a series of cases of P. knowlesi malaria in Songkhla and Narathiwat provinces of Southern Thailand. To our knowledge, this is the first report of P. knowlesi malaria in Songkhla Province, and the first from the Thai-Malaysian border area with blood smear evidence. The travel history of all patients revealed travel to an area inhabited by wild monkeys.
Plasmodium knowlesi infection is considered extremely uncommon in Thailand. During October 2017 and September 2018, a total of 10 cases of P. knowlesi malaria from the four border provinces (Songkhla, Yala, Narathiwat, and Satun) were reported for the first time by the Thai NMCP, despite the fact that the current national reporting system had been deployed since 2012. These cases forewarn the potential emerging threat of P. knowlesi in the southernmost area of Thailand. It is noteworthy that all reported P. knowlesi cases in this study were mistakenly diagnosed as P. vivax or P. falciparum during admission to the clinics or hospitals, suggesting that the hidden burden of knowlesi malaria might be much higher. It is important that blood smears of all suspected cases of P. knowlesi in Thailand and Peninsular Malaysia are confirmed by expert microcopy and molecular diagnosis to closely track the disease burden in the near future.
Table 1.
Summary of confirmed Plasmodium knowlesi malaria cases
| Case no. | Age (years) | Gender | Ethnicity | Residence | Traveled to | Initial malaria diagnosis | Expert microscopy | PCR-based diagnosis | Date | |
|---|---|---|---|---|---|---|---|---|---|---|
| Nested PCR | Quantitative polymerase chain reaction | |||||||||
| 1 | 22 | M | Thai | Na Thawi, Songkhla | Baan Na Ka, Kedah state of Malaysia | Plasmodium vivax | P. knowlesi | P. knowlesi | P. knowlesi | November 2017 |
| 2 | 45 | M | Thai | Jor I-rong, Narathiwat | Garuntun, Malaysia | P. vivax | P. knowlesi | P. knowlesi | P. knowlesi | January 2018 |
| 3 | 50 | M | Thai | Na Thawi, Songkhla | Baan Keun Nam, Kedah state of Malaysia | P. vivax | P. knowlesi | P. knowlesi | P. knowlesi | February 2018 |
| 4 | 48 | F | Thai | Sadao, Songkhla | Malaysian border | P. vivax | P. knowlesi | P. knowlesi | P. knowlesi | April 2018 |
| 5 | 32 | M | Thai | Sadao, Songkhla | Satun, Thailand–Malaysian border | Plasmodium falciparum | P. knowlesi | P. knowlesi | P. knowlesi | April 2018 |
| 6 | 35 | M | Thai | Saba Yoi, Songkhla | Baan Keun Nam, Kedah state of Malaysia | P. vivax | P. knowlesi | P. knowlesi | P. knowlesi | April 2018 |
F = female; M = male; PCR = polymerase chain reaction.
REFERENCES
- 1.White NJ, 2008. Plasmodium knowlesi: the fifth human malaria parasite. Clin Infect Dis 46: 172–173. [DOI] [PubMed] [Google Scholar]
- 2.Singh B, Daneshvar C, 2013. Human infections and detection of Plasmodium knowlesi. Clin Microbiol Rev 26: 165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abeyasinghe R, 2016. Plasmodium knowlesi Current Status and the Request for Review by an Evidence Review Group Committee. Malaria Policy Advisory Committee. Geneva, Switzerland: World Health Organization. [Google Scholar]
- 4.Marchand RP, Culleton R, Maeno Y, Quang NT, Nakazawa S, 2011. Co-infections of Plasmodium knowlesi, P. falciparum, and P. vivax among humans and Anopheles dirus mosquitoes, southern Vietnam. Emerg Infect Dis 17: 1232–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Veeranoot Nissapatorn YLAL, 2010. Plasmodium knowlesi: Old Story but New Fact in Southeast Asia. Joint International Tropical Medicine Meeting, December 1–3, 2010. Kuala Lumpur, Malaysia: Department of Parasitology, Faculty of Medicine, University of Malaya. [Google Scholar]
- 6.Traipattanakul JCD, Trakulhun K, Phiboonbanakit D, Mungthin M, 2014. A first case of Plasmodium knowlesi malaria in Phramongkutklao Hospital. J Infect Dis Antimicrob Agents 31: 91–100. [Google Scholar]
- 7.Bird EM, et al. 2016. Transfusion-transmitted severe Plasmodium knowlesi malaria in a splenectomized patient with beta-thalassaemia major in Sabah, Malaysia: a case report. Malar J 15: 357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chin W, Contacos PG, Collins WE, Jeter MH, Alpert E, 1968. Experimental mosquito-transmission of Plasmodium knowlesi to man and monkey. Am J Trop Med Hyg 17: 355–358. [DOI] [PubMed] [Google Scholar]
- 9.Chin W, Contacos PG, Coatney GR, Kimball HR, 1965. A naturally acquired quotidian-type malaria in man transferable to monkeys. Science 149: 865. [DOI] [PubMed] [Google Scholar]
- 10.Cox-Singh J, Singh B, 2008. Knowlesi malaria: newly emergent and of public health importance? Trends Parasitol 24: 406–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Luchavez J, Espino F, Curameng P, Espina R, Bell D, Chiodini P, Nolder D, Sutherland C, Lee K-S, Singh B, 2008. Human infections with Plasmodium knowlesi, the Philippines. Emerg Infect Dis 14: 811–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fan L, Lee SY, Koay E, Harkensee C, 2013. Plasmodium knowlesi infection: a diagnostic challenge. BMJ Case Rep 2013: bcr2013009558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eede PVD, Van HN, Van Overmeir C, Vythilingam I, Duc TN, Hung LX, Manh HN, Anné J, D’Alessandro U, Erhart A, 2009. Human Plasmodium knowlesi infections in young children in central Vietnam. Malar J 8: 249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iwagami M, et al. 2018. First case of human infection with Plasmodium knowlesi in Laos. PLoS Negl Trop Dis 12: e0006244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ghinai I, et al. 2017. Malaria epidemiology in central Myanmar: identification of a multi-species asymptomatic reservoir of infection. Malar J 16: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jongwutiwes S, Putaporntip C, Iwasaki T, Sata T, Kanbara H, 2004. Naturally acquired Plasmodium knowlesi malaria in human, Thailand. Emerg Infect Dis 10: 2211–2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Putaporntip C, Hongsrimuang T, Seethamchai S, Kobasa T, Limkittikul K, Cui L, Jongwutiwes S, 2009. Differential prevalence of Plasmodium infections and cryptic Plasmodium knowlesi malaria in humans in Thailand. J Infect Dis 199: 1143–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cordina CJ, Culleton R, Jones BL, Smith CC, MacConnachie AA, Coyne MJ, Alexander CL, 2014. Plasmodium knowlesi: clinical presentation and laboratory diagnosis of the first human case in a Scottish traveler. J Travel Med 21: 357–360. [DOI] [PubMed] [Google Scholar]
- 19.Froeschl G, Beissner M, Huber K, Bretzel G, Hoelscher M, Rothe C, 2018. Plasmodium knowlesi infection in a returning German traveller from Thailand: a case report on an emerging malaria pathogen in a popular low-risk travel destination. BMC Infect Dis 18: 148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kantele A, Marti H, Felger I, Müller D, Jokiranta TS, 2008. Monkey malaria in a European traveler returning from Malaysia. Emerg Infect Dis 14: 1434–1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Centers for Disease Control and Prevention , 2009. Simian malaria in a U.S. traveler–New York, 2008. MMWR Morb Mortal Wkly Rep 58: 229–232. [PubMed] [Google Scholar]
- 22.Kuo M-C, Chiang T-Y, Chan C-W, Tsai W-S, Ji D-D, 2009. A case report of Simian malaria, Plasmodium knowlesi, in a Taiwanese traveler from Palawan Island, the Philippines. Taiwan Epidemiol Bull 25: 178–191. [Google Scholar]
- 23.Tanizaki R, et al. 2013. First case of Plasmodium knowlesi infection in a Japanese traveller returning from Malaysia. Malar J 12: 128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Link L, Bart A, Verhaar N, van Gool T, Pronk M, Scharnhorst V, 2012. Molecular detection of Plasmodium knowlesi in a Dutch traveler by real-time PCR. J Clin Microbiol 50: 2523–2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jongwutiwes S, Buppan P, Kosuvin R, Seethamchai S, Pattanawong U, Sirichaisinthop J, Putaporntip C, 2011. Plasmodium knowlesi malaria in humans and macaques, Thailand. Emerg Infect Dis 17: 1799–1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sermwittayawong N, Singh B, Nishibuchi M, Sawangjaroen N, Vuddhakul V, 2012. Human Plasmodium knowlesi infection in Ranong province, southwestern border of Thailand. Malar J 11: 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.ThaiMoPH , 2017. Thailand Malaria Elimination Program, Thailand: Ministry of Public Health, Available at: http: http://203.157.41.215/malariar10/index_newversion.php. Accessed November 1, 2018. [Google Scholar]
- 28.Yorsaeng R, Saeseu T, Chotivanich K, Felger I, Wampfler R, Cui L, Mueller I, Sattabongkot J, Nguitragool W, 2019. Indigenous Plasmodium malariae infection in an endemic population at the Thai-Myanmar border. Am J Trop Med Hyg 100: 1164–1169. [DOI] [PMC free article] [PubMed] [Google Scholar]