Abstract
Objective
To investigate the effects of lactoferrin (LF) on subjective skin conditions in winter.
Design
A preliminary, randomized, double-blinded, placebo-controlled trial.
Setting and subjects
Healthy adults in Japan.
Interventions
Intake of placebo, 200 mg, or 600 mg of LF for 12 weeks in winter.
Endpoints
Changes in the scores of subjective skin conditions.
Results
Three hundred and forty-six subjects were randomized. Nine subjects (placebo, n=0; 200 mg, n=5; 600 mg, n=4) withdrew consent, and 7 subjects (placebo, n=4; 200 mg, n=2; 600 mg, n=1) were lost to follow-up, resulting in 330 for a full analysis set.
Outcomes
Changes in the scores of moisture were greater in the 600 mg group than in the placebo group. Changes in the scores of moisture were greater in the 200 mg and 600 mg groups, and of texture were greater in the 600 mg group than in the placebo group in female subjects.
Conclusion
Intake of LF may improve moisture or texture of skin in winter.
Keywords: lactoferrin, winter, skin, moisture, texture
Introduction
Cold and dry environmental conditions negatively affect the skin. People exposed to harsh weather during the winter may experience dry and itchy skin, or deterioration of already existing dermatoses.3 Major protective measures include moisturizing of the skin;6 however, other protective measures are insufficient and, thus, desired.
Lactoferrin (LF) is an iron-binding glycoprotein found in the milk of most mammals,8 and shows various biological functions.4 LF derived from bovine milk has been used to fortify food products and skin care products.14 In previous clinical trials, LF has been shown to improve dermatosis such as tinea pedis,16 acne vulgaris,2,7,9 plaque psoriasis,12 and atopic dermatitis.13 Therefore, intake of LF may be good for skin health. However, the effects of LF on the skin conditions of healthy people in winter have not yet been examined. Therefore, we herein preliminary investigated whether the intake of LF exerts positive effects on the subjective skin conditions of healthy adults in winter, as part of another trial to investigate the effects of LF on infectious diseases in winter.
Materials and Methods
Trial Design and Ethical Approval
This was a preliminary, randomized (1:1:1), double-blinded, placebo-controlled, parallel-group comparative trial conducted at the Department of Preventive Medicine and Public Health, Shinshu University School of Medicine, Matsumoto, Japan, between November 2015 and March 2016, in accordance with the current revision of the Declaration of Helsinki15 and Ethical Guidelines for Medical and Health Research Involving Human Subjects (2015).11 The protocol and informed consent form were approved by the Institutional Review Board (IRB) at Shinshu University School of Medicine on November 4, 2015 (approval number: 3271).
Subjects, Eligibility, and Exclusion Criteria
Eligible subjects were all healthy adults aged 20 years or more and working for kindergartens and nursery schools in Nagano prefecture, Japan. Exclusion criteria were a milk allergy, pregnancy, plan of resignation, a history of serious disorders in the liver, kidneys, heart, lungs, gastrointestinal tract, blood, endocrine system, or metabolic system, the habitual consumption of lactoferrin, and being judged as inappropriate to participate in this trial by the principal investigator (breast-feeding, chronic diseases, etc.).
Intervention
After IRB approval, investigators explained the details of this trial in accordance with the informed consent form, and written informed consent and background information were obtained from subjects. Three hundred and forty-six subjects were allocated into 3 groups. The test food and a diary were given before the start of the intervention. Subjects in each group were instructed to swallow 6 tablets per day (placebo, 200 mg of LF, or 600 mg of LF) with water at bedtime during the intervention period (12 weeks). Subjects were also instructed to record the intake of tablets, and physical changes in the diary. All subjects started and finished the intake of the test food and diary records on the same day. In the 7 days before (week 0) and in the last 7 days (week 12) of the intervention period, subjects answered questionnaires of subjective skin conditions. They subjectively evaluated moisture, resilience, texture, smoothness, transparency, pore, gloss, oiliness, wrinkle, and sag with scores between −3 (bad) and +3 (good). Changes in the scores of subjective skin conditions from week 0 to week 12 were calculated (Change in the score in each subjective skin condition = score on week 12 – score on week 0).
Endpoints
The endpoints were the changes in the scores of subjective skin conditions. Adverse events were evaluated with Revised National Cancer Institute – Common Toxicity Criteria (NCI-CTCAE) Version 4.0, and any adverse event for which a causal relationship to the intake of the test foods could not be ruled out was defined as an adverse drug reaction.
Sample Size
This trial was part of another trial to investigate the effects of LF on infectious diseases in winter. Therefore, we estimated the incidence of infectious diseases as 20%, the expected intake of LF to reduce it to 6.7% (one-third) with a type 1 error of 0.05 and a power of 80%, calculated a sample size of 306 (102 in each group), estimated a dropout rate of 33% (one-third), and set a target sample size of 450 (150 in each group) including dropouts.
Randomization, Allocation, and Blinding
The allocation manager, a researcher unrelated to this trial used computer-generated lists of random numbers block-randomized with a block size of 3 (1:1:1 ratio), prepared allocation tables for each nursery schools and kindergartens, and numbered test foods consecutively in accordance with the lists. He concealed the allocation tables from investigators, sealed them in an opaque envelope, and kept them until code breaking, and investigators and subjects were blinded during this period. Investigators enrolled subjects, assigned order numbers to them, and gave test foods with the corresponding numbers to them. Test foods were round tablets with a red-orange color, a weight of 250 mg, a diameter of 9.1 mm, and a thickness of 4.8 mm. The tablet for the 600 mg group contained 100 mg (600 mg/6 tablets) of bovine LF (purity [≥96%] by a HPLC method) derived from cheese whey (identical to the commercial supplement “Lactoferrin Original”, Morinaga Milk Industry, Japan), while that for the 200 mg group and placebo group contained 33.3 mg (200 mg/6 tablets) and 0 mg of LF, respectively. The tablet for the 600 mg group consisted of maltitol, indigestible dextrin, dextrin, hydrogenated rapeseed oil, LF, and guar gum. In the tablets for the 200 mg group and placebo group, LF was replaced with dextrin, and the color tone was adjusted with pigment. The allocation manager evaluated the characteristics of tablets, including their appearance, solidness, smell, and taste, as well as the packages of the 3 types of test foods at both allocation and code breaking, and confirmed them to be indistinguishable. Code breaking was performed after locking of the database and statistical analysis plan.
Statistical Analysis
In accordance with the closed testing procedure, the Mann–Whitney U-test was used to analyze the changes in the scores of subjective skin conditions (the placebo group and the 600 mg group were compared, and when a significant difference was observed between groups, the placebo group and the 200 mg group were compared). The Jonckheere-Terpstra trend test was used to analyze the dose–response relationship. Statistical analyses were performed with EZR software (Saitama Medical Center, Jichi Medical University, Saitama, Japan).5 P values <0.05 were considered to be significant.
Results
Three hundred and fifty subjects provided informed consent, 4 subjects were excluded (3 due to habitual consumption of lactoferrin and 1 due to being judged as inappropriate to participate), and a total of 346 subjects were enrolled (placebo, n=116; 200 mg, n=113; 600 mg, n=117) (Table 1).
Table 1.
Baseline Demographic of the Subjects
| Total | Placebo | 200 mg | 600 mg | |
|---|---|---|---|---|
| Subjects, n | 346 | 116 | 113 | 117 |
| Female, % | 95.1 | 94.0 | 93.8 | 97.4 |
| Age, year | 40.1 (12.9) | 40.3 (13.2) | 40.4 (13.1) | 39.7 (12.5) |
Note: Age is shown as the mean (standard deviation).
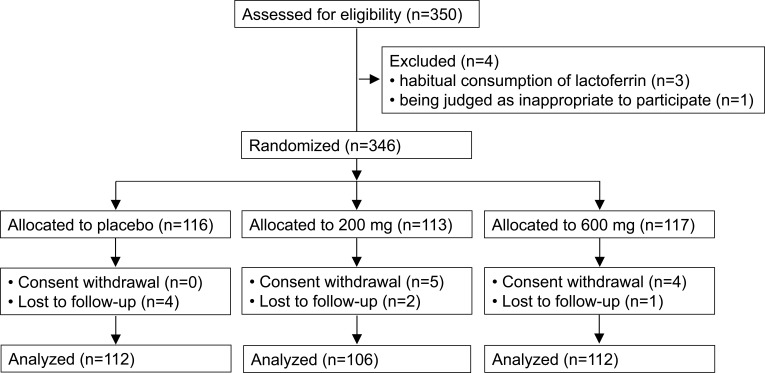
The mean (standard deviation [SD]) age was 40.1 years (12.9) and the proportion of females was 95.1%. After randomization, 9 subjects withdrew consent, and 7 subjects were lost to follow-up. We used a full analysis set (FAS) of the data (330 subjects) for the primary analysis (Figure 1).
Figure 1.
CONSORT flow diagram of subjects.
The pre-intervention period was between November and December 2015 (after IRB approval and trial registration). The intervention period was between December 2015 and March 2016 (12 weeks), and was completed as scheduled.
In the FAS of the data, 4, 7, and 5 subjects were lost in the placebo group, 200 mg group, and 600 mg group, respectively (n=112; n=106; n=112). The mean (SD) intake rates (%) of the test foods were 79.6 (18.7), 82.5 (20.3), and 82.7 (22.1) in each group, and were not significantly different (p=0.455). Baseline scores of subjective skin conditions in the FAS subjects are shown in Table 2. As endpoints, changes in the scores of moisture were significantly greater in the 600 mg group than in the placebo group (Table 3). No significant differences were observed in other scores. As 95% of subjects were women, female subjects were also analyzed. Changes in the scores of moisture showed a significant trend, and were significantly greater in the 200 mg and 600 mg groups. Changes in the scores of texture were also significantly greater in the 600 mg group than in the placebo group (Table 4).
Table 2.
Baseline Scores of Subjective Skin Conditions in the FAS Subjects
| Placebo | 200 mg | 600 mg | |
|---|---|---|---|
| Moisture | −0.46 (1.01) | −0.38 (1.20) | −0.62 (0.96) |
| Resilience | −0.66 (1.11) | −0.63 (1.07) | −0.79 (0.99) |
| Texture | −0.72 (1.05) | −0.56 (1.14) | −0.78 (0.91) |
| Smoothness | −0.44 (1.13) | −0.37 (1.15) | −0.45 (0.91) |
| Transparency | −0.85 (1.00) | −0.70 (1.10) | −0.92 (1.00) |
| Pore | −0.83 (1.03) | −0.88 (0.98) | −0.93 (0.97) |
| Gloss | −0.71 (1.04) | −0.50 (1.13) | −0.76 (0.88) |
| Oiliness | −0.30 (0.88) | −0.26 (0.94) | −0.43 (0.86) |
| Wrinkle | −0.71 (1.13) | −0.68 (1.07) | −0.66 (1.15) |
| Sag | −0.83 (1.11) | −0.83 (1.00) | −0.74 (1.10) |
Note: Scores represent the mean (standard deviation).
Table 3.
Changes in the Scores of Subjective Skin Conditions in the FAS Subjects
| Placebo | 200 mg | 600 mg | P Valuea | P Valueb | P for Trend | |
|---|---|---|---|---|---|---|
| Moisture | 0.21 (1.02) | 0.41 (0.99) | 0.49 (1.15) | 0.092 | 0.048 | 0.064 |
| Resilience | 0.30 (1.06) | 0.40 (1.03) | 0.55 (1.04) | 0.393 | 0.064 | 0.077 |
| Texture | 0.33 (1.11) | 0.35 (0.98) | 0.49 (0.95) | 0.586 | 0.082 | 0.104 |
| Smoothness | 0.22 (1.15) | 0.26 (1.11) | 0.37 (1.03) | 0.869 | 0.389 | 0.414 |
| Transparency | 0.35 (1.09) | 0.39 (0.91) | 0.47 (1.02) | 0.859 | 0.571 | 0.591 |
| Pore | 0.39 (0.93) | 0.47 (1.05) | 0.48 (1.00) | 0.731 | 0.688 | 0.710 |
| Gloss | 0.36 (1.13) | 0.33 (1.11) | 0.49 (0.97) | 0.883 | 0.148 | 0.195 |
| Oiliness | 0.23 (0.98) | 0.22 (1.08) | 0.25 (1.12) | 0.847 | 0.914 | 0.912 |
| Wrinkle | 0.31 (0.98) | 0.32 (1.01) | 0.24 (1.02) | 0.870 | 0.695 | 0.725 |
| Sag | 0.40 (0.93) | 0.45 (1.03) | 0.29 (1.08) | 0.782 | 0.289 | 0.330 |
Notes: Scores represent the mean (standard deviation). aPlacebo vs 200 mg; bplacebo vs 600 mg.
Table 4.
Changes in the Scores of Subjective Skin Conditions in Female Subjects
| Placebo | 200 mg | 600 mg | P Valuea | P Valueb | P for Trend | |
|---|---|---|---|---|---|---|
| Moisture | 0.17 (1.03) | 0.43 (0.99) | 0.50 (1.16) | 0.038 | 0.023 | 0.034 |
| Resilience | 0.30 (1.08) | 0.43 (1.06) | 0.57 (1.04) | 0.326 | 0.056 | 0.069 |
| Texture | 0.32 (1.13) | 0.37 (1.00) | 0.51 (0.95) | 0.385 | 0.046 | 0.063 |
| Smoothness | 0.20 (1.16) | 0.27 (1.14) | 0.38 (1.03) | 0.734 | 0.267 | 0.295 |
| Transparency | 0.34 (1.11) | 0.39 (0.91) | 0.49 (1.01) | 0.797 | 0.478 | 0.498 |
| Pore | 0.39 (0.95) | 0.48 (1.07) | 0.50 (0.99) | 0.677 | 0.628 | 0.655 |
| Gloss | 0.36 (1.14) | 0.35 (1.14) | 0.51 (0.96) | 0.772 | 0.125 | 0.171 |
| Oiliness | 0.23 (0.97) | 0.20 (1.10) | 0.25 (1.13) | 0.927 | 0.893 | 0.891 |
| Wrinkle | 0.31 (0.98) | 0.33 (1.02) | 0.25 (1.02) | 0.949 | 0.760 | 0.785 |
| Sag | 0.42 (0.92) | 0.45 (1.05) | 0.30 (1.08) | 0.874 | 0.290 | 0.326 |
Notes: Scores represent the mean (standard deviation). aPlacebo vs 200 mg; bplacebo vs 600 mg.
The numbers of subjects with adverse events were 58, 68, and 64 in the placebo group, 200 mg group, and 600 mg group, respectively, and no significant differences were observed between groups (P=0.302). Major events included hay fever, menstrual pain, transient fatigue, and adverse drug reactions were not observed.
Discussion
We herein investigated whether the intake of LF exerts positive effects on subjective skin conditions of healthy adults in winter. In Japan, almost all people working for nursery schools and kindergartens are women, therefore 95.1% of subjects were also women. In the FAS of the data, changes in the scores of moisture were significantly greater in the 600 mg group than in the placebo group. In the analysis of female subjects, changes in the scores of moisture showed significant trend, and were significantly greater in the 200 mg and 600 mg groups, and changes in the scores of texture were also significantly greater in the 600 mg group than in the placebo group. In a previous trial, the effects of LF on acne vulgaris were more clearly observed in women, and an association with the menstrual cycle was suggested.2 This may also be relevant to the results in the present trial. The incidence of adverse events was similar between groups, and adverse drug reactions were not observed. These results suggest that the intake of 200–600 mg/day of LF is safe and efficacious for improving subjective skin conditions such as moisture and texture in healthy adults in winter.
In previous clinical trials, the effects of LF on skin illnesses were reported;2,7,9,12,13,16 however, this is the first report suggesting the positive effects of LF on skin conditions in health subjects.
In the present trial, scores of moisture and texture were improved in the LF group. LF is reported to suppress the increase of transepidermal water loss and reduction in skin hydration induced by UV irradiation in hairless mice.10 As the mechanisms of action, LF is considered to reduce IL-1β (inflammatory cytokine) levels enhanced by UV irradiation, and prevent skin damage. Inflammatory cytokine, IL-1α, is reported to be higher in the epidermis of hairless mice kept in a low-humidity environment.1 Therefore, the dry environment in winter may cause inflammation of skin and increase transepidermal water loss, which may be suppressed by LF.
This preliminary trial is part of another trial to investigate the effects of LF on infectious diseases in winter. The effects of oral LF were evaluated based on subjective scoring, and objective measures, such as skin moisture and transepidermal water loss, were not determined. In addition, the use of cosmetics, lotion, or cream was not also controlled. These are the limitations of the present study, and further studies that consider these limitations are required to confirm the reliability of the outcomes obtained herein.
Conclusions
The intake of LF improved subjective scores of skin moisture in healthy adults, and subjective scores of skin moisture and texture in women in winter. Further investigation using objective measures are needed to confirm the efficacy of LF for improving skin conditions in healthy subjects.
Acknowledgments
Authors thank all subjects, directors in kindergartens and nursery schools, municipal officials in Matsumoto-city, Ikeda-town, Minowa-town, Yamagata-village, and Chikuhoku-village, Matsumoto Regional Health Industry Promotion Council, and Matsumoto City Medical Association for contribution in this trial.
This study was registered at the University Hospital Medical Information Network (UMIN) Clinical Trials Registry in Japan on November 11, 2015 under the registration number UMIN000019752. Full details on the protocol and dataset used in the present study (in Japanese) are available from the corresponding author on reasonable request.
Funding Statement
Morinaga Milk Industry provided research funds and the test foods.
Author Contributions
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
HO, Momoko Miyakawa, MT, KY, and FA are employees of Morinaga Milk Industry. The authors report no other conflicts of interest in this work.
References
- 1.Ashida Y, Ogo M, Denda M. Epidermal interleukin-1alpha generation is amplified at low humidity: implications for the pathogenesis of inflammatory dermatoses. Br J Dermatol. 2001;144(2):238–243. doi: 10.1046/j.1365-2133.2001.04007.x [DOI] [PubMed] [Google Scholar]
- 2.Chan H, Chan G, Santos J, Dee K, Co JK. A randomized, double-blind, placebo-controlled trial to determine the efficacy and safety of lactoferrin with vitamin E and zinc as an oral therapy for mild to moderate acne vulgaris. Int J Dermatol. 2017;56(6):686–690. doi: 10.1111/ijd.13607 [DOI] [PubMed] [Google Scholar]
- 3.Engebretsen KA, Johansen JD, Kezic S, Linneberg A, Thyssen JP. The effect of environmental humidity and temperature on skin barrier function and dermatitis. J Eur Acad Dermatol Venereol. 2016;30(2):223–249. doi: 10.1111/jdv.13301 [DOI] [PubMed] [Google Scholar]
- 4.García-Montoya IA, Cendón TS, Arévalo-Gallegos S, Rascón-Cruz Q. Lactoferrin a multiple bioactive protein: an overview. Biochimica Biophysica Acta Gen Subj. 2012;1820(3):226–236. doi: 10.1016/j.bbagen.2011.06.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013;48(3):452–458. doi: 10.1038/bmt.2012.244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kikuchi K, Kobayashi H, Hirao T, Ito A, Takahashi H, Tagami H. Improvement of mild inflammatory changes of the facial skin induced by winter environment with daily applications of a moisturizing cream. Dermatology. 2003;207(3):269–275. doi: 10.1159/000073089 [DOI] [PubMed] [Google Scholar]
- 7.Kim J, Ko Y, Park Y-K, Kim N-I, Ha W-K, Cho Y. Dietary effect of lactoferrin-enriched fermented milk on skin surface lipid and clinical improvement of acne vulgaris. Nutrition. 2010;26(9):902–909. doi: 10.1016/j.nut.2010.05.011 [DOI] [PubMed] [Google Scholar]
- 8.Masson PL, Heremans JF. Lactoferrin in milk from different species. Comp Biochem Physiol. 1971;39(1):119–129. [DOI] [PubMed] [Google Scholar]
- 9.Mueller EA, Trapp S, Frentzel A, Kirch W, Brantl V. Efficacy and tolerability of oral lactoferrin supplementation in mild to moderate acne vulgaris: an exploratory study. Curr Med Res Opin. 2011;27:793–797. doi: 10.1185/03007995.2011.557720 [DOI] [PubMed] [Google Scholar]
- 10.Murata M, Satoh T, Wakabayashi H, Yamauchi K, Abe F, Nomura Y. Oral administration of bovine lactoferrin attenuates ultraviolet B-induced skin photodamage in hairless mice. J Dairy Sci. 2014;97(2):651–658. doi: 10.3168/jds.2013-7153 [DOI] [PubMed] [Google Scholar]
- 11.Ogasawara K. 8. Revised “ethical guidelines for medical and health research involving human subjects.”. Jpn J Radiol Technol. 2017;73(5):397–402. doi: 10.6009/jjrt.2017_JSRT_73.5.397 [DOI] [PubMed] [Google Scholar]
- 12.Saraceno R, Gramiccia T, Chimenti S, Valenti P, Pietropaoli M, Bianchi L. Topical lactoferrin can improve stable psoriatic plaque. Giornale Italiano Di Dermatologia E Venereologia. 2014;149(3):335–340. [PubMed] [Google Scholar]
- 13.Tong PL, West NP, Cox AJ, et al. Oral supplementation with bovine whey-derived Ig-rich fraction and lactoferrin improves SCORAD and DLQI in atopic dermatitis. J Dermatol Sci. 2017;85(2):143–146. doi: 10.1016/j.jdermsci.2016.11.009 [DOI] [PubMed] [Google Scholar]
- 14.Wakabayashi H, Yamauchi K, Takase M. Lactoferrin research, technology and applications. Int Dairy J. 2006;16(11):1241–1251. doi: 10.1016/j.idairyj.2006.06.013 [DOI] [Google Scholar]
- 15.World Medical Association Declaration of Helsinki. Ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191. doi: 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 16.Yamauchi H, Yamazaki W, Kuwata T, Yamaguchi H. Oral administration of bovine lactoferrin for treatment of tinea pedis. A placebo-controlled, double-blind study. Mycoses. 2000;43(5):197–202. doi: 10.1046/j.1439-0507.2000.00571.x [DOI] [PubMed] [Google Scholar]