Abstract
Purpose
To evaluate the safety and efficacy of MicroPulse® transscleral cyclophotocoagulation (µP-TSCPC) up to 24-months follow-up using a standardized fixed protocol in patients with various types of glaucoma.
Methods
Prospective, nonrandomized, non-comparative interventional case series of µP-TSCPC performed by a single glaucoma surgeon at tertiary hospitals between May 2017 and May 2019.
Results
A total of 71 eyes of 68 patients (39 males, 29 females) aged 60.0 years (13–89 years) were treated with µP-TSCPC. The most common diagnosis was neovascular glaucoma. The safety index of corrected distance visual acuity (CDVA) was 1.0, i.e. there was no significant change in CDVA postoperatively compared to baseline CDVA. The median baseline IOP was 35.0 mmHg (21.0–70.0 mmHg), which was reduced to 16 mmHg (8–32 mmHg) at 12 months/last follow-up postoperatively (p<0.001, Wilcoxon signed-rank test). The median reduction in IOP was 52% (0.0–89%) at 12 months/last follow-up postoperatively compared to baseline. The median number of medications was 5 (3–5) at baseline compared to 4 (2–4) at 12 months/last follow-up postoperatively (p<0.001, Wilcoxon signed-rank test). The percent of eyes treated with systemic glaucoma medication was 62% (44) at baseline compared to 0% (0) at 12 months/last follow-up postoperatively. The postoperative surgical success of 90%, 91.4%, and 95.7% at 2 weeks, 3 months, and 6 months respectively then remains unchanged. No significant adverse events or complications were observed.
Conclusion
µP-TSCPC demonstrated good efficacy and safety profiles with minimal vision-threatening complications in treating a variety of glaucoma types.
Keywords: glaucoma, ciliary body, transscleral cyclophotocoagulation, MicroPulse diode laser
Introduction
Glaucoma is a progressive, degenerative, optic neuropathy.1 It is known to cause irreversible blindness with a 10% risk of becoming legally blind during one’s lifetime.2 Managing IOP plays a crucial role in the care of glaucoma, as it is the only modifiable factor in the progression of this disease. Currently, glaucoma therapies are designed as topical medications, incisional drainage surgery, and laser therapy. In most cases, topical medications are the initial intervention. While medications are a safe method by which to decrease IOP, patients need to have a high level of compliance in order for medications to be effective. Unfortunately, research has shown that compliance is generally poor and limited by side effects and high cost.3
As glaucoma progresses, filtration surgery is sometimes necessary to control IOP and preserve the residual optic nerve’s integrity. While it has been well established that IOP reduction has positive outcomes after a glaucoma filtration surgery, the long-term complication risks, such as infectious endophthalmitis, bleb-related complications, and hypotony, remain a concern.4
Finally, laser treatments work by lowering the IOP either by increasing the aqueous drainage or by lowering the aqueous production, as in transscleral cyclophotocoagulation (TSCPC). In TSCPC, laser is transmitted via the sclera to the melanin in the pigment cell of the ciliary body epithelium.5 Two TSCPC methods exist: traditional continuous-wave (CW-TSCPC) and more recently, MicroPulse® TSCPC (µP-TSCPC) (Iridex, Mountain View, CA, USA).
CW-TSCPC is utilized to directly disrupt the ciliary body that produces aqueous humor. This procedure can cause significant destruction to the adjacent tissue, including the ciliary stroma and muscle.6 As such, it may cause serious complications, such as vision loss, hyphema, macular edema, hypotony, persistent inflammation, phthisis bulbi, and sympathetic ophthalmia. Thus, traditional CW-TSCPC is typically reserved for advanced glaucoma with a poor visual outcome.7,8
µP-TSCPC has been shown to decrease IOP with fewer complications than CW-TSCPC.9 MicroPulse® technology (Iridex) separates a continuous-wave beam into repetitive short “ON” pulses separated by longer “OFF” periods that allow the tissue to cool to prevent thermal build-up and reduce the magnitude of tissue damage as seen in traditional, continuous-wave laser.
Previous studies have demonstrated µP-TSCPC is an effective method to reduce medications and IOP in eyes with various types of glaucoma, while exhibiting an excellent safety profile.10,11
Our study objective was to evaluate the safety and efficacy of µP-TSCPC at up to 24-months follow-up using a standardized fixed protocol in patients with various types of glaucoma. To our knowledge, this is the first study published on a patient population from Saudi Arabia and represents the largest cohort of neovascular glaucoma (NVG) patients (24 eyes; 33.8%) within a single µP-TSCPC study.
Methods
The study design was a prospective, nonrandomized, non-comparative interventional case series of µP-TSCPC performed by a single glaucoma surgeon (AH) at King Fahad Hospital of the University in Al Khobar and Al Mana General Hospital. This study was approved by a research ethics committee at King Fahad Hospital of the University in Al Khobar and Al Mana General Hospital, and all participants were provided with a written informed consent form that informed them of the risks and benefits of the study.
The patients recruited for this study met the following inclusion criteria: uncontrolled IOP > 21 mmHg despite using maximum tolerated medical therapy with or without oral acetazolamide, and with or without prior surgical intervention. Prior to enrollment, patients were informed of non-incisional µP-TSCPC, incisional surgery treatment options, and associated risks, and had opted for µP-TSCPC. Patients were followed for up to 24 months (from May 2017 to May 2019).
µP-TSCPC was performed in the main ocular operating room under local anesthesia (lidocaine hydrochloride 2%: bucaine 0.5%) in the form of a subtenon, peribulbar, or retrobulbar injection based on ocular axial length. The Cyclo G6® Glaucoma Laser (Iridex) was used in its MicroPulse treatment mode. The MicroPulse P3® Probe (Iridex) was connected to the Cyclo G6 laser console and used to deliver 2200 mW for 120 s for each 180-degree arc. Xylocaine gel was applied over the 360° of the conjunctiva prior to external illuminated fiber optics being applied to retro illuminate the ciliary body to identify its exact location. The MicroPulse P3 probe was placed adjacent to the limbal margin and held perpendicular to the surface, which positioned the fiber optic 3 mm away from the limbal margin. The probe was held with a firm, steady pressure over the conjunctiva while continuously sliding it back and forth for 10 times (each sweep in one direction was about 12 s) over 5 clock hours in the superior 180° and then repeated in the inferior 180°. Caution was taken to avoid thinned sclera, cystic blebs, and tube devices. Post µP-TSCPC, patients received a subconjunctival steroid injection, and then an ocular patch was applied. The postoperative regimen consisted of topical steroid every 2 hrs and tapered over 6 weeks, atropine for a week including a combination of dorzolamide and timolol (Cosopt; Merck, Kenilworth, NJ).
Patient data collected included age; sex; preoperative diagnosis; previous ocular surgeries; preoperative and postoperative medication number use; preoperative and postoperative visual acuity corrected distance visual acuity (CDVA) converted from Snellen into logarithm of minimum angle of resolution (LogMAR); preoperative and postoperative IOP measured by the Goldmann applanation tonometer; and postoperative complications collected over 24 months at baseline, 1 day, 2 weeks, and at 1, 3, 6, 9, 12, 18, and 24 months.
Success was defined as a change in IOP between 6 and 21 mmHg or 30% reduction in baseline IOP without vision loss of “light perception,” with no secondary glaucoma intervention, and without an increase in the number of medications. Failure was defined as IOP <6 mmHg (hypotony) or IOP >21 mmHg, vision loss of “light perception,” reoperation (defined as secondary surgical intervention) for glaucoma, or an increase in the number of medications. An IOP spike was defined as an IOP increase greater than or equal to 10 mmHg during the whole postoperative period. For the purpose of analysis, CDVA was converted from Snellen into LogMAR. The safety index was defined as postoperative CDVA/baseline CDVA in decimal.
The statistical analysis was performed using IBM’s SPSS for Windows (version 22). Figures were created using Microsoft Excel® 2013 (Microsoft Corporation, Redmond, WA, USA). The normality of the data was assessed using the Shapiro–Wilk test. The preoperative and postoperative IOP and the number of glaucoma medications were compared with the Wilcoxon signed-rank test. The surgical success, IOP spike, µP-TSCPC retreatment, and reoperation for glaucoma were analyzed using the Kaplan–Meier curve. A cox regression was performed in order to investigate influential factors. A p value less than 0.05 was considered statistically significant.
Results
A total of 71 eyes of 68 patients (39 males, 29 females) aged 60.0 years (13–89 years) were treated with µP-TSCPC. The demographic characteristics of the study patients are shown in Table 1.
Table 1.
Patient Demographic Characteristics
| Variable | Data (n=71) |
|---|---|
| Number of eyes (n) | 71 |
| Number of patients (n) | 68 |
| Age (year) | 60.0 years (13–89 years) |
| Gender % (n) | |
| Males | 57.4% (39) |
| Females | 42.6% (29) |
| Follow-up | 12 months (3–24 months) |
Table 2 summarizes the distribution of baseline diagnosis and corresponding surgical success for all study patients.
Table 2.
Patient Baseline Diagnosis And Corresponding Surgical Success
| Diagnosis | Percent Eyes (n) | Surgical Success % (n) |
|---|---|---|
| Neovascular glaucoma (NVG) | 33.8% (24) | 91.7% (22) |
| Primary open-angle glaucoma | 21.1% (15) | 93.3% (14) |
| Secondary glaucoma | 19.7% (14) | 85.7% (12) |
| Keratoplasty | 8.5% (6) | 35.7% (5) |
| Aphakia | 4.2% (3) | 21.4% (3) |
| Keratoprosthesis | 1.4% (1) | 7.1% (1) |
| Cyst excision | 1.4% (1) | 7.1% (1) |
| Iridocorneal endothelial syndrome | 1.4% (1) | 7.1% (1) |
| Trauma | 1.4% (1) | 7.1% (1) |
| Unknown etiology | 1.4% (1) | 7.1% (1) |
| Chronic angle closure glaucoma | 12.7% (9) | 100% (9) |
| Pseudoexfoliation glaucoma | 5.6% (4) | 75% (3) |
| Microphthalmos | 2.8% (2) | 100% (2) |
| Uveitic glaucoma | 2.8% (2) | 100% (2) |
| Congenital glaucoma | 1.4% (1) | 100% (1) |
Secondary glaucoma included three aphakia glaucoma, one post trauma, one iridocorneal endothelial syndrome, six keratoplasty glaucoma, one keratoprosthesis, and one cyst excision. The median cup-to-disc ratio was 0.9 (0.3–1.0).
Table 3 shows that 33.8% (24) eyes had prior surgical history, of which 5.6% (4) eyes had glaucoma surgery, 8.5% (6) eyes had combined surgery, and 19.7% (14) eyes had other ocular surgery. About two-thirds [66.2% (47)] of eyes treated with µP-TSCPC had no prior surgical intervention.
Table 3.
Patient Prior Surgical History
| Type Of Surgery | Number Of Eyes | Percent Eyes (n) | Surgical Success %(n) |
|---|---|---|---|
| Glaucoma surgery | 5.6% (4) | 100% (4) | |
| AVI+ECP+trabeculectomy | (1) | ||
| Trabeculectomy | (3) | ||
| Combined surgery | 8.5% (6) | 100% (5) | |
| PKP+trabeculectomy | (2) | ||
| PKP+AVI+CW-CPC | (1) | ||
| DASEK+trabeculectomy | (1) | ||
| Cataract+trabeculectomy | (1) | ||
| Keratoprosthesis+AVI | (1) | ||
| Other ocular surgery | 19.7% (14) | 92.95% (13) | |
| PKP | (4) | ||
| Globe rupture repair | (1) | ||
| Cataract | (6) | ||
| PKP+cataract | (1) | ||
| DASEK+cataract | (1) | ||
| Cyst excision | (1) | ||
| None | 66.2% (47) | 91.5% (43) |
Abbreviations: AVI, Ahmed valve implantation; ECP, endoscopic cyclophotocoagulation; PKP, penetrating keratoplasty; CW-CPC, continuous wave-cyclophotocoagulation; DASEK, descemet automated stripping endothelial keratoplasty.
The lens status was not determined except for aphakia (n=3 eyes). The safety index was 1.0, i.e. there was no significant change in CDVA postoperatively compared to baseline CDVA. The median baseline and 12 months/last follow-up postoperative CDVA of the treated eyes was 2.0 LogMAR (0.0–5.0 LogMAR) which is equivalent to count fingers (20/20-light perception) in Snellen notation.
Table 4 summarizes the CDVA at baseline, 2 weeks, 1 month, 3 months, 6 months, 9 months, and 12 months/last follow-up.
Table 4.
Patient Baseline And Postoperative CDVA
| Time | Eyes (N) | Corrected Distance Visual Acuity (LogMAR) | ||
|---|---|---|---|---|
| Median | Minimum | Maximum | ||
| Baseline | 71 | 2.00 | 0.00 | 5.00 |
| 2 weeks | 70 | 2.00 | 0.20 | 5.00 |
| 1 month | 68 | 2.00 | 0.20 | 5.00 |
| 3 months | 71 | 2.00 | 0.00 | 5.00 |
| 6 months | 67 | 2.00 | 0.00 | 5.00 |
| 9 months | 62 | 2.00 | 0.00 | 5.00 |
| 12 months/Last follow-up | 71 | 2.00 | 0.00 | 5.00 |
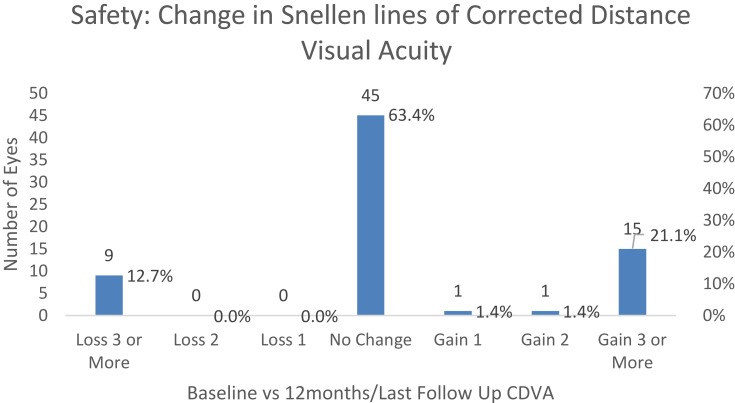
Figure 1 shows the number of lines gained/lost in postoperative CDVA compared to baseline CDVA.
Figure 1.
Lines gained/lost in postoperative CDVA compared to baseline CDVA.
The median CDVA of the fellow eyes was 0.40 LogMAR (0.0–5.0 LogMAR) which is equivalent to 20/50 (20/20-light perception) in Snellen notation. No loss of light perception occurred postoperatively.
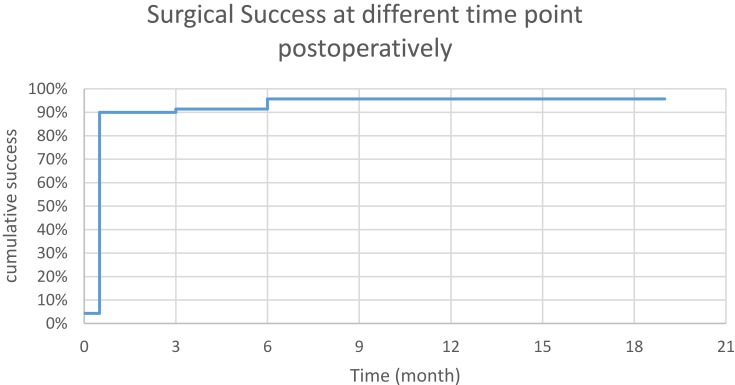
The Kaplan–Meier curve indicates postoperative surgical success of 90%, 91.4%, and 95.7% at 2 weeks, 3 months, and 6 months, respectively, then remains unchanged (Figure 2).
Figure 2.
Surgical success at different time points postoperatively.
The median time to success was 2 weeks. The surgical success per diagnosis is shown in Table 2. There was no statistical difference in surgical success among primary open-angle glaucoma, chronic angle closure glaucoma, NVG, and secondary glaucoma (p=0.854, Pearson chi-square). The surgical success was 100% in cases with prior glaucoma surgery or combined surgery (Table 3). Cox regression shows that age (p=0.604), gender (p=0.759), baseline number of medication (p=0.603), baseline IOP (p=0.630), and prior glaucoma surgery (p=0.574) were not influential factors on surgical success. Table 5 summarizes IOP at baseline, 2 weeks, 1 month, 3 months, 6 months, 9 months, and 12 months/last follow-up.
Table 5.
Baseline And Postoperative IOP
| Time | N | IOP (mmHg) | ||
|---|---|---|---|---|
| Median | Minimum | Maximum | ||
| Baseline | 70 | 35.0 | 21.0 | 70.0 |
| 2 weeks | 70 | 10.0 | 4.0 | 40.0 |
| 1 month | 67 | 13.0 | 6.0 | 47.0 |
| 3 months | 70 | 16.0 | 8.0 | 42.0 |
| 6 months | 66 | 16.0 | 8.0 | 36.0 |
| 9 months | 62 | 16.0 | 8.0 | 38.0 |
| 12 months/last follow-up | 70 | 16.0 | 8.0 | 32.0 |
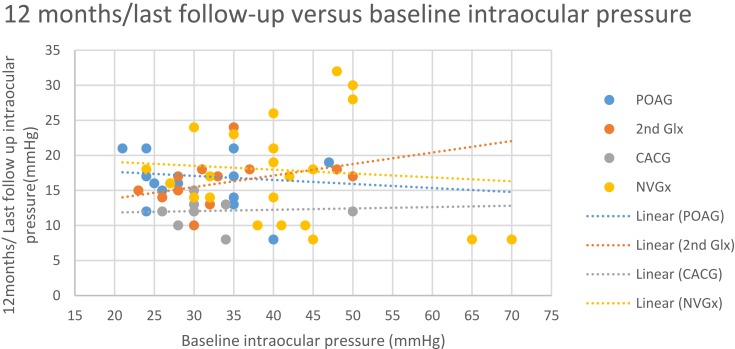
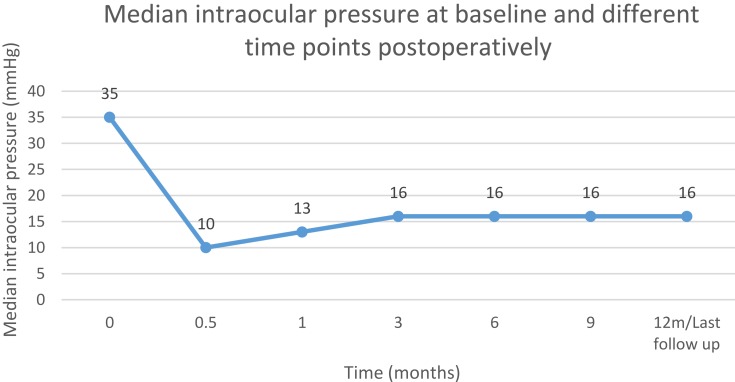
The median baseline IOP was 35.0 mmHg (21.0–70.0 mmHg), which was reduced to 16.0 mmHg (8.0–32.0 mmHg) at 12 months/last follow-up postoperatively (p<0.001, Wilcoxon signed-rank test). The median reduction in IOP was 52% (0.0–89%) at 12 months/last follow-up postoperatively compared to baseline. Linear regression indicates that reduction in IOP after µP-TSCPC at 12 months/last follow-up is expected to be 41.9% (95% CI: 37.5–46.3%) of baseline IOP (p<0.001) in future cases with similar characteristics to those in this study. Figure 3 plots baseline IOP versus 12 months/last follow-up IOP and indicates a statistically insignificant correlation between baseline and 12 months IOPs
Figure 3.
Final IOP versus baseline IOP.
Figure 4 shows median baseline IOP and postoperative IOP at 2 weeks, 1 month, 3 months, 6 months, 9 months, and 12 months/last follow-up, respectively.
Figure 4.
Baseline and postoperative IOP.
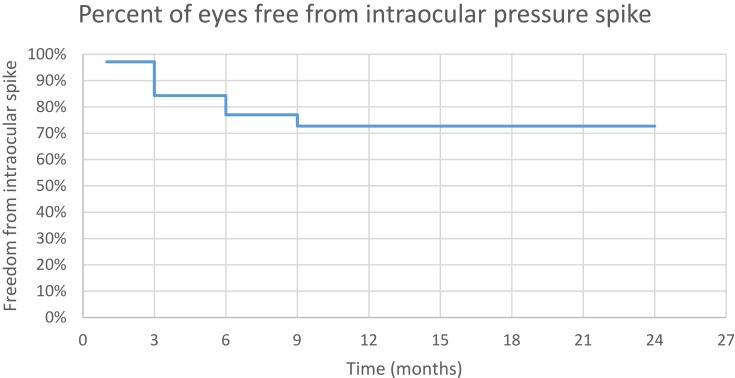
Kaplan–Meier curve indicates that 84.3% of eyes were free from IOP spike at 3 months, 77% of eyes free at 6 months, and 72.7% of free at 9 months postoperatively (Figure 5).
Figure 5.
Freedom from IOP spike.
The mean survival time to occurrence of IOP spike was 18.7 months (95% CI: 16.6–20.7 months). The number of medications was increased by one medication in seven eyes with IOP spike. Two eyes with IOP spike received µP-TSCPC retreatment. Three eyes with IOP spike received other surgical interventions (two Ahmed valve implantation and one deep sclerectomy after 3 months postoperatively). Seven eyes with IOP spike resolved spontaneously without increase of medication, µP-TSCPC retreatment, or other surgical intervention.
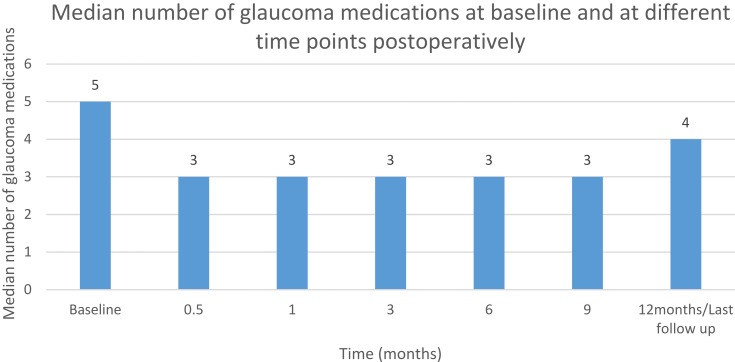
The median number of medications was 5 (3–5) at baseline compared to 4 (2–4) at 12 months/last follow-up postoperatively (p<0.001, Wilcoxon signedrank test). Table 6 summarizes the number of glaucoma medications prescribed postoperatively (Figure 6).
Table 6.
Baseline And Postoperative Glaucoma Medications
| Time | N | Number of Glaucoma Medications | ||
|---|---|---|---|---|
| Median | Minimum | Maximum | ||
| Baseline | 71 | 5.0 | 3.0 | 5.0 |
| 2 weeks | 70 | 3.0 | 0.0 | 4.0 |
| 1 month | 68 | 3.0 | 0.0 | 4.0 |
| 3 months | 71 | 3.0 | 0.0 | 5.0 |
| 6 months | 66 | 3.0 | 0.0 | 5.0 |
| 9 months | 62 | 3.0 | 0.0 | 4.0 |
| 12 months/last follow-up | 71 | 4.0 | 2.0 | 4.0 |
Figure 6.
Baseline and postoperative glaucoma medications.
The percent of eyes treated with acetazolamide (Diamox) Tab 250 mg PO was 62% (44) at baseline compared to 0% (0) at 12 months/last follow-up postoperatively. However, one eye received systemic medication for treatment of IOP spike; the systemic medication was discontinued in the subsequent visit.
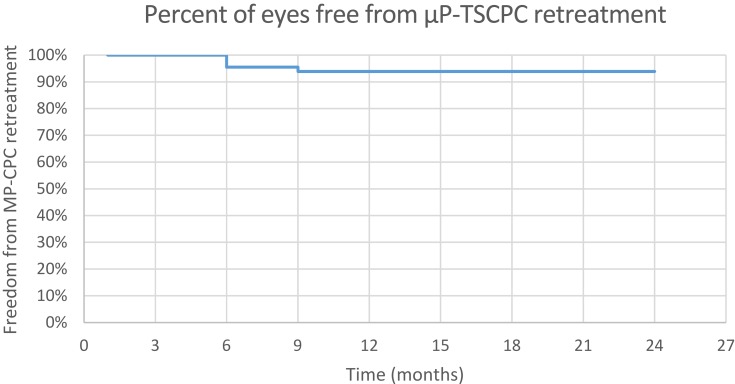
The median of number µP-TSCPC treatments was 1 (1–2), i.e. 94.4% (67) of eyes received one session of µP-TSCPC; only 5.6% (4) eyes received µP-TSCPC retreatment. The Kaplan–Meier curve indicates that 95.5% of eyes were free from µP-TSCPC retreatment at 6 months and 93.9% of eyes free at 9 months postoperatively (Figure 7).
Figure 7.
Freedom from µP-TSCPC retreatment.
The survival time to µP-TSCPC retreatment was 22.9 months (95% CI: 21.9–24 months). Only 5.6% (4) eyes had glaucoma reoperation. The Kaplan–Meier curve indicates that 94.4% were free from glaucoma reoperation at 3 months postoperatively (Figure 8).
Figure 8.
Freedom from reoperation for glaucoma surgery.
The survival time to glaucoma reoperation was 22.8 months (95% CI: 21.7–23.9 months).
Complications included one eye with inflammation and four eyes with tonic pupil (dilated with loss of accommodation) postoperatively, which eventually resolved after 1 month. There was no development of phthisis bulbi, hypotony, hyphema, vision loss of light perception, macular edema, severe pain, or corneal edema.
Discussion
Traditionally, our major concern with CW-TSCPC was the risk of potential vision-threatening complications. CW-TSCPC has shown in the histological specimen to be destructive to the surrounding structure of ciliary body, stroma, and muscle – supporting why it has been reserved as a last treatment resort for advanced uncontrolled glaucoma with poor visual prognosis.7,8 However, the novel method of µP-TSCPC has gained a more advantageous role in the treatment of glaucoma due to its reduced risk of complications and proven efficacy.10–12
In our study, surgical success rates post µP-TSCPC were 90%, 91.4%, and 95.7% at 2 weeks, 3 months, and 6 months, respectively. These results represent higher success rates than previous studies most likely attributable to higher total laser energy delivered and our patients’ elevated IOP baseline, in which most patients achieved a 30% reduction. In the prospective, non-comparative study done by Tan et al,10 the success rate (defined as IOP <21 mm Hg or a 30% IOP reduction) was 80% after 18 months. Zaarour et al13 showed a success rate (IOP between 6 and 21 mmHg or a >20% IOP reduction compared with baseline with or without IOP-lowering agents) of 81.4% at the 6-month follow-up and 73.3% at the 12-month follow-up. However, Williams et al14 found a lower success rate (defined as an IOP between 6 and 21 mmHg or a 20% reduction from baseline without an increase in glaucoma medication over baseline) of 75% at 3 months and 66% at 6 months. In our cases, patients’ IOP had significantly reduced by 52% at 12 months/last follow-up postoperatively. This number demonstrates a greater reduction than previous reports most likely due to our elevated IOP baseline. The retrospective chart review study by Emanuel et al11 showed an IOP reduction from 27.7 mmHg preoperatively to 16.3 mmHg at 1 month postoperatively, a 41.2% reduction. IOP further lowered to 14.6 mmHg, 13.0 mmHg, and 11.1 mmHg at months 3, 6, and 12, respectively. Also, Kuchar et al15 showed a mean IOP reduction of 40.1% at last follow-up (6–7 months), but Zaarour et al13 found a lower reduction rate of IOP of 25.5% at 1 month, and 35.4% at 15 months follow-up.
Our study demonstrated a major, but temporary, decrease in the topical anti-glaucoma drops. Medications were reduced from a baseline median of 5 (range, 3–5) to 4 (range, 2–4) at 12 months/last follow-up postoperatively, which corresponds to other reports. Kuchar et al15 showed decreased in anti-glaucoma medication number from 2.6 preoperatively to 1.9 postoperatively at 2 months follow-up. Moreover, Emanuel et al11 reported a decrease of antiglaucoma medications from 3.3 preoperatively to 1.9, 2.0, 2.0, and 2.3 at months 1, 3, 6, and 12, respectively. In addition, it is important to note that all 44 eyes (62%) being treated with oral acetazolamide at baseline no longer needed the tablets at 12 months/last follow-up. The results are similar to those achieved by Tan et al.10 Zaarour et al13 also found a significant decrease in the number of acetazolamide tablets over all the follow-up periods.
We did not observe any major complications, such as phthisis bulbi, hypotony, hyphemia, vision loss of light perception, macular edema, severe pain, or corneal edema. We did, however, observe one eye that developed inflammation. Our rate of inflammation is less than reported in other studies. This may be explained by our steroid treatment protocol and four eyes with tonic pupil (dilated with loss of accommodation), which eventually resolved after 1 month. In previous reports, the complication rates were found to be higher. Emanuel et al11 had five cases of persistent hypotony, three cases of hyphema, and one case of choroidals. Their postoperative inflammation was 86% at 1 week, which may have been due to their mean treatment session time (319 s), divided between the two hemispheres. Tan et al10 also found a higher complication rate after µP-TSCPC. Zaarour et al13 had no serious complications, and their 23% rate of postoperative inflammation completely cleared.
In our data, retreatment was needed in only 5.6%, but in Zaarour et al's13 study, they found 8% needed a retreatment after 6 months.
In conclusion, µP-TSCPC performed by one surgeon (AH) at fixed parameters showed promising results with consistent reduction in IOP and decrease of anti-glaucoma medications at last follow-up.
In our experience, µP-TSCPC demonstrated a good safety profile with minimal vision-threatening complications in treating a variety of glaucoma types.
We find µP-TSCPC is an encouraging treatment option for patients as a primary procedure in cases of high IOP or medication intolerance. A 91.5% success rate was achieved in about two-thirds (66.2%) of our patients who were on maximum tolerated medical therapy with no surgical intervention.
Additionally, µP-TSCPC can be used as a temporary treatment session for patients with high IOP refractory to the maximum tolerated medical therapy before proceeding to incisional glaucoma surgery. Lowering IOP prior to incisional surgery can help decrease the postoperative risks associated with an elevated IOP. Furthermore, the efficacy and safety of µP-TSCPC for patients who previously underwent other glaucoma surgery was very promising. It was an ideal treatment option for our patients with failed incisional surgeries and very high IOP, where additional incisional surgery would have been too risky. In post keratoplasty cases, µP-TSCPC treatment was effective in lowering IOP without graft-associated complications such as graft rejection or even failure.
Our study further supports the growing clinical evidence in the literature that demonstrates the safety and effectiveness of µP-TSCPC for treating a variety of glaucoma types. Limitations of the study include short follow-up duration and moderate number of data obtained. Further studies are required to find exactly the longevity of the µP-TSCPC effect and its possible late complications.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Weinreb RN, Aung T, Medeiros FA. The pathophysiology and treatment of glaucoma: a review. JAMA. 2014;311:1901–1911. doi: 10.1001/jama.2014.3192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006;90:262–267. doi: 10.1136/bjo.2005.081224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Prum BE Jr, Rosenberg LF, Gedde SJ, et al. Primary open-angle glaucoma preferred practice pattern guidelines. Ophthalmology. 2016;123:P41–P111. doi: 10.1016/j.ophtha.2015.10.053 [DOI] [PubMed] [Google Scholar]
- 4.Gedde SJ, Herndon LW, Brandt JD, Budenz DL, Feuer WJ, Schiffman JC; Tube Versus Trabeculectomy Study Group. Postoperative complications in the Tube Versus Trabeculectomy (TVT) study during five years of follow-up. Am J Ophthalmol. 2012;153:804–814. doi: 10.1016/j.ajo.2011.10.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ndulue JK, Rahmatnejad K, Sanvicente C, et al. Evolution of cyclo-photocoagulation. J Ophthalmic Vis Res. 2018;13:55–61. doi: 10.4103/jovr.jovr_190_17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pantcheva MB, Kahook MY, Schuman JS, et al. Comparison of acute structural and histopathological changes of the porcine ciliary processes after endoscopic cyclophotocoagulation and transscleral cyclophotocoagulation. Clin Exp Ophthalmol. 2007;35:270–274. doi: 10.1111/ceo.2007.35.issue-3 [DOI] [PubMed] [Google Scholar]
- 7.Schlote T, Derse M, Rassman K, et al. Efficacy and safety of contact trans-scleral diode laser cyclophoto-coagulation for advanced glaucoma. J Glaucoma. 2001;10:294–301. doi: 10.1097/00061198-200108000-00009 [DOI] [PubMed] [Google Scholar]
- 8.Ramli N, Htoon HM, Ho CL, et al. Risk factors for hypotony after transscleral diode cyclophotocoagulation. J Glaucoma. 2012;21:169–173. doi: 10.1097/IJG.0b013e318207091a [DOI] [PubMed] [Google Scholar]
- 9.Aquino MC, Barton K, Tan AM, et al. Micropulse versus continuous wave transscleral diode cyclophotocoagulation in refractory glaucoma: a randomized exploratory study. Clin Exp Ophthalmol. 2015;43(1):40–46. doi: 10.1111/ceo.12360 [DOI] [PubMed] [Google Scholar]
- 10.Tan AM, Chockalingam M, Aquino MC, et al. Micropulse transscleral diode laser cyclophotocoagulation in the treatment of refractory glaucoma. Clin Exp Ophthalmol. 2010;38:266–272. doi: 10.1111/j.1442-9071.2010.02238.x [DOI] [PubMed] [Google Scholar]
- 11.Emanuel ME, Grover DS, Fellman RL, et al. Micropulse cyclophotocoagulation: initial results in refractory glaucoma. J Glaucoma. 2017;26(8):726–729. doi: 10.1097/IJG.0000000000000715 [DOI] [PubMed] [Google Scholar]
- 12.Abdelrahman AM, El Sayed YM. Micropulse versus continuous wave transscleral cyclophotocoagulation in refractory pediatric glaucoma. J Glaucoma. 2018;27(10):9005. [DOI] [PubMed] [Google Scholar]
- 13.Zaarour K, Abdelmassih Y, Arej N, et al. Outcomes of micropulse transscleral cyclophotocoagulation in uncontrolled glaucoma patients. J Glaucoma. 2019;28:270–275. doi: 10.1097/IJG.0000000000001174 [DOI] [PubMed] [Google Scholar]
- 14.Williams AL, Moster MR, Rahmatnejad K, et al. Clinical efficacy and safety profile of micropulse transscleral cyclophotocoagulation in refractory glaucoma. J Glaucoma. 2018;27:445–449. doi: 10.1097/IJG.0000000000000934 [DOI] [PubMed] [Google Scholar]
- 15.Kuchar S, Moster MR, Reamer CB, et al. Treatment outcomes of micropulse transscleral cyclophotocoagulation in advanced glaucoma. Lasers Med Sci. 2016;31:393–396. doi: 10.1007/s10103-015-1856-9 [DOI] [PubMed] [Google Scholar]