Abstract
This feature article focuses on the discrepancy between the distribution of axon diameters within the primate corticospinal tract, determined neuroanatomically, and the distribution of axonal conduction velocities within the same tract, determined electrophysiologically. We point out the importance of resolving this discrepancy for a complete understanding of corticospinal functions, and discuss the various explanations for the mismatch between anatomy and physiology.
Keywords: antidromic, axon, corticospinal, monkey, recurrent inhibition
Innocenti et al. (2018) recently reported on the diversity of corticofugal projections in the primate brain. These authors emphasized the glaring discrepancy between anatomical and electrophysiological estimates of axon sizes and conduction velocities in the primate corticospinal tract (Humphrey and Corrie 1978; Firmin et al. 2014). The corticospinal tract exhibits a 100-fold difference in the diameters of its constituent axons, although how that is reflected in the diverse function of the tract is unknown. A major hindrance in understanding those functions is the almost complete lack of information about the numerous corticospinal neurons with fine axons. Pyramidal tract neurons (PTNs), whose axons travel in the pyramidal tract, and then descend further into the spinal cord, can be identified by antidromic activation from the pyramid, and such identification has been used in many studies to study the function of corticospinal neurons (e.g., Evarts 1968; Lemon et al. 1986; Umilta et al. 2007; Kraskov et al. 2009). However, most of these studies have been heavily biased towards recordings from larger neurons with fast axons, and accordingly, little is known about the slow PTNs. As Innocenti et al. comment, such a bias means that “half a century of electrophysiological recordings might have told us only a small fraction of what the brain does”.
Does this discrepancy really matter? Yes, it does matter because we are talking about the great majority of small fibers that make up one of the most important descending pathways (Lemon 2008). The enigma of the slow fibers needs to be solved! Since they are known to be more resistant to trauma than the larger, faster fibers (Blight 1991), they could be important in therapies designed to promote functional recovery after stroke or spinal injury. In the search for identifying the nature of the slow PTNs, Firmin et al. (2014) made a first step by defining the scale of the discrepancy between anatomy and physiology. They estimated that most of what we know about the neurophysiology of PTNs, including their role in movement preparation and execution, is entirely based on recordings gathered from cells giving rise to the larger PT fibers, estimated to comprise around 3% of the total tract. In the monkey this would still amount to over 18 000 fibers, but leaves us ignorant of the function of the remaining ~575 000! In many accounts of the motor system, the corticospinal output is considered as a homogenous population, although there is good evidence that, in addition to its involvement in motor control, this system is also involved in a variety of other functions, and this range of functions, such as descending control of afferent input, may be reflected in the almost 100-fold difference in primate corticospinal axon diameters (Lemon 2008).
Innocenti et al. (2018) documented the range of axon diameters in cortical projections arising from different cortical areas in the macaque monkey. They labeled corticofugal axons by cortical injections of biotinylated dextran amine (BDA) and measured these axons at the level of the internal capsule, pons, pyramidal tract, and lateral corticospinal tract, and compared their histological measurements with diffusion MRI tractography data from macaque and vervet monkey. In reviewing their findings in relation to earlier work, Innocenti et al. highlight the discrepancy between anatomy and electrophysiology at both ends of the axon size distribution: very large and very small.
Large fibers: a characteristic of many primates, including humans, is the presence of large fibers within the corticospinal tract, although it has long been known that these fibers form a relatively tiny proportion of the tract as a whole (only 8% of fibers in humans are >4 μm; (Lassek 1948)). Nevertheless large fibers dominate descending volleys in the corticospinal tract, excited by stimulation of the cortex or pyramid, and recorded from the surface of the spinal cord. These volleys have conduction velocities up to 70–85 m/s (Kernell and Chien-Ping 1967; Maier et al. 2002; Shimazu et al. 2004).
Single PTNs can be identified by antidromic invasion resulting from stimulation of the pyramidal tract, and a high proportion of these PTNs send axons to the spinal cord in the corticospinal tract. Humphrey and Corrie (1978) reported that macaque PTNs could conduct at up to 65 m/s, and our estimates, based on many years of recording antidromic responses from PTNs in awake macaques, suggest that the fastest PTNs conduct at velocities above 70 m/s, with the fastest PTN conducting at 94 m/s (Firmin et al. 2014). However, it should be stressed that the precision of these estimates is problematic because of the short conduction distance (~47 mm) from pyramid to cortex, and the brevity of the shortest antidromic latencies (ADLs; 0.5–0.7 ms).
A Hursh factor of 6.0, which relates the diameter of a myelinated axon to its conduction velocity (Hursh 1939), predicts that the fastest conducting axons should have diameters of around 12 μm. A very small number of axons with diameters in this range were reported by Häggqvist (1937) at light microscope level, and we confirmed this at the EM level (Firmin et al. 2014, Fig 2A). Innocenti et al. (2018) also found relatively few large axons, and accordingly they computed the maximum conduction velocity at around 67 m/s. However, because the largest axons constitute such a tiny proportion of the total, unless large numbers of fibers are sampled, it is quite possible that the largest fibers will be missed. Other methodological differences and the manner of correction for tissue shrinkage could well account for the variation across different studies in terms of the largest axon reported.
Small fibers: The discrepancy between anatomy and physiology is far more serious at the small end of the fiber spectrum. The primate corticospinal tract, as in other species, is dominated by the presence of many small fibers, with diameters around 1 μm (Häggqvist 1937; Firmin et al. 2014; Innocenti et al. 2018). In contrast, recordings of antidromically identified cell bodies giving rise to these fine fibers are either rare or completely missing from published studies, so that the distribution of ADLs of PTNs is dominated by values around 1.0 ms, corresponding to a fast conduction time between pyramidal tract and cortex (Firmin et al. 2014). The domination of the latency distribution by these fast PTNs is particularly clear in primary motor cortex (area 4) but is also seen in other cortical areas, such as supplementary motor area and premotor cortex (Macpherson et al. 1982; Firmin et al. 2014). Given the huge numbers of fine fibers in the tract, with a peak diameter at around 1 μm, one would expect to record many PTNs with ADLs of around 5–10 ms (for a Hursh factor of 6, equal to axons with outer diameters of 1.5 down to 0.8 μm). However, such responses are relatively rare, constituting only a few percent of the recorded responses (Humphrey and Corrie 1978; Firmin et al. 2014).
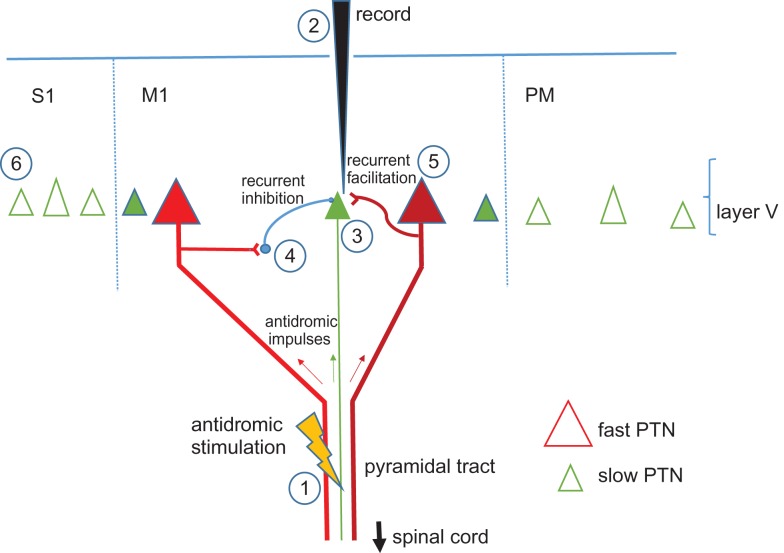
There are at least six different factors that might explain the lack of responses from slow PTNs (Fig. 1).
- ARE FINE AXONS IN THE PYRAMIDAL TRACT ACTIVATED BY TEST STIMULI?
- The first issue is whether antidromic stimuli actually excite axons belonging to slow PTNs (1 in Fig. 1). It is well-known that thin axons have higher thresholds than thick axons, so it is possible that search stimuli of a few hundred μA, while suprathreshold for fast PT fibers, are ineffective in activating slow ones (Swadlow 1998). Firmin et al. (2014) determined the threshold for 799 PTNs recorded in macaque M1, area F5 and the SMA. We looked at the threshold for the small number of slow PTNs with ADLs >5 ms, and estimated conduction velocities of <10/ms (only 14 PTNs), and found that it ranged from 30 to 300 μA, similar to the range found for fast fibers. Across the whole population of PTNs, there was only a weak correlation (r = 0.22) between ADL and the threshold for activating an antidromic response. The reason for this is likely to be that distance of the fiber from the stimulating electrodes inserted into the tract is a more important factor determining threshold than is fiber diameter. Future studies may need to test stronger and wider stimulation pulses to investigate whether fine PT fibers are excited.
- DOES ELECTRODE RECORDING BIAS MEAN THAT SLOW PTNs ARE MISSED?
- Assuming that slow axons are excited, why are slow PTNs missing from the recorded neurons? Most investigators have attributed this result to the well-known bias of extracellular recording methods towards stable recording from neurons with large somas and presumably large, fast axons (2 in Fig. 1: Towe and Harding 1970; Humphrey and Corrie 1978). Other factors, including cell-packing density, can contribute to this bias. Correcting the observed distribution of ADLs and estimated conduction velocities for this bias provided a good fit between the velocity and axon diameter distribution, with a peak at around 10 m/s, corresponding to an axon diameter of around 1.5 μm. Interestingly, several studies using glass micropipettes to record from PTNs reported a small but significant population of PTNs with slowly-conducting axons(<10 m/s; Towe et al. 1968 (cat); Humphrey and Corrie 1978 (macaque); Mediratta and Nicoll 1983 (rat)). Firmin et al. (2014), who used metal microelectrodes, which are probably more biased towards large neurons, reported <2% of macaque M1 PTNs with estimated conduction velocities <10 m/s. The introduction of fine intracortical probes, with multiple, high-density contacts, such as NeuroNexus or Neuropixels probes, may well allow the smaller neurons giving rise to fine axons to be recorded as discriminable single units.
- DO ANTIDROMIC IMPULSES IN THIN AXONS FAIL TO INVADE THEIR PARENT CELL BODIES?
- Failure to invade the soma-dendritic membrane (3 in Fig. 1) has been reported for some CNS neurons (Lipski 1981; Swadlow 1998), and if the smaller PTNs failed in this way, this would explain the difficulty of identifying them with the antidromic method. If this proves to be a significant problem for identification of slow PTNs, newer methodology, will be needed to locate them. One possibility is to infect cells retrogradely with viral vectors expressing optogenetic constructs (Tervo et al. 2016); any cell responding to the appropriate light wavelength is then proven to project to the region injected with the virus. Optogenetic depolarization of slow PTNs might also promote antidromic invasion.
- DOES RECURRENT INHIBITION BLOCK ANTIDROMIC INVASION OF SLOW PTNs?
- Failure to invade could also be influenced by the degree of synaptic inhibition of the PTN. Innocenti et al. (2018) made the interesting suggestion that the lack of recordings from slow PTNs might be due to recurrent inhibition (RI) of these neurons from collaterals of faster conducting axons activated by the stimuli applied to the pyramidal tract. Antidromic impulses in axons of fast PTNs could invade their collaterals, which then engage powerful inhibitory circuits (4 in Fig. 1) which inhibit other pyramidal cells, including slow PTNs (Berger et al. 2010).
- This idea is certainly worth investigating, although a number of points might suggest that RI may not block antidromic identification of slow PTNs. First, this type of RI is frequency dependent (Silberberg and Markram 2007) and effective inhibition requires repetitive stimuli; single stimuli applied to the pyramidal tract may not be effective in recruiting RI sufficient to block antidromic invasion (Phillips 1959; Stefanis and Jasper 1964; Takahashi et al. 1967). Second, the onset of RI in pyramidal cells has a long latency: in the rat in vitro study cited by Innocenti et al. (2018) the recurrent IPSPs, recorded intracellularly from one pyramidal neuron following intracellular repetitive stimulation of a neighboring pyramidal neuron did not begin until over 100 ms after the onset of stimulation. Of course this is a very localized stimulus. However, even in vivo stimulation of the entire pyramid evokes RI with a relatively long latency (>10 ms and up to 40 ms; (Stefanis and Jasper 1964; Takahashi et al. 1967)). This would be too late to block antidromic invasion at latencies of 5–10 ms. Third, it must be added that while there is evidence for RI in fast PTNs, none of the investigations to date has looked at RI in slow PTNs.
- RI might be expected to be more effective in the awake state than under anesthesia (Stefanis and Jasper 1964), which might block the recurrent inhibitory synapses. However, in the awake macaque, antidromic invasion of PTNs is highly reproducible from shock-to-shock, and we have seen little or no evidence for occasional failure of antidromic responses, which is what might be expected if RI was effective in blocking antidromic invasion of the recorded PTN. RI might also be reduced by local cortical injections of bicuculline, or muscimol, as suggested by Innocenti et al. (2018), and this should reveal more antidromic responses from slow PTNs.
- It is known that antidromic stimulation in the awake monkey can pause or suppress spontaneous activity in some PTNs (Stefanis and Jasper 1964), which would be expected if RI were present, and we have seen some evidence for such pauses in the awake macaque. However, these effects are generally not as strong as the synaptic facilitation of activity that often begins 4–5 ms after a single PT shock. The origin of this facilitation is unknown: it could be due to recurrent facilitation (see below) or spread of stimulating current to the adjacent medial lemniscus.
- DO RECURRENT FACILITATION AND DISCHARGE HISTORY PREVENT STABLE ANTIDROMIC RESPONSES?
- Stimulation of the pyramidal tract can also lead to recurrent facilitation of PTNs through axon collaterals of corticospinal neurons (5 in Fig. 1; Phillips 1959; Takahashi et al. 1967; Ghosh and Porter 1988; Thomson et al. 1993). Early recurrent synaptic excitation and discharge of slow PTNs from collaterals of faster PTNs could also block antidromic responses in the slow PTNs by colliding the antidromic spike before it reached the slow PTNs. A related problem is that while antidromic responses in a given PTN usually have a very constant latency, with jitter of <0.1 ms, slow PTNs may show higher jitter when the antidromic impulse is set up after spontaneous activity in the same neuron (Swadlow 1998). Two mechanisms might contribute here: (a) variable levels of recurrent synaptic excitation slowing or facilitating antidromic activation; (b) conduction in the slow axons being in impulse-dependent partial refractory or supernormal periods, during which conduction is respectively slower or faster than in a resting axon (Swadlow 1998). These effects are particularly marked in slowly-conducting fibers. Variation in either the probability or latency of responses may result in them being incorrectly dismissed as being synaptic, rather than antidromic in nature, especially as, when testing for antidromic excitation, synaptically evoked responses are often observed around the latencies expected for slow axons.
- ARE THERE SLOW PTNs IN PRIMARY MOTOR CORTEX?
- In 1937 G. Häggqvist was the first to demonstrate the preponderance of fine fibers in the macaque pyramidal tract. Interestingly, he suggested that these fine fibers arose from outside areas 4 and 6 (6 in Fig. 1). In four cases, he made large lesions of these areas in the left precentral gyrus. Animals were sacrificed 2 months later and fibers counted and measured in both the left and right pyramids. Whereas larger fibers were no longer present after the lesion, the fine fiber (<3 μm) count looked identical on both sides, suggesting that fine fibers arise outside areas 4 and 6. However, given the very large numbers of fine fibers, it may be that he was unable to detect differences in their number before and after lesion. Häggqvist’s conclusion has been questioned by Innocenti et al. (2018), who found plenty of fine corticospinal fibers labeled after BDA injections in area 4 (M1). At the level of the pyramid, these authors give the mean values for the inner diameter of axons in two different animals as 1.36 and 1.18 μm, with the median values 1.09 and 0.94 μm (Innocenti et al. (2018), Fig. 5), which clearly demonstrates the preponderance of small axons in their sample. Nevertheless, it would be invaluable to compare electrophysiological investigations in M1 with non-primary areas such premotor cortex or supplementary motor area, as well as cingulate motor or postcentral areas. Innocenti et al. (2018) have suggested that the timing differences between corticofugal outputs from different cortical areas could interact at spinal levels to update the final corticospinal input to spinal centres.
Figure 1.
Six different factors that might explain the relative lack of slow pyramidal tract neurons (PTNs) in recordings from motor areas. 1: failure to excite slow axons, electrical stimulation of the pyramidal tract to set up antidromic volleys activates large, fast axons (in red) but may not activate slow, thin axons (green) originating from slow PTNs (green triangle). 2: recording bias towards larger neurons may result in most antidromic effects being recorded in large, fast PTNs (red triangles). 3: failure of antidromic impulses to invade the soma and dendrites of small PTNs. 4: recurrent inhibition (RI), antidromic impulses in axons of large fast PTNs (red) results in impulses in intracortical collaterals which activate local inhibitory interneurons (blue) terminating on slow PTNs (green). RI prevents antidromic impulses in slow fibers from invading the cell bodies and dendrites of slow PTNs (green) 5: recurrent facilitation through excitatory collateral from fast PTNs to slow PTNs causes synaptic discharge of slow PTNs, colliding antidromic impulses in slow fibers before they can reach their parent slow PTNs. 6: absence of PTNs with slow, thin axons in M1, possible that these fibers originate mostly from cortex outside area 4 (M1) such as postcentral gyrus (S1) or premotor areas (PM).
All of the discussion above has been based on a simple relationship between conduction velocity and nerve fiber diameter, the Hursh factor, which allows us to characterize the corticospinal fibers in very broad terms. However, if we wish properly to resolve the discrepancy between the anatomical and physiological measurements for the fine axons, more detailed comparisons may be required that will raise various fundamental issues, including the importance of the g-ratio (the ratio between the diameter of an axon and its myelinated diameter), and how it relates to conduction velocity for fine axons in the CNS (Ritchie 1982), such as those of slow PTNs. Further questions relate to the effect of collateralization on conduction velocity, which might impact on the constancy of axon diameter along the entire length of corticospinal axons (Innocenti et al. 2018) and, last but not least, the internodal distance, that is assumed to be constant along the axon, but which might instead change, especially for longer axons.
In conclusion, there are a number of possible explanations for the discrepancy in the distribution of corticospinal axon sizes/conduction velocities determined by anatomical versus electrophysiological means. Until the neurons giving rise to these numerous fine fibers can be identified antidromically, their activity in relation to behavior and other functions cannot be investigated. We agree with Innocenti et al. (2018) that this discrepancy needs to be resolved.
Notes
Conflict of Interest: None declared.
References
- Berger TK, Silberberg G, Perin R, Markram H. 2010. Brief bursts self-inhibit and correlate the pyramidal network. PLoS Biol. 8:e1000473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blight AR. 1991. Morphometric analysis of a model of spinal cord injury in guinea pigs, with behavioral evidence of delayed secondary pathology. J Neurol Sci. 103:156–171. [DOI] [PubMed] [Google Scholar]
- Evarts EV. 1968. Relation of pyramidal tract activity to force exerted during voluntary movement. J Neurophysiol. 31:14–27. [DOI] [PubMed] [Google Scholar]
- Firmin L, Field P, Maier MA, Kraskov A, Kirkwood PA, Nakajima K, Lemon RN, Glickstein M. 2014. Axon diameters and conduction velocities in the macaque pyramidal tract. J Neurophysiol. 112:1229–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh S, Porter R. 1988. Morphology of pyramidal neurones in monkey motor cortex and the synaptic actions of their intracortical axon collaterals. J Physiol. 400:593–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphrey DR, Corrie WS. 1978. Properties of pyramidal tract neuron system within a functionally defined subregion of primate motor cortex. J Neurophysiol. 41:216–243. [DOI] [PubMed] [Google Scholar]
- Hursh JB. 1939. Conduction velocity and diameter of nerve fibers. Am J Physiol. 127:131–139. [Google Scholar]
- Häggqvist G. 1937. Faseranalytische studien über die pyramidenbahn. Acta Psychiatr Scand. 12:457–466. [Google Scholar]
- Innocenti GM, Caminiti R, Rouiller EM, Knott G, Dyrby TB, Descoteaux M, Thiran J-P. 2018. Diversity of cortico-descending projections: histological and diffusion MRI characterization in the monkey. Cereb Cortex. 10.1093/cercor/bhx363. [DOI] [PubMed] [Google Scholar]
- Kernell D, Chien-Ping WU. 1967. Responses of the pyramidal tract to stimulation of the baboon’s motor cortex. J Physiol. 191:653–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraskov A, Dancause N, Quallo MM, Shepherd S, Lemon RN. 2009. Corticospinal neurons in macaque ventral premotor cortex with mirror properties: a potential mechanism for action suppression? Neuron. 64:922–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassek AM. 1948. The pyramidal tract: basic considerations of corticospinal neurons. Res Pub Assoc Res Nerv Ment Dis. 27:106–128. [PubMed] [Google Scholar]
- Lemon RN. 2008. Descending pathways in motor control. Annu Rev Neurosci. 31:195–218. [DOI] [PubMed] [Google Scholar]
- Lemon RN, Mantel GW, Muir RB. 1986. Corticospinal facilitation of hand muscles during voluntary movement in the conscious monkey. J Physiol. 381:497–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipski J. 1981. Antidromic activation of neurones as an analytic tool in the study of the central nervous system. J Neurosci Methods. 4:1–32. [DOI] [PubMed] [Google Scholar]
- Macpherson JM, Wiesendanger M, Marangoz C, Miles TS. 1982. Corticospinal neurones of the supplementary motor area of the monkey. Exp Brain Res. 48:81–88. [DOI] [PubMed] [Google Scholar]
- Maier MA, Armand J, Kirkwood PA, Yang H-W, Davis JN, Lemon RN. 2002. Differences in the corticospinal projection from primary motor cortex and supplementary motor area to macaque upper limb motoneurons: an anatomical and electrophysiological study. Cereb Cortex. 12:281–296. [DOI] [PubMed] [Google Scholar]
- Mediratta NK, Nicoll JAR. 1983. Conduction velocities of corticospinal axons in the rat studied by recording cortical antidromic responses. J Physiol. 336:545–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips CG. 1959. Actions of antidromic pyramidal volleys on single Betz cells in the cat. Quart J Exp Physiol. 44:1–25. [DOI] [PubMed] [Google Scholar]
- Ritchie JM. 1982. On the relation between fibre diameter and conduction velocity in myelinated nerve fibres. Proc Roy Soc B. 217:29–35. [DOI] [PubMed] [Google Scholar]
- Shimazu H, Maier MA, Cerri G, Kirkwood PA, Lemon RN. 2004. Macaque ventral premotor cortex exerts powerful facilitation of motor cortex outputs to upper limb motoneurons. J Neurosci. 24:1200–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silberberg G, Markram H. 2007. Disynaptic inhibition between neocortical pyramidal cells mediated by Martinotti cells. Neuron. 53:735–746. [DOI] [PubMed] [Google Scholar]
- Stefanis C, Jasper H. 1964. Recurrent collateral inhibition in pyramidal tract neurons. J Neurophysiol. 27:855–877. [DOI] [PubMed] [Google Scholar]
- Swadlow HA. 1998. Neocortical efferent neurons with very slowly conducting axons: strategies for reliable antidromic identification. J Neurosci Methods. 79:131–141. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Kubota K, Uno M. 1967. Recurrent facilitation in cat pyramidal tract cells. J Neurophysiol. 30:22–34. [Google Scholar]
- Tervo DG, Hwang BY, Viswanathan S, Gaj T, Lavzin M, Ritola KD, Lindo S, Michael S, Kuleshova E, Ojala D, et al. 2016. A designer AAV variant permits efficient retrograde access to projection neurons. Neuron. 92:372–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson AM, Deuchars West DC. 1993. Large, deep layer pyramid-pyramid single axon EPSPs in slices of rat motor cortex display paired pulse and frequency-dependent depression, mediated presynaptically and self-facilitation, mediated postsynaptically. J Neurophysiol. 70:2354–2369. [DOI] [PubMed] [Google Scholar]
- Towe AL, Harding GW. 1970. Extracellular microelectrode sampling bias. Exp Neurol. 29:366–381. [DOI] [PubMed] [Google Scholar]
- Towe AL, Whitehorn D, Nyquist JK. 1968. Differential activity among wide-field neurons of the cat postcruciate cerebral cortex. Exp Neurol. 20:497–521. [DOI] [PubMed] [Google Scholar]
- Umilta MA, Brochier T, Spinks RL, Lemon RN. 2007. Simultaneous recording of macaque premotor and primary motor cortex neuronal populations reveals different functional contributions to visuomotor grasp. J Neurophysiol. 98:488–501. [DOI] [PubMed] [Google Scholar]