Abstract
Analgesics are the most commonly consumed drugs worldwide. Evidence that analgesics increase kidney cancer risk has been mixed. We investigated the association between renal cell carcinoma (RCC) and analgesic use in a large population-based case-control study and a post-trial observational cohort study. Findings were used to update a recent meta-analytic review. We analyzed data from 1,217 RCC cases and 1,235 controls in the US Kidney Cancer Study and 98,807 participants in the US Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial (PLCO: n=137 RCCs). Self-reported acetaminophen, aspirin, and non-steroid anti-inflammatory drug (NSAID) use and duration information was assessed in relation to RCC. For the US Kidney Cancer Study, we calculated odds ratios (ORs) and 95% confidence intervals (CIs) using unconditional logistic regression. For PLCO, we computed hazard ratios (HRs) and 95%CIs using Cox regression. Among case-control participants, RCC risk was associated with over-the-counter acetaminophen use (OR=1.35, 95%CI=1.01–1.83). There was a positive trend with increasing duration (P-trend=0.01), with a two-fold risk for use ≥10 years (OR=2.01, 95%CI=1.30–3.12). No association with prescription acetaminophen use was detected. In PLCO, acetaminophen use was also associated with increased RCC risk (HR=1.68, 95%CI=1.19–2.39), although elevated risk was absent among the few long-term users. No association with RCC risk was detected for aspirin or NSAIDs use in either study. An association between acetaminophen use and kidney cancer was supported by meta-analytic cohort (n=4; summary relative risk=1.34; 95%CI=1.13–1.59; P-heterogeneity=0.40) and case-control (n=9, summary OR=1.20; 95%CI=1.01–1.42; P-heterogeneity=0.05) findings. In brief, acetaminophen use may increase the risk of developing RCC.
Keywords: Renal Cell Carcinoma, Kidney Cancer, Acetaminophen, Analgesics, Meta-Analysis
INTRODUCTION
Analgesics, used to reduce pain or fever, are the most commonly consumed drugs in the world (1, 2). Acetaminophen, ibuprofen, and aspirin are the most frequently used over-the-counter (OTC) and prescription drugs in the United States (US) (2, 3). For over two decades, analgesic consumption and availability has increased in the US (1-3). According to US National Health Interview Survey data, regular aspirin use increased nearly 60% between 2005 and 2010 (4). US Food and Drug Administration data also showed an approximate 30% increase in acetaminophen sales between 2004 and 2008 (5). While epidemiological studies have shown a protective effect against various cancers for several of these drugs (6, 7), findings have been inconsistent for kidney cancer (8-13).
Kidney cancer is the seventh- and ninth-most commonly diagnosed malignancy among US men and women, respectively, with an estimated 64,000 cases expected in 2015 (14-16). The most common form, renal cell carcinoma (RCC), accounts for over 80% of kidney cancers (17, 18). Although primary RCC risk factors such as male sex, African American race, family history of kidney cancer, cigarette smoking, hypertension, and excess body weight have been established, these factors account for only about half of cases (17, 18).
To gain a better understanding of the relationship between analgesic use and RCC risk, we analyzed OTC and prescription drug history information from participants in a large US population-based case-control study of Caucasians and African Americans. Because self-reported information on analgesic use obtained post-diagnosis could be subject to recall bias, we further examined this relationship in the prospective US Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial (PLCO). Findings from both studies were subsequently used to update a recent meta-analytic review.
METHODS
US Kidney Cancer Study
Details regarding recruitment and data collection in the US Kidney Cancer Study have been described previously (19, 20). Briefly, between 2002 and 2007 we enrolled white and black participants, 20 to79 years old, from Detroit, Michigan and Chicago, Illinois with histologically confirmed incident RCC (International Classification of Diseases for Oncology (ICD-O), Third Edition, Code 64.9). Controls, selected from the general population, were frequency-matched to cases on age, sex, race (self-reported), and area of residence. Controls <65 years were identified from Department of Motor Vehicle (DMV) records. Controls aged 65 to 79 were identified from Centers for Medicare and Medicaid Services (CMS) files.
Although blacks have higher RCC rates than whites (18), in the current study more whites were diagnosed with RCC given the greater number of white residents. To increase enrollment of blacks, all eligible black cases were invited to participate, whereas only a subset of potentially eligible white cases were recruited. To further increase power for blacks, our targeted control:case matching ratio was 2:1 for blacks and 1:1 for whites. Among the 1,918 eligible cases identified, we were able to contact 1,571 cases for enrollment and recruit 1,217 cases for participation. Among the 2,718 potentially eligible controls identified, we were able to contact 2,269 controls for enrollment and recruit 1,235 for participation. Institutional Review Boards at collaborating institutions approved study procedures and all subjects provided written informed consent prior to data collection.
Data Collection
Demographics, diet, occupational history, smoking history, as well as medical and medication history data were collected using in-home, computer-assisted personal interviews. Approximately 60% of the cases were interviewed within six months of their diagnosis, and 84% were interviewed within one year of their diagnosis. Participants were queried about their use of the following medications for pain or fever, two years prior to the interview: OTC acetaminophen; prescription acetaminophen; OTC non-steroidal anti-inflammatory drugs (NSAIDs) including aspirin; and prescription NSAIDs. Data on aspirin use for a heart regimen was also collected. Subjects were shown medication cards with names of different OTC and prescribed products. “Regular” use of OTC drugs was defined as use for at least once a week for three months or longer, at least two years prior to the interview. Regular use of prescription drugs and aspirin for a heart regimen was defined as use once a week for one month or longer, at least two years prior to interview. For participants acknowledging regular use, information on start age or year, stop age or year, and duration of use was obtained. The “once per week” criterion was not maintained for the duration-response analyses. Whether participants took the drug “regularly” throughout the entire duration that they used the product is unknown. Additionally, data were not collected on frequency or dose of these medications.
Statistical Analysis
We assessed ever/never and duration of acetaminophen and NSAIDs use separately for OTC and prescription drugs; subsequently, we examined OTC and prescription use combined. Categories for duration of use were classified as: no regular use (referent), <1 year, 1 to <5 years, 5 to <10 years, and ≥10 years.
For analytic purposes, we developed a set of sample weights to reduce potential biases arising from differential sampling rates for controls and cases, from survey non-response, and from deficiencies in coverage of the population at risk by DMV and CMS files for control selection. Sample weights for controls also included a post-stratification adjustment so that the weighted control distribution across matching variables corresponded exactly to the weighted case distribution (21). We compared the sample-weighted frequency distribution of selected characteristics and known RCC risk factors between cases and controls using a Wald F-test (22). Unconditional logistic regression using post-stratified weights was used to calculate odds ratios (ORs) and 95% confidence intervals (CIs) associated with analgesic use. We performed trend tests by modeling the median of the exposure-response categories for duration of use as ordinal variables and applying the Wald Chi-Square test (22). Standard errors were estimated using the jackknife replicate weight method (23).
We adjusted regression models for sex, race, smoking (ever/never), age (20–44, 45–54, 55–64, 65–74, ≥75 years), body mass index (BMI; <25, 25 to <30, and ≥30 kg.m−2), hypertension (yes/no), family history of cancer (no, yes non-kidney, yes kidney), education (<12 years, high school graduate, some college, college graduate), center (Detroit/Chicago), and dialysis treatment (ever/never). We conducted subgroup analyses by race, sex, median age (<60 years/≥60 years), BMI (<30 kg.m−2 / ≥30 kg.m−2), hypertension (ever/never), smoking (ever/never), family history of cancer (yes/no), tumor grade (Fuhrman Nuclear Grade: I and II vs. III and IV), and histologic RCC subtypes (clear cell/papillary). Interactions were tested using a t-test and Wald test.
We conducted analyses in the US Kidney Cancer Study with SAS 9.2 and STATA 10.0 using procedures for sample weighted data. All analyses were also performed without sample weighting and results were similar to those presented (data not shown). Statistical significance was determined at a two-sided P-value<0.05.
The Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial (PLCO)
The multi-center randomized PLCO trial was designed to evaluate prostate, lung, colorectal, and ovarian cancer screening effectiveness on disease-specific mortality (24, 25). Between 1993 and 2001, nearly 155,000 men and women, aged 55 to 74, were enrolled from ten US centers. Anthropometric, medical, and lifestyle questionnaire data were collected at baseline. Between 2006 and 2007, a supplemental questionnaire was mailed to participants to gather additional information on family history of cancer, medical history, physical activity, and gender-specific health factors.
Our analysis excluded participants who had not returned both the baseline and supplemental questionnaires (n=52,345), had questionnaires completed by proxy (n=3,177), had reported a previous kidney cancer at baseline or were diagnosed with kidney cancer prior to completion of the supplemental questionnaire (n=408), or had missing follow-up data (n=163). Our final population included 98,807 subjects. PLCO participants were followed-up for all cancer diagnoses by annually mailed questionnaires, with subsequent confirmation through medical records. We identified 137 incident RCC (ICD-O, Second Edition, Code C649) cases from the date of supplemental questionnaire completion through December 31, 2009. Censoring events included death, loss to follow-up, renal pelvis or kidney cancer in situ diagnosis, or end of follow-up.
Data Collection
In addition to demographic factors, participants were asked the number of years they took acetaminophen, NSAIDs (excluding aspirin), and aspirin (including baby aspirin) at least once per week in the supplemental questionnaire. Data on strength/dose was collected only for aspirin. The questionnaire did not distinguish between OTC and prescription forms or provide a list of common prescription products that contain these medications in combination with other drugs.
Statistical Analysis
We examined acetaminophen, NSAIDs, and aspirin use separately. Questionnaire data for duration of use was collected using the following categories: no regular use (referent), <10 years, 10 to <20 years, and ≥20 years. Given the small number of subjects with ≥20 years of use, we combined the upper two categories. Aspirin strength/dose was assessed as 81mg or 325mg.
We conducted analyses using SAS 9.2. Statistical significance was determined at a two-sided P-value<0.05. We calculated hazard ratios (HRs) and 95% CIs relating risk factors to RCC incidence using Cox proportional hazards models, with attained age (in days) as the time metric. We performed trend tests with ordinal variables by treating each category as a continuous term (0, 1, 2…) in the model; tests were based on the Wald statistics. We assessed proportional hazards assumptions by adding interaction terms between age and each analgesic exposure in the Cox models; no evidence of violations against proportionality was found. Final models were adjusted for sex, BMI (<25, 25 to <30, and ≥30kg.m−2), hypertension (ever/never), race/ethnicity (white, black, Hispanic, Asian/Native Indian/Alaskan Pacific Islander), center, smoking (ever/never), and education (<12th grade, 12th grade or completed high school, post-high school training, some college, college, post-college graduate). We conducted subgroup analyses by sex, median age (<70 years/70+ years), hypertension (ever/never), smoking (ever/never), and BMI (<30 kg.m−2 / ≥30 kg.m−2). Lastly, we repeated analyses excluding the first-two years of follow-up after completion of the supplemental questionnaire (54 cases remained) to evaluate the possibility of bias introduced by early symptoms of cancer influencing reported analgesic use.
Meta-Analyses
To follow up on our acetaminophen findings, we conducted a meta-analysis of relevant cohort and case-control studies. Studies written in English through December 2013 were identified in PubMed using the following key words: analgesic use and (kidney) cancer risk, paracetamol use and (kidney) cancer risk, and acetaminophen use and (kidney) cancer risk. References from identified publications were reviewed for additional studies. Including our current study, four cohort (11-13) and 12 case-control (26-36) studies on kidney cancer risk and acetaminophen use were identified. To ensure thoroughness of our meta-analysis, we designed and reported findings according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guideline (Supplemental Figure 1) (37).
Meta-analyses were conducted for cohort and case-control studies separately, using STATA version 10.0. For several studies, risk estimates were not provided; where possible, we calculated crude risk estimates using the number of subjects provided (i.e., unexposed/exposed) (11, 26, 28, 30, 31). Sensitivity analyses excluding these crude risk estimates from analyses did not significantly alter findings. The data were combined using random-effects models to estimate the summary relative risks (SRRs) or odds ratios (SORs) and 95% CIs. Cochrane’s Q test and Higgin’s I2 statistic were used to evaluate heterogeneity among the studies, while publication bias was assessed by Begg’s and Egger’s tests and through evaluation of funnel plots (38, 39).
RESULTS
US Kidney Cancer Study
Cases and controls were comparable with regard to sex and age, but cases had a higher BMI, prevalence of hypertension, history of smoking, a lower education level, and were more likely to have a family history of kidney cancer, and to have undergone dialysis treatment (Supplemental Table 1). Stratified analyses showed comparable distribution of characteristics by race and sex (data not shown).
Table 1 displays associations between analgesic use and RCC risk. Ever OTC acetaminophen use was associated with increased RCC risk (OR=1.35, 95% CI=1.01– 1.83). A positive trend with increasing duration of OTC acetaminophen use (P-trend=0.01) was also observed; subjects with ≥10 years of use had a two-fold increase in risk (OR=2.01, 95% CI=1.30–3.12). Findings for prescription acetaminophen were null. A borderline significant trend (P-trend=0.07) was observed for OTC and prescription acetaminophen use combined, with a significantly elevated risk for ≥10 years of use (OR=1.54, 95% CI=1.09– 2.16). RCC risk was not associated with OTC or prescription NSAIDs or with aspirin use for a heart regimen. Similar findings are shown by sex and race, with no evidence of interaction (Supplemental Table 2). Results for analgesic use did not differ (P-interaction>0. 05) by BMI, median age, smoking status, hypertension, tumor grade, or RCC histologic subtypes (data not shown).
Table 1.
Weighted RCC risk and “regular” use of analgesics in the US Kidney Cancer Study
| Analgesics | Case, n | Control, n | ORa | 95% CIa | |
|---|---|---|---|---|---|
| ACETAMINOPHEN USE AND DURATION | |||||
| Over the Counter | |||||
| No | 1081 | 1144 | 1.00 | ||
| Yes | 133 | 90 | 1.35 | 1.01 | 1.83 |
| <1year | 12 | 12 | 1.08 | 0.44 | 2.64 |
| 1 to <5 years | 24 | 24 | 0.79 | 0.41 | 1.53 |
| 5 to <10 years | 27 | 16 | 1.37 | 0.71 | 2.65 |
| 10+ years | 63 | 34 | 2.01 | 1.30 | 3.12 |
| P-trend | 0.01 | ||||
| Prescription | |||||
| No | 1014 | 1038 | 1.00 | ||
| Yes | 198 | 190 | 0.96 | 0.74 | 1.24 |
| <1year | 64 | 80 | 0.88 | 0.63 | 1.22 |
| 1 to <5 years | 55 | 50 | 1.19 | 0.74 | 1.93 |
| 5 to <10 years | 27 | 19 | 1.13 | 0.59 | 2.19 |
| 10+ years | 43 | 30 | 1.09 | 0.65 | 1.82 |
| P-trend | 0.67 | ||||
| Over the Counter or Prescription | |||||
| No | 937 | 986 | 1.00 | ||
| Yes | 275 | 242 | 1.09 | 0.87 | 1.37 |
| <1year | 61 | 80 | 0.86 | 0.61 | 1.21 |
| 1 to <5 years | 64 | 62 | 1.03 | 0.67 | 1.59 |
| 5 to <10 years | 37 | 30 | 1.06 | 0.59 | 1.89 |
| 10+ years | 92 | 57 | 1.54 | 1.09 | 2.16 |
| P-trend | 0.07 | ||||
| NSAIDs (INCLUDING ASPIRIN) USE AND DURATION | |||||
| Over the Counter | |||||
| No | 1076 | 1114 | 1.00 | ||
| Yes | 137 | 120 | 1.01 | 0.76 | 1.34 |
| <1year | 16 | 11 | 1.28 | 0.58 | 2.81 |
| 1 to <5 years | 34 | 30 | 0.94 | 0.56 | 1.57 |
| 5 to <10 years | 23 | 22 | 0.89 | 0.43 | 1.85 |
| 10+ years | 53 | 49 | 1.01 | 0.67 | 1.53 |
| P-trend | 0.93 | ||||
| Prescription | |||||
| No | 1031 | 1032 | 1.00 | ||
| Yes | 182 | 197 | 0.87 | 0.66 | 1.16 |
| <1year | 50 | 63 | 0.78 | 0.53 | 1.15 |
| 1 to <5 years | 56 | 64 | 0.95 | 0.61 | 1.48 |
| 5 to <10 years | 32 | 29 | 0.91 | 0.50 | 1.69 |
| 10+ years | 40 | 31 | 1.14 | 0.65 | 2.01 |
| P-trend | 0.86 | ||||
| Over the Counter and Prescription | |||||
| No | 927 | 970 | 1.00 | ||
| Yes | 283 | 261 | 1.07 | 0.86 | 1.35 |
| <1year | 56 | 60 | 1.04 | 0.70 | 1.54 |
| 1 to <5 years | 80 | 72 | 1.14 | 0.78 | 1.64 |
| 5 to <10 years | 46 | 33 | 1.50 | 0.85 | 2.64 |
| 10+ years | 88 | 74 | 1.15 | 0.83 | 1.57 |
| P-trend | 0.20 | ||||
| ASPIRIN FOR HEART REGIMEN USE AND DURATION | |||||
| No | 786 | 879 | 1.00 | ||
| Yes | 401 | 325 | 1.15 | 0.92 | 1.42 |
| <1year | 37 | 47 | 0.75 | 0.43 | 1.29 |
| 1 to <5 years | 149 | 125 | 1.09 | 0.81 | 1.47 |
| 5 to <10 years | 100 | 74 | 1.22 | 0.85 | 1.75 |
| 10+ years | 96 | 71 | 1.25 | 0.89 | 1.75 |
| P-trend | 0.12 | ||||
Abbreviations: BMI- body mass index; CI- confidence interval; n- number; NSAIDs- non-steroid anti-inflammatory drugs; OR- odds ratio; OTC- over-the-counter; RCC- renal cell carcinoma.
The following data are unknown: OTC acetaminophen use (3 cases, 1 control) and duration (10 cases, 5 controls), prescription acetaminophen use (5 cases, 7 controls) and duration (14 cases, 18 controls), OTC NSAIDs use (4 cases, 1 control) and duration (15 cases, 9 controls), prescription NSAIDs use (4 cases, 6 controls) and duration (8 cases and 16 controls), and aspirin for a heart regimen use (30 cases, 31 controls) and duration (49 cases, 39 controls).
Due to missing start age or year, stop age or year, or duration of use data the following are unknown: overall acetaminophen duration (26 cases, 20 controls) and overall NSAIDs duration (20 cases, 26 controls).
Weighted logistic regression adjusted for sex, race, hypertension, BMI, smoking status, age, education, study center, family history of cancer, and dialysis treatment.
PLCO
Among the 98,807 PLCO participants examined, 82% reported regular analgesic use (aspirin, 62.0%; non-aspirin NSAIDs, 30 9%; acetaminophen, 32.7%). Compared to non-analgesic users, participants with regular use typically had a higher BMI and were more likely to be white, to smoke, and to be hypertensive (Supplemental Table 3).
An elevated RCC risk was observed among regular acetaminophen users (HR=1.68, 95% CI=1.19– 2.39) (Table 2). Risk was elevated among participants with <10 years of use (HR=2.09, 95% CI=1.39–3.14) but not for those with ≥10 years (HR=1.08, 95% CI=0.55–2.10). Findings for NSAIDs and aspirin were null. Results for analgesic use did not differ (P-interaction>0.05) by sex, race, BMI, median age, smoking status, hypertension, clear cell RCC histologic subtype, or after excluding the first-two years of follow-up (data not shown).
Table 2.
RCC risk and “regular” use of analgesics in the PLCO Screening Trial
| Analgesics | Cases, n | HR | 95% CI | |
|---|---|---|---|---|
| RCC Cases | 137 | |||
| ACETAMINOPHEN USE AND DURATION | ||||
| No | 73 | 1.00 | ||
| Yes | 59 | 1.68 | 1.19 | 2.39 |
| <10 years | 36 | 2.09 | 1.39 | 3.14 |
| 10+ years | 10 | 1.08 | 0.55 | 2.10 |
| P-trend | 0.06 | |||
| NSAIDs (EXCLUDING ASPIRIN) USE AND DURATION | ||||
| No | 88 | 1.00 | ||
| Yes | 41 | 1.03 | 0.71 | 1.51 |
| <10 years | 33 | 1.43 | 0.95 | 2.15 |
| 10+ years | 4 | 0.43 | 0.16 | 1.17 |
| P-trend | 0.69 | |||
| ASPIRIN USE, DURATION AND STRENGTH | ||||
| No | 51 | 1.00 | ||
| Yes | 84 | 0.82 | 0.58 | 1.17 |
| <10 years | 44 | 0.80 | 0.54 | 1.20 |
| 10+ years | 29 | 0.83 | 0.52 | 1.32 |
| P-trend | 0.38 | |||
| 325mg strength | 32 | 0.87 | 0.56 | 1.36 |
| 81mg strength | 48 | 0.80 | 0.54 | 1.20 |
| P-trend | 0.29 | |||
Abbreviations: BMI- body mass index; CI- confidence interval; HR- Hazard Ratios; n- number; NSAIDs- non-steroid anti-inflammatory drugs; PLCO- Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial; RCC- renal cell carcinoma.
The following data are unknown: acetaminophen use (5 cases) and duration (18 cases), NSAIDs use (9 cases) and duration (13 cases), and aspirin use (2 cases), duration (13 cases) and strength (6 cases).
Cox proportional hazard regression models adjusted for: BMI, education, race, smoking status, hypertension and center.
Meta-Analysis
Four cohort (11-13) and 12 case-control (26-36) studies were identified from the scientific literature. Supplemental Table 4 briefly describes each study. Three case-control studies (29-31) were excluded because the study populations were subsets of a large international study (32). Thus, 13 studies (four cohort, nine case-control) were included in our meta-analysis.
A forest plot in Figure 1 summarizes study-specific and summary associations between acetaminophen use and kidney cancer. The acetaminophen-kidney cancer SRR association for all studies combined was 1.25 (95% CI=1.10–1.41), with no evidence of between-study heterogeneity (P=0.08; I2=37.73) or publication bias (P-Begg’s=0.54; P-Egger’s=0.42). The summary effect was slightly stronger for cohort (SRR=1.34, 95% CI=1.13–1.59; P-heterogeneity=0.40; I2<0.01) than case-control (SOR=1.20, 95% CI=1.01–1.42; P-heterogeneity=0.05; I2=48.61) findings. Publication bias was not detected for cohort (P-Begg’s>0.99; P-Egger’s=0.59) or case-control (P-Begg’s=0.68; P-Egger’s=0.49) studies. When restricting analysis to studies limited to RCC risk (n=11), the summary effect estimate remained significantly elevated for acetaminophen use (SRR=1.25, 95% CI=1.09–1.45; P-heterogeneity=0.05; I2=46.01; P-Begg’s=0.70; P-Egger’s=0.49).
Figure 1.
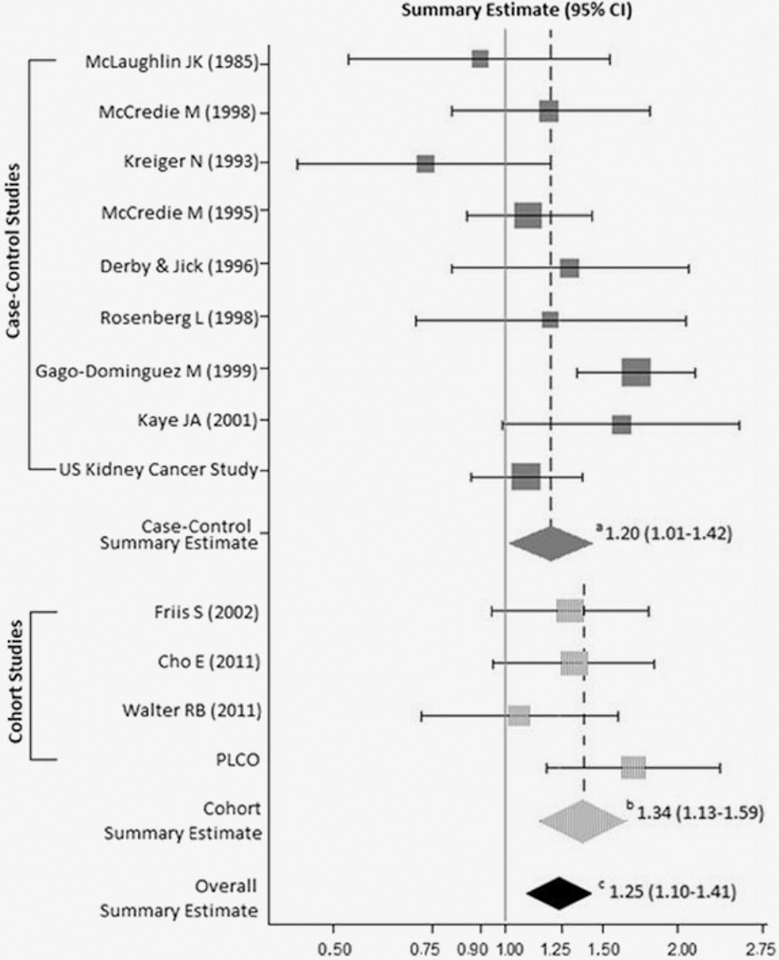
Associations between regular use of acetaminophen and kidney cancer risk. aCase-control meta-analytic summary estimate. bCohort meta-analytic summary estimate. cCombined overall (case-control and cohort) meta-analytic summary estimate.
DISCUSSION
Our investigation of RCC risk and analgesic use in the US Kidney Cancer Study and the PLCO Screening Trial suggests that acetaminophen use may be associated with increased RCC risk. In the US Kidney Cancer Study, risk increased significantly with duration of OTC acetaminophen use, with a two-fold risk among subjects with at least ten years of use; this association was null among prescription acetaminophen users. In PLCO, risk was significantly elevated among ever acetaminophen users. Long-term acetaminophen use was not associated with risk, although RCC case numbers with ten or more years of use was small (n=10). Our meta-analytic findings support an association between acetaminophen use and kidney cancer, where comparable summary estimates were observed for cohort and case-control studies. Similar meta-analytic findings were observed when we restricted analyses to studies that examined RCC risk. Use of NSAIDs and aspirin was not associated with RCC risk in either of the two studies.
Three published cohort studies have investigated the association between acetaminophen use and kidney cancer risk (11-13). The largest of these studies reported a marginally significant 30% increase in RCC risk among acetaminophen users in the US Nurse’s Health Study and Health Professionals Follow-up Study; but, no trend with duration of use was reported (12). In a second US cohort study, no relationship with ever or duration of acetaminophen use and RCC was shown (11). Among prescription acetaminophen users in a Danish cohort, a non-significantly elevated standardized incidence ratio of 1.3 was observed for RCC, with a significant positive trend with increasing numbers of prescriptions (P=0.04) (13). A meta-analysis of these cohort studies by Choueiri et al. showed a non-significant elevated kidney cancer risk of 1.19 (10). After incorporating our PLCO study results, the strength of pooled cohort findings increased, revealing a significant association between acetaminophen use and kidney cancer. For case-control studies, Choueiri et al. showed a significantly elevated summary effect for kidney cancer risk and acetaminophen use (RR=1.30, 95% CI=1.13–1.49) (10). After we excluded three case-control studies (29-31) that were subsets of a large international study (32) from Choueiri’s review (10), pooled case-control findings for OTC or prescription acetaminophen use and kidney cancer risk remained elevated both with (SOR=1.20, 95% CI=1.01– 1.42; P-heterogeneity=0.05) and without (SOR=1.22, 95% CI= 0.99–1.49; P-heterogeneity=0.05) inclusion of our US Kidney Cancer Study findings, although borderline evidence of heterogeneity was detected. The Choueiri et al. review also examined the effect of increased intake and duration on the acetaminophen-kidney cancer association. The overall RR across case-control and cohort studies combined (RR=1.28, 95% CI=1.15–1.44) increased to 1.68 (95% CI=1.22– 2.30) for high acetaminophen intake, but no relationship with duration was observed.
Although individual epidemiological study results have been inconsistent for acetaminophen use and kidney cancer risk, meta-analytic findings, which are more generalizable and less likely affected by small sample size, reveal a significant positive association that is biologically plausible (10). Approximately 90% of ingested acetaminophen is hepatically converted to nontoxic sulfates and glucuronide conjugates that are excreted in urine. The remaining amounts are oxidized in the liver and kidneys to reactive electrophilic N-acetyl-p-benzoquinoneimine (NAPQI) (40). At normal therapeutic doses, trace amounts of reactive NAPQI are formed. However, at higher doses, glutathione levels deplete, inhibiting formation of conjugates for excretion (40, 41). NAPQI is then available to form protein adducts, bind covalently to kidney and liver DNA, and disrupt cellular homeostasis (40, 41). Consequently, renal tubular necrosis may develop. Furthermore, long-term acetaminophen exposure at therapeutic doses has been correlated with chronic renal disease and analgesic nephropathy, and is thought to be dependent on prostaglandin endoperoxide synthesis (PGES), which exhibits a high affinity for acetaminophen (40). In the kidneys, PGES metabolically activates acetaminophen to NAPQI, increasing formation of reactive intermediates that cause tissue damage.
In the US Kidney Cancer Study, long-term OTC acetaminophen users had a statistically significant two-fold elevated RCC risk compared to non-users, but no association was observed among long-term prescription acetaminophen users. In the US, acetaminophen prescription products are typically found in combination with another analgesic, like codeine or hydrocodone. As these products have some dependence liability and/or abuse potential, they are generally prescribed for limited periods of time. Therefore, participants who reported long-term use of prescription acetaminophen products may have been taking these drugs on an “as needed” basis or for acute flare-ups rather than on a regular basis. These issues might also explain the null finding among long-term acetaminophen users in the PLCO study, which did not distinguish between OTC and prescription use. Alternately, the null association for long-term acetaminophen users in PLCO may be related to small case numbers.
Neither the US Kidney Cancer Study nor PLCO revealed any associations between RCC risk and use of NSAIDs (including aspirin) for pain or fever (US Kidney), use of aspirin as part of a heart regimen (US Kidney), use of aspirin for any indication (PLCO), or use of non-aspirin NSAIDs (PLCO). To date, two meta-analytic reviews on aspirin use and kidney cancer risk have been reported (9, 10). Both reviews observed non-significantly elevated risk, ranging from 1.1 to 1.2 for cohort and case-control studies, with significant heterogeneity between studies. For non-aspirin NSAIDs use, only one meta-analytic review has been published (10). Statistically significant 25% and 31% increased summary risk estimates were reported for all studies combined (n=5) and for cohort studies (n=2), respectively. A non-significant 1.14 summary estimate was observed for case-control studies. No significant evidence of heterogeneity was detected across the studies.
Our investigation of analgesic use and kidney cancer risk has several strengths. Strengths of both studies include their population-based design, data collection on specific types of analgesics, and the ability to control for known RCC risk factors as well as potential confounders like family history of cancer and education (a surrogate for socioeconomic status). Additional strengths of the US Kidney Cancer Study include the ability to assess risk for whites and blacks separately and inclusion of only histologically-confirmed RCC cancers. PLCO additional strengths include its large size and prospective design. Both studies are limited by a lack of data on analgesic dose and reliance on self-reported data. The potential misclassification of exposure due to inaccurate reporting would be non-differential in PLCO, where data were collected prior to diagnosis. However, since RCC can present as abdominal pain, it is possible that individuals may have used analgesics to treat early symptoms of RCC before diagnosis. Nonetheless, our analysis restricted to PLCO participants diagnosed greater than two years after completion of the supplementary questions does not suggest such bias. For the US Kidney Cancer Study, data on indication for analgesic use was not collected from participants and may have confounded results. Furthermore, inconsistencies in the results for OTC versus prescription drug use as well as for duration-response analyses may be related to the imprecise definition of “regular” drug use in both the US Kidney Cancer Study (“at least once a week for three months or longer” for OTC drugs and “at least once a week for one month or longer” for prescription drugs and aspirin) and in PLCO (“at least once per week”). The available exposure information in these studies did not allow for evaluation of intensity of use or assessment of consistency of use, and exposure misclassification in our definitions of regular use is likely to have biased our results towards the null. Another limitation of the US Kidney Cancer Study is the lack of statistical power to detect associations for analyses stratified by race and sex. The response rate among controls was not optimal, but the sample weights included adjustments for differential non-response rates among demographic categories that may have reduced bias in analyses due to non-response (21). An additional limitation of PLCO is the lack of data on OTC versus prescription analgesic use. With regard to our meta-analytic review, publication bias can potentially lead to summary estimates biased away from the null, although evidence of publication bias was not detected. Furthermore, variability of acetaminophen use definitions across studies and the inability to have a homogeneous definition of “regular” use was a limitation. However, our meta-analytic study did show consistently elevated summary risk estimates for both cohort and case-control studies with no significant evidence of heterogeneity. In addition, we were able to update previous meta-analytic findings by calculating summary risk estimates that incorporated our US Kidney Cancer case-control and PLCO cohort results.
In summary, findings from our population-based case-control study, multi-center randomized trial, and meta-analysis suggest that acetaminophen use may increase RCC risk. No evidence for an association between aspirin and NSAIDs use and RCC risk was observed.
Supplementary Material
Acetaminophen use and kidney cancer risk literature search process
Novelty and Impact:
Analgesics, like acetaminophen, aspirin, and non-steroid anti-inflammatory drugs (NSIADs), are the most commonly consumed drugs worldwide; yet, evidence that analgesics increase renal cell carcinoma (RCC) risk has been mixed. To better understand the relationship, the association between analgesic use and RCC was evaluated in a case-control study, and cohort study, and a meta-analytic review. Evidence from all three study designs suggests that acetaminophen use is associated with increased RCC risk. Associations with aspirin or NSAIDs were null.
ACKNOWLEDGMENTS:
This research was supported by the Intramural Research Program of the National Cancer Institute (NCI), Division of Cancer Epidemiology and Genetics (DCEG), National Institutes of Health (NIH), Department of Health and Human Services (DHHS). The PLCO research was supported by contracts from the Division of Cancer Prevention, NCI, NIH, DHHS.
This research was supported by the Intramural Research Program of the NCI, Division of Cancer Epidemiology and Genetics, National Institutes of Health. The PLCO research was supported by contracts from the Division of Cancer Prevention, National Cancer Institute, NIH, DHHS.
Abbreviations Used:
- BMI
body mass index
- CI
confidence interval
- CMS
Centers for Medicare and Medicaid Services
- DMV
Department of Motor Vehicle
- HR
hazard ratio
- ICD-O
International Classification of Diseases for Oncology
- NAPQI
N-acetyl-p-benzoquinoneimine
- NSAIDs
non-steroid anti-inflammatory drugs
- PGES
prostaglandin endoperoxide synthesis
- PLCO
Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial
- OR
odds ratio
- OTC
over-the-counter
- RCC
renal cell carcinoma
- RR
relative risk
- SOR
summary odds ratio
- SRR
summary relative risk
- US
United States
Footnotes
Author’s disclosures of potential conflicts of interest: non for all authors.
REFERENCES
- 1.Roumie CL, Griffin MR. Over-the-counter analgesics in older adults: a call for improved labelling and consumer education. Drugs Aging 2004;21(8):485–98. PMID: . [DOI] [PubMed] [Google Scholar]
- 2.Chandok N, Watt KD. Pain management in the cirrhotic patient: the clinical challenge. Mayo Clin Proc 2010;85(5):451–8. PMID: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kaufman DW, Kelly JP, Rosenberg L, Anderson TE, Mitchell AA. Recent patterns of medication use in the ambulatory adult population in the United States: the Slone Survey. JAMA 2002:287(3):337–44. PMID: . [DOI] [PubMed] [Google Scholar]
- 4.Zhou Y, Boudreau DM, Freedman AN. Trends in the use of aspirin and nonsteroidal anti-inflammatory drugs in the general U.S. population. Pharmacoepidemiol Drug Saf 2014;23(1):43–50. PMID: . [DOI] [PubMed] [Google Scholar]
- 5.Pan GJD. Acetaminophen- Background and Overview: U.S. Food and Drug Administration, 2009. update. http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/DrugSafetyandRiskManagementAdvisoryCommittee/UCM175767.pdf.
- 6.Cuzick J, Thorat MA, Bosetti C, Brown PH, Burn J, Cook NR, et al. Estimates of benefits and harms of prophylactic use of aspirin in the general population. Ann Oncol. 2015;26:47–57. PMID: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brasky TM, Liu J, White E, Peters U, Potter JD, Walter RB, et al. Non-steroidal Anti-inflammatory Drugs and Cancer Risk in Women: Results from the Women’s Health Initiative. Int J Cancer 2014;135(8):1869–83. PMID: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu W, Park Y, Purdue MP, Giovannucci E, Cho E. A large cohort study of nonsteroidal anti-inflammatory drugs and renal cell carcinoma incidence in the National Institutes of Health-AARP Diet and Health Study. Cancer Causes Control 2013;24(10);1865–73. PMID: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bosetti C, Rosato V, Gallus S, Cuzick J, La Vecchia C. Aspirin and cancer risk: a quantitative review to 2011. Ann Oncol 2012;23(6):1403–15. PMID: . [DOI] [PubMed] [Google Scholar]
- 10.Choueiri TK, Je Y, Cho E. Analgesic use and the risk of kidney cancer: A meta-analysis of epidemiologic studies. Int J Cancer 2014;134(2):384–96. PMID: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Walter RB, Brasky TM, White E. Cancer risk associated with long-term use of acetaminophen in the prospective VITamins and lifestyle (VITAL) study. Cancer Epidemiol Biomarkers Prev 2011;20(12):2637–41. PMID: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cho E, Curhan G, Hankinson SE, Kantoff P, Atkins MB, Stampfer M, Choueiri TK. Prospective evaluation of analgesic use and risk of renal cell cancer. Arch Intern Med 2011;171(16):1487–93. PMID: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Friis S, Nielsen GL, Mellemkjaer L, McLaughlin JK, Thulstrup AM, Blot WJ, et al. Cancer risk in persons receiving prescriptions for paracetamol: a Danish cohort study. Int J Cancer 2002;97(1):96–101. PMID: . [DOI] [PubMed] [Google Scholar]
- 14.Centers for Diseases Control and Prevention. Top 10 Cancers Among Men. Atlanta, Georgia: USA.gov, 2011. update. http://www.cdc.gov/Features/dsMenTop10Cancers/ [Google Scholar]
- 15.Centers for Diseases Control and Prevention. Top 10 Cancers Among Women. Atlanta, Georgia: USA.gov, 2011. update. http://www.cdc.gov/Features/dsWomenTop10Cancers/ [Google Scholar]
- 16.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin 2014;64(1):9–29. PMID: . [DOI] [PubMed] [Google Scholar]
- 17.Chow WH, Dong LM, Devesa SS. Epidemiology and risk factors for kidney cancer. Nat Rev Urol 2010;7(5):245–57. PMID: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lipworth L, Tarone RE, Lund L, McLaughlin JK. Epidemiologic characteristics and risk factors for renal cell cancer. Clin Epidemiol 2009;1:33–43. PMID: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Colt JS, Schwartz K, Graubard BI, Davis F, Ruterbusch J, DiGaetano R, et al. Hypertension and risk of renal cell carcinoma among white and black Americans. Epidemiology 2011;22(6):797–804. PMID: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Purdue MP, Moore LE, Merino MJ, Boffetta P, Colt JS, Schwartz KL, et al. An investigation of risk factors for renal cell carcinoma by histologic subtype in two case-control studies. Int J Cancer 2013;132(11):2640–7. PMID: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li Y, Graubard BI, DiGaetano R. Weighting methods for population-based case-control studies with complex sampling. J Royal Stat Soc Series C (Applied Statistics) 2011;60:165–85. [Google Scholar]
- 22.Korn EL, Graubard BI. Analysis of Helath Surveys. New York: John Wiley & Sons; 1999. [Google Scholar]
- 23.Rust KF, Rao JN. Variance estimation for complex surveys using replication techniques. Stat Methods Med Res 1996;5(3):283–310. PMID: . [DOI] [PubMed] [Google Scholar]
- 24.Prorok PC, Andriole GL, Bresalier RS, Buys SS, Chia D, Crawford ED, et al. Design of the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial. Control Clin Trials 2000;21(6 Suppl):273S–309S. PMID: . [DOI] [PubMed] [Google Scholar]
- 25.Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial. U.S. Department of Health and Human Services, National Institutes of Health, National Cancer Institute, Division of Cancer Prevention: USA.gov. http://prevention.cancer.gov/plco [Google Scholar]
- 26.McLaughlin JK, Blot WJ, Mehl ES, Fraumeni JF Jr. Relation of analgesic use to renal cancer: population-based findings. Natl Cancer Inst Monogr 1985;69:217–22. PMID: . [PubMed] [Google Scholar]
- 27.McCredie M, Stewart JH. Does paracetamol cause urothelial cancer or renal papillary necrosis? Nephron 1988;49(4):296–300. PMID: . [DOI] [PubMed] [Google Scholar]
- 28.Kreiger N, Marrett LD, Dodds L, Hilditch S, Darlington GA. Risk factors for renal cell carcinoma: results of a population-based case-control study. Cancer Causes Control 1993;4(2):101–10. PMID: . [DOI] [PubMed] [Google Scholar]
- 29.McCredie M, Stewart JH, Day NE. Different roles for phenacetin and paracetamol in cancer of the kidney and renal pelvis. Int J Cancer 1993;53(2):245–9. PMID: . [DOI] [PubMed] [Google Scholar]
- 30.Mellemgaard A, Niwa S, Mehl ES, Engholm G, McLaughlin JK, Olsen JH. Risk factors for renal cell carcinoma in Denmark: role of medication and medical history. Int J Epidemiol 1994;23(5):923–30. PMID: . [DOI] [PubMed] [Google Scholar]
- 31.Chow WH, McLaughlin JK, Linet MS, Niwa S, Mandel JS. Use of analgesics and risk of renal cell cancer. Int J Cancer 1994;59(4):467–70. PMID: . [DOI] [PubMed] [Google Scholar]
- 32.McCredie M, Pommer W, McLaughlin JK, Stewart JH, Lindblad P, Mandel JS, et al. International renal-cell cancer study. II. Analgesics. Int J Cancer 1995;60(3):345–9. PMID: . [DOI] [PubMed] [Google Scholar]
- 33.Derby LE, Jick H. Acetaminophen and renal and bladder cancer. Epidemiology 1996;7(4):358–62. PMID: . [DOI] [PubMed] [Google Scholar]
- 34.Rosenberg L, Rao RS, Palmer JR, Strom Bl, Zauber A, Warshauer ME, et al. Transitional cell cancer of the urinary tract and renal cell cancer in relation to acetaminophen use (United States). Cancer Causes Control 1998;9(1):83–8. PMID: . [DOI] [PubMed] [Google Scholar]
- 35.Gago-Dominguez M, Yuan JM, Castelao JE, Ross RK, Yu MC. Regular use of analgesics is a risk factor for renal cell carcinoma. Br J Cancer 1999;81(3):542–8. PMID: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kaye JA, Myers MW, Jick H. Acetaminophen and the risk of renal and bladder cancer in the general practice research database. Epidemiology 2001;12(6):690–4. PMID: . [DOI] [PubMed] [Google Scholar]
- 37.Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group (2009). Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6:e1000097 PMID: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002;21(11):1539–58. PMID: . [DOI] [PubMed] [Google Scholar]
- 39.Hayashino Y, Noguchi Y, Fukui T. Systematic evaluation and comparison of statistical tests for publication bias. J Epidemiol 2005;15(6):235–43. PMID: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bessems JG, Vermeulen NP. Paracetamol (acetaminophen)-induced toxicity: molecular and biochemical mechanisms, analogues and protective approaches. Crit Rev Toxicol 2001;31(1):55–138. PMID: . [DOI] [PubMed] [Google Scholar]
- 41.McGill MR, Jaeschke H. Metabolism and disposition of acetaminophen: recent advances in relation to hepatotoxicity and diagnosis. Pharm Res 2013;30(9):2174–87. PMID: . [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Acetaminophen use and kidney cancer risk literature search process


