Abstract
Background:
Prostate cancer (PCa) is a common health problem in elderly. RAGE (Receptor for advanced glycation end products) is overexpressed in multiple human cancers. SOX2 (Sex-determining region Y box 2) also functions as an oncoprotein and promotes cancer progression but the mechanisms involved remain largely unknown.
Aim:
The current study investigated the expression patterns of RAGE and SOX2 in benign and malignant prostate samples in correlation with the histopathological findings in order to evaluate their role as prognostic markers or therapeutic targets.
Methods:
Immunohistochemical staining for RAGE and SOX2 antibodies was applied on 87 prostatic biopsies [16 of prostatitis, 20 of benign prostatic hyperplasia (BPH) and 51 of PCa].
Results:
Expression of RAGE and SOX2 (percentage of positive cells) was significantly higher in PCa lesions compared with prostatitis (p<0.01) and BPH (p<0.0001) and was also significantly higher in prostatitis compared with BPH lesions (p<0.01). Also, percentage of positive RAGE and SOX2 cells showed a significant stepwise increase from Gleason Grade 3 to Grade 5 and were significantly higher in high Gleason Scores (≥8) compared to lower Scores (≤7) with statistical significance (p=0.001).
Conclusion:
RAGE and SOX2 were up-regulated in prostate cancer lesions, mainly in advanced grades, suggesting an active role of both antigens in the development and progression of prostate cancer and expecting the possibility of their use as therapeutic targets.
Key Words: RAGE, SOX2, prostate, cancer, immunohistochemistry
Introduction
Prostate cancer (PCa) is a common health problem that in the majority of cases starts to develop at the age of 50 years, reaching its peak at 60–70 years of age (Boyle et al., 2003). While highly curable if localized, patients with metastatic disease have a 5-year survival rate of only 31% (Takahashi et al., 2007). As no reliable means of identifying patients at risk of metastatic dissemination are currently available (Briganti et al., 2014). A better understanding of the mechanisms underlying the spread of prostate cancer cells should aid the development of treatment strategies that improve outcomes for patients with advanced disease (Bae et al., 2016).
RAGE (Receptor for advanced glycation end products) is a cell surface molecule and a member immunoglobulin superfamily (Alexiou et al., 2010). RAGE has multiple ligands; its major one is advanced glycation end products (AGEs) and AGE-RAGE interaction is implicated in development, homeostasis, inflammation, neurodegenerative disorders, tumors, and pathogenesis of various diseases (Yan et al., 2009). It is likely that abnormal stimulation of AGE-RAGE affects cell cycle genes controlling the G1/S phase transition, increases the number of cells in the S phase of the cell cycle (DNA synthesis phase) and decreases the percentage of cells in G1 phase (cell growth phase) (Kim et al., 2008), so this interaction can be involved in the development, growth and metastasis of a number of tumor types including prostate cancer (Sims et al., 2010).
The embryonic stemness gene SOX2 [Sex-determining region Y box 2] is one of the key members of the Sox family that encodes a series of transcription factors. SOX2 functions as an oncoprotein and promotes cancer progression and focused on its positive contribution to many physiological processes of cancer cells, such as proliferation and growth, cellular migration and invasion, maintenance of stemness, anti-apoptotic activity, chemoresistance, metastasis and tumorigenesis, however, the mechanisms involved remain largely unknown, both in prostate and in other cancers (Weina and Utikal, 2014).
In this study, we analyzed immunohistochemical expression of SOX2 and RAGE expression in benign and malignant prostatic tissue samples and investigated their correlation with Gleason Grade, Gleason Score and Grade Group of prostate cancer cases.
Materials and Methods
We collected archival prostatic specimens and clinical pathological data related to 87 patients who had chronic urologic complaint and came to seek medical advice at Urology Department, Theodor Bilharz Research Institute, in the interval from January 2016 to December 2017, where clinical examination and radiological investigations were done, then cystoscopic or transrectal ultrasound-guided biopsies were taken.
Routine histopathological processing and diagnosis were made at Pathology Department, Theodor Bilharz Research Institute, using hematoxylin and eosin stain. Pathology reporting sheet includes diagnosis (benign: hyperplasia/ prostatitis or malignant) and scoring of malignant cases according to Gleason’s criteria. Then immunohistochemical studies were applied on archival prostatic tissue using RAGE and SOX2 antibodies.
Prostate cancer patients under study did not receive hormone therapy, che¬motherapy or radiotherapy. Serum PSA (Serum prostate-specific antigen) levels were measured for all studied cases.
This study was approved by our institutional ethics committee.
Study groups were categorized as: Prostatitis: 16 cases; Benign prostatic hyperplasia (BPH): 20 cases; and Prostate cancer (PCa): 51 cases.
Prostate cancer samples were further classified according to Gleason Classifications (Moch et al, 2016):
• Gleason Grade (Grades 1-5): According to degree of tumor differentiation.
• Gleason Score (Scores 2-10): Determined by adding up the two most prevalent Gleason Grade numbers.
• Gleason Grade Group (Groups 1-5): A newly categorized grading system that is based on Gleason score.
Immunohistochemical technique
Tissue sections were processed into 3 μm thick sections. Unmasking of the antigen was performed with 10 ml sodium citrate buffer, pH 6.0, at 90oC for 30 minutes. Sections were incubated in 0.03% hydrogen peroxide for 10 minutes at room temperature to remove endogenous peroxidase activity and then in blocking serum (0.04% bovine serum albumin, A2153, Sigma-Aldrich, Shanghai, China, and 0.5% normal goat serum X0907, Dako Corporation, Carpinteria, CA, USA, in PBS) for 30 minutes at room temperature. Sections were incubated overnight at 4°C in humid chamber with the primary antibodies: Anti-RAGE antibody (A11) (Santa Cruz Biotechnology, Catalog no. sc- 80652, CA, USA) and Anti- SOX2 antibody (Abcam, Catalog no. ab97959, Cambridge, MA, USA) at an optimal dilution of 1:100. Sections were then washed three times for 5 min in PBS. Non-specific staining was blocked 5% normal serum for 30 min at room temperature. Finally, staining was developed with diaminobenzidine substrate and sections were counterstained with hematoxylin. PBS replaced RAGE and SOX2 antibody in negative controls.
Interpretation of immunostaining
The expression of RAGE and SOX2 was semi-quantitatively estimated as brown membrano-cytoplasmic immunostaining (for RAGE) and brown nuclear immunostaining (for SOX2), which was calculated as the proportion of positive cells to the total number of cells calculated in 10 successive high power fields (percentage of positive cells).
The sections were examined by using light microscope [Scope A1, Axio, Zeiss, Germay]. Photomicrographs were taken using a microscope-camera [AxioCam, MRc5, Zeiss, Germany].
Statistical analysis
SPSS for Windows, version 20, was used for statistical analysis (IBM Corporation, Armonk, NY, USA). Results were given as mean ±SD. Distribution of negative and positive cases was studied with cross tables (Pearson’s Chi square-test). Spearman rank correlation coefficient was used to investigate a possible correlation of RAGE or SOX2 expression with tumor grade. The comparisons of quantitative variables were performed between two groups using ANOVA and student t-tests. A p value of < 0.05 was considered of statistical significance.
Results
In our study, the mean ages of PCa and BPH were 68.30 and 68.97 years respectively which showed significant difference with the mean age of prostatitis cases (65.33years) with p=0.05. Mean of serum PSA level was higher in PCa lesions (72.88 mg/ml) compared with prostatitis (20.55 mg/dl ) and PBH (10.90 mg/dl) with significant differences (p<0.01 and <0.0001 respectively) (Table 1).
Table 1.
Differences in Mean Age, Mean PSA Level, RAGE Expression and SOX2 Expression among Studied Groups
| Diagnosis | Age Mean±S.D. | PSA Mean±S.D. | Percentage of positive cells Mean±S.D. |
|
|---|---|---|---|---|
| RAGE expression | SOX2 expression | |||
| Prostatitis (16) | 65.33±4.50 | 20.53±9.86 | 33.91±6.17 | 50.00±16.73 |
| BPH (20) | 68.30±4.05* | 10.90±2.03** | 18.57±4.35** | 37.00±10.59** |
| PCa (51) | 68.97±5.33* | 72.88±20.39**## | 88.33±1.43##** | 70.56±17.56##** |
*, Significant difference compared to prostatitis (p=0.05); **, High significant difference compared to prostatitis (p=0.01); ##, High significant difference compared to BPH (p=0.0001).
Expression of RAGE and SOX2 (percentage of positive cells) was significantly higher in PCa lesions compared with prostatitis (p<0.01) and BPH (p<0.0001) and also was significantly higher in prostatitis compared with BPH lesions (p<0.01) (Table 1) (Figure 1 and 2)
Figure 1.
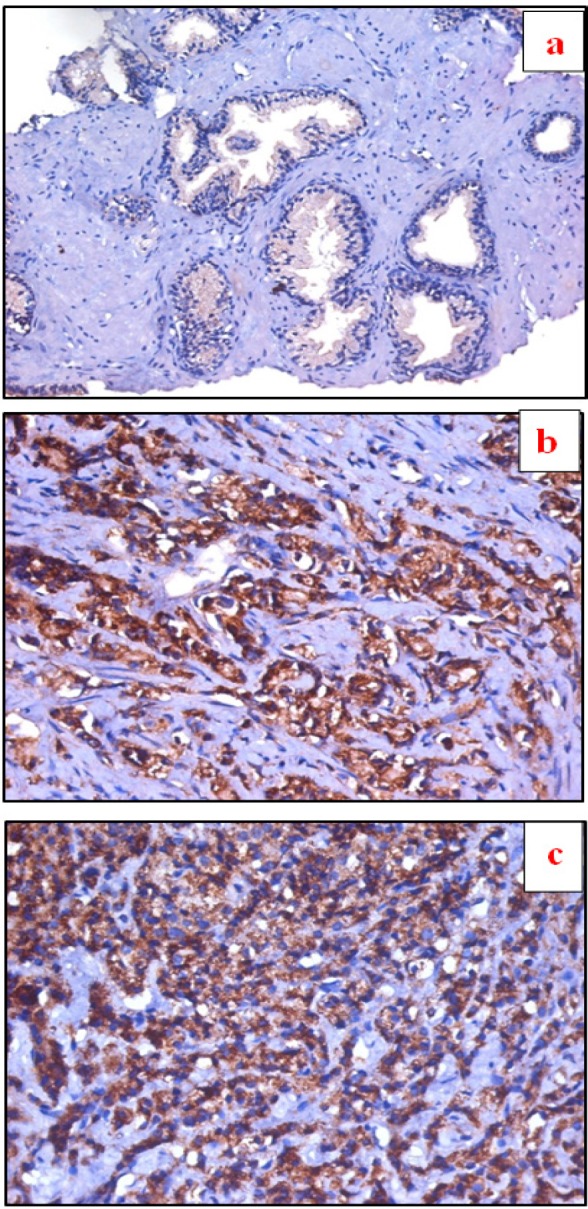
a, Section in Benign prostatic hyperplasia, showing negative RAGE expression in prostatic glands (IHC for RAGE, DAB X100); b, Section in Prostatic adenocarcinoma, Gleason Grade 3, showing moderate membrano-cytoplasmic RAGE expression in prostatic glands (IHC for RAGE, DAB X100); c, Section in Prostatic adenocarcinoma, Gleason Grade 5, with marked membrano-cytoplasmic positive expression for RAGE (IHC for RAGE, DAB X100)
Figure 2.
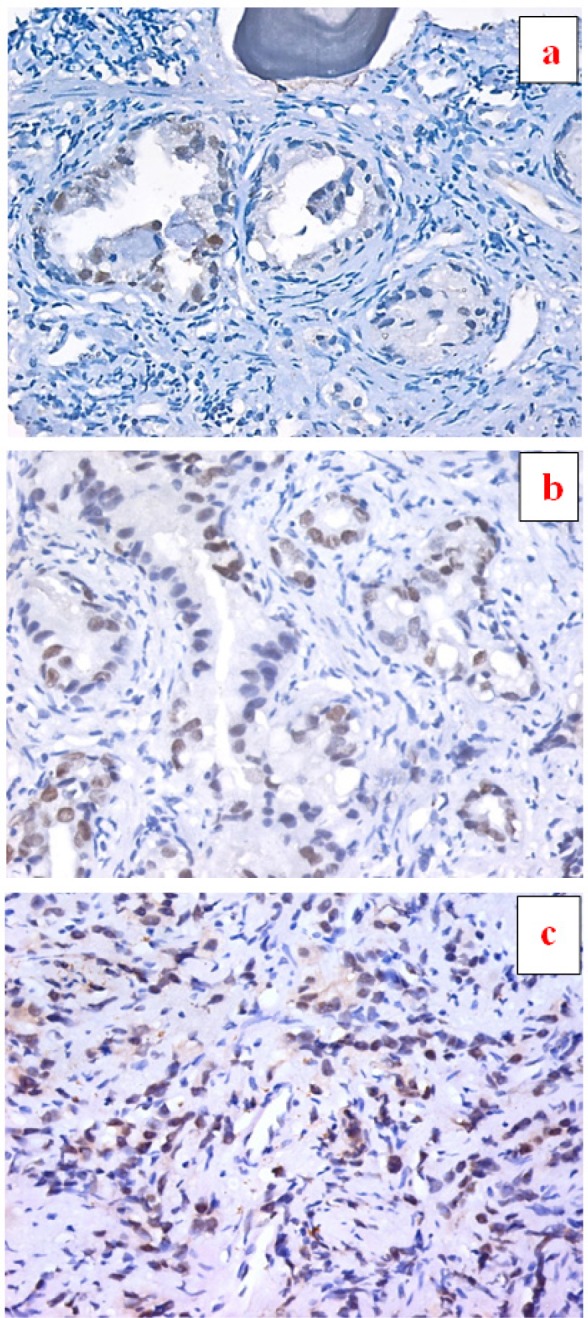
a, Section in Benign prostatic hyperplasia, showing prostatic acini lined by a double layer of epithelial cells with discrete positive nuclear expression for SOX2 in epithelial cells (IHC for SOX2, DAB X 200); b, Section in Prostatic adenocarcinoma, Gleason Grade 3, showing scattered malignant prostatic acini lined by a single layer of epithelial cells with moderate positive nuclear expression for SOX2 in epithelial cells (IHC for SOX2, DAB X 200); c, Section in Prostatic adenocarcinoma, Gleason Grade 5, showing infiltrating single files of epithelial cells with moderate nuclear expression for SOX2 (IHC for SOX2, DAB X100)
By comparing the different grades of PCa according to Gleason’s classification; percentage of positive RAGE and SOX2 cells showed a significant stepwise increase from Gleason Grade 3 to Grade 5. Also, by comparing Gleason Score of PCa; expression of RAGE and SOX2 showed significantly increase in high Gleason Scores (≥8) compared to lower Scores (≤7) with statistical significance (p=0.001). Furthermore, by comparing the different Gleason Grade Groups of PCa according to the new Gleason classification; expression of RAGE and SOX2 was significantly increased in high Gleason Grade Groups (≥3) compared to lower Groups (≤2) with statistical significance; being tested by ANOVA (Table 2).
Table 2.
Relation of Gleasons' Scores to RAGE and SOX2 Expression
| Item | Percentage of positive cells Mean±S.D. |
|
|---|---|---|
| RAGE expression | SOX2 expression | |
| Gleason Score | ||
| Grade 3 (32) | 84.37±9.65 | 64.28±17.62 |
| Grade 4 (12) | 95.00±4.26^ | 80.00±11.18^ |
| Grade 5 (7) | 100.00±0.00 @ | 100.00±0.00 @ |
| Gleason Total Score | ||
| Score 6 (25) | 82.85±9.37 | 64.13±17.16 |
| Score 7 (7) | 92.50±2.76* | 67.85±18.67 |
| Score 8 (13) | 100.00±0.00** | 79.09±10.44** |
| Score 9 (3) | 97.50±2.67** | 95.00±0.00** |
| Score 10 (3) | 100.00±0.00** | 100.00±0.00** |
| Gleason Grade Group | ||
| Grade Group 1 (25) | 82.85±9.37 | 64.13±17.16 |
| Grade Group 2 (7) | 92.50±7.76# | 58.33±27.53 |
| Grade Group 3 (12) | 93.12±3.88## | 75.01±5.77# |
| Grade Group 4 (2) | 100.00±0.00 @ | 83.11±10.15 |
| Grade Group 5 (5) | 97.50±2.67## | 95.00±0.0 @ |
| P value ANOVA | 0.0001 | 0.005 |
^, High significant difference with score 3 (p<0.01); *, Significant difference with total score 6 (p<0.05); **, High significant difference with total score 6 (p<0.001); #, Significant difference with Grade Group 1 (p<0.05); ##, High significant difference with Grade Group 1 (p<0.001); @, t-test could not be performed (as S.D. = Zero).
Our study showed a significant positive correlation between RAGE and SOX2 percentage of positive cells with different examined Gleason’s classifications (Gleason Grade, Score and Grade Group), but no significant correlation with PSA level (Table 3).
Table 3.
Correlations between Expression of RAGE and SOX2 with Different Studied Parameters of PCa
| Spearman's rho | PSA | Gleason Grade | Gleason Sore | Gleason Grade Group | |
|---|---|---|---|---|---|
| RAGE % of positive cells | Correlation Coefficient | 0.559** | 0.528** | 0.759** | 0.492** |
| N | 87 | 51 | 51 | 51 | |
| SOX2 % of positive cells | Correlation Coefficient | 0.406** | 0.504** | 0.521** | 0.529** |
| N | 87 | 51 | 51 | 51 |
*, Correlation is significant at the 0.05 level (two-tailed); **, Correlation is significant at the 0.01 level (two-tailed); %, percentage; N, number of cases.
Discussion
In Egypt, according to Registry Program 2008-2011, prostate cancer is the sixth cancer among male malignancies, with an incidence of 4.27% (Ibrahim et al., 2014).
Serum prostate-specific antigen (PSA) is a protein produced by normal and malignant prostate gland and is a widely used parameter for early detection and monitoring of prostate cancer. Consistent with the fact that in addition to prostate cancer, prostatitis and BPH can cause elevation in man’s PSA level; we found an increase in mean PSA value in prostate cancer compared with prostatitis and BPH. Naz et al., (2004) observed that a majority of prostate cancer cases had serum PSA >20 ng/ml and 50% of BPH cases had serum PSA in the gray zone (4.1-20 ng/ml). Meanwhile, PSA levels did not show significant correlations with RAGE or SOX2 expression in our studied prostatic lesions.
The AGE-RAGE interaction plays a crucial part in the development of prostate cancer. Ishiguro et al., (2005) stated that inhibiting this interaction has potential as a new molecular target for cancer prevention or therapy. Several reports had revealed an association between the expression of RAGE and development of cancers, as Aboushousha et al., (2016) found that RAGE expression was up-regulated in human gastric adenocarcinoma and Kuniyasu et al., (2003) described that RAGE production was enhanced in metastatic compared to non-metastatic prostate cancer and healthy prostate tissue.
In our study, we found significant increase in mean percentage of RAGE positive cells in prostate cancer tissue compared with prostatitis and BPH, which matches results of Lu et al., (2010) who reported a significant higher expression of RAGE in the prostate cancer than in normal prostate tissue by immunohistochemistry, western blot analysis and real-time quantitative PCR. Ishiguro et al., (2005) found that RAGE mRNA expression was up-regulated in prostate cancer tissues and also found that untreated primary prostate cancer tissue and hormone-refractory prostate cancer tissue showed significantly higher RAGE mRNA expression than normal prostate tissues. In a study performed by Aboushousha et al., (2018) they reported a significant higher RAGE expression in hepatic dysplasia and hepatocellular carcinoma lesions than in non-tumor hepatic tissue. Similarly, Amornsupak et al., (2017) found stronger RAGE immunoreactivity in the breast cancer tissue sections than in that in the benign disease tissue. Collectively, these findings suggest a role played by RAGE in development and growth of cancer lesions.
According to our results, RAGE expression showed a positive significant correlation with each of Gleason Grade, Gleason Score and Gleason Grade Group. We found a significant increase in mean percentage of RAGE positive cells in higher Gleason Grades (≥4) compared with lower Grade 3, and in higher Gleason Scores (≥ 8) compared with lower Scores (≤7) and also in higher Gleason Grade Groups ((≥3) compared with lower Groups (≤2). RAGE overexpression had been reported in advanced grades and stages in diverse types of malignant tumors. Aboushousha et al., (2016) found a significant correlation between RAGE expression with advanced stages and lymph node metastasis of gastric adenocarcinoma. Nankali et al., (2016) reported higher RAGE expression in high-stage and aggressive breast cancer tumors. Sasahira et al., (2005) demonstrated higher RAGE expression in colorectal adenomas with severe atypia. In contrast; Aboushousha et al., (2018) detected higher expression of RAGE in low grades of hepatocellular carcinoma compared with high grades, moreover, Ishiguro et al., (2005) did not find a significant difference in RAGE mRNA expression according to both grade and stage in their prostate cancer samples.
SOX2, an important transcription factor, has been shown to be over-expressed in most human cancer types, including prostate cancer. It plays a key role in cell self-renewal, differentiation, proliferation and apoptosis in stem cells and in cancer cells (Lin et al., 2012).
Bae et al., (2010) and Jia et al., (2011) reported an increase in percentage of SOX2 positive cells in prostate neoplastic tissue compared with BPH, which matches our results. In our study, we demonstrated a significant higher percentage of SOX2 positive cells in prostate cancer compared with prostatitis and BPH lesions; however, Ugolkov et al., (2011) reported an increase in expression of SOX2 in both benign and malignant prostate tissue, but only in a small percentage of cells (10%). So it is possible that SOX2 participates in the development and regulation of both hyperplastic and malignant prostatic tissues. Meanwhile Russo et al., (2016) demonstrated distinct SOX2 expression in the basal cell layer of normal prostate glands and its absence in most of the neoplastic epithelia, with the exception of a few foci of low-grade and high-grade prostate cancer, which agrees with the notion that prostates lose basal cells during cancer progression. Taken together, these controversial findings have indicated that the dynamics of SOX2 expression in prostate cancer are largely unknown and there is a need for further studies to elucidate its role in prostate cancer progress.
In accordance with previous studies (Bae et al., 2010; Jia et al., 2011; Kregel et al., 2013) which reported a correlation between SOX2 expression and both of Gleason Grade and Gleason Score, we found a statistical significant increase in percentage of SOX2 positive cells in higher Gleason Grades, Gleason Scores and Gleason Grade Groups compared with lower ones. Since a higher Gleason grade indicates a worse prognosis, so it is suggested that SOX2 may contribute to the tumorigenesis of prostate cancer and may play an important role in the clinical progress of prostate cancer. Bae et al., (2016) postulated that, the expression of SOX2 in prostate tumors has been thought to promote a less differentiated embryonic stem cell tumor phenotype; that confers a worse disease prognosis.
In the present study, we found a positive significant correlation between percentage of SOX2 positive cells and each of Gleason Grade, Gleason Score and Gleason Grade Group. Many previous studies reported an association between SOX2 expression and advanced stages in several human tumors. Yang et al., (2013) reported that SOX2 expression was associated with clinical stage and lymph node status in patients with small cell lung cancer. Tang et al., (2013) suggested that SOX2 expression was significantly associated with clinical stage, lymph node metastasis and recurrence in laryngeal squamous cell carcinoma. Zhang et al., (2010) reported that patients with strong SOX2 expression showed deeper invasion and advanced clinical stages compared to patients with low SOX2 expression in gastric cancer. Huang et al., (2014) reported a close association between SOX2 expression with clinicopathological parameters in breast cancer; including high histological grade, large tumor size, molecular subtypes, negative hormone receptor status and high proliferation index.
In conclusion, RAGE and SOX2 were up-regulated in prostate cancer; mainly in cancers with a worse prognosis which have higher Gleason Grade, higher Gleason Score and higher Gleason Grade Group. So, we suggest a role played by RAGE and SOX2 in the progression of prostate cancer that they can be used as predictive tools or therapeutic targets in prostate cancer.
References
- Aboushousha T, Badawy A, Moussa M, et al. Evaluation of RAGE (Receptor for Advanced Glycation End-products) expression in gastric carcinoma of Egyptian patients in relation to Helicobacter pylori infection. Annal Pathol Lab Med. 2016;3:259–68. [Google Scholar]
- AboushoushaT , Mamdouh S, Hamdy H, et al. Immunohistochemical and biochemical expression patterns of TTF-1, RAGE, GLUT-1 and SOX2 in HCV-associated Hepatocellular Carcinomas. Asian Pac J Cancer Prev. 2018;19:219–27. doi: 10.22034/APJCP.2018.19.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexiou P, Chatzopoulou M, Pegklidou K, Demopoulos VJ. RAGE: a multi-ligand receptor unveiling novel insights in health and disease. Curr Med Chem. 2010;17:2232–52. doi: 10.2174/092986710791331086. [DOI] [PubMed] [Google Scholar]
- Amornsupak K, Jamjuntra P, Warnnissorn M, et al. High ASMA+ fibroblasts and low cytoplasmic HMGB1+ breast cancer cells predict poor prognosis. Clin Breast Cancer. 2017;17:441–52. doi: 10.1016/j.clbc.2017.04.007. [DOI] [PubMed] [Google Scholar]
- Bae K-M, Su Z, Frye C, et al. Expression of pluripotent stem cell reprogramming factors by prostate tumor initiating cells. J Urol. 2010;183:2045–53. doi: 10.1016/j.juro.2009.12.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae K-M, Dai Y, Vieweg J, Siemann DW. Hypoxia regulates SOX2 expression to promote prostate cancer cell invasion and sphere formation. Am J Cancer Res. 2016;5:1078–88. [PMC free article] [PubMed] [Google Scholar]
- Boyle P, Severi G, Giles GG. The epidemiology of prostate cancer. Urol Clin North Am. 2003;2:209–17. doi: 10.1016/s0094-0143(02)00181-7. [DOI] [PubMed] [Google Scholar]
- Briganti A, Suardi N, Gallina A, et al. Predicting the risk of bone metastasis in prostate cancer. Cancer Treat Rev. 2014;40:3–11. doi: 10.1016/j.ctrv.2013.07.001. [DOI] [PubMed] [Google Scholar]
- Huang YH, Luo MH, Ni YB, et al. Increased SOX2 expression in less differentiated breast carcinomas and their lymph node metastases. Histopathology. 2014;64:494–03. doi: 10.1111/his.12257. [DOI] [PubMed] [Google Scholar]
- Ibrahim AM, Khaled HM, Mikhail NN, Baraka H, Kamel H. Cancer incidence in Egypt: Results of the Nationa population-based cancer registry program. J Cancer Epidemiol. 2014 doi: 10.1155/2014/437971. Article ID 437971, 18 pages. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishiguro H, Nakaigawa N, Miyoshi Y, et al. Receptor for advanced glycation end products (RAGE) and its ligand, amphoterin are overexpressed and associated with prostate cancer development. Prostate. 2005;64:92–100. doi: 10.1002/pros.20219. [DOI] [PubMed] [Google Scholar]
- Jia X, Li X, Xu Y, et al. SOX2 promotes tumorigenesis and increases the anti-apoptotic property of human prostate cancer cell. J Mol Cell Biol. 2011;3:230–8. doi: 10.1093/jmcb/mjr002. [DOI] [PubMed] [Google Scholar]
- Kim JY, Park HK, Yoon JS, et al. Advanced glycation end product (AGE)-induced proliferation of HEL cells via receptor for AGE-related signal pathways. Int J Oncol. 2008;33:493–501. [PubMed] [Google Scholar]
- Kregel S, Kiriluk KJ, Rosen AM, et al. Sox2 is an androgen receptor-repressed gene that promotes castration-resistant prostate cancer. PLoS One. 2013;8:e53701. doi: 10.1371/journal.pone.0053701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuniyasu H, Chihara Y, Kondo H, Ohmori H, Ukai R. Amphoterin induction in prostatic stromal cells by androgen deprivation is associated with metastatic prostate cancer. Oncol Rep. 2003;10:1863–8. [PubMed] [Google Scholar]
- Lin F, Lin P, Zhao D, et al. Sox2 targets cyclinE, p27 and survivin to regulate androgen-independent human prostate cancer cell proliferation and apoptosis. Cell Prolif. 2012;45:207–16. doi: 10.1111/j.1365-2184.2012.00812.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu B, Song XL, Jia LY, Song FL, Zhao SC, et al. Differential expressions of the receptor for advanced glycation end products in prostate cancer and normal prostate. Zhonghua Nan Ke Xue. 2010;16:405–9. [PubMed] [Google Scholar]
- Moch H, Humphrey PA, Ulbright TM, Reute VE. Tumors of the urinary tract, in ‘WHO classification of tumors of the urinary system and male genital organs. 4th Edition, Eds . Lyon: international agency for research on cancer; 2016. pp. 78–133. [Google Scholar]
- Naz S, Ahmad S, Ghafoor F, Butt NS, Akhtar MW. Free and total prostate specific antigen in benign prostate hyperplasia and prostate cancer. J Coll Physicians Surg Pak. 2004;14:69–71. [PubMed] [Google Scholar]
- Nankali M, Karimi J, Goodarzi MT, et al. Increased expression of the receptor for advanced glycation end-products (RAGE) is associated with advanced breast cancer stage. Oncol Res Treat. 2016;39:622–8. doi: 10.1159/000449326. [DOI] [PubMed] [Google Scholar]
- Russo MV, Esposito S, Tupone MG, et al. SOX2 boosts major tumor progression genes in prostate cancer and is a functional biomarker of lymph node metastasis. Oncotarget. 2016;15:12372–85. doi: 10.18632/oncotarget.6029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasahira T, Akama Y, Fujii K, Kuniyasu H. Expression of receptor for advanced glycation end products and HMGB1/amphoterin in colorectal adenomas. Virchows Arch. 2005;446:411–5. doi: 10.1007/s00428-005-1210-x. [DOI] [PubMed] [Google Scholar]
- Sims GP, Rowe DC, Rietdijk ST, Herbst R, Coyle AJ. HMGB1 and RAGE in inflammation and cancer. Annu Rev Immunol. 2010;28:367–88. doi: 10.1146/annurev.immunol.021908.132603. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Tanabe K, Ohnuki M, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–72. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- Tang XB, Shen XH, Li L, Zhang YF, Chen GQ. SOX2 overexpression correlates with poor prognosis in laryngeal squamous cell carcinoma. Auris Nasus Larynx. 2013;40:481–6. doi: 10.1016/j.anl.2013.01.003. [DOI] [PubMed] [Google Scholar]
- Ugolkov AV, Eisengart LJ, Luan C, Yang XJ. Expression analysis of putative stem cell markers in human benign and malignant prostate. Prostate. 2011;71:18–25. doi: 10.1002/pros.21217. [DOI] [PubMed] [Google Scholar]
- Weina K, Utikal J. SOX2 and cancer: current research and its implications in the clinic. Clin Transl Med. 2014;3:19–29. doi: 10.1186/2001-1326-3-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan S, Ramasamy R, Schmidt A. Receptor for AGE (RAGE) and its ligands–cast into leading roles in diabetes and the inflammatory response. J Mol Med. 2009;87:235–47. doi: 10.1007/s00109-009-0439-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang F, Gao Y, Geng J, et al. Elevated expression of SOX2 and FGFR1 in correlation with poor prognosis in patients with small cell lung cancer. Int J Clin Exp Pathol. 2013;6:2846–54. [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Yu H, Yang Y, et al. SOX2 in gastric carcinoma, but not Hath1, is related to patients’ clinicopathological features and prognosis. J Gastrointest Surg. 2010;14:1220–6. doi: 10.1007/s11605-010-1246-3. [DOI] [PubMed] [Google Scholar]


