Abstract
Purpose
It has been reported that circulating levels of IgG antibodies against p16, CD25 and FOXP3 proteins were significantly changed in patients with lung cancer, breast cancer and esophageal cancer. However, different peptide fragments appear to trigger different immune responses. This work aimed to analyze the alteration of plasma IgG for p16-derived peptide antigen called p16a, CD25-derived peptide antigen called CD25a and a FOXP3-derived antigen in hepatocellular carcinoma (HCC).
Patients and methods
An enzyme-linked immunosorbent assay (ELISA) was developed in-house to detect plasma IgG to p16a, CD25a and FOXP3 in 119 patients with HCC and 132 control subjects.
Results
Circulating levels of IgG antibodies for all three peptide antigens were significantly higher in HCC patients than control subjects (P<0.001 for all 3 assays); male patients mainly contributed to increase (P<0.01 for all 3 assays). Further analysis showed that plasma anti-p16a, anti-CD25a and anti-FOXP3 IgG levels were increased mainly in patients with intermediate and late-stage HCC (P<0.01 for both assays). Receiver operating characteristic (ROC) curve analysis showed that with a specificity of >95%, the area under the ROC curve (AUC) was 0.62 with 11.4% sensitivity for anti-p16a assay, 0.68 with 14.3% sensitivity for anti-CD25a IgG assay and 0.64 with 10.1% sensitivity for anti-FOXP3 assay. Of the three groups of HCC patients, group 3 (BCLC stage C+D) showed the best sensitivity for the detection of plasma anti-p16a and anti-FOXP3 IgG levels with an AUC of 0.66 and 0.65.
Conclusion
Circulating IgG antibody to p16a, CD25a and FOXP3 proteins may be a useful biomarker for assessment of HCC prognosis of this malignancy, especially in male patients with HCC.
Keywords: autoantibody, p16, CD25, FOXP3, hepatocellular carcinoma
Introduction
Liver cancer was the fourth leading cause of cancer-related deaths in 2015 following lung, colorectal, and stomach cancer.1 The most common type of liver cancer is hepatocellular carcinoma (HCC).2 In our recent studies, we found that circulating IgG antibodies against linear peptide antigens derived from p16 protein, interleukin 2 (IL-2) receptor α-subunit (also called CD25) and forkhead/winged-helix transcription factor box P3 (FOXP3) were significantly changed in liver cancer,3 non-small cell lung cancer (NSCLC),4–8 breast cancer9,10 and esophageal cancer.11–13 Therefore, circulating IgG antibodies for these target molecules may be either diagnostic or prognostic values for solid tumors.
While the reports on circulating IgG antibodies against CD25-derived peptide antigens in NSCLC showed inconsistent results,4–8 further investigation suggested that the immune system had different responses to distinct CD25-derived peptides. For example, a significant low anti-CD25b IgG level was observed in patients with an early-stage NSCLC but anti-CD25a IgG levels were significantly increased7 in this malignancy. Interestingly, our previous study revealed that anti-CD25b IgG levels were significantly increased in patients with HCC.3 In this study, therefore, we attempted to confirm if anti-CD25a IgG levels were significantly changed in HCC. Because circulating IgG for both p16 and FOXP3-derived peptide antigens have been found to be associated with several types of solid cancer,7,8 it is important to see if circulating IgG antibodies for these 2 self-antigens could serve as biomarkers for clinical assessment of HCC.
Materials and Methods
Subjects
The study cohort consisted of 251 participants, of whom 119 were diagnosed with HCC at the Second Hospital of Jilin University, Changchun, China, and 132 were used as control subjects. These 119 HCC patients aged 54.7±9.7 years consisted of 102 males and 17 females; their blood samples were taken during the first hospitalization and before any anticancer treatment was received. HCC staging was made based on the Barcelona Clinic Liver Cancer (BCLC) staging system,14 and these 119 patients with HCC were classified into three subgroups: group 1 (stage 0+A), group 2 (stage B) and group 3 (stage C+D). These 132 healthy control subjects (106 males and 26 females), aged 54.9±8.6 years, were recruited from local communities, and they were included in this study based on the following criteria: (1) they had no any history of liver cancer and other malignancies; (2) they had no any severe autoimmune conditions, such as autoimmune thyroid disease, pernicious anemia, type-1 diabetes, celiac disease, ankylosing spondylitis, systemic lupus erythematosus, rheumatoid arthritis, multiple sclerosis and inflammatory bowel diseases. All the subjects were of Chinese Han origin and all provided informed written consent to take part in the study as approved by the Institutional Review Boards of the Second Hospital of Jilin University and conformed to the Declaration of Helsinki.
Antibody Testing
Linear peptide antigens were used to develop an in-house enzyme-linked immunosorbent assay (ELISA) for the detection of anti-p16a, anti-CD25a and anti-FOXP3 IgG antibodies in plasma as described in our previous studies.7,8,15,16 The peptide sequences used in this study are given in Table 1. The in-house ELISA was then developed with these linear peptides as described in previous reports.7,8 Briefly, the synthetic peptides were dissolved in 67% acetic acid to 5mg/mL, respectively, and diluted with the coating buffer (0.1 M phosphate buffer containing 0.15 M NaCl and 10 mM EDTA, pH 7.2) at 20 µg/mL to coat Maleimide-activated 96-well microplates (Thermo Scientific, Shanghai, China) according to the manufacturer’s instructions. Just prior to use, the antigen-coated plates were washed twice with 200 μL Wash Buffer (phosphate-buffered saline (PBS; P4417, Sigma-Aldrich, Shanghai, China)) containing 0.05% Tween-20. A 50 μL plasma sample diluted 1:200 in Assay Buffer (PBS) containing 0.5% bovine serum albumin (BSA) was added to each well, while 50 μL Assay Buffer was added to the negative control (NC) wells, and 50 μL positive control (PC) sample was added as well. The binding reaction system was incubated at room temperature for 1.5 hrs, followed by washing and incubation with 50 μL peroxidase-conjugated goat anti-human IgG antibody (ab98567, Abcam, Guangzhou, China), diluted 1:50,000 in Assay Buffer at 4°C for 1 hr. The color development was made by adding 50 μL Stabilized Chromogen (SB02, Life Technologies, Beijing, China) and terminated after 20 mins by adding 25 μL Stop Solution (SS04, Life Technologies). The optical density (OD) at 450 nm was measured on a microplate reader with a reference wavelength of 620nm. All the samples were tested in duplicate and the specific binding ratio (SBR) was used to represent the relative levels of plasma IgG antibodies. The SBR was computed as follows:
Table 1.
Information for Peptide Antigens Derived from Three Antigens
| Antigen | Sequence{N→C} | NCBIA Accession | Position{aa} |
|---|---|---|---|
| p16a | CGFLDTLVVLHRAGARLDVRDAWGRLPVD | NP_000068 | 89–102 |
| CD25a | KPGHCREPPPWENEATERIYHFVVGQMVY | NP_000408 | 99–126 |
| FOXP3 | CDWFTRMFAFFRNHPATWKNAIRHNLSLHKD | NP_001107849 | 331–358 |
SBR= (ODSample - ODNC)/(ODPC - ODNC)
To minimize the intra-assay deviation, the ratio of the difference between the duplicated OD values of each sample to their sum was used to assess the precision of ELISA. If the ratio was >10%, the test of the sample was treated as invalid and not used for data analysis.
Data Analysis
The mean ± SD in SBR was used to present the data. The Mann–Whitney U-test was used to examine the differences in plasma IgG levels between the patient and the control groups and Pearson’s correlation analysis was used to examine the correlation between plasma IgG levels and clinical parameters (BCLC stages, serum bilirubin, albumin and prothrombin time). Receiver operating characteristic (ROC) curve analysis was used to calculate the area under the ROC curve (AUC) with 95% confidence interval (CI) and the sensitivity of the IgG assay against a specificity of >95% (Figure 1). A p-value of <0.017 was considered to be significant as three antigens were tested in this study.
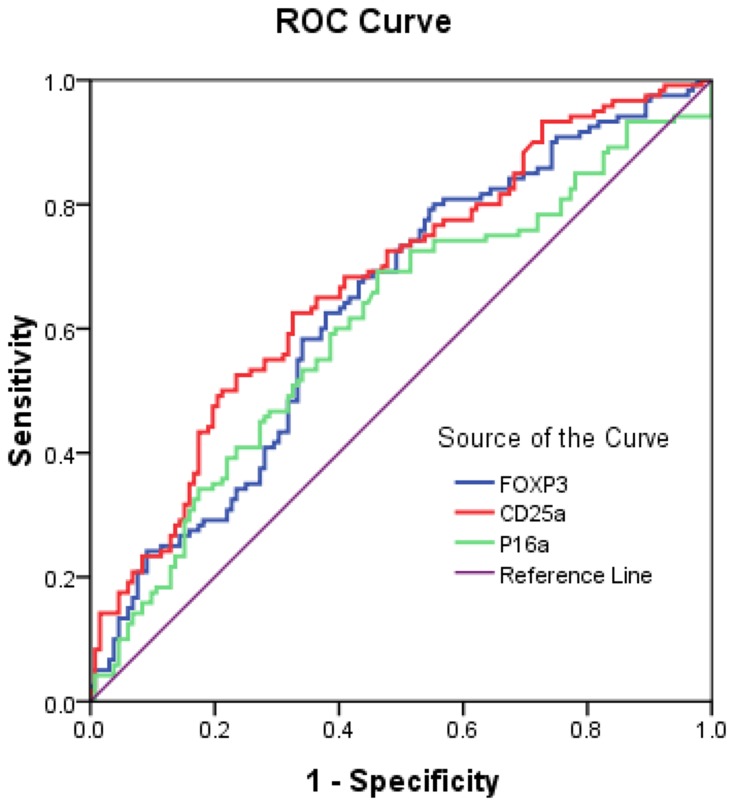
Figure 1.
ROC curve analysis of circulating levels of anti-p16a, anti-CD25a and anti-FOPX3 IgG antibodies in hepatocellular carcinoma.
Results
The levels of plasma IgG antibodies to p16a, CD25a and FOXP3 were compared between HCC patients and control subjects (Table 2). When compared with control subjects, patients with HCC had a significant higher level of anti-p16a IgG (Z= 3.51, P=0.0004), anti-CD25a IgG (Z=−3.834, P<0.001) and anti-FOXP3 IgG (Z=−4.959, P<0.001); the male patients primarily contributed to the increased IgG levels (Z=−2.86, P=0.004 for anti-p16a IgG, Z=−5.210, P<0.001 for anti-CD25a IgG and Z=−4.060, P<0.001 for anti-FOXP3 IgG, respectively).
Table 2.
Comparison of Circulating IgG Levels for 3 Target Peptide Antigens Between HCC Patients and Control Subjects
| Group | Patients (n) | Control (n) | Z | P* |
|---|---|---|---|---|
| p16a | ||||
| Male | 1.92±0.93 (102) | 1.60±0.67 (106) | −2.86 | 0.004 |
| Female | 1.86±0.46 (17) | 1.53±0.49 (26) | −2.36 | 0.018 |
| Both | 1.91±0.87 (119) | 1.59±0.64 (132) | −3.51 | 0.0004 |
| CD25a | ||||
| Male | 1.04±0.14 (102) | 0.93±0.12 (106) | −5.210 | <0.001 |
| Female | 1.01±0.13 (17) | 0.99±0.13 (26) | −0.621 | 0.535 |
| Both | 1.04±0.14 (119) | 0.95±0.13 (132) | −3.834 | <0.001 |
| FOXP3 | ||||
| Male | 1.09±0.22 (102) | 0.97±0.20 (106) | −4.060 | <0.001 |
| Female | 0.98±0.17 (17) | 1.00±0.23 (26) | −0.248 | 0.804 |
| Both | 1.08±0.22 (119) | 0.97±0.21 (132) | −4.959 | <0.001 |
Note: *P-value of <0.017 is considered statistically significant as 3 peptide antigens were tested.
Further analysis showed that increased levels of plasma anti-p16a, anti-CD25a and anti-FOXP3 IgG levels were mainly shown in groups 2 and 3 (Table 3), in which patients with advanced and terminal stage HCC had the highest anti-p16a, and anti-FOXP3 IgG levels (Z=3.38, P=0.0008 in group 2 and Z=−3.038, P=0.002 in group 3, respectively). Plasma anti-CD25a IgG levels were slightly higher in group 1 patients (stage 0+A) than controls (Z=−2.317, P=0.020), although there were no significant correlations between BCLC stages and plasma IgG levels (P>0.05 for all 3 assays), but anti-CD25a IgG levels were positively correlated with anti-FOXP3 IgG levels (r=0.766, P<0.001) (Table 4). Moreover, plasma anti-CD25a and anti-FOXP3 IgG levels were negatively correlated with albumin and positively correlated with prothrombin time in HCC patients.
Table 3.
Analysis of Circulating IgG Levels for 3 Target Peptide Antigens in HCC Patients at Different Stages
| Stage | Patients (n) | Control (n) | Z | P |
|---|---|---|---|---|
| p16a | ||||
| Group 1 | 1.66±0.58 (25) | 1.59±0.64 (132) | 0.97 | 0.331 |
| Group 2 | 1.86±0.79 (43) | 1.59±0.64 (132) | 2.51 | 0.012 |
| Group 3 | 2.08±1.03 (51) | 1.59±0.64 (132) | 3.36 | 0.0008 |
| CD25a | ||||
| Group 1 | 1.00±0.13 (25) | 0.95±0.13 (132) | −2.317 | 0.020 |
| Group 2 | 1.05±0.12 (43) | 0.95±0.13 (132) | −4.603 | <0.001 |
| Group 3 | 1.04±0.15 (51) | 0.95±0.13 (132) | −3.228 | <0.001 |
| FOXP3 | ||||
| Group 1 | 1.05±0.20 (25) | 0.97±0.21 (132) | −2.044 | 0.044 |
| Group 2 | 1.06±0.16 (43) | 0.97±0.21 (132) | −2.797 | 0.005 |
| Group 3 | 1.11±0.26 (51) | 0.97±0.21 (132) | −3.038 | 0.002 |
Notes: The antibody levels are expressed as mean ± SD in SBR. Group 1= BCLC stage 0+A, Group 2 = BCLC stage B, Group 3 = BCLC stage C+D.
Table 4.
Correlation Between IgG Levels and Biochemical Parameters in the Circulation
| Parameters | P16a | CD25a | FOXP3 | |||
|---|---|---|---|---|---|---|
| r | P | r | P | r | P | |
| BCLC stages | 0.119 | 0.197 | 0.060 | 0.514 | 0.098 | 0.287 |
| Total bilirubin | 0.048 | 0.632 | 0.027 | 0.790 | 0.006 | 0.951 |
| ALb | −0.194 | 0.051 | −0.416 | <0.001 | −0.475 | <0.001 |
| PT | 0.048 | 0.637 | 0.227 | 0.024 | 0.357 | <0.001 |
ROC curve analysis showed that with a specificity of >95%, the anti-p16a IgG assay had an AUC of 0.62 with 11.4% sensitivity, the anti-CD25a IgG assay had an AUC of 0.68 with 14.3% sensitivity and the anti-FOXP3 IgG assay had an AUC of 0.64 with 10.1% sensitivity (Tables 5–7). Of these 3 groups of HCC patients, group 3 (BCLC stage C+D) showed the best sensitivity for detection of plasma anti-p16a IgG with an AUC of 0.66 (Table 5) and anti-FOXP3 IgG with an AUC of 0.65 (Table 7); group 2 (BCLC stage B) showed the best sensitivity for plasma anti-CD25a detection with an AUC of 0.73 (Table 6). The combination of plasma IgG antibodies for all 3 peptide antigens showed an AUC of 0.71 with 61.5% sensitivity against specificity of 71.5%, PPV of 66.1% and NPV of 67.4%. The combination of anti-CD25a IgG and anti-FOXP3 IgG showed an AUC of 0.681 with 63.0% sensitivity against specificity of 67.4%, PPV of 63.6% and NPV of 66.9% (Table 8).
Table 5.
ROC Curve Analysis of Circulating Anti-P16a IgG Levels in HCC
| Group | AUC | SEa | 95% CI | Sensitivity (%)b |
|---|---|---|---|---|
| 1 | 0.56 | 0.061 | 0.44–0.68 | 0.0 |
| 2 | 0.62 | 0.048 | 0.53–0.72 | 8.9 |
| 3 | 0.66 | 0.049 | 0.56–0.75 | 19.6 |
| Overall | 0.62 | 0.035 | 0.55–0.69 | 11.4 |
Notes: aStandard error; bagainst a specificity of 96.2%. Group 1 = BCLC stage 0+A, Group 2 = BCLC stage B and Group 3 = BCLC stage C+D.
Table 7.
ROC Curve Analysis of Circulating Anti-FOXP3 IgG Levels in HCC
| Group | AUC | SEa | 95% CI | Sensitivity (%)b |
|---|---|---|---|---|
| 1 | 0.63 | 0.059 | 0.51–0.74 | 12.0 |
| 2 | 0.64 | 0.044 | 0.56–0.73 | 4.7 |
| 3 | 0.65 | 0.002 | 0.55–0.74 | 13.7 |
| Overall | 0.64 | 0.035 | 0.57–0.71 | 10.1 |
Notes: aStandard error; bagainst a specificity of 96.2%. Group 1 = BCLC stage 0+A, Group 2 = BCLC stage B and Group 3 = BCLC stage C+D.
Table 6.
ROC Curve Analysis of Circulating Anti-CD25a IgG Levels in HCC
| Group | AUC | SEa | 95% CI | Sensitivity (%)b |
|---|---|---|---|---|
| 1 | 0.65 | 0.061 | 0.53–0.77 | 8.0 |
| 2 | 0.73 | 0.041 | 0.65–0.82 | 14.0 |
| 3 | 0.65 | 0.045 | 0.57–0.74 | 17.6 |
| Overall | 0.68 | 0.033 | 0.62–0.75 | 14.3 |
Notes: aStandard error; bagainst a specificity of 96.2%. Group 1 = BCLC stage 0+A, Group 2 = BCLC stage B and Group 3 = BCLC stage C+D.
Table 8.
Analysis of Combined IgG Antibodies for 3 Target Peptide Antigens in HCC
| Combination | AUC | Cut-Off value | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) |
|---|---|---|---|---|---|---|
| CD25a+FOXP3 | 0.681 (0.615,0.747) | 0.493 | 63.0 | 67.4 | 63.6 | 66.9 |
| CD25a+FOXP3+P16a | 0.707 (0.642,0.772) | 0.514 | 61.5 | 71.5 | 66.1 | 67.4 |
Discussion
The present study demonstrated that circulating levels of IgG antibodies for p16a, CD25a and FOPX3 were significantly higher in HCC patients than control subjects, and the male patients mainly contributed to this increase (Table 2). Further analysis showed that increased levels of plasma IgG for these 3 peptide antigens were mainly shown in patients with the intermediate and late-stage HCC (Table 3). These observations suggest that anti-p16, anti-CD25a and anti-FOXP3 IgG antibodies have a prognostic value for HCC, consistent with our previous report on the alteration of anti-CD25b IgG levels in HCC.3 Because men are three times more likely to develop HCC than women, circulating IgG antibodies to these 3 protein-derived peptide antigens may contribute to the gender disparity of HCC.
The profile of circulating anti-p16 IgG levels in HCC patients was quite similar to a number of previous studies that revealed an increase in anti-p16 IgG levels in several other types of cancer.10,13,17,18 Meanwhile, another two studies applied an ELISA made from recombinant p16 protein to measure circulating anti-p16 IgG levels and found that HCC patients had significantly higher levels of anti-p16 IgG than control subjects19,20 What mechanism is involved in raising anti-p16 IgG levels in cancer remains unknown, but high expression of p16 protein in cancer tissues may be one of the most possible reasons,21,22 although a study reported lack of p16 expression in patients with HCC.23
Based on the present study, CD25+FOXP3+Treg cells could regulate the function of tumor-associated factors and liver biochemical parameters (Table 4). CD25 is a transmembrane protein present on the activated lymphocytes, especially Treg cells.24 FOXP3 is a member of the forkhead/winged-helix family and a transcription factor that is specifically expressed in Treg cells.25,26 Treg cells are critical immunomodulators in the immune system and may play a major role in the development of HCC;27–29 the increased number of Treg cells has been found to be related to HCC stages,30–32 and FOXP3 has also been found to contribute to HCC progression. For example, overexpression and low methylation of FOXP3 are involved in the oncogenic and progression of HCC.33 Accordingly, the alteration of anti-CD25a and anti-FOXP3 IgG levels in HCC patients may directly affect the function of the Treg cells, and increased release of CD25 and FOXP3 molecules may stimulate autoreactive B cells to secrete antibodies against these two molecules.34
The combination of these 3 target molecules could increase the in-house ELISA sensitivity although the ELISA specificity was not ideal (Table 8). Further study is needed to improve the specificity for diagnostic purpose. Nevertheless, this work has provided an interesting clue that increased levels of circulating IgG antibodies for p16, CD25 and anti-FOXP3 could serve as a useful biomarker for assessment of HCC prognosis instead of early diagnosis of this malignancy.
There are a few limitations to this study. First, clinical information regarding biochemical examinations was incomplete, especially the alpha-fetoprotein (AFP) levels that were not collected. Second, clinical follow-up was not performed, so that the correlation between survival time and plasma IgG levels could not be estimated. Third, the sample size used in this study was rather small; the female sample number was much smaller than the male sample number, which may lead to an underpowered test. Accordingly, this work cannot draw a firm conclusion. Further replication of this initial finding with large sample size and detailed clinical information remains needed.
Conclusions
This study has confirmed that circulating IgG antibodies to p16, CD25 and FOXP3 are significantly increased in HCC patients, especially in late-stage HCC. These autoantibodies may be useful biomarkers for assessment of HCC prognosis, which would provide an in-depth insight into the prevention and treatment of HCC in cancer patients.
Acknowledgments
We thank all the patients and control subjects for their participation in this study. This work was supported by Hailanshen Biomedical &Technology Ltd, Shenzhen, China.
Abbreviations
AUC, area under the ROC curve; CI, confidence interval; FOXP3, forkhead box P3; HCC, hepatocellular carcinoma; IL2RA, interleukin 2 receptor; NC, negative control; OD, optical density; PC, positive control; ROC, receiver operating characteristic; SBR, specific binding ratio; Tregs, regulatory T-lymphocytes.
Author Contributions
According to the IMCJE guidelines, all authors have met the following conditions:
Substantial contributions to conception and design, data acquisition, or data analysis and interpretation;
Final approval of the version to be published;
Drafting the article or critically revising it for important intellectual content;
Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of the work are appropriately investigated and resolved.
Jiaxin Wang designed and performed laboratory work, and drafted the manuscript. Yanjun Wang and Yangchun Xu carried out recruitment of HCC patients and control subjects as well as collected clinical information. Guizhen Zhang and Xuan Zhang conceived of this study and carried out the data analysis, and edited the manuscript. All authors reviewed the results and approved the final version of the manuscript.
Funding
There was no funding support for this study.
Disclosure
The authors declare that they have no conflict of interest.
References
- 1.Mortality GBD. Causes of Death C. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388(10053):1459–1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Petrick JL, Braunlin M, Laversanne M, Valery PC, Bray F, McGlynn KA. International trends in liver cancer incidence, overall and by histologic subtype, 1978–2007. Int J Cancer. 2016;139(7):1534–1545. doi: 10.1002/ijc.30211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang J, Xu Y, Zhao H, Zhang X. Change of circulating antibodies against CD25-derived peptide antigen in hepatocellular carcinoma. J Cancer Res Ther. 2017;13(5):813–816. doi: 10.4103/jcrt.JCRT_823_17 [DOI] [PubMed] [Google Scholar]
- 4.Wang W, Leiguang Y, Xiaomei L, Guan S, Sun S, Wang M. Circulating IgG antibody against FOXP3 may be a potential biomarker for lung cancer. Adv Lung Cancer. 2013;2(4):79–83. doi: 10.4236/alc.2013.24011 [DOI] [Google Scholar]
- 5.Ye L, Li X, Sun S, et al. A study of circulating anti-CD25 antibodies in non-small cell lung cancer. Clin Transl Oncol. 2013;15(8):633–637. doi: 10.1007/s12094-012-0980-2 [DOI] [PubMed] [Google Scholar]
- 6.Chen C, Wang W, Meng Q, Wu N, Wei J. Further study of circulating IgG antibodies to CD25-derived peptide antigens in nonsmall cell lung cancer. FEBS Open Bio. 2016;6(3):211–215. doi: 10.1002/2211-5463.12034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhao H, Zhang X, Han Z, Xie W, Yang W, Wei J. Alteration of circulating natural autoantibodies to CD25-derived peptide antigens and FOXP3 in non-small cell lung cancer. Sci Rep. 2018;8(1):9847. doi: 10.1038/s41598-018-28277-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhao H, Zhang X, Han Z, Wang Y. Circulating anti-p16a IgG autoantibodies as a potential prognostic biomarker for non-small celllung cancer. FEBS Open Bio. 2018;8(11):1875–1881. doi: 10.1002/feb4.2018.8.issue-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu T, Song YN, Shi QY, Liu Y, Bai XN, Pang D. Study of circulating antibodies against CD25 and FOXP3 in breast cancer. Tumour Biol. 2014;35(4):3779–3783. doi: 10.1007/s13277-013-1500-x [DOI] [PubMed] [Google Scholar]
- 10.Chen C, Huang Y, Zhang C, Liu T. Circulating antibodies to p16 protein-derived peptides in breast cancer. Mol Clin Oncol. 2015;3(3):591–594. doi: 10.3892/mco.2015.485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ye L, Guan S, Zhang C, et al. Circulating autoantibody to FOXP3 may be a potential biomarker for esophageal squamous cell carcinoma. Tumour Biol. 2013;34(3):1873–1877. doi: 10.1007/s13277-013-0729-8 [DOI] [PubMed] [Google Scholar]
- 12.Guan S, Liu B, Zhang C, Lee KH, Sun S, Wei J. Circulating autoantibody to CD25 may be a potential biomarker for early diagnosis of esophageal squamous cell carcinoma. Clin Transl Oncol. 2013;15(10):825–829. doi: 10.1007/s12094-013-1007-3 [DOI] [PubMed] [Google Scholar]
- 13.Jin Y, Guan S, Liu L, Sun S, Lee KH, Wei J. Anti-p16 autoantibodies may be a useful biomarker for early diagnosis of esophageal cancer. Asia Pac J Clin Oncol. 2015;11(4):e37–e41. doi: 10.1111/ajco.12198 [DOI] [PubMed] [Google Scholar]
- 14.Llovet JM, Fuster J, Bruix J. Barcelona-clinic liver cancer G. The Barcelona approach: diagnosis, staging, and treatment of hepatocellular carcinoma. Liver Transpl. 2004;10(2):S115–S120. doi: 10.1002/lt.20034 [DOI] [PubMed] [Google Scholar]
- 15.Hallford P, St Clair D, Halley L, Mustard C, Wei J. A study of type-1 diabetes associated autoantibodies in schizophrenia. Schizophr Res. 2016;176(2–3):186–190. doi: 10.1016/j.schres.2016.07.020 [DOI] [PubMed] [Google Scholar]
- 16.Whelan R, St Clair D, Mustard CJ, Hallford P, Wei J. Study of novel autoantibodies in schizophrenia. Schizophr Bull. 2008;44(6):1341–1349. doi: 10.1093/schbul/sbx175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huangfu M, Liu L, Xu S, et al. Detecting of p16 autoantibody as a potential early diagnostic serum biomarker in patients with cervical cancer. Clin Lab. 2016;62(6):1117–1120. doi: 10.7754/Clin.Lab.2015.151024 [DOI] [PubMed] [Google Scholar]
- 18.Zhang C, Ye L, Guan S, et al. Autoantibodies against p16 protein-derived peptides may be a potential biomarker for non-small cell lung cancer. Tumor Biol. 2014;35(3):2047–2051. doi: 10.1007/s13277-013-1271-4 [DOI] [PubMed] [Google Scholar]
- 19.Looi K, Megliorino R, Shi FD, Peng XX, Chen Y, Zhang JY. Humoral immune response to p16, a cyclin-dependent kinase inhibitor in human malignancies. Oncol Rep. 2006;16(5):1105–1110. [PubMed] [Google Scholar]
- 20.Zhang JY, Megliorino R, Peng XX, Tan EM, Chen Y, Chan EK. Antibody detection using tumor-associated antigen mini-array in immunodiagnosing human hepatocellular carcinoma. J Hepatol. 2007;46(1):107–114. doi: 10.1016/j.jhep.2006.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tong J, Sun X, Cheng H, et al. Expression of p16 in non-small cell lung cancer and its prognostic significance: a meta-analysis of published literatures. Lung Cancer. 2011;74(2):155–163. doi: 10.1016/j.lungcan.2011.04.019 [DOI] [PubMed] [Google Scholar]
- 22.Henshall SM, Quinn DI, Lee CS, et al. Overexpression of the cell cycle inhibitor p16INK4A in high-grade prostatic intraepithelial neoplasia predicts early relapse in prostate cancer patients. Clin Cancer Res. 2001;7(3):544–550. [PubMed] [Google Scholar]
- 23.Csepregi A, Ebert MP, Röcken C, et al. Promoter methylation of CDKN2A and lack of p16 expression characterize patients with hepatocellular carcinoma. BMC Cancer. 2010;10:317. doi: 10.1186/1471-2407-10-317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Erfani N, Mehrabadi SM, Ghayumi MA, et al. Increase of regulatory T cells in metastatic stage and CTLA-4 over expression in lymphocytes of patients with non-small cell lung cancer (NSCLC). Lung Cancer. 2012;77(2):306–311. doi: 10.1016/j.lungcan.2012.04.011 [DOI] [PubMed] [Google Scholar]
- 25.Sakaguchi S, Miyara M, Costantino CM, Hafler DA. FOXP3+ regulatory T cells in the human immune system. Nat Rev Immunol. 2010;10(7):490–500. doi: 10.1038/nri2785 [DOI] [PubMed] [Google Scholar]
- 26.Sakaguchi S, Vignali DA, Rudensky AY, Niec RE, Waldmann H. The plasticity and stability of regulatory T cells. Nat Rev Immunol. 2013;13(6):461–467. doi: 10.1038/nri3464 [DOI] [PubMed] [Google Scholar]
- 27.Sharma S, Khosla R, David P, et al. CD4+CD25+CD127(low) regulatory T cells play predominant anti-tumor suppressive role in hepatitis B virus-associated hepatocellular carcinoma. Front Immunol. 2015;6:49. doi: 10.3389/fimmu.2015.00049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhou Y, Wang B, Wu J, et al. Association of preoperative EpCAM circulating tumor cells and peripheral treg cell levels with early recurrence of hepatocellular carcinoma following radical hepatic resection. BMC Cancer. 2016;16:506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.He X, Qu F, Zhou F, et al. High leukocyte mtDNA content contributes to poor prognosis through ROS-mediated immunosuppression in hepatocellular carcinoma patients. Oncotarget. 2016;7(16):22834–22845. doi: 10.18632/oncotarget.8071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang ZQ, Yang ZY, Zhang LD, et al. Increased liver-infiltrating CD8+FoxP3+ regulatory T cells are associated with tumor stage in hepatocellular carcinoma patients. Hum Immunol. 2010;71(12):1180–1186. doi: 10.1016/j.humimm.2010.09.011 [DOI] [PubMed] [Google Scholar]
- 31.Shen X, Li N, Li H, Zhang T, Wang F, Li Q. Increased prevalence of regulatory T cells in the tumor microenvironment and its correlation with TNM stage of hepatocellular carcinoma. J Cancer Res Clin Oncol. 2010;136(11):1745–1754. doi: 10.1007/s00432-010-0833-8 [DOI] [PubMed] [Google Scholar]
- 32.Greten TF, Ormandy LA, Fikuart A, et al. Low-dose cyclophosphamide treatment impairs regulatory T cells and unmasks AFP-specific CD4+ T-cell responses in patients with advanced HCC. J Immunother. 2010;33(2):211–218. doi: 10.1097/CJI.0b013e3181bb499f [DOI] [PubMed] [Google Scholar]
- 33.Carsetti R, Rosado MM, Wardmann H. Peripheral development of B cells in mouse and man. Immunol Rev. 2004;197:179–191. doi: 10.1111/imr.2004.197.issue-1 [DOI] [PubMed] [Google Scholar]
- 34.Shi JY, Ma LJ, Zhang JW, et al. FOXP3 Is a HCC suppressor gene and acts through regulating the TGF-beta/Smad2/3 signaling pathway. BMC Cancer. 2017;17(1):648. doi: 10.1186/s12885-017-3633-6 [DOI] [PMC free article] [PubMed] [Google Scholar]