Abstract
The inappropriate use of antimicrobials has resulted in the selection of resistant strains. Thus, a great number of studies have focused on the investigation of new antimicrobial agents. The use of zinc oxide nanoparticles (ZnO NPs) to optimise the fight against microbial resistance has been receiving increased attention due to the non-specific activity of inorganic antimicrobial agents. The small particle size and the high surface area of ZnO NPs can enhance antimicrobial activity, causing an improvement in surface reactivity. In addition, surface modifiers covering ZnO NPs can play a role in mediating antimicrobial activity since the surface properties of nanomaterials alter their interactions with cells; this may interfere with the antimicrobial effect of ZnO NPs. The possibility of using surface modifiers with groups toxic to microorganisms can improve the antimicrobial activity of ZnO NPs. Understanding the exact toxicity mechanisms is crucial to elucidating the antimicrobial activity of ZnO NPs in bacteria and fungi. Therefore, this review aims to describe the mechanisms of ZnO NPs toxicity against fungi and bacteria and how the different structural and physical-chemical characteristics of ZnO NPs can interfere in their antimicrobial activity.
Keywords: nanoparticles, zinc oxide, antimicrobial activity, toxicity mechanisms
Introduction
The inappropriate use of antimicrobials has resulted in the emergence of multidrug-resistant strains, causing an increase in infectious disease and mortality.1,2 Bacterial resistance may be generated by genetic mutations arising from adaptive responses or via horizontal gene transfer (HGT).3 HGT can occur by conjugation, which requires cell–cell contact across the cell surface pili or adhesins (elements present in the cellular structure of some bacteria), through which the DNA is transferred by plasmids from the donor cell to the recipient cell. For example, β-lactamases have been found to be transmitted by plasmids and are capable of being transferred between bacterial species.4 In the same way, the prolonged and intense use of antifungal compounds has led to decreased sensitivity and increased numbers of strains resistant to miconazole, fluconazole and amphotericin-B. These drugs are still considered the best standards for the treatment of severe mycoses, but severe adverse effects such as chronic toxicity limit the use of these drugs due to renal function impairment.5 Another important resistance mechanism is biofilm production. Biofilms are found adhered to a solid surface and are composed of an array that covers a community of bacterial cells and protects them from the action of antibiotics.6
Commonly used antimicrobials act by inhibiting essential components to maintain microbial metabolism, such as cell wall synthesis (beta-lactams, glycopeptides), proteins (aminoglycosides, macrolides, chloramphenicol), DNA (quinolones, coumarins), RNA (rifamycins) and intermediate metabolism (sulphonamides and trimetropine).7 However, one of the main challenges of biomedical researchers is to develop antibacterial drugs that are able to combat biofilm-forming and multidrug-resistant strains.
Based on this problem, the use of nanomaterials as antimicrobial agents is justified. This is because the areas in which antibiotics fail can be filled by using nanomaterials in a number of ways: overcoming the mechanisms of microbial resistance through membrane disruption or impeding biofilm formation, or stopping microbial growth using multiple mechanisms of action simultaneously. This is due to the fact that most antibiotic resistance mechanisms do not correspond to the same pathways in which nanomaterials act.8 In this sense, the use of inorganic nanoparticles can be an alternative to minimise microbial resistance and to reduce toxicity to human cells.9 Recent advances in nanotechnology have enabled the production of particles with various sizes, shapes and a large surface area relative to volume, allowing for interactions with bacterial cells. This has led to the development of new biocidal agents.10
Zinc oxide (ZnO), titanium dioxide and silver are used in different areas to control microbial growth.11 However, ZnO has greater advantages because it has the highest photocatalytic efficiency among all the inorganic photocatalytic materials and is more biocompatible than titanium dioxide. Additionally, ZnO has greater selectivity, better durability and heat resistance, it can be used to combat a diversity of microorganisms, such as S. aureus,9,12,13 E. coli14 and C. albicans.15–17
ZnO is a semi-conductor presenting a high bandgap of 3.4 eV and binding energy of 60 meV, leading to peculiar electrical and optical properties.18–20 As an antimicrobial agent, ZnO has been investigated on the microscale and nanoscale.17 A decrease in ZnO particle size causes changes in the electrical, optical and chemical properties; some authors believe that these changes are caused by the quantum confinement surface, thus enabling new applications.21 For instance, when the size of ZnO is reduced to the nanoscale, its activity against microorganisms is increased.17 Particularly in terms of antimicrobial applications, among the advantages of using an inorganic material rather than an organic material are reduced toxicity, improved durability, lower resistance and good selectivity.2,22 Recent studies have demonstrated that ZnO NPs are efficient at combating multidrug-resistant bacteria23 and preventing biofilm formation.24
The mechanism of ZnO NP toxicity depends on the modification of the surface, the intrinsic physicochemical properties of ZnO and the medium used to disperse the NPs. Several studies have investigated the mechanisms of action of ZnO NPs on bacteria and fungi, but these studies have not been conclusive.12,25–28
As previously mentioned, the use of ZnO NPs may be a promising alternative to minimising microbial resistance. In addition, it is possible to produce ZnO NPs with various sizes, shapes and surface modifications. Therefore, this review aims to summarise the mechanisms of action described in the literature and how the physicochemical properties and surface modifications of ZnO may alter their antimicrobial activity. Thus, this review may help to clarify the most desirable ZnO NP characteristics to achieve the optimal antimicrobial effect.
Mechanism Of Antibacterial Activity Of ZnO NPs
To better understand how the structural parameters of ZnO NPs could alter their antimicrobial activity, this section will describe the main mechanisms of action found in the literature. It is important to highlight that many studies have addressed this theme. However, as nanoparticles act in a non-specific manner, more than one mechanism may explain their activity, which makes the interpretation of the main mechanism responsible for antimicrobial activity more difficult.
ZnO aqueous suspensions allow for chemical interactions between hydrogen peroxide and the proteins of the cell membrane. Thus, the different chemical species formed explain the different antimicrobial activities.29,30
The proposed mechanisms are (i) the production of reactive oxygen species (ROS);2,12,29–31 (ii) the loss of cellular integrity following contact between ZnO NPs and the cell wall;32,33 (iii) the release of Zn2+ ions16,34,35 and (iv) ZnO NPs internalisation.32
The production of ROS by metal oxide NPs is one of the mechanisms responsible for antimicrobial activity most commonly reported in the literature. ROS include superoxide anions (O2−), hydroxyl radicals (HO−2) and hydrogen peroxide (H2O2),2,31 which can cause the destruction of cellular components such as DNA, proteins and lipids.29,30
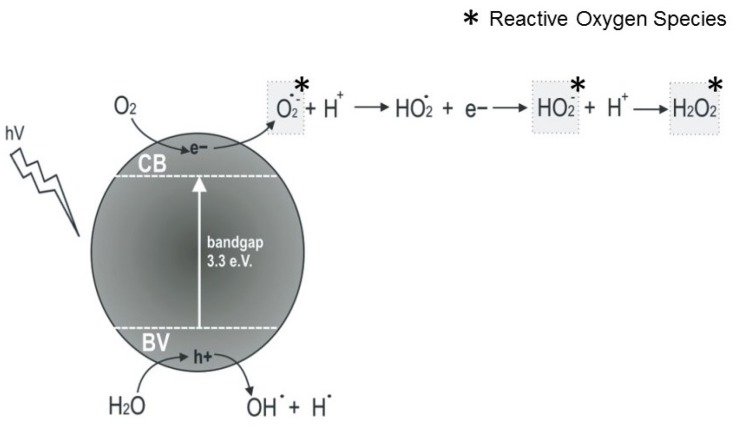
The ROS production mechanisms driven by ZnO NPs are described in Figure 1. As a semiconductor, the electronic structure of the ZnO consists of a conduction band (CB) and a valence band (VB). Photon radiation (hv) with greater energy than the bandgap, ie, higher than 3.3 eV, is immediately absorbed and electrons leave the VB by passing to the CB, which initiates a series of photoreactions.36 Positive holes (h+) are formed in the VB and, in turn, there are free electrons (e−) in the CB. These electron pairs initiate various reactions. The “holes” separate water molecules into OH− and H+. Dissolved oxygen molecules react with the free electron pair of the CB and are transformed into superoxide radical anions (O2−), which react with H+ and generate (HO2) radicals that after colliding with the electrons (e_) are transformed into hydroxyl peroxide anions (HO2−). These, in turn, can react with hydrogen ions and produce hydrogen peroxide (H2O2) that can penetrate into the cell and kill microorganisms.2,30,31,36
Figure 1.
Formation of reactive oxygen species by ZnO NPs.
Raghupathi and co-workers12 attributed the increased antibacterial activity of ZnO NPs with increased ROS production by NPs after exposure to UV light. However, some studies have shown that ROS can be created even without light exposure.37,38 Hirota et al38 developed ZnO ceramics with sustainable antibacterial activity. The analyses of electron spin resonance and chemical photoluminescence showed that the antibacterial activity of ZnO could be attributed to superoxide anion production on the ZnO surface, even under dark conditions. The bacterial cell wall has negative charges, like hydroxyl radicals and superoxides, so they cannot penetrate the membrane, but direct contact can cause damage. Therefore, these species can be found only outside the bacterium. Conversely, hydrogen peroxide is able to pass through the cell wall and can be internalised in the bacterial cell, triggering cell death.2,31,33
D’Água et al39 showed that bacteria that were more sensitive to hydrogen peroxide were also the more sensitive to ZnO NPs. Similar results were obtained with less sensitive bacteria. In this way, the authors suggested that peroxide hydrogen could be the mechanism responsible for the antibacterial activity of ZnO NPs.
Kadiyala et al40 recently studied the antibacterial mechanism of ZnO NPs against methicillin-resistant S. aureus. Contrary to studies demonstrating that the antimicrobial activity is dependent on the ROS or Zn ions, in this study, ROS toxicity was not the principal mediator of antibacterial activity. The most relevant parameters to explain the activity of ZnO NPs were mechanisms that involved pyrimidine, sugar metabolism and amino acid biosynthesis.
Another mechanism that may be responsible for damage to microorganisms is photoconductivity, which is a process of photo-induction.36 Due to its semiconductor properties, ZnO has high photocatalytic efficiency; this can contribute to its antimicrobial effect.17,31 Therefore, when ZnO is submitted to UV light, the antimicrobial effect can be optimised due to the improvement in conductivity, which activates the interactions between ZnO and bacterial cells. Moreover, when the UV light is turned off, conductivity persists.17
Another mechanism that can kill bacteria is the release of Zn2+ ions. When ZnO NPs are in solution, partial dissolution results in the release of Zn2+ ions, which have antimicrobial activity. Therefore, the dissolution of ZnO NPs contributes to its antimicrobial activity16,34 by decreasing amino acid metabolism and perturbing the enzymatic system.17
Under acidic conditions, ZnO NPs dissolve and produce Zn2+ ions. Based on this dissolution, Cho et al41 conducted studies on rats and showed that ZnO NPs remain intact at neutral or biological pH. However, they rapidly dissolve under acidic conditions (pH 4.5), for example, in a microorganism’s lysosomes, leading to death due by binding to the biomolecules inside the bacterial cell and inhibiting its growth.
Li et al35 evaluated the toxicity of ZnO NPs in E. coli, correlating the release of Zn2+ according to the medium used to disperse them. The media used were ultrapure water, 0.85% NaCl, phosphate-buffered saline (PBS), minimal Davis (MD) and Luria-Bertani (LB). The results showed that a smaller concentration of Zn2+ in the medium resulted in lower toxicity of NPs against E. coli. For example, in PBS, precipitate generation was observed; zinc complexes like citrate-zinc and amino acid-zinc were formed in MD and LB, respectively. These species drastically reduced the Zn2+ ion concentration, which resulted in lower toxicity in these media. The authors concluded that toxicity of ZnO NPs was found in the following order: ultrapure water > NaCl > MD > LB > PBS.
In contrast, Li and co-workers42 measured the antibacterial activity of ZnO NPs according to ROS production and Zn2+ ions released by the ZnO NP suspensions. The researchers observed that, in the case of a Zn2+ concentration of about 1 mg/L, there was no inhibition of E. coli. However, the growth rate of E. coli decreased with increasing ROS levels, showing that the released Zn2+ ions had a smaller effect.
Baek and An27 also obtained results contrasting those presented by Li et al.35 The release of free ions by various NPs was studied, including ZnO NPs. For this purpose, they used strains of E. coli, B. subtilis and S. aureus. The maximum fraction of metal ions that can be released by NPs was used in the experiment. At a concentration of 125 mg/mL of ZnO NPs, 6.8 mg/mL of zinc ions were released; thus, the bacteria were placed in contact with the ions and the antibacterial activity was assessed. The results showed that, in the evaluation of free ion activity, inhibition was low (from 2% to 3% for all strains tested), but the ZnO NPs showed high toxicity for all strains tested. Thus, the authors suggested that NPs activity may be related to intrinsic metal particle toxicity.
Another possible toxicity mechanism of ZnO NPs corresponds to the interaction of the ZnO particle with the cell wall, resulting in the loss of bacterial integrity.16,32 Brayner et al32 evaluated the toxicological impact of ZnO NPs against E. coli. The results showed that the bacteria cells were damaged, leading to disorganisation of the membrane; therefore, there was an increase in membrane permeability, leading to the accumulation of ZnO NPs in the bacterial cell membrane as well as the internalisation of NPs. Similar results were observed by Lallo da Silva et al43 with S. aureus, ie, after contact with ZnO NPs, cells have holes in the membrane.
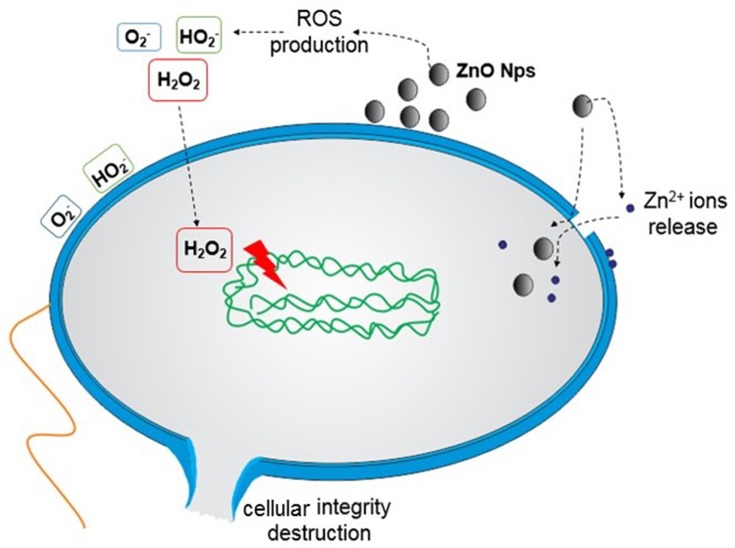
As previously reported, the toxicity of ZnO NPs is not always dependent on their internalisation in the bacterial cell. ZnO NPs can cause changes in the environment close to the bacteria, ie, producing ROS or increasing the solubility of ZnO NPs, which can induce cellular damage.25,44 Figure 2 schematically shows the possible toxicity mechanisms of ZnO NPs.
Figure 2.
Suggested mechanisms of action of ZnO NPs against bacteria. (1) ZnO NPs release Zn2+ ions, which can be internalised into the bacterial cell and disrupt the enzymatic system. (2) ROS production (causing the destruction of cellular components such as DNA, proteins and lipids): O2− and HO2− (do not penetrate the membrane, but direct contact causes damage) and H2O2 (internalised). (3) Internalisation within the bacteria cell and direct contact cause damage such as the loss of cellular integrity.
Possible mechanisms of the antifungal activity of ZnO NPs are also described in the literature. ZnO NPs may enter the cell by diffusion and endocytosis; once in contact with the cytoplasm, they interfere in the functioning of mitochondria, promoting the release of ROS and Zn2+. These released ions can penetrate the membrane and reach the DNA, causing nuclear damage like irreversible chromosome damage, which induces cell death.28
He et al45 studied the antifungal activity and ZnO NPs against B. cinerea and P. expansum. Scanning electron microscopy (SEM) and Raman spectroscopy were used to assess morphological changes and cellular composition of hyphae after treatment with ZnO NPs. Inhibition of the both fungi was achieved; however, P. expansum was more sensitive. For B. cinerea, hyphae malformation was observed, suggesting inhibition by affecting cell function and causing increased nucleic acids derived from the hyphae in response to oxidative stress. In contrast, ZnO NPs inhibited the development of conidiophores and conidia of P. expansum. These differences in susceptibility may have been caused by the inherent tolerance of each fungus or differences in morphological growth. Indeed, P. expansum grows more densely on the surface of the agar, leading to better exposure to ZnO NPs.
Lipovsky et al46 studied the antifungal activity of ZnO NPs mediated by ROS against Candida albicans. Histidine removes oxygen radicals, and therefore it was added into the culture of C. albicans in order to assess the inhibitory effect on toxicity mediated by ZnO NPs. The results showed that 5 nM histidine inhibited the antifungal effect of ZnO. Thus, the authors suggested that the release of ROS in aqueous media may be responsible for cell death. In addition, in contact with visible light, ZnO NPs provoked an increase in the cell death rate.
It has been found that the antimicrobial activity of ZnO NPs is proportional to the NPs concentration;14,15,28 moreover, the level of ROS released by ZnO NPs is also dose-dependent.28
Pasquet et al26 suggested that the antimicrobial activity of ZnO NPs in fungi may be lower when compared to bacteria since the fungus has the ability to develop pores in hostile conditions. ZnO demonstrated only fungistatic activity at all NPs concentration used and it did not decrease the initial population. In contrast, a study performed by Eskandari et al47 showed that ZnO nanorods not only decrease the growth of C. albicans but also have fungicidal activity.
ZnO NPs have Gram-positive and Gram-negative antibacterial activity and antifungal activity;48 however, the strength of the effect depends on the sensitivity of the microorganism.49 A study performed by Shinde et al50 on the antimicrobial activity of ZnO microspheres (MS-ZnO) against S. aureus and E. coli suggested that the difference in susceptibility of MS-ZnO in the two bacteria may be explained by the differences in their cell wall. The cell wall of Gram-positive bacteria consists of a thick layer of peptidoglycan (20–80 nm), which provides a physical barrier that serves to protect the environment of the cell. This thick layer of peptidoglycan also is responsible for fixing polymers such as teichoic and lipoteichoic acids and surface proteins.49,51 Gram-negative bacteria have a cell wall with a complex organisation. There is a thin peptidoglycan layer and an external membrane that contain lipopolysaccharides. The external membrane and peptidoglycan are linked by lipoproteins. The peptidoglycan layer is located in the periplasmic space that is formed between the external membrane and the cytoplasmic membrane.25,32,52 The cell wall is responsible for maintaining the osmotic pressure and the shape of the cell. NPs of various sizes can easily pass through the layer of peptidoglycan and therefore are highly capable of causing damage.17 The peptidoglycan layer contains repeated units of amino acids and carbohydrates. Thus, ZnO NPs can interact with carboxylic acid and amino groups and thereby inhibit cellular processes. However, the peptidoglycan layer of Gram-negative bacteria is thinner than the membrane of Gram-positive bacteria, so rupture of the cell membrane is easier.50
The difference between the components of Gram-positive and Gram-negative cell walls was elucidated in a study conducted by Tayel and co-workers.53 These researchers suggested that Gram-positive bacteria are more susceptible to attack by ZnO NPs than Gram-negative bacteria. The minimum inhibitory concentration (MIC) of ZnO NPs observed by Reddy et al54 in S. aureus, a Gram-positive bacterium, and E. coli, a Gram-negative bacterium, was 1 mg/mL and 3.4 mg/mL, respectively, which shows that the inhibition of Gram-negative bacteria requires higher concentrations of ZnO NPs. This is likely because the peptidoglycan layer that surrounds Gram-positive bacteria can promote ZnO attack inside the cell, while the cell wall components of Gram-negative bacteria, such as lipopolysaccharides, can counter this attack. Similar results were found by d’Agua et al,39 using the agar diffusion method to evaluate the antibacterial activity of textiles containing ZnO NPs. In this study, the authors showed that Gram-positive bacteria were more sensitive to peroxide hydrogen than Gram-negative bacteria.
Bacteria present the intrinsic ability to produce some substances that induce resistance to oxidative stress. Sphaphyloxantin is a carotenoid produced by the membrane of S. aureus that functions as an antioxidant. Thus, DNA, proteins and lipids are better protected against oxidative stress due to the presence of sphaphyloxantin.55 Cytochrome oxidase is an enzyme present in some bacteria that can interfere in the antibacterial activity of ZnO NPs because it directly reduces the amount of oxygen present in the water. In the absence of cytochrome oxidase, oxygen reduction makes use of catalase, which involves more stages. This discussion was approached by Pasquet et al,26 who observed better activity of ZnO NPs in E. coli than P. aeruginosa, which possesses both cytochrome oxidase and catalase whereas E. coli possesses only catalase. Thus, E. coli catalase is involved in oxygen reduction in water whereas P. aeruginosa catalase is readily available for H2O2 reduction because it is supplemented by cytochrome oxidase. The bacterial growth rate is another factor that can change the bacterial tolerance to ZnO NPs. Bacteria with a slow growth are less susceptible to the action of antibiotics compared to those with rapid growth.25,56
Based on the studies described in this section, it can be concluded that the positive aspects of using ZnO NPs to combat microbial growth make them promising alternatives for combating multidrug-resistant organisms. This is due to the fact that NPs act in a non-specific manner and can thus act on pathways that antibiotics fail to target. Moreover, the main mechanisms of antibiotic resistance are irrelevant to nanoparticles, because the mechanisms do not necessarily involve NPs penetration. For these reasons, it is likely that ZnO NPs will be less likely to result in the selection of resistant bacteria, compared to antibiotics.
However, as a negative aspect, precisely because it involves many mechanisms of antimicrobial activity, it is difficult to elucidate all the ways in which ZnO NPs act on bacteria and fungi. According to the literature review on this topic, it is suggested that ROS production may be responsible for most of the toxicity. However, it is important to emphasise that studies have shown new mechanisms, as demonstrated by Kadiyala et al40 and Tiwari et al.23
The Influence Of The Physicochemical Parameters Of ZnO NPs On Their Antimicrobial Activity
This section covers the structural parameters that may influence the antimicrobial activity of ZnO NPs, focussing on the relationship between the morphology and size of nanoparticles with antimicrobial activity. The control of these parameters is important because the antimicrobial activity of ZnO NPs depends on their intrinsic physicochemical properties. Thus, the toxicity of ZnO NPs can be altered depending on their morphology and size. Based on these properties, some studies have assessed the impact of the morphology and size of ZnO NPs on their antimicrobial activities.57,58 The morphology control of the ZnO NPs is one of the most important factors for their production; therefore, the reaction parameters should be standardised to ensure the shape and size of the product.50
Stanković et al58 studied the influence of ZnO NPs shape when synthesised by the hydrothermal method using different types of stabilising agents on the antibacterial activity of ZnO NPs against E. coli and S. aureus. The shape of the particles was investigated by field emission scanning electron microscopy FE SEM that showed hexagonal prisms 1 nm in length and 100 nm in diameter, ellipses with a length of 500–600 nm and a diameter of 100 nm and spherical particles with diameters around 30 nm. ZnO crystal growth depends on the type of the stabilising agent used in the synthesis. Better antibacterial activity was observed with spherical particles synthesised with polyvinyl alcohol (PVA), which presented a larger surface area and smaller size of 30 m2.g−1 and 25.70 nm, respectively. In addition, they observed different morphologies for powders prepared with polyvinyl pyrrolidone (PVP) and poly (L-glutamic acid; PGA) presenting greater than 100% differences in surface area. This feature led to different antibacterial activity in S. aureus and E. coli. ZnO powers exhibited almost the same level of antibacterial activity for E. coli, while in the case of S. aureus, the functional dependence was more strongly related to the value of the surface area measurements. This occurred because E. coli cells are more elongated than S. aureus cells and they come into contact with a larger number of spherical particles.
Another study conducted by Telebian et al57 synthesised ZnO NPs by the same method of Stanković et al. However, they used other solvents that resulted in different morphologies, ie, 1-hexanol, ethylene glycol and water formed rod-, flower- and spherical morphologies, respectively. Antimicrobial activity was assessed against S. aureus and E. coli by the colony count method. The bacteria were exposed to samples for 20 mins, aiming to evaluate the bactericidal effect under dark and UV light conditions. The results showed that ZnO nanoflowers had better photocatalytic activity than ZnO nanorods and nanospheres. Inactivation under UV light and dark conditions increased in the following order: flower-like > spheres > rods.
Pasquet et al26 evaluated the antimicrobial activity of ZnO NPs in C. albicans, A. brasiliensis, E. coli, P. aeruginosa and S. aureus. The challenge test was used in this study to assess the relationship between antimicrobial activity and the structural parameters of ZnO NPs. Three samples of ZnO NPs were characterised in terms of crystal size, surface area, porosity and aggregated particles in aqueous medium and in Muller-Hinton broth. The antimicrobial activity was evaluated by disc diffusion and MIC measurements with the NPs for each microorganism tested. The results showed that the best activity was observed for smaller ZnO crystals and the antimicrobial activity was dependent on the surface area. The photocatalytic effect was more dependent on the crystallinity than the specific area. The size of the agglomerates was not a relevant parameter, because smaller sizes were obtained and resulted in lower activities against the strains tested. However, the formation of agglomerates should be avoided, in order to minimise sedimentation and to maintain the homogeneity of the suspension. Thus, these authors concluded that the most important parameters to explain antimicrobial activity are a small crystal size and high porosity with large pores.
Raghupathi et al12 studied the effect of particle size of ZnO in terms of antibacterial activity. The results showed that the viability of the cell significantly decreased with decreasing particle size (from 212 nm to 12 nm). When the particle size was larger than 100 nm, the inhibition of methicillin-sensitive S. aureus was incomplete. In this case, ZnO NPs had a bacteriostatic function. The particles smaller than 12 nm inhibited not only growth but also killed S. aureus. The results of confocal microscopy showed that the treatment of bacteria with small-sized NPs led to an increase in cell death, probably due to rupture of the bacterial cell wall.
Padmavathy and Vijayaraghavan2 studied the antibacterial activity of ZnO NPs synthesised by two routes that resulted in different particle sizes; therefore, these researchers correlated the influence of NPs size with antibacterial activity. Disk diffusion assays showed that the inhibition zone was increased with smaller sized NPs, which may be related to the ability of these particles to rupture the bacterial membrane. The MIC and MBC assays were performed using E. coli. At concentrations below 1 mM, no significant antibacterial activity was observed in any sample, with 1 mM ZnO NPs, the activity was bacteriostatic, and concentrations of 5–100 mM were bactericidal. The bactericidal activity found at high and low concentrations was better for a suspension of 12 nm ZnO than for 45 nm and 2 µm NPs.2
A study conducted by Dutta et al59 aimed to evaluate the changes in oxygen vacancies and the effect of ZnO NPs size with antibacterial activity. The results show that one of the major factors responsible for regulating the mechanism of toxicity is the difference in oxygen vacancies, with modification of ZnO properties, as well as particle size, since smaller particles have s larger surface area in relation to volume and can penetrate into the bacterial cell. Furthermore, the increase in oxygen vacancies was responsible for positively loading ZnO NPs, which enabled interactions with the bacterial cell wall, which has a negative charge.
Shinde et al50 studied the antibacterial activity of ZnO microspheres (ZnO-MS) against S. aureus and E. coli. The MIC was found at a concentration of 5 mg/L for both bacteria. As the concentration increased, complete inhibition was achieved at 75 mg/mL. The ZnO-MS with a smaller size (20.20 nm) had better antibacterial activity. Jones et al60 bought ZnO NPs in various sizes and performed MIC assays. For ZnO NPs with a smaller size (8 nm), the MIC was 80 µg/mL for S. aureus in Muller-Hilton medium, while that for larger NPs (50–70 nm) was calculated to be 1.2 mg/mL. Lallo da Silva et al43 produced ZnO NPs with a size of 5 nm by the sol–gel method; the size of these NPs was increased (21–38 nm) with heat treatment. Antibacterial assays were performed to calculate the MIC (Muller-Hilton medium) and MBC against S. aureus and E. coli. The results showed that the smaller NPs had better antibacterial activity. The MIC (S. aureus) for to the smaller NPs was 78 µg/mL. The size influenced the mechanism of action because the 5 nm NPs had bactericidal activity and the large NPs had bacteriostatic activity.
Based on these findings, it was possible to observe that there was excellent agreement in the MIC values for S. aureus between studies. Lallo da Silva et al43 produced ZnO NPs with varied sizes (5 nm and 21–38 nm). When we compared the same sizes with the same bacteria and medium used to perform the MIC assay, the same MIC values were found. For example, the particles synthesised by Arakha et al1 with a size of 39 nm and the ZnO NPs produced by Lallo da Silva et al (38.25 nm) both had an MIC value of 0.1041 mg/mL. It was also possible to correlate the antibacterial activity of the ZnO NPs synthesised by Lallo da Silva et al43 with the data presented by Sharma et al.61 The ZnO NPs synthesised by Sharma et al61 had a size of 20 nm, while the ZnO NPs produced by Lallo da Silva et al had a size of 21 nm. The MIC value obtained for S. aureus in both cases was 0.31 mg/mL. Comparing the 5 nm ZnO NPs synthesised by Lallo da Silva et al43 with the 8 nm ZnO NPs used in the experiments performed by Jones et al, we can observe almost the same MIC for S. aureus (around 80 µg/mL). These findings show once again the importance of ZnO NP size on their antibacterial activity.
A study recently conducted by Zhang et al62 on hexagonal faceted ZnO quantum dots (Qdots) showed that the photocatalytic activity could be explained in three ways. These results could be also applied to the antimicrobial properties. Because ZnO Qdots are very small nanoparticles, they have a high specific surface area, which enables more contact with the microorganism surface. Another reason is related to the hexagonal faceted morphology. Due to its unsaturated oxygen coordination and positive charge, the (001) face is able to adsorb oxygen molecules and OH− ions. This results in a higher rate of H2O2 and OH• radical production, and hence increased antimicrobial activity.
Table 1 provides a summary of the different studies used in this review and the main conclusions. As we can see, the most relevant parameter for excellent activity was the small size of the NPs. It was found that ZnO NPs with a smaller size are found at a higher concentration in the blood than larger ones (19 and >100 nm).63 In the same way, the better activity of smaller sized NPs may be attributed the need for more smaller particles to cover the bacterial colony, which results in the generation of higher concentrations of ROS released on the surface of the colony, promoting bacterial death more efficiently.2 In addition, smaller NPs can penetrate the bacterial membrane more easily due to the high interfacial area.17 In addition, ZnO NPs dissolution into Zn2+ ions has been reported as being a size-dependent phenomenon44,64,65 and as previously mentioned, Zn2+ ions may be responsible for the antimicrobial activity of ZnO NPs. In summary, although NP morphology is a very important parameter, all the antimicrobial mechanisms of ZnO NPs are size-dependent. Thus, ZnO NPs with a very small size may result in optimised antimicrobial activity.
Table 1.
Summary Of The More Relevant Results Found In The Review
| MOs Tested | Methods Used | Size | Shape | Conclusions | Reference |
|---|---|---|---|---|---|
| S. aureus and E. coli | Colony count method | i) Length 1 um and diameter 100 nm; ii) Length 500–600 nm and diameter 100 nm; iii) 30 nm | i) Prism; ii) ellipse. iii) spherical | Better activity was observed for NPs with a smaller size and spherical morphology | 58 |
| S. aureus and E. coli | Colony count method | i) 45 nm; ii) 76 nm; iii) 65 nm | i) Flower-like, ii) hexagonal-rod, iii) spherical | Better activity was observed for NPs with a smaller size and flower-like morphology | 57 |
| C. albicans, A. braseliensis, E. coli, P.aeruginosa and S. aureus | Disk diffusion and MIC | i) 14.7 nm; ii) 17.5 nm; iii) 76.2 nm | i) Platelet; ii) platelet, iii) rod-like | Greater antimicrobial activity was found for NPs with a small crystal size, high porosity and larger pores | 26 |
| S. aureus; S. epidermidis; S. pyogenes; E. faecalis; B. subtilis; B. cereus; E. coli, P. vulgaris, S. typhimurium, S. flexneri, P. alcaligenes, and E. aerogenes | Culture turbidity, and colony count method | 12 to 212 nm | Not defined | Better results were found with smaller NPs | 12 |
| E. coli | Disk diffusion, MIC and MBC | 12 nm, 45 nm and 2 µm | Spherical | The antibacterial activity was higher for ZnO suspension of 12 nm | 2 |
| E. coli | Disk diffusion and MIC | 25 to 300 nm | Spherical | Size, oxygen defects and surface modification of ZnO NPs play critical roles in toxicological activity of ZnO NPs | 59 |
| S. aureus and E. coli | Disk diffusion and MIC | 20.2 nm, 27.1 nm and 36.8 nm | Spherical | Smaller size exhibits better antibacterial activity | 50 |
| S. aureus and E. coli | MIC and MBC | 5 nm to 38 nm | Spherical | Smaller size exhibits better antibacterial activity and the size influences the type of activity | 43 |
Zinc Oxide Surface Modification And Doping: Relationship With Antimicrobial Activity
Although the uses of ZnO NPs are diverse, their instability in water limits their biological applications, considering that biomolecules such as DNA, proteins and peptides are present in aqueous media. Therefore, surface modifications to ZnO NPs are performed in order to improve the dispersion of biomolecules. For these reasons, different strategies have been explored in an attempt to change the ZnO NPs surface to make them stable in water.66
Surface modifications enhanced the dispersion of ZnO NPs and resulted in less aggregation. This finding was demonstrated by Aditya et al,67 who dispersed ZnO NPs in an ionic liquid. This enhanced the bactericidal efficacy against S. epidermidis because ZnO NPs in ionic liquids showed elevated production of ROS.
In addition to surface modifications used to improve stability as mentioned earlier, it is possible to design systems in which the surface of ZnO NPs is altered in order to optimise their antimicrobial activity. For example, this can be done using organosilanes. Organosilanes can be formed by different organic functional groups and are precursors of corresponding organosilanols or organosilsesquioxanes. They are inorganic-organic hybrid materials that can be applied to different substances to modify their surface by attaching oxides by silyation.68 Thus, for the stabilisation of ZnO NPs, researchers have modified their surface with organosilanes, because these molecules bind covalently to the hydroxylated surface of NPs, making them stable in water by creating a barrier that protects the NPs in the nucleus and allows the dispersion of NPs in water.69,70
The surface topography of a material can change bacterial behaviour. This occurs because surface properties affect cellular adhesion and influence cellular metabolism.71 A study conducted by Jana et al72 showed that αFe2O3-ZnO nanocomposites in the nanoscale and microscale had differences in terms of the roughness of the surface morphology. Thus, it was concluded that surface roughness is very important for antibacterial activity. The surface properties of nanomaterials alter interactions with the cell and can interfere with the antimicrobial effect of ZnO NPs.49
Doping of metal oxides is one strategy to improve their photocatalytic properties and can result in changes to the surface area due to the introduction of dopant ions. Thus, the presence of impurities in semiconductor NPs can lead to dramatic changes in their structural, electrical and optical properties. The introduction of dopants during the nanostructure formation process will cause new crystal growth conditions, inducing drastic changes in internal defects and morphology.73–75 In these defects, enhanced oxygen vacancy favours photocatalytic reactions, and therefore a higher amount of ROS is produced.76,77
The presence of dopant ions in ZnO NPs has been used in some studies to assess their relationships with the antimicrobial activity of ZnO NPs.61,78 The presence of these dopant ions can change the size of ZnO NPs.79 This characteristic is important for the antimicrobial effect of NPs. In addition, higher ROS concentrations result in greater antimicrobial activity by ZnO NPs; ROS generation is a major mechanism responsible for ZnO NPs antimicrobial activity. The abovementioned toxicity mechanisms, such as the production of oxygen radicals, Zn2+ ions and the interaction of ZnO NPs with the cell membrane, are affected by surface coatings,80 and these surface characteristics may help in interactions between NPs and the cell wall of bacteria and fungi.81
Based on the relevant information presented in this section, the next items will be discussed in order to show the influence of antimicrobial activity obtained with the introduction of dopants or surface modifiers (with a focus on organosilanes) in ZnO NPs. The discussion about these parameters is very important because ZnO NPs structural changes result in new properties that can enhance the antimicrobial activity of ZnO NPs. Understanding how doping and surface modifications alter the properties of ZnO NPs is already a challenge. However, applying these findings to antimicrobial activity may be an excellent alternative to achieve ZnO NPs-based systems with excellent antimicrobial properties.
Doped ZnO NPs
Sharma et al61 studied the antimicrobial activities of doped ZnO NPs against fungi, Gram-positive and Gram-negative bacteria using standard isolates (S) and clinical isolates (C), by the microdilution method. The researchers used pure doped ZnO NPs with 1% and 10% Fe, Mn, Cu and Co and correlated the synergistic effect of NPs with antimicrobial agents such as ciprofloxacin (Cip), ampicillin (Amp), fluconazole (Flu) and amphotericin B (Amp B). The results showed that there were significant differences in the MIC values for undoped and doped NPs. Better antimicrobial activity was obtained with 10% dopant than for 1% doped and undoped NPs. Synergistic interactions were observed with Cip and Amp for NPs with 10% dopant. For fungi, only an additive effect was found with Flu and Amp B. The presence of dopants can accelerate ROS formation due to the synergistic effect of Mn, Cu, Co and Fe, which improve the photocatalytic activity of ZnO NPs.
Based on this improvement in the activity of ciprofloxacin when associated with ZnO NPs, some researchers assumed that ZnO NPs may interfere with the pumping activity of NorA protein in S. aureus. NorA protein mediates hydrophilic fluoroquinolone efflux to the cell, conferring resistance to the microorganism. Recent reports have suggested that metallic oxide NPs are able to induce faster transfer of electrons to the active sites of enzymes, so ZnO NPs could interfere with the pumping activity of this protein (overexpression of the protein is related to cases of microbial resistance to the action of fluoroquinolones in S. aureus and E. coli).61,82–84 Another explication can be attributed to ZnO NPs relationship with the increased absorption of antibiotics in S. aureus cells, which is mediated by the Omf protein (responsible for the permeation of quinolones through the cell membrane).61,82,85
Some antibiotics are able to form complexes with metal ions, such as Co, Ni and Cu, as shown by Patel et al, who demonstrated the ability of ciprofloxacin to form a complex with chelating agents. In this way, it is possible that the fluorine atom reacts with the Zn atom, stabilising the combination of Cip-ZnO.86
Ravichandran et al87 doped ZnO NPs with Cu and graphene. No change in antibacterial activity was observed with ZnO:Cu. It is important to highlight that the concentration of Cu (less than 1%) was less than that in the studies conducted by Sharma et al.61 Thus, differences in the concentration of a dopant can result in different activities.
Ravichandran et al87 found enhanced antibacterial properties with ZnO:Cu:graphene. The researchers attributed the improved antibacterial activity due to the smaller crystallite size. This was explained because, with a smaller size, the number of ZnO NPs per volume increases, as well as the surface area; this results in greater generation of H2O2.
Some studies have evaluated the effect of silver (Ag) doped ZnO NPs.88,89 Bechambi et al showed that the antibacterial activity of Ag-doped ZnO NPs was better than undoped ones. The authors attributed these increases in antibacterial activity to the presence of Ag that can interact with bacterial cells and adhere to bacterial cell walls. Thus, Ag doping affects the adhesion properties of ZnO and influences its inactivation behaviour. In contrast to this study, Kumar et al88 observed that undoped ZnO NPs had better antibacterial activity than Ag-doped ZnO NPs against S. aureus and E. coli. They discussed this result with the formation of Ag2O and subsequent loss of oxygen vacancies obtained with doped Ag ZnO compared to undoped ZnO, which negatively affected the antibacterial properties.
Research conducted by Rekha et al78 assessed the antibacterial activity of ZnO NPs doped with Mn, using the disk diffusion method. The X-ray diffraction results indicated that Mn2+ ions replaced Zn2+ ions with no change in the wurtzite structure. Mn-doped ZnO NPs exhibited better antibacterial activity than undoped ZnO NPs against Gram-positive and Gram-negative bacteria.
Sehmi et al90 evaluated the bactericidal activity of ZnO, Mg-doped ZnO, and MgO NPs in clinical isolates. These materials had promising antibacterial activity against S. aureus and E. coli. However, Mg-doped ZnO did not show strong antibacterial activity.
Guo and co-workers31 studied the antimicrobial activity of Ta-doped ZnO NPs and showed that the MIC was influenced by the Ta+5 ions incorporated into ZnO; this had a strong influence on the antibacterial effect of ZnO NPs against P. aeruginosa, E. coli, S. aureus and B. subtilis. Possible mechanisms of antibacterial activity were proposed since Ta-doped NPs showed more effective bactericidal efficacy than pure ZnO. This effect was attributed to the synergistic effect of enhanced surface bioactivity and increased electrostatic force due to the incorporation of Ta+.
Hammed et al91 studied the antibacterial activity of undoped ZnO NPs and ZnO NPs doped with neodymium (Nd) against enzymes called extended-spectrum beta-lactamases (ESBL), produced by E. coli and Klebsiella pneumoniae. ESBL confer resistance to many antibiotics such as penicillin and cephalosporin. The results showed that Nd-doped ZnO NPs presented higher antibacterial activity than pure ZnO NPs.
Bomila et al76 synthesised dual doped ZnO NPs with rare earth elements such as cerium (Ce), lanthanum (La) and gadolinium (Gd). There was an increase in ROS achieved by Ce-La dual-doped ZnO NPs compared to NOP doped with the other ions. However, the particle size of this sample was smaller than the other doped ZnO NPs. Additionally, Ce-La ZnO NPs had more lattice defects and higher photocatalytic efficiency. Thus, the researchers attributed these characteristics to the enhancement of antibacterial activity.
ZnO NPs were dual doped with Sn and Cu by Vignesh et al.92 The results showed that dual-doped ZnO NPs induced larger zones of inhibition in antibacterial assays than pure ZnO and Sn:ZnO and Cu:ZnO. The authors suggested that, although the antibacterial activity was attributed the higher amount of ROS produced by dual doped ZnO NPs and the deposition of NPs on the surface of the bacteria, a decrease in crystallite size was also one of the reasons for the enhanced antibacterial activity.
In this section, we showed that doping ZnO NPs may be one way to enhance antibacterial properties due to increases in oxygen vacancies and ROS production. However, not all doping strategies resulted in better activity, even when using the same dopant. This shows that the improvement of activity does not depend solely on the dopant but also on the structural characteristics of ZnO NPs. Therefore, it is important to control the reaction in order to produce doped nanoparticles with large quantities of oxygen vacancies and with small sizes. Doped ZnO NPs with small sizes showed the best antibacterial activity.
Surface Modified ZnO NPs: Focus On Organosilanes
Farouk et al93 studied ZnO containing 3-glycidyloxypropyltrimethoxysilane (GPTMS) as an antibacterial agent for textile industries. The sol-gel method proposed by Spanhel and Anderson,94 with some modifications, was used to produce ZnO NPs and the disc diffusion assay provided information about antibacterial activity. The zone of inhibition was observed, and its diameter was used to measure the antibacterial activity of ZnO NPs with GPTMS. The test was carried out against M. luteus and E. coli. The results showed that cotton samples containing ZnO modified with GPTMS after 5 hrs of treatment had 98.8% less contamination with E. coli and 97.3% less contamination with M. luteus. The polymer alone provided no inhibition, so it can be concluded that the modified ZnO NPs have promising antibacterial activity in textile materials.
Lallo da Silva et al43 studied ZnO NPs modified by GPTMS to investigate the antibacterial activity against E. coli and S. aureus. The ZnO NPs could be dispersed in water and the NPs presented excellent antibacterial activity at low concentrations. For example, NPs at 0.1367 mg/mL had bactericidal activity against S. aureus.
Li et al95 modified ZnO NPs with aminoethylaminopropyl-trimethoxy silane (KH550) and γ-glycidoxypropyl-trimethoxysilane (here, referred to as KH560). Then, modified ZnO were coupled with polyethylene (HDPE) composite films for the evaluation of antibacterial activity. The results showed that HDPE did not present any activity against S. aureus and E. coli. On the other hand, surface modification of ZnO with HDPE improved the antibacterial activity compared to unmodified ZnO-HDPE. KH560-modified ZnO-HDPE had better activity than KH550-modified ZnO-HDPE.
Soumya et al96 modified ZnO NPs with 3-(aminopropyl)trimethoxy silane and then provided them with poly(methyl methacrylate) (PMMA) polymeric coatings for application to cotton textiles. The results showed that the textiles were resistant to the growth of the fungi Aspergillus flavus and Aspergillus niger, and the coating also made the textile less prone to water absorption.
Kamonkhantikul et al97 incorporated PMMA with ZnO NPs modified with methacryloxypropyltrimethoxysilane. The silane-ZnO NPs had greater antifungal activity against C. albicans than unmodified NPs. The difference in the antifungal activity may have been due to the better distribution of silane-ZnO NPs in PMMA compared to unmodified NPs. The higher surface energy present in unmodified NPs may have resulted in a low surface-to-volume ratio and consequently poor dispersion. Hydroxyl groups in the ZnO NPs react with silane groups, which increases the distance between ZnO NPs. In this way, better distribution of ZnO NPs in PMMA was expected. This can maintain particle size; moreover, with a high surface-to-volume ratio, there is increased contact between ZnO NPs and C. albicans, which enhanced the antifungal effect.
Leung et al49 evaluated the antibacterial activity of various silane-based surface modified ZnO NPs in a light environment using the Gram-positive bacteria B. atrophaeus and E. faecium and the Gram-negative bacterium E. coli. According to the results, different surface modifications of ZnO NPs can affect the release of Zn2 + and the production of ROS and thereby affect antibacterial activity. Bacteria died with or without surface modifications. The increase in Zn2 + release was not related to increased activity, but lower Zn2+ concentrations were associated with better antibacterial properties. Silane surface modifiers containing amino groups caused changes in the cell structure, ie, deforming it, which indicated a change in membrane permeability and suggested antibacterial activity.
Cepin et al98 synthesised ZnO nanocrystals and functionalised them with amino and ionic-liquid silane anchoring groups to obtain a hybrid material. Two ionic liquids containing silanes were selected, which contained in their chemical structure one, two or three amino groups. These amino groups have attractive antimicrobial properties, especially those containing quaternary amine since the positive charges are attracted to the negative charges of the bacterial membrane. Studies were performed in relation to the MIC of ZnO nanocrystals without modification. For unmodified ZnO, the results showed that a concentration of 0.125 g/L inhibited 50% of the bacterial growth and a concentration of 0.25 g/L completely inhibited the growth of S. aureus and E. coli. The authors concluded that ZnO nanocrystals with modified surfaces containing ionic liquids and amino groups have excellent antimicrobial activity, inactivating 100% of the bacteria at low concentrations. However, not all changes decreased the MIC values compared to pristine ZnO.
Milović et al99 sought to elucidate issues of the antimicrobial effect and the practical use of antimicrobial coatings. The bacterial resistance developed against polycationic coatings was tested using E. coli and S. aureus. The results showed notable bactericidal action of amino-glass slides obtained by chemical derivatisation with N-hexyl, methyl-polyethylenimine, resulting from the rupture of bacterial cell membranes. Additionally, the rare bacterial survivors did not develop resistance. The authors suggested that they probably survived by avoiding contact with the immobilised polycationic coating.
A study conducted by Arakha et al1 showed modifications to ZnO NPs resulting in two types of surface with opposite potential. A correlation was established between the zeta potential of bacterial cells, the surface charge of the NPs and the antibacterial effect. The results showed that positively charged particles showed higher antimicrobial activity against Gram-positive and Gram-negative bacteria in relation to negatively charged NPs, considering that a repulsive force is established with the bacterial surface that also has negative charges.
Ozkan et al100 studied the bactericidal activity of polydimethylsiloxane (PDMS) coated with crystal violet, which possess antibacterial activity, in the presence of ZnO NPs. This activity was compared with a polymer containing crystal violet without ZnO NPs and only ZnO NPs. The analysis of antibacterial activity was performed using E. coli and S. aureus. The results showed that the developed material had better antibacterial activity than pure ZnO.100
In addition to silanes, ZnO NPs are associated with other compounds to achieve optimised antibacterial activity. In studies performed by Sehmi et al,90 it was demonstrated that oleate-capped ZnO NPs combined with crystal violet were biocompatible and significantly reduced the numbers of S. aureus, possibly due to the oleate ligand itself. Additionally, ZnO capped with di(octyl)phosphinic acid (DOPA) showed excellent antibacterial activity against E. coli without a photosensitiser or white-light activation. However, regarding antibacterial activity against strains of methicillin-resistant S. aureus (MRSA), better antibacterial activity was obtained with ZnO NPs prepared with crystal violet and DOPA. The smaller size of the ZnO-DOPA NPs (∼2–4 nm) compared to that of ZnO_OA NPs (∼18 nm) resulted in better antibacterial activity.
Although there are many methods for modifying the surface of ZnO NPs, the use of organosilanes has become one of the most widely used methods. The major advantage of using silanes is their rapid covalent bond formation, which results in a thin layer on the surface of the ZnO. This covalent bonding is responsible for stabilising the monolayer, making further modification easy without compromising the integrity of the surface film.101
In a study conducted by Soumya et al,96 it was observed that silane-modified ZnO NPs had a very narrow size distribution with an average particle size of 220 nm. In contrast, without modification, the size distribution was bimodal, and in this case, the particles were in the range of 255–970 nm. The wide size distribution clearly indicated that, in the absence of silane, ZnO nanoaggregates were obtained.
In summary, this section has shown that different silanes can be used to modify ZnO NPs. Although there are many strategies for performing ZnO surface modification, the use of silanes has many advantages. In addition to the rapid reaction, there are many silanes on the market that allow nanoparticles to be dispersed in hydrophilic or hydrophobic media, improving their stabilisation. However, the main advantage of using these silanes is the size control of the formed NPs. For example, GPTMS-modified ZnO NPs showed greater resistance to growth when subjected to high temperatures compared to unmodified NPs.43 This control of nanoparticle growth coupled with a better size distribution results in a system with better antimicrobial properties, enabling the formation of nanoparticles with optimised antimicrobial properties, since obtaining small NPs is one of the requirements for improving antimicrobial activity.
Conclusion
The lack of a standard in relation to the microbial strains used in these studies and the methodologies for microbiological testing are sources of uncertainty. However, in this review, it was possible to see that the most important mechanism to explain the antimicrobial activity of ZnO NPs is ROS production.
Some researchers have shown that the different physical and chemical parameters can change the antimicrobial activity of ZnO NPs; the most relevant parameter found in our review regarding antimicrobial activity was the size of ZnO NPs. The importance of size as the most relevant parameter is supported by the same MIC values found in studies performed by different researchers using nanoparticles of the same size. In addition, when studies compared nanoparticles with different morphologies, generally those that were more toxic to microorganisms were also smaller.
The use of dopants can increase the activity of ZnO NPs. As the proportion of dopants increases, NPs tend to decrease in size. In addition, in general, larger amounts of ROS are produced, which results in better activity. Surface modifications of ZnO NPs, in general, lead to improved antimicrobial activity, reducing the MIC of these NPs against the tested microorganisms. This happens due to the improved size distribution and growth resistance of ZnO NPs. These findings once again support the need for nanoparticle size control to achieve optimal antimicrobial activity.
The limitations and future outlook consist of better understanding the antibacterial activity of ZnO NPs, which is a challenge because studies continually reveal new mechanisms, which shows that not all existing mechanisms are understood and there is a need to better explore this area. The lack of a standard in relation to the microbial strains used in these studies and the methodologies for microbiological tests has led to conflicting results. There are many studies on ROS toxicity, but only the intracellular inhibitory mechanisms and protein synthesis, gene expression and metabolism of bacterial cells. Thus, as future perspectives, it is important that further research be conducted in this regard.
Acknowledgments
The authors thank São Paulo Research Foundation (FAPESP), Coordination for the Improvement of Higher Education (CAPES) – Finance Code 001. We also thank the Scientific Support and Development Program of School of Pharmaceutical Sciences (PADC- UNESP).
Disclosure
The authors have declared no conflict of interest in this work.
References
- 1.Arakha M, Saleem M, Mallick BC, Jha S. The effects of interfacial potential on antimicrobial propensity of ZnO nanoparticle. Sci Rep. 2015;5. doi: 10.1038/srep09578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Padmavathy N, Vijayaraghavan R. Enhanced bioactivity of ZnO nanoparticles—an antimicrobial study. Sci Technol Adv Mater. 2016;9:035004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Derewacz DK, Goodwin CR, McNees CR, McLean JA, Bachmann BO. Antimicrobial drug resistance affects broad changes in metabolomic phenotype in addition to secondary metabolism. Proc Natl Acad Sci. 2013;110(6):2336–2341. doi: 10.1073/pnas.1218524110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.von Wintersdorff CJH, Penders J, van Niekerk JM, et al. Dissemination of antimicrobial resistance in microbial ecosystems through horizontal gene transfer. Front Microbiol. 2016;7. doi: 10.3389/fmicb.2016.00173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carrillo-Munoz AJ, Giusiano G, Ezkurra PA, Quindós G. Antifungal agents: mode of action in yeast cells. Rev Esp Quimioter. 2006;19(2):130–139. [PubMed] [Google Scholar]
- 6.Landini P, Antoniani D, Burgess JG, Nijland R. Molecular mechanisms of compounds affecting bacterial biofilm formation and dispersal. Appl Microbiol Biotechnol. 2010;86(3):813–823. doi: 10.1007/s00253-010-2468-8 [DOI] [PubMed] [Google Scholar]
- 7.Hooper DC. Mechanisms of action of antimicrobials: focus on fluoroquinolones. Clin Infect Dis. 2001;32(Supplement 1):S9–S15. doi: 10.1086/319370 [DOI] [PubMed] [Google Scholar]
- 8.Wang L, Hu C, Shao L. The antimicrobial activity of nanoparticles: present situation and prospects for the future. Int J Nanomedicine. 2017;12:1227. doi: 10.2147/IJN.S121956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rai M. Nanobiotecnologia verde: biossínteses de nanopartículas metálicas e suas aplicações como nanoantimicrobianos. Ciência E Cultura. 2013;65(3):44–48. doi: 10.21800/S0009-67252013000300014 [DOI] [Google Scholar]
- 10.Durairaj B, Muthu S, Xavier T. Antimicrobial activity of Aspergillus niger synthesized titanium dioxide nanoparticles. Adv Appl Sci Res. 2015;6:45–48. [Google Scholar]
- 11.Firouzabadi FB, Noori M, Edalatpanah Y, Mirhosseini M. ZnO nanoparticle suspensions containing citric acid as antimicrobial to control Listeria monocytogenes, Escherichia coli, Staphylococcus aureus and Bacillus cereus in mango juice. Food Control. 2014;42:310–314. doi: 10.1016/j.foodcont.2014.02.012 [DOI] [Google Scholar]
- 12.Raghupathi KR, Koodali RT, Manna AC. Size-dependent bacterial growth inhibition and mechanism of antibacterial activity of zinc oxide nanoparticles. Langmuir. 2011;27(7):4020–4028. doi: 10.1021/la104825u [DOI] [PubMed] [Google Scholar]
- 13.Manoharan C, Pavithra G, Dhanapandian S, Dhamodaran P, Shanthi B. Properties of spray pyrolised ZnO: Sn thin films and their antibacterial activity. Spectrochim Acta Part A. 2015;141:292–299. doi: 10.1016/j.saa.2015.01.051 [DOI] [PubMed] [Google Scholar]
- 14.Liu Y, He L, Mustapha A, Li H, Hu ZQ, Lin M. Antibacterial activities of zinc oxide nanoparticles against Escherichia coli O157: H7. J Appl Microbiol. 2009;107(4):1193–1201. doi: 10.1111/j.1365-2672.2009.04303.x [DOI] [PubMed] [Google Scholar]
- 15.Janaki AC, Sailatha E, Gunasekaran S. Synthesis, characteristics and antimicrobial activity of ZnO nanoparticles. Spectrochim Acta Part A. 2015;144:17–22. doi: 10.1016/j.saa.2015.02.041 [DOI] [PubMed] [Google Scholar]
- 16.Pasquet J, Chevalier Y, Couval E, Bouvier D, Bolzinger M-A. Zinc oxide as a new antimicrobial preservative of topical products: interactions with common formulation ingredients. Int J Pharm. 2015;479(1):88–95. doi: 10.1016/j.ijpharm.2014.12.031 [DOI] [PubMed] [Google Scholar]
- 17.Sirelkhatim A, Mahmud S, Seeni A, et al. Review on zinc oxide nanoparticles: antibacterial activity and toxicity mechanism. Nano-Micro Lett. 2015;7(3):219–242. doi: 10.1007/s40820-015-0040-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zheng MJ, Zhang LD, Li GH, Shen WZ. Fabrication and optical properties of large-scale uniform zinc oxide nanowire arrays by one-step electrochemical deposition technique. Chem Phys Lett. 2002;363(1):123–128. doi: 10.1016/S0009-2614(02)01106-5 [DOI] [Google Scholar]
- 19.Jagadish C, Pearton SJ. Zinc Oxide Bulk, Thin Films and Nanostructures: Processing, Properties, and Applications. Elsevier; 2011. [Google Scholar]
- 20.Talam S, Karumuri SR, Gunnam N. Synthesis, characterization, and spectroscopic properties of ZnO nanoparticles. ISRN Nanotechnol. 2012;2012. doi: 10.5402/2012/372505 [DOI] [Google Scholar]
- 21.Ahmad M, Zhu J. ZnO based advanced functional nanostructures: synthesis, properties and applications. J Mater Chem. 2011;21(3):599–614. doi: 10.1039/C0JM01645D [DOI] [Google Scholar]
- 22.Rana S, Rawat J, Sorensson MM, Misra RDK. Antimicrobial function of Nd 3+-doped anatase titania-coated nickel ferrite composite nanoparticles: a biomaterial system. Acta Biomater. 2006;2(4):421–432. doi: 10.1016/j.actbio.2006.03.005 [DOI] [PubMed] [Google Scholar]
- 23.Tiwari V, Mishra N, Gadani K, Solanki PS, Shah N, Tiwari M. Mechanism of anti-bacterial activity of zinc oxide nanoparticle against carbapenem resistant Acinetobacter baumannii. Front Microbiol. 2018;9:1218. doi: 10.3389/fmicb.2018.01218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alves MM, Bouchami O, Tavares A, et al. New insights into antibiofilm effect of a nanosized ZnO coating against the pathogenic methicillin resistant Staphylococcus aureus. ACS Appl Mater Interfaces. 2017;9(34):28157–28167. doi: 10.1021/acsami.7b02320 [DOI] [PubMed] [Google Scholar]
- 25.Hajipour MJ, Fromm KM, Ashkarran AA, et al. Antibacterial properties of nanoparticles. Trends Biotechnol. 2012;30(10):499–511. doi: 10.1016/j.tibtech.2012.06.004 [DOI] [PubMed] [Google Scholar]
- 26.Pasquet J, Chevalier Y, Couval E, et al. Antimicrobial activity of zinc oxide particles on five micro-organisms of the challenge tests related to their physicochemical properties. Int J Pharm. 2014;460(1):92–100. doi: 10.1016/j.ijpharm.2013.10.031 [DOI] [PubMed] [Google Scholar]
- 27.Baek Y-W, An Y-J. Microbial toxicity of metal oxide nanoparticles (CuO, NiO, ZnO, and Sb 2 O 3) to Escherichia coli, Bacillus subtilis, and Streptococcus aureus. Sci Total Environ. 2011;409(8):1603–1608. doi: 10.1016/j.scitotenv.2011.01.014 [DOI] [PubMed] [Google Scholar]
- 28.Shoeb M, Singh BR, Khan JA, et al. ROS-dependent anticandidal activity of zinc oxide nanoparticles synthesized by using egg albumen as a biotemplate. Adv Nat Sci. 2013;4(3):035015. [Google Scholar]
- 29.Applerot G, Lellouche J, Perkas N, Nitzan Y, Gedanken A, Banin E. ZnO nanoparticle-coated surfaces inhibit bacterial biofilm formation and increase antibiotic susceptibility. RSC Adv. 2012;2(6):2314–2321. doi: 10.1039/c2ra00602b [DOI] [Google Scholar]
- 30.Sawai J, Shoji S, Igarashi H, et al. Hydrogen peroxide as an antibacterial factor in zinc oxide powder slurry. J Ferment Bioeng. 1998;86(5):521–522. doi: 10.1016/S0922-338X(98)80165-7 [DOI] [Google Scholar]
- 31.Guo B-L, Han P, Guo L-C, et al. The antibacterial activity of Ta-doped ZnO nanoparticles. Nanoscale Res Lett. 2015;10(1):1–10. doi: 10.1186/1556-276X-10-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brayner R, Ferrari-Iliou R, Brivois N, Djediat S, Benedetti MF, Fiévet F. Toxicological impact studies based on Escherichia coli bacteria in ultrafine ZnO nanoparticles colloidal medium. Nano Lett. 2006;6(4):866–870. doi: 10.1021/nl052326h [DOI] [PubMed] [Google Scholar]
- 33.Zhang L, Jiang Y, Ding Y, Povey M, York D. Investigation into the antibacterial behaviour of suspensions of ZnO nanoparticles (ZnO nanofluids). J Nanopart Res. 2007;9(3):479–489. doi: 10.1007/s11051-006-9150-1 [DOI] [Google Scholar]
- 34.Brunner TJ, Wick P, Manser P, et al. In vitro cytotoxicity of oxide nanoparticles: comparison to asbestos, silica, and the effect of particle solubility. Environ Sci Technol. 2006;40(14):4374–4381. doi: 10.1021/es052069i [DOI] [PubMed] [Google Scholar]
- 35.Li M, Zhu L, Lin D. Toxicity of ZnO nanoparticles to Escherichia coli: mechanism and the influence of medium components. Environ Sci Technol. 2011;45(5):1977–1983. doi: 10.1021/es102624t [DOI] [PubMed] [Google Scholar]
- 36.Seven O, Dindar B, Aydemir S, Metin D, Ozinel MA, Icli S. Solar photocatalytic disinfection of a group of bacteria and fungi aqueous suspensions with TiO 2, ZnO and Sahara desert dust. J Photochem Photobiol A. 2004;165(1):103–107. doi: 10.1016/j.jphotochem.2004.03.005 [DOI] [Google Scholar]
- 37.Adams LK, Lyon DY, Alvarez PJJ. Comparative eco-toxicity of nanoscale TiO 2, SiO 2, and ZnO water suspensions. Water Res. 2006;40(19):3527–3532. doi: 10.1016/j.watres.2006.08.004 [DOI] [PubMed] [Google Scholar]
- 38.Hirota K, Sugimoto M, Kato M, Tsukagoshi K, Tanigawa T, Sugimoto H. Preparation of zinc oxide ceramics with a sustainable antibacterial activity under dark conditions. Ceram Int. 2010;36(2):497–506. doi: 10.1016/j.ceramint.2009.09.026 [DOI] [Google Scholar]
- 39.d’Água RB, Branquinho R, Duarte MP, et al. Efficient coverage of ZnO nanoparticles on cotton fibres for antibacterial finishing using a rapid and low cost in situ synthesis. New J Chem. 2018;42(2):1052–1060. doi: 10.1039/C7NJ03418K [DOI] [Google Scholar]
- 40.Kadiyala U, Turali-Emre ES, Bahng JH, Kotov NA, VanEpps JS. Unexpected insights into antibacterial activity of zinc oxide nanoparticles against methicillin resistant Staphylococcus aureus (MRSA). Nanoscale. 2018;10(10):4927–4939. doi: 10.1039/c7nr08499d [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cho W-S, Duffin R, Howie SEM, et al. Progressive severe lung injury by zinc oxide nanoparticles; the role of Zn 2+ dissolution inside lysosomes. Part Fibre Toxicol. 2011;8(1):27. doi: 10.1186/1743-8977-8-27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li Y, Zhang W, Niu J, Chen Y. Mechanism of photogenerated reactive oxygen species and correlation with the antibacterial properties of engineered metal-oxide nanoparticles. ACS Nano. 2012;6(6):5164–5173. doi: 10.1021/nn300934k [DOI] [PubMed] [Google Scholar]
- 43.Lallo da Silva B, Caetano BL, Chiari-Andréo BG, Pietro RCLR, Chiavacci LA. Increased antibacterial activity of ZnO nanoparticles: influence of size and surface modification. Colloids Surf B. 2019;177:440–447. doi: 10.1016/j.colsurfb.2019.02.013 [DOI] [PubMed] [Google Scholar]
- 44.Heinlaan M, Ivask A, Blinova I, Dubourguier H-C, Kahru A. Toxicity of nanosized and bulk ZnO, CuO and TiO 2 to bacteria Vibrio fischeri and crustaceans Daphnia magna and Thamnocephalus platyurus. Chemosphere. 2008;71(7):1308–1316. doi: 10.1016/j.chemosphere.2007.11.047 [DOI] [PubMed] [Google Scholar]
- 45.He L, Liu Y, Mustapha A, Lin M. Antifungal activity of zinc oxide nanoparticles against Botrytis cinerea and Penicillium expansum. Microbiol Res. 2011;166(3):207–215. doi: 10.1016/j.micres.2010.03.003 [DOI] [PubMed] [Google Scholar]
- 46.Lipovsky A, Nitzan Y, Gedanken A, Lubart R. Antifungal activity of ZnO nanoparticles—the role of ROS mediated cell injury. Nanotechnology. 2011;22(10):105101. doi: 10.1088/0957-4484/22/10/105101 [DOI] [PubMed] [Google Scholar]
- 47.Eskandari M, Haghighi N, Ahmadi V, Haghighi F, Mohammadi SR. Growth and investigation of antifungal properties of ZnO nanorod arrays on the glass. Physica B. 2011;406(1):112–114. doi: 10.1016/j.physb.2010.10.035 [DOI] [Google Scholar]
- 48.Kairyte K, Kadys A, Luksiene Z. Antibacterial and antifungal activity of photoactivated ZnO nanoparticles in suspension. J Photochem Photobiol B. 2013;128:78–84. doi: 10.1016/j.jphotobiol.2013.07.017 [DOI] [PubMed] [Google Scholar]
- 49.Leung YH, Chan CMN, Ng AMC, et al. Antibacterial activity of ZnO nanoparticles with a modified surface under ambient illumination. Nanotechnology. 2012;23(47):475703. doi: 10.1088/0957-4484/23/47/475703 [DOI] [PubMed] [Google Scholar]
- 50.Shinde VV, Dalavi DS, Mali SS, Hong CK, Kim JH, Patil PS. Surfactant free microwave assisted synthesis of ZnO microspheres: study of their antibacterial activity. Appl Surf Sci. 2014;307:495–502. doi: 10.1016/j.apsusc.2014.04.064 [DOI] [Google Scholar]
- 51.Scott JR, Barnett TC. Surface proteins of gram-positive bacteria and how they get there. Annu Rev Microbiol. 2006;60:397–423. doi: 10.1146/annurev.micro.60.080805.142256 [DOI] [PubMed] [Google Scholar]
- 52.Cabeen MT, Jacobs-Wagner C. Bacterial cell shape. Nat Rev Microbiol. 2005;3(8):601–610. doi: 10.1038/nrmicro1205 [DOI] [PubMed] [Google Scholar]
- 53.Tayel AA, El‐Tras WF, Moussa S, et al. Antibacterial action of zinc oxide nanoparticles against foodborne pathogens. J Food Saf. 2011;31(2):211–218. doi: 10.1111/jfs.2011.31.issue-2 [DOI] [Google Scholar]
- 54.Reddy KM, Feris K, Bell J, Wingett DG, Hanley C, Punnoose A. Selective toxicity of zinc oxide nanoparticles to prokaryotic and eukaryotic systems. Appl Phys Lett. 2007;90(21):213902. doi: 10.1063/1.2742324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Clauditz A, Resch A, Wieland K-P, Peschel A, Götz F. Staphyloxanthin plays a role in the fitness of Staphylococcus aureus and its ability to cope with oxidative stress. Infect Immun. 2006;74(8):4950–4953. doi: 10.1128/IAI.00204-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Brown MRW, Allison DG, Gilbert P. Resistance of bacterial biofilms to antibiotics a growth-rate related effect? J Antimicrobial Chemother. 1988;22(6):777–780. doi: 10.1093/jac/22.6.777 [DOI] [PubMed] [Google Scholar]
- 57.Talebian N, Amininezhad SM, Doudi M. Controllable synthesis of ZnO nanoparticles and their morphology-dependent antibacterial and optical properties. J Photochem Photobiol B. 2013;120:66–73. doi: 10.1016/j.jphotobiol.2013.01.004 [DOI] [PubMed] [Google Scholar]
- 58.Stanković A, Dimitrijević S, Uskoković D. Influence of size scale and morphology on antibacterial properties of ZnO powders hydrothemally synthesized using different surface stabilizing agents. Colloids Surf B. 2013;102:21–28. doi: 10.1016/j.colsurfb.2012.07.033 [DOI] [PubMed] [Google Scholar]
- 59.Dutta RK, Sharma PK, Bhargava R, Kumar N, Pandey AC. Differential susceptibility of Escherichia coli cells toward transition metal-doped and matrix-embedded ZnO nanoparticles. J Phys Chem B. 2010;114(16):5594–5599. doi: 10.1021/jp1004488 [DOI] [PubMed] [Google Scholar]
- 60.Jones N, Ray B, Ranjit KT, Manna AC. Antibacterial activity of ZnO nanoparticle suspensions on a broad spectrum of microorganisms. FEMS Microbiol Lett. 2008;279(1):71–76. doi: 10.1111/j.1574-6968.2007.01012.x [DOI] [PubMed] [Google Scholar]
- 61.Sharma N, Jandaik S, Kumar S. Synergistic activity of doped zinc oxide nanoparticles with antibiotics: ciprofloxacin, ampicillin, fluconazole and amphotericin B against pathogenic microorganisms. Anais Da Academia Brasileira De Ciências. 2016;88:1689–1698. doi: 10.1590/0001-3765201620150713 [DOI] [PubMed] [Google Scholar]
- 62.Zhang L, Yin L, Wang C, Lun N, Qi Y. Sol− Gel Growth of hexagonal faceted ZnO prism quantum dots with polar surfaces for enhanced photocatalytic activity. ACS Appl Mater Interfaces. 2010;2(6):1769–1773. doi: 10.1021/am100274d [DOI] [PubMed] [Google Scholar]
- 63.Tuomela S, Autio R, Buerki-Thurnherr T, et al. Gene expression profiling of immune-competent human cells exposed to engineered zinc oxide or titanium dioxide nanoparticles. PLoS One. 2013;8(7):e68415. doi: 10.1371/journal.pone.0068415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wong SWY, Leung PTY, Djurišić AB, Leung KMY. Toxicities of nano zinc oxide to five marine organisms: influences of aggregate size and ion solubility. Anal Bioanal Chem. 2010;396(2):609–618. doi: 10.1007/s00216-009-3249-z [DOI] [PubMed] [Google Scholar]
- 65.Pasquet J, Chevalier Y, Pelletier J, Couval E, Bouvier D, Bolzinger M-A. The contribution of zinc ions to the antimicrobial activity of zinc oxide. Colloids Surf A. 2014;457:263–274. doi: 10.1016/j.colsurfa.2014.05.057 [DOI] [Google Scholar]
- 66.Ghasemi Y, Peymani P, Afifi S. Quantum dot: magic nanoparticle for imaging, detection and targeting. Acta Bio Medica. 2009;80(2):156–165. [PubMed] [Google Scholar]
- 67.Aditya A, Chattopadhyay S, Jha D, Gautam HK, Maiti S, Ganguli M. Zinc oxide nanoparticles dispersed in ionic liquids show high antimicrobial efficacy to skin-specific bacteria. ACS Appl Mater Interfaces. 2018;10(18):15401–15411. doi: 10.1021/acsami.8b01463 [DOI] [PubMed] [Google Scholar]
- 68.Chen Q, Yakovlev NL. Adsorption and interaction of organosilanes on TiO 2 nanoparticles. Appl Surf Sci. 2010;257(5):1395–1400. doi: 10.1016/j.apsusc.2010.08.036 [DOI] [Google Scholar]
- 69.Aboulaich A, Tilmaciu C-M, Merlin C, et al. Physicochemical properties and cellular toxicity of (poly) aminoalkoxysilanes-functionalized ZnO quantum dots. Nanotechnology. 2012;23(33):335101. doi: 10.1088/0957-4484/23/33/335101 [DOI] [PubMed] [Google Scholar]
- 70.Jana NR, Earhart C, Ying JY. Synthesis of water-soluble and functionalized nanoparticles by silica coating. Chem Mater. 2007;19(21):5074–5082. doi: 10.1021/cm071368z [DOI] [Google Scholar]
- 71.Mitik-Dineva N, Wang J, Truong VK, et al. Escherichia coli, Pseudomonas aeruginosa, and Staphylococcus aureus attachment patterns on glass surfaces with nanoscale roughness. Curr Microbiol. 2009;58(3):268–273. doi: 10.1007/s00284-008-9320-8 [DOI] [PubMed] [Google Scholar]
- 72.Jana TK, Jana SK, Kumar A, et al. The antibacterial and anticancer properties of zinc oxide coated iron oxide nanotextured composites. Colloids Surf B. 2019;177:512–519. doi: 10.1016/j.colsurfb.2019.02.041 [DOI] [PubMed] [Google Scholar]
- 73.Kumar RS, Sathyamoorthy R, Sudhagar P, Matheswaran P, Hrudhya CP, Kang YS. Effect of aluminum doping on the structural and luminescent properties of ZnO nanoparticles synthesized by wet chemical method. Physica E. 2011;43(6):1166–1170. doi: 10.1016/j.physe.2011.01.022 [DOI] [Google Scholar]
- 74.Brehm JU, Winterer M, Hahn H. Synthesis and local structure of doped nanocrystalline zinc oxides. J Appl Phys. 2006;100(6):064311. doi: 10.1063/1.2349430 [DOI] [Google Scholar]
- 75.Liu Y, Lian J. Optical and electrical properties of aluminum-doped ZnO thin films grown by pulsed laser deposition. Appl Surf Sci. 2007;253(7):3727–3730. doi: 10.1016/j.apsusc.2006.08.012 [DOI] [Google Scholar]
- 76.Bomila R, Suresh S, Srinivasan S. Synthesis, characterization and comparative studies of dual doped ZnO nanoparticles for photocatalytic applications. J Mater Sci. 2019;30(1):582–592. [Google Scholar]
- 77.Bomila R, Srinivasan S, Gunasekaran S, Manikandan A. Enhanced photocatalytic degradation of methylene blue dye, opto-magnetic and antibacterial behaviour of pure and La-doped ZnO nanoparticles. J Supercond Nov Magn. 2018;31(3):855–864. doi: 10.1007/s10948-017-4261-8 [DOI] [Google Scholar]
- 78.Rekha K, Nirmala M, Nair MG, Anukaliani A. Structural, optical, photocatalytic and antibacterial activity of zinc oxide and manganese doped zinc oxide nanoparticles. Physica B. 2010;405(15):3180–3185. doi: 10.1016/j.physb.2010.04.042 [DOI] [Google Scholar]
- 79.Liu Y, Ai K, Yuan Q, Lu L. Fluorescence-enhanced gadolinium-doped zinc oxide quantum dots for magnetic resonance and fluorescence imaging. Biomaterials. 2011;32(4):1185–1192. doi: 10.1016/j.biomaterials.2010.10.022 [DOI] [PubMed] [Google Scholar]
- 80.Hsu A, Liu F, Leung YH, et al. Is the effect of surface modifying molecules on antibacterial activity universal for a given material? Nanoscale. 2014;6(17):10323–10331. doi: 10.1039/c4nr02366h [DOI] [PubMed] [Google Scholar]
- 81.Fakhroueian Z, Dehshiri AM, Katouzian F, Esmaeilzadeh P. In vitro cytotoxic effects of modified zinc oxide quantum dots on breast cancer cell lines (MCF7), colon cancer cell lines (HT29) and various fungi. J Nanopart Res. 2014;16(7):1–14. doi: 10.1007/s11051-014-2483-2 [DOI] [Google Scholar]
- 82.Banoee M, Seif S, Nazari ZE, et al. ZnO nanoparticles enhanced antibacterial activity of ciprofloxacin against Staphylococcus aureus and Escherichia coli. J Biomed Mater Res Part B. 2010;93(2):557–561. doi: 10.1002/jbm.b.31615 [DOI] [PubMed] [Google Scholar]
- 83.Yu J-L, Grinius L, Hooper DC. NorA functions as a multidrug efflux protein in both cytoplasmic membrane vesicles and reconstituted proteoliposomes. J Bacteriol. 2002;184(5):1370–1377. doi: 10.1128/jb.184.5.1370-1377.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Neyfakh AA, Borsch CM, Kaatz GW. Fluoroquinolone resistance protein NorA of Staphylococcus aureus is a multidrug efflux transporter. Antimicrob Agents Chemother. 1993;37(1):128–129. doi: 10.1128/aac.37.1.128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sikong L, Kongreong B, Kantachote D, Sutthisripok W. Photocatalytic activity and antibacterial behavior of Fe3+-doped TiO2/SnO2 nanoparticles. Energy Res J. 2010;1(2):120–125. doi: 10.3844/erjsp.2010.120.125 [DOI] [Google Scholar]
- 86.Patel MN, Chhasatia MR, Gandhi DS. DNA-interaction and in vitro antimicrobial studies of some mixed-ligand complexes of cobalt (II) with fluoroquinolone antibacterial agent ciprofloxacin and some neutral bidentate ligands. Bioorg Med Chem Lett. 2009;19(10):2870–2873. doi: 10.1016/j.bmcl.2009.03.078 [DOI] [PubMed] [Google Scholar]
- 87.Ravichandran K, Chidhambaram N, Gobalakrishnan S. Copper and Graphene activated ZnO nanopowders for enhanced photocatalytic and antibacterial activities. J Phys Chem Solids. 2016;93:82–90. doi: 10.1016/j.jpcs.2016.02.013 [DOI] [Google Scholar]
- 88.Kumar V, Prakash J, Singh JP, et al. Role of silver doping on the defects related photoluminescence and antibacterial behaviour of zinc oxide nanoparticles. Colloids Surf B. 2017;159:191–199. doi: 10.1016/j.colsurfb.2017.07.071 [DOI] [PubMed] [Google Scholar]
- 89.Bechambi O, Chalbi M, Najjar W, Sayadi S. Photocatalytic activity of ZnO doped with Ag on the degradation of endocrine disrupting under UV irradiation and the investigation of its antibacterial activity. Appl Surf Sci. 2015;347:414–420. doi: 10.1016/j.apsusc.2015.03.049 [DOI] [Google Scholar]
- 90.Sehmi SK, Noimark S, Pike SD, et al. Enhancing the antibacterial activity of light-activated surfaces containing crystal violet and ZnO nanoparticles: investigation of nanoparticle size, capping ligand, and dopants. ACS Omega. 2016;1(3):334–343. doi: 10.1021/acsomega.6b00017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hameed ASH, Karthikeyan C, Ahamed AP, et al. In vitro antibacterial activity of ZnO and Nd doped ZnO nanoparticles against ESBL producing Escherichia coli and Klebsiella pneumoniae. Sci Rep. 2016;6:24312. doi: 10.1038/srep24312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Shanmugam V, Jeyaperumal KS. Investigations of visible light driven Sn and Cu doped ZnO hybrid nanoparticles for photocatalytic performance and antibacterial activity. Appl Surf Sci. 2018;449:617–630. doi: 10.1016/j.apsusc.2017.11.167 [DOI] [Google Scholar]
- 93.Farouk A, Moussa S, Ulbricht M, Schollmeyer E, Textor T. ZnO-modified hybrid polymers as an antibacterial finish for textiles. Textile Res J. 2014;84(1):40–51. doi: 10.1177/0040517513485623 [DOI] [Google Scholar]
- 94.Spanhel L, Anderson MA. Semiconductor clusters in the sol-gel process: quantized aggregation, gelation, and crystal growth in concentrated zinc oxide colloids. J Am Chem Soc. 1991;113(8):2826–2833. doi: 10.1021/ja00008a004 [DOI] [Google Scholar]
- 95.Li YN, Xu WM, Zhang GQ. Effect of Coupling Agent on Nano-Zno Modification and Antibacterial Activity of Zno/HDPE Nanocomposite Films. IOP Publishing; 2015:012054. [Google Scholar]
- 96.Soumya S, Kumar SN, Mohamed AP, Ananthakumar S. Silanated nano ZnO hybrid embedded PMMA polymer coatings on cotton fabrics for near-IR reflective, antifungal cool-textiles. New J Chem. 2016;40(8):7210–7221. doi: 10.1039/C6NJ00353B [DOI] [Google Scholar]
- 97.Kamonkhantikul K, Arksornnukit M, Takahashi H. Antifungal, optical, and mechanical properties of polymethylmethacrylate material incorporated with silanized zinc oxide nanoparticles. Int J Nanomedicine. 2017;12:2353. doi: 10.2147/IJN.S132116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Čepin M, Jovanovski V, Podlogar M, Orel ZC. Amino-and ionic liquid-functionalised nanocrystalline ZnO via silane anchoring–an antimicrobial synergy. J Mater Chem B. 2015;3(6):1059–1067. doi: 10.1039/C4TB01300J [DOI] [PubMed] [Google Scholar]
- 99.Milović NM, Wang J, Lewis K, Klibanov AM. Immobilized N‐alkylated polyethylenimine avidly kills bacteria by rupturing cell membranes with no resistance developed. Biotechnol Bioeng. 2005;90(6):715–722. doi: 10.1002/bit.20454 [DOI] [PubMed] [Google Scholar]
- 100.Ozkan E, Ozkan FT, Allan E, Parkin IP. The use of zinc oxide nanoparticles to enhance the antibacterial properties of light-activated polydimethylsiloxane containing crystal violet. RSC Adv. 2015;5(12):8806–8813. doi: 10.1039/C4RA13649G [DOI] [Google Scholar]
- 101.Jaramillo AF, Baez-Cruz R, Montoya LF, et al. Estimation of the surface interaction mechanism of ZnO nanoparticles modified with organosilane groups by Raman spectroscopy. Ceram Int. 2017;43(15):11838–11847. doi: 10.1016/j.ceramint.2017.06.027 [DOI] [Google Scholar]