Abstract
Background
The dysregulation of microRNAs (miRNAs) has been linked with male infertility. miR-509-5p is highly expressed in testis and exerts suppressive effects on multiple types of human cancers.
Objectives
Yet, whether miR-509-5p is connected with male infertility and plays a role in testicular germ cell tumor (TGCT) have not been explored.
Materials and methods
This study detected miR-509-5p expression in germ cells from MA patients, and further characterize its functional roles in the proliferation and apoptosis of TGCT cells in vitro.
Results
We report that miR-509-5p is downregulated in germ cells from infertile men with maturation arrest (MA), which implies an inverse association between miR-509-5p level and male infertility. In addition, miR-509-5p suppresses proliferation and induces apoptosis of TGCT cells in vitro, suggesting that it exhibits tumor-suppressive effects on TGCT. Mechanistically, miR-509-5p targets the mouse double minute 2 (MDM2), an oncogenic factor in TGCT, and moreover, restored expression of MDM2 rescues miR-509-5p suppressive effects on TGCT cells, demonstrating that miR-509-5p suppresses TGCT cells through targeting MDM2.
Conclusion
Collectively, these results implicate that miR-509-5p may participate in the pathogenesis of male infertility and TGCT through regulating proliferation and apoptosis, two critical cellular activities for spermatogenesis and TGCT tumorigenesis.
Keywords: male infertility, testicular germ cell tumor, miR-509-5p, proliferation, apoptosis, MDM2
Introduction
Infertility is a common reproductive problem, and it has been estimated that 60–80 million couples are currently suffering from it, accounting for 10–15% couples worldwide.1 Among them, approximately 50% of cases are caused by male factors, with most of which diagnosed to be idiopathic, although the underlying etiological mechanisms of male infertility disorder are still far from clear.2 The nonobstructive azoospermia (NOA) is characterized by the absence of sperm resulting from abnormal progression of spermatogenesis, and represents 10–20% of male infertility and remains the most challenging infertility to treat.3 Particularly, maturation arrest (MA) is a common histopathological subtype of NOA and leads to severe male infertility and limited pregnancy rate.4 Until now, the comprehensive investigations on MA are still scarce and the causative factors leading to it are very obscure. To improve the clinical outcome of MA, its physiopathology needs to be elucidated at a molecular level for pursuing the therapeutic targets.
Spermatogenesis is an elaborated multistep programme dictating the differentiation of spermatogonial stem cells into spermatozoa throughout the reproductive period of adult males.5 Spermatogenesis consists of stages of mitotic proliferating spermatogonia, meiotic spermatocytes, and haploid-differentiated spermatids, and a dynamic balance between cell proliferation and apoptosis is required for maintaining the number of spermatozoa.6 Accordingly, abnormally increased apoptosis of spermatozoa and decreased mitotic activity of spermatogonia have been linked with male infertility.7–9 The dysregulated apoptosis and proliferation also contribute to the pathogenesis of testicular germ cell tumor (TGCT), which represent the leading malignancies in 15- to −35-year-old males.10,11
In addition, spermatogenesis process is orchestrated by a complicated network of transcriptional and post-transcriptional regulation.12 In recent years, the microRNAs (miRNAs)-regulated gene expression has emerged to be important factor for spermatogenesis and male infertility.13,14 miRNAs are a class of small non-coding RNAs regulating the targeted mRNA molecules through binding with the 3ʹ-untranslated region (3ʹ-UTR) sequence.15 The abnormal expression of some miRNAs is present in male infertility, which holds the potential to serve as molecular biomarkers for disease diagnosis, such as miR-34c-5p, miR-122, miR-I9b, and let-7a.16 In fact, miRNAs control every step of spermatogenesis.14 For instance, the depletion of male germ cell-specific Dicer1, an enzyme producing miRNAs,17 causes infertility as a result of meiotic and spermiogenic defects.18 Although a few specific miRNAs, such as miR-38319 and miR-320a,20 have been associated with male infertility, our understanding of the role of miRNAs in male infertility is still very limited.
Previous studies have shown that miR-509-5p exerts tumor-suppressive effects on various cancers, including renal cell carcinoma,21 pancreatic cancer cells,22 and breast cancer.23 Besides, miR-509-5p displays high expression level in testis.24 Whereas whether miR-509-5p has a possible connection with male infertility and plays a role in TGCT remain unclear. This study aimed to detect miR-509-5p expression in germ cells from MA patients, and further characterize its functional roles in the proliferation and apoptosis of TGCT cells in vitro.
Materials And Methods
Testicular Tissues
Testicular tissues were collected from infertile male patients with maturation arrest (MA) (aged 22–38 years, n = 12) and normal individuals (aged 23–35, n = 8) who underwent orchiectomy for prostate carcinoma at the Linyi Central Hospital. The diagnosis for MA was based on a standard pathological criteria, in which spermatogenic defects were confirmed by sertoli-cell-only syndrome and spermatogenic arrest appearing in spermatogonia or primary spermatocytes. On the other hand, histological examination confirmed the normal spermatogenesis in control individuals, who had no medical history of infertility. The patient consent was written informed consent, and it was conducted in accordance with the Declaration of Helsinki. This study was approved by the Institutional Review Boards of Linyi Central Hospital.
Cell Culture And Transfection
Cell lines of NT2, NCCIT, and HEK293T were obtained from the American type culture collection (ATCC). The morphology was periodically confirmed and the short tandem repeat (STR) profiling method was used to authenticate cell lines. NT2 and HEK293T cell lines were cultured in DMEM medium and NCCIT cell line was cultured in RPMI1640 medium added with 10% fetal bovine serum and 100 U/mL penicillin/streptomycin (Life Technologies) at 37°C with an atmosphere of 5% CO2. Cells passaged for 3–7 times were used for experiments. The transfection of control mimic, miR-509-5p mimic, control inhibitor or miR-509-5p inhibitor (Genepharma, Shanghai, China), pcDNA3.1 vector (Invitrogen) or pcDNA3.1-MDM2 was conducted using Lipofectamine 2000 Reagent (Invitrogen) following the manufacturer’s protocols. The transfection efficiency was confirmed by measuring mRNA level or protein level of targets following 2 days of transfection.
Quantitative Real-Time PCR Analysis
RNAs were isolated from the testicular tissues and TGCT cells with TRIzol reagent (Invitrogen) and purified RNAs were synthesized into complimentary cDNAs by using PrimeScript RT reagent kit (TaKaRa). Quantitative real-time PCR (qRT-PCR) was carried out with StepOne real-time PCR system (Applied Biosystems) and SYBR Premix Ex Taq II Kit (Takara). β-actin served to be an internal control. For the analysis of miR-509-5p, small RNAs were isolated by mirPremier miRNA isolation kit (Sigma-Aldrich). miR-509-5p expression was measured by TaqMan miRNA assays (Applied Biosystems). U6 snRNA served to be a normalization. The sequences of gene-specific primer pairs are available when request.
Western Blotting Analysis
Protein samples were isolated from the testicular tissues and TGCT cells using RIPA buffer (Beyotime, P0013B) containing the protease inhibitor cocktail (Roche). Then, protein samples were resoluted by SDS-PAGE, and transferred to nitrocellulose membranes (Amersham Biosciences). Membranes were sequentially probed with primary and secondary antibodies. Blots were visualized by Enhanced Chemiluminescence System (Amersham Biosciences), and quantified using the ImageJ software. Antibody sources are listed as below: cleaved caspase 3 (1:1000, Cell Signaling Technology, 9661), MDM2 (1:500, Abcam, ab16895), β-actin (1:2000, Proteintech, 66009-1-Ig), goat anti-rabbit IgG (1:5000, Novus, NB730-H) and goat anti-mouse IgG (1:5000, Novus, NBP2-30347H).
Cell Proliferation And Apoptosis
Cell proliferation of TGCT cells was determined by Cell Counting Kit-8 kit (Beyotime, C0038). Each condition was allocated five parallel wells. The absorbance at 450 nm was recorded by Epoch 2 Microplate Spectrophotometer (Biotek Instruments). Apoptosis was determined using Annexin V-FITC Apoptosis Detection Kit (BioLegend, 640914) and following flow cytometry analysis with FACScalibur flow cytometer (BD Biosciences). Apoptotic cells were defined as Annexin V positive cells. Data were analyzed by FlowJo (Tree Star).
Luciferase Reporter Assay
HEK293T cells were cotransfected 100 nM control mimic or 100 nM miR-509-5p mimic, or 100 nM control inhibitor or miR-509-5p inhibitor with 2 µg dual luciferase reporter pGL3-basic plasmids (Promega) containing either wild-type or mutant MDM2 3ʹ-UTR of MDM2 using Lipofectamine 2000. The mutation of binding sites was introduced using the Quikchange site-directed mutagenesis kit (Stratagene, La Jolla, CA, USA) following the manufacturer’s instructions. The simultaneous transfection of 20 ng Renilla vector was used as a transfection control. At 36 h after transfection, luciferase activity was assessed by Dual Luciferase Reporter Assay System (Promega). The Renilla luciferase activity served as a normalization.
Statistical Analysis
Data are shown as the mean ± SD. The differences between two sets of data were assessed by one-way ANOVA test, while the correlation was evaluated using Pearson’s correlation analysis. Comparisons were considered to be statistically significant if P values are less than 0.05.
Results
miR-509-5p Is Downregulated And Negatively Associated With Proliferation In Germ Cells From Infertile Men With Maturation Arrest
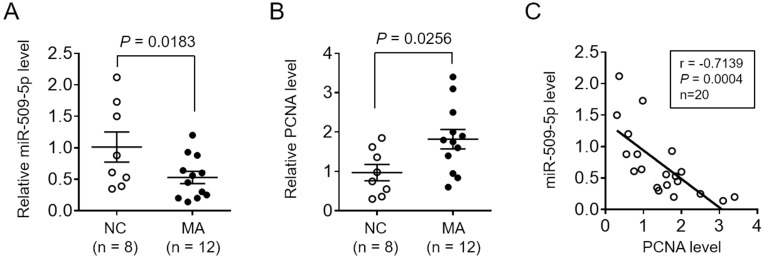
To test whether miR-509-5p is associated with male infertility, we first measured its expression level in testicular biopsy specimens collected from normal controls (NC, n = 8) and men with maturation arrest (MA, n = 12). qRT-PCR analysis showed that compared with NC specimens, miR-509-5p expression was markedly decreased in MA patients (Figure 1A, P = 0.0183). It has been demonstrated that the expression of the proliferating cell nuclear antigen (PCNA), an indicator of proliferating activity in testes, was elevated in tubules of MA patients than those with focal spermatogenesis,25 and that PCNA expression was also upregulated in germ cells from MA patients compared with normal controls.19 Consistently, we also noticed that PCNA expression significantly increased in testicular biopsy specimens from MA patients relative to NC, as shown by qRT-PCR analysis (Figure 1B, P = 0.0256). To check whether miR-509-5p has a correlation with germ cell proliferation, the expression levels of miR-509-5p and PCNA in NC and MA groups were pooled together, and analyzed by the Pearson’s correlation analysis. As a result, a strong reverse correlation was observed between miR-509-5p and PCNA levels in testicular samples (Figure 1C, r = −0.7139, P = 0.0004). Altogether, these observations reveal a downregulated miR-509-5p expression in testicular samples from MA patients, which is accompanied by increased proliferating activity.
Figure 1.
miR-509-5p is decreased and reversely correlated with PCNA expression in germ cells from infertile men with maturation arrest. (A-B) miR-509-5p level (A) and PCNA level (B) in the testes of normal controls (NC, n = 8) and infertile men with maturation arrest (MA, n = 12) were determined by qRT-PCR analysis. U6 snRNA and β-actin were used as internal controls, respectively. Each symbol represents the mean value from 3 replicates. P values are shown above. (C) The correlation of miR-509-5p level and PCNA level shown as in (A-B) was analyzed by the Pearson’s correlation analysis. r = −0.7139; P = 0.0004; n = 20.
miR-509-5p Retards Proliferation And Induces Apoptosis Of Testicular Germ Cell Tumor Cells
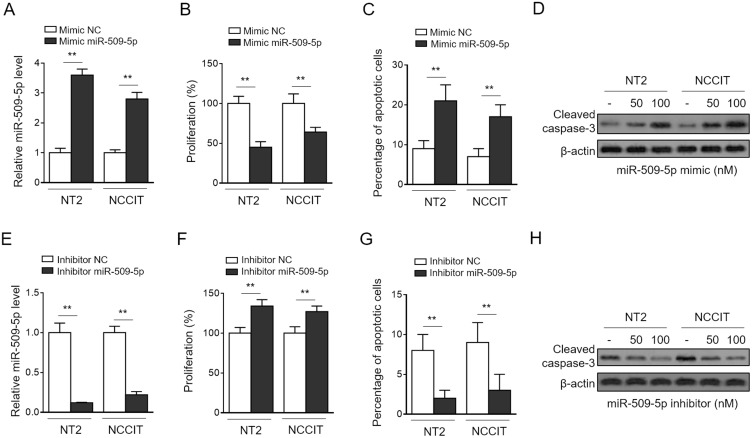
Until now, the function of miR-509-5p has been largely connected with numerous suppressive effects on malignant properties of human cancers, including proliferation, apoptosis, migration, and invasion.21,22,26 To provide a useful clue on how miR-509-5p may participate in regulating germ cell proliferation and other processes, we next evaluated its functional roles using two cell lines of testicular germ cell tumor (TGCT), NT-2 and NCCIT, cultured in vitro. To our knowledge, whether miR-509-5p affects the proliferation and apoptosis of TGCT cells has not been characterized. Through transfecting the synthetic miR-509-5p mimic into NT-2 and NCCIT cells (Figure 2A), we found that miR-509-5p overexpression led to a remarkable suppression in cell proliferation rate, as determined by cell proliferation assay CCK-8 (Figure 2B). Moreover, the analysis of annexin V/PI double staining showed that miR-509-5p overexpression also induced apoptosis in both NT-2 and NCCIT cells (Figure 2C). This finding was further strengthened by increased level of cleaved caspase-3 in miR-509-5p-overexpressing cells (Figure 2D). To confirm these effects of miR-509-5p, we inhibited miR-509-5p via transfecting the antisense oligonucleotides (Figure 2E). In concert with results obtained by miR-509-5p overexpression, its inhibition markedly resulted in increased cell proliferation (Figure 2F) and decreased apoptosis (Figure 2G–H) in NT-2 and NCCIT cells. Hence, these findings indicate that miR-509-5p functions to suppress cell proliferation and induce apoptosis in TGCT cells, at least in vitro. Further, given its downregulation and the reverse correlation with proliferating activity in testicular samples from MA patients, we suppose that miR-509-5p may be functionally involved in male infertility pathogenesis through regulating germ cell proliferation and apoptosis.
Figure 2.
miR-509-5p inhibits proliferation and induces apoptosis of testicular germ cell tumor cells. (A-C) TGCT cell lines NT2 and NCCIT were transfected with negative control mimic (NC mimic) or 100 nM miR-509-5p mimic. After 3 days, cells were harvested for following analyses. (A) miR-509-5p level was determined by qRT-PCR analysis (n = 3). U6 snRNA level was used as an internal control. (B) Cell proliferation was assessed using CCK-8 assay (n = 5). (C) Cell apoptosis was analyzed by FACS (n = 5). (D) NT2 and NCCIT were transfected with NC mimic, 50 mM or 100 nM miR-509-5p mimic. After 3 days, the cleaved caspases 3 was detected by Western blotting. (E-H) NT2 and NCCIT were transfected with negative control inhibitor (NC inhibitor) or miR-509-5p inhibitor. After 3 days, the miR-509-5p level (E), cell proliferation (F), and cell apoptosis (G-H) were analyzed as in (A-D). Data are mean ± SD. **P < 0.01.
MDM2 Is Targeted By miR-509-5p
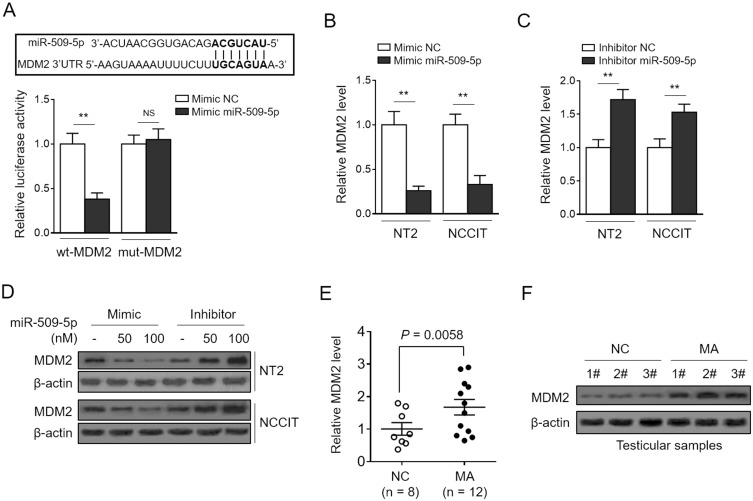
To understand how miR-509-5p elicits tumor-suppressive effects on TGCT cells, the tool of TargetScan algorithm was applied to predict the binding targets.27 Among these potential targets, we focused on the mouse double minute 2 (MDM2) (Figure 3A, upper panel), a well-defined oncogene,28 since it plays a critical role in modulating the proliferation and apoptosis of TGCT cells.29 We initially validated the direct target of MDM2 by miR-509-5p using the luciferase reporter assay, as shown by miR-509-5p-suppressed luciferase activity of reporter construct containing the wild-type 3ʹ-UTR MDM2, but not the mutant one (Figure 3A, bottom panel). Consistently, miR-509-5p overexpression reduced and its inhibition increased the mRNA (Figure 3B and C) and protein (Figure 3D) levels of MDM2 in NT-2 and NCCIT cells, therefore proving that miR-509-5p targets and suppresses MDM2 expression in TGCT cells. Furthermore, we wondered whether MDM2 expression is also reversely correlated with miR-509-5p in testicular samples from MA patients. Indeed, by analyzing the same testicular samples, we discovered that just contrary to miR-509-5p downregulation (Figure 1A), MDM2 expression was significantly elevated in testicular samples from MA patients at both mRNA (Figure 3E) and protein levels (Figure 3F), as compared with NC. These data point to a possibility that, in vivo, miR-509-5p may also negatively regulate MDM2 expression in the testes.
Figure 3.
miR-509-5p targets MDM2. (A, upper panel) The putative binding sites for miR-509-5p in MDM2 3ʹ-UTR was predicted by TargetScan algorithms. (A, bottom panel) NC mimic or miR-509-5p mimic was cotransfected with luciferase reporter construct with either wild-type (wt-MDM2) or mutant MDM2 3ʹ-UTR (mut-MDM2) into HEK293 cells. The luciferase activity assayed at 36 h after transfection (n = 5). (B-D) NT2 and NCCIT were transfected with NC mimic or miR-509-5p mimic, or NC inhibitor or miR-509-5p inhibitor. The mRNA level (B-C) and protein level (D) of MDM2 were determined by qRT-PCR analysis and Western blotting analysis, respectively. (E-F) The mRNA level (E) and protein level (F) of MDM2 in the testes of normal controls (NC, n = 8) and infertile men with maturation arrest (MA, n = 12) were determined. Data are mean ± SD. **P < 0.01; NS, not significant.
Enforced Expression Of MDM2 Rescues miR-509-5p Effects On Testicular Germ Cell Tumor Cells
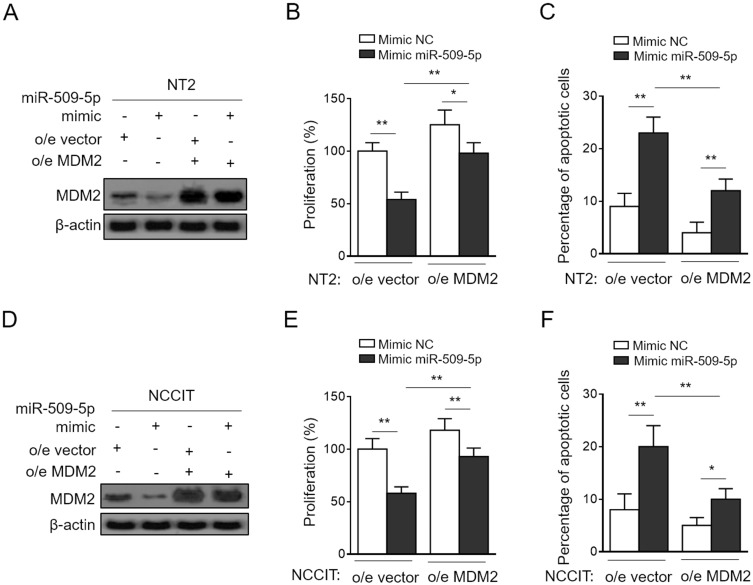
Finally, to clarify whether MDM2 mediates miR-509-5p effects on TGCT cells, we performed the enforced expression of MDM2 in NT-2 and NCCIT cells transfected with synthetic miR-509-5p mimic so as to restore its suppressed expression (Figure 4A and D). Importantly, in pace with the restored MDM2 expression, the miR-509-5p-suppressed proliferation (Figure 4B) and -induced apoptosis (Figure 4C) in NT-2 cells were considerably recovered. Additionally, similar results could be obtained using NCCIT cells (Figure 4E and F). Therefore, a conclusion could be drawn that MDM2 is a critical downstream effector through which miR-509-5p imposes anti-proliferative and pro-apoptosis effects on TGCT cells. Besides, in consideration of the altered expression levels of miR-509-5p and MDM2 in testicular samples from MA patients, the miR-509-5p/MDM2 axis may also have functional relevance to disease pathology, which is of interest and clinical significance to be addressed by further investigations.
Figure 4.
MDM2 restoration rescues miR-509-5p effects on testicular germ cell tumor cells. (A-C) NT2 cells were transfected with NC mimic or 100 nM miR-509-5p mimic in combination with pcDNA3.1-vector or pcDNA3.1-MDM2. (A) The protein level of MDM2 was determined by Western blotting analysis. (B) Cell proliferation was determined by CCK-8 method (n = 5). (C) Cell apoptosis was assessed by FACS analysis (n = 5). (D-F) NCCIT cells were treated as in (A). The protein level of MDM2 (D), cell proliferation (E), and cell apoptosis (F) were analyzed as in (A-C). Data are mean ± SD. *P < 0.05; **P < 0.01.
Discussion
We found that miR-509-5p was decreased and its level was negatively associated with the proliferation activity in germ cells from infertile men with MA. We also discovered that miR-509-5p not only suppressed proliferation but also induced apoptosis of TGCT cells in vitro, and further proved that MDM2, as a downstream target of miR-509-5p, is vital for mediating these roles of miR-509-5p. Thus, our study may identify miR-509-5p as a novel candidate miRNA that is associated with the pathogenesis of male infertility due to MA, and also provide a mechanistic insight into the previously unrecognized tumor suppressive roles of miR-509-5p in TGCT.
PCNA is essential for DNA duplication during cell cycle progression, and it has been demonstrated to serve as a useful molecular marker for evaluating germ cell proliferation kinetics and the testes spermatogenic function of male infertility.30–32 We found that PCNA expression was significantly upregulated in testicular biopsy specimens from MA patients, and meanwhile, miR-509-5p expression was downregulated and negatively correlated well with PCNA. Further, we demonstrated that miR-509-5p exhibited an anti-proliferative activity in TGCT cells. According to these clues, we suppose it is very possible that serving as a negative regulator of proliferation, miR-509-5p downregulation may contribute to the hyperactive germ cell proliferation which eventually causes spermatogenic arrest and male infertility. However, the limitation is that it remains unclear whether miR-509-5p affects meiotic spermatocytes or haploid differentiated spermatids only based on our available data. Further investigations on revealing the cellular distribution of miR-509-5p in testicular tissues would be helpful to fully address miR-509-5p functions in different stages of spermatogenesis.
On the other hand, for clinical diagnosis of male infertility, the diagnostic tools are limited and the frequently used method is semen analysis which offers an almost crude estimation.33 There is a need of additional diagnostic biomarkers for evaluating male fertility. It has been proposed that the altered expression levels of miRNAs in male infertility may serve as potential good biomarkers for diagnosis.16 The significant downregulation of miR-509-5p in male infertility with MA implies that it may be useful in assisting the diagnosis of this subtype of male infertility. Whether miR-509-5p expression displays alteration in other subtypes of male infertility is uncertain, which needs to be clarified in the future.
We then show that miR-509-5p limits proliferation and induces apoptosis of NT-2 and NCCIT cells through targeting MDM2, therefore extending its tumor-suppressive activities into TGCT. We found that MDM2 expression was induced in testicular samples from MA patients, which hints that MDM2 may play a role in its pathology. The genetic variants in MDM2 have been found to be associated with male infertility.34 It is interesting to investigate the mechanistic connection of MDM2 to male infertility. Typically, TGCT is derived from the germ cells of testis.35 Besides, it is known that the male infertility is a risk factor for the development of testicular cancer, including TGCT.36 Our findings of miR-509-5p downregulation and reverse correlation with germ cell proliferation in MA patients combined with its tumor-suppressive activities in TGCT cells may offer a new clue to understand the connection between male infertility and TGCT.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Kumar N, Singh AK. Trends of male factor infertility, an important cause of infertility: a review of literature. J Hum Reprod Sci. 2015;8(4):191–196. doi: 10.4103/0974-1208.170370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Agarwal A, Mulgund A, Hamada A, Chyatte MR. A unique view on male infertility around the globe. Reprod Biol Endocrinol. 2015;13:37. doi: 10.1186/s12958-015-0032-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weedin JW, Bennett RC, Fenig DM, Lamb DJ, Lipshultz LI. Early versus late maturation arrest: reproductive outcomes of testicular failure. J Urol. 2011;186(2):621–626. doi: 10.1016/j.juro.2011.03.156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hung AJ, King P, Schlegel PN. Uniform testicular maturation arrest: a unique subset of men with nonobstructive azoospermia. J Urol. 2007;178(2):608–612. discussion 612. doi: 10.1016/j.juro.2007.03.125 [DOI] [PubMed] [Google Scholar]
- 5.Griswold MD. Spermatogenesis: the commitment to meiosis. Physiol Rev. 2016;96(1):1–17. doi: 10.1152/physrev.00013.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Russell LD, Chiarini-Garcia H, Korsmeyer SJ, Knudson CM. Bax-dependent spermatogonia apoptosis is required for testicular development and spermatogenesis. Biol Reprod. 2002;66(4):950–958. doi: 10.1095/biolreprod66.4.950 [DOI] [PubMed] [Google Scholar]
- 7.Pareek TK, Joshi AR, Sanyal A, Dighe RR. Insights into male germ cell apoptosis due to depletion of gonadotropins caused by GnRH antagonists. Apoptosis. 2007;12(6):1085–1100. doi: 10.1007/s10495-006-0039-3 [DOI] [PubMed] [Google Scholar]
- 8.Takagi S, Itoh N, Kimura M, Sasao T, Tsukamoto T. Spermatogonial proliferation and apoptosis in hypospermatogenesis associated with nonobstructive azoospermia. Fertil Steril. 2001;76(5):901–907. doi: 10.1016/s0015-0282(01)02732-7 [DOI] [PubMed] [Google Scholar]
- 9.Steger K, Aleithe I, Behre H, Bergmann M. The proliferation of spermatogonia in normal and pathological human seminiferous epithelium: an immunohistochemical study using monoclonal antibodies against Ki-67 protein and proliferating cell nuclear antigen. Mol Hum Reprod. 1998;4(3):227–233. doi: 10.1093/molehr/4.3.227 [DOI] [PubMed] [Google Scholar]
- 10.Reuter VE. Origins and molecular biology of testicular germ cell tumors. Mod Pathol. 2005;18(Suppl 2):S51–S60. doi: 10.1038/modpathol.3800309 [DOI] [PubMed] [Google Scholar]
- 11.Sheikine Y, Genega E, Melamed J, Lee P, Reuter VE, Ye H. Molecular genetics of testicular germ cell tumors. Am J Cancer Res. 2012;2(2):153–167. [PMC free article] [PubMed] [Google Scholar]
- 12.Bettegowda A, Wilkinson MF. Transcription and post-transcriptional regulation of spermatogenesis. Philosophical transactions of the Royal Society of London Series B. Biological Sciences. 2010;365(1546):1637–1651. doi: 10.1098/rstb.2009.0196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Papaioannou MD, Nef S. microRNAs in the testis: building up male fertility. J Androl. 2010;31(1):26–33. doi: 10.2164/jandrol.109.008128 [DOI] [PubMed] [Google Scholar]
- 14.Kotaja N. MicroRNAs and spermatogenesis. Fertil Steril. 2014;101(6):1552–1562. doi: 10.1016/j.fertnstert.2014.04.025 [DOI] [PubMed] [Google Scholar]
- 15.Carthew RW, Sontheimer EJ. Origins and mechanisms of miRNAs and siRNAs. Cell. 2009;136(4):642–655. doi: 10.1016/j.cell.2009.01.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khazaie Y, Nasr Esfahani MH. MicroRNA and male infertility: a potential for diagnosis. Int J Fertil Steril. 2014;8(2):113–118. [PMC free article] [PubMed] [Google Scholar]
- 17.Wienholds E, Koudijs MJ, van Eeden FJ, Cuppen E, Plasterk RHA. The microRNA-producing enzyme Dicer1 is essential for zebrafish development. Nat Genet. 2003;35(3):217–218. doi: 10.1038/ng1251 [DOI] [PubMed] [Google Scholar]
- 18.Romero Y, Meikar O, Papaioannou MD, et al. Dicer1 depletion in male germ cells leads to infertility due to cumulative meiotic and spermiogenic defects. PLoS One. 2011;6(10):e25241. doi: 10.1371/journal.pone.0025241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lian J, Tian H, Liu L, et al. Downregulation of microRNA-383 is associated with male infertility and promotes testicular embryonal carcinoma cell proliferation by targeting IRF1. Cell Death Dis. 2010;1:e94. doi: 10.1038/cddis.2010.70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lu M, Tian H, Cao YX, et al. Downregulation of miR-320a/383-sponge-like long non-coding RNA NLC1-C (narcolepsy candidate-region 1 genes) is associated with male infertility and promotes testicular embryonal carcinoma cell proliferation. Cell Death Dis. 2015;6:e1960. doi: 10.1038/cddis.2015.267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang WB, Pan ZQ, Yang QS, et al. Tumor suppressive miR-509-5p contributes to cell migration, proliferation and antiapoptosis in renal cell carcinoma. Ir J Med Sci. 2013;182(4):621–627. doi: 10.1007/s11845-013-0941-y [DOI] [PubMed] [Google Scholar]
- 22.Li X, Li Y, Wan L, Chen R, Chen F. miR-509-5p inhibits cellular proliferation and migration via targeting MDM2 in pancreatic cancer cells. Onco Targets Ther. 2017;10:4455–4464. doi: 10.2147/OTT.S130378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Song YH, Wang J, Nie G, et al. MicroRNA-509-5p functions as an anti-oncogene in breast cancer via targeting SOD2. Eur Rev Med Pharmacol Sci. 2017;21(16):3617–3625. [PubMed] [Google Scholar]
- 24.Ludwig N, Leidinger P, Becker K, et al. Distribution of miRNA expression across human tissues. Nucleic Acids Res. 2016;44(8):3865–3877. doi: 10.1093/nar/gkw116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bar-Shira Maymon B, Yogev L, Yavetz H, et al. Spermatogonial proliferation patterns in men with azoospermia of different etiologies. Fertil Steril. 2003;80(5):1175–1180. doi: 10.1016/s0015-0282(03)02161-7 [DOI] [PubMed] [Google Scholar]
- 26.Wang P, Deng Y, Fu X. MiR-509-5p suppresses the proliferation, migration, and invasion of non-small cell lung cancer by targeting YWHAG. Biochem Biophys Res Commun. 2017;482(4):935–941. doi: 10.1016/j.bbrc.2016.11.136 [DOI] [PubMed] [Google Scholar]
- 27.Lewis BP, Shih IH, Jones-Rhoades MW, Bartel DP, Burge CB. Prediction of mammalian microRNA targets. Cell. 2003;115(7):787–798. doi: 10.1016/s0092-8674(03)01018-3 [DOI] [PubMed] [Google Scholar]
- 28.Freedman DA, Wu L, Levine AJ. Functions of the MDM2 oncoprotein. Cell Mol Life Sci. 1999;55(1):96–107. doi: 10.1007/s000180050273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bauer S, Muhlenberg T, Leahy M, et al. Therapeutic potential of Mdm2 inhibition in malignant germ cell tumours. Eur Urol. 2010;57(4):679–687. doi: 10.1016/j.eururo.2009.06.014 [DOI] [PubMed] [Google Scholar]
- 30.Schlatt S, Weinbauer GF. Immunohistochemical localization of proliferating cell nuclear antigen as a tool to study cell proliferation in rodent and primate testes. Int J Androl. 1994;17(4):214–222. doi: 10.1111/j.1365-2605.1994.tb01245.x [DOI] [PubMed] [Google Scholar]
- 31.Salama N, Tsuji M, Tamura M, Kagawa S. Proliferating cell nuclear antigen in testes of infertile men with varicocele–preliminary results of interrelationship with sperm count before and after varicocelectomy. Scand J Urol Nephrol. 2003;37(1):48–52. doi: 10.1080/00365590310008695 [DOI] [PubMed] [Google Scholar]
- 32.Tanaka H, Fujisawa M, Okada H, Arakawa S, Kamidono S. Assessment of germ-cell kinetics in the testes of patients with varicocele using image analysis of immunostained proliferating cell nuclear antigen. Br J Urol. 1996;78(5):769–771. doi: 10.1046/j.1464-410x.1996.19619.x [DOI] [PubMed] [Google Scholar]
- 33.Bieniek JM, Drabovich AP, Lo KC. Seminal biomarkers for the evaluation of male infertility. Asian J Androl. 2016;18(3):426–433. doi: 10.4103/1008-682X.175781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huang C, Liu W, Ji GX, et al. Genetic variants in TP53 and MDM2 associated with male infertility in Chinese population. Asian J Androl. 2012;14(5):691–694. doi: 10.1038/aja.2012.39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gilbert D, Rapley E, Shipley J. Testicular germ cell tumours: predisposition genes and the male germ cell niche. Nat Rev Cancer. 2011;11(4):278–288. doi: 10.1038/nrc3021 [DOI] [PubMed] [Google Scholar]
- 36.Hotaling JM, Walsh TJ. Male infertility: a risk factor for testicular cancer. Nat Rev Urol. 2009;6(10):550–556. doi: 10.1038/nrurol.2009.179 [DOI] [PubMed] [Google Scholar]