Abstract
Background:
Prevalence of multidrug-resistant microorganisms (MDROs) continues to increase, while infection control gaps in healthcare settings facilitate their transmission between patients. In this setting, 5 distinct yet interlinked pathways are responsible for transmission. The complete transmission process is still not well understood. Designing and conducting a single research study capable of investigating all 5 complex and multifaceted pathways of hospital transmission would be costly and logistically burdensome. Therefore, this scoping review aims to synthesize the highest-quality published literature describing each of the 5 individual potential transmission pathways of MDROs in the healthcare setting and their overall contribution to patient-to-patient transmission.
Methods:
In 3 databases, we performed 2 separate systematic searches for original research published during the last decade. The first search focused on MDRO transmission via the HCW or the environment to identify publications studying 5 specific transmission pathways: (1) patient to HCW, (2) patient to environment, (3) HCW to patient, (4) environment to patient, and (5) environment to HCW. The second search focused on overall patient-to-patient transmission regardless of the transmission pathway. Both searches were limited to transmission of methicillin-resistant Staphylococcus aureus (MRSA), vancomycin-resistant Enterococcus, multidrug-resistant A. baumannii, and carbapenem-resistant Enterobacteriaceae. After abstract screening of 5,026 manuscripts, researchers independently reviewed and rated the remaining papers using objective predefined criteria to identify the highest quality and most influential manuscripts.
Results:
High-quality manuscripts were identified for all 5 routes of transmission. Findings from these studies were consistent for all pathways; however, results describing the routes from the environment/HCW to a noncolonized patient were more limited and variable. Additionally, most research focused on MRSA, instead of other MDROs. The second search yielded 10 manuscripts (8 cohort studies) that demonstrated the overall contribution of patient-to-patient transmission in hospitals regardless of the transmission route. For MRSA, the reported cross-transmission was as high as 40%.
Conclusions:
This scoping review brings together evidence supporting all 5 possible transmission pathways and illustrates the complex nature of patient-to-patient transmission of MDROs in hospitals. Our findings also confirm that transmission of MDROs in hospitals occurs frequently, suggesting that ongoing efforts are necessary to strengthen infection prevention and control to prevent the spread of MDROs. (Received 6 November 2018; accepted 13 December 2018)
The prevalence of multidrug-resistant microorganisms (MDROs) continues to increase in healthcare settings in the United States. For instance, methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-resistant Enterococcus (VRE) colonization among intensive care unit (ICU) patients have been reported as high as 16% and 25% respectively in US hospitals.1 Likewise, multidrug-resistant Acinetobacter baumannii and carbapenem-resistant Enterobacteriacea (CRE) colonization rates have been reported to be as high as 41%2 and 45%3 in hospitalized patients. On average, 21 VRE or MRSA new acquisitions per1,000 patient days at risk have been observed in ICU settings across the United States.4
The continuing emergence of MDROs and their spread across healthcare settings are believed to be due to multiple causal factors, including antibiotic use and transmission due to gaps in infection control.5 Although low or moderate levels of health care worker (HCW) carriage of MDRO have been observed,6,7 HCW hands, gloves, and gown contamination have been consistently reported.8–10 Moreover, HCW apparel (ie, scrubs, and white coats) and the hospital environment have been shown to carry a high burden of MDROs.6,11,12,13 Therefore, HCWs and the healthcare environment can function as intermediate transmission vectors of MDROs among patients.
Although hospital settings are commonly considered hot spots for MDRO transmission, the complete transmission process is still not well understood. Furthermore, the relative importance of each potential transmission pathway in the overall patient-to-patient transmission process in healthcare settings is still controversial. The design and implementation of a single research study to appropriately investigate all potential pathways simultaneously and their intercorrelations is largely infeasible due to cost and logistics. Nevertheless, the lack of clear evidence and understanding of the complexity and multifaceted nature of MDRO transmission in hospitals has resulted in disagreements regarding the most appropriate infection prevention measures needed to prevent transmission of these organisms in healthcare settings. For example, this leads to large discussions about the pros and cons of contact precautions.
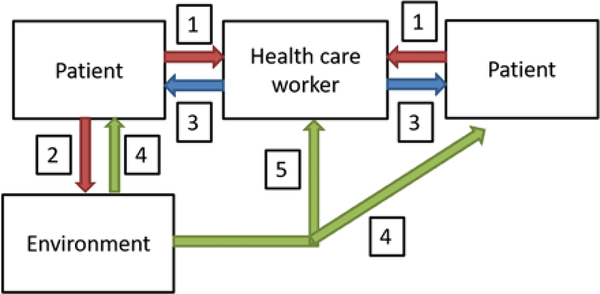
This scoping review aims to fill this research gap by synthesizing the existing evidence on MDRO hospital transmission. We have summarized the best evidence on each of the 5 following potential transmission routes: (1) patient to HCW, (2) patient to environment, (3) HCW to patient, (4) environment to patient, and (5) environment to HCW (Fig. 1). Additionally, we have synthesized findings from high-quality manuscripts that demonstrate the overall contribution of patient-to-patient transmission in hospitals irrespective of the transmission routes.
Fig. 1.
Main transmission pathways involved in hospital transmission of multidrug-resistant organisms (MDROs).
Methods
We performed a scoping review, which is a widespread methodology to systematically search, select, and summarize existing evidence on a specific subject to identify research gaps.14,15 We chose this methodology instead of a traditional systematic review because it provided the flexibility to perform a general preliminary assessment of the existing literature on the complex and multifaceted topic of transmission of MDROs in the hospital setting.
Study identification and selection
We performed 2 separate searches in PubMed, EMBASE, and Cochrane Central Register of Controlled Trials (CENTRAL). The first search focused on MDRO transmission via the HCW or the environment to identify research studying specific transmission pathways (patient to HCW, patient to environment, HCW to patient, environment to patient, or environment to HCW). We used the following search terms: multidrug-resistant organism AND healthcare personnel OR environment OR equipment AND disease transmission OR cross-transmission OR nosocomial transmission. The second search focused on overall patient-to-patient transmission regardless of whether the study specifically evaluated the transmission pathway. The following search terms were included: multidrug-resistant organism AND disease transmission OR cross-transmission OR nosocomial transmission OR patient-to-patient transmission. Both searches identified terms in the title, abstract, and keywords fields. A medical librarian developed each search strategy.
Only original research manuscripts written in English and published during the last decade (2007–2017) were eligible for inclusion. Letters to the editor, guidelines, mathematical models, case studies, or outbreak investigations were excluded. Additionally, only research studies conducted in the acute-care setting among adult populations focusing on the following MDROs were considered: CRE, MRSA, VRE, and/or multidrug-resistant A. baumannii. Finally, to ensure that manuscripts satisfactorily demonstrated the transmission of the same multidrug-resistant strain across individuals, only manuscripts using modern molecular laboratory techniques such as whole-genome sequence (WGS) or pulsed-field gel electrophoresis (PFGE) were included. When studying the transmission pathways, manuscripts able to establish directionality of transmission (ie, patient to HCW vs HCW to patient) were preferred.
One researcher (N.B.) screened all titles and abstracts for eligibility. After abstract screening, the remaining papers were independently reviewed and rated by 3 researchers (N.B., A.D.H., and L.O.) using predefined objective criteria to identify the highest-quality manuscripts. The following main criteria used for manuscript evaluation were extracted: type of study design, sample size, and discriminatory power of the molecular technique used to demonstrate relatedness between strains. Disagreements were resolved by consensus. The best manuscripts from each search were summarized using a standardized template to synthesize the existing evidence on hospital transmission of MDROs. Additionally, the evidence level of consistency across each transmission route was categorized as either high, moderate, or low based on the level of agreement across studies.
Results
Potential transmission routes
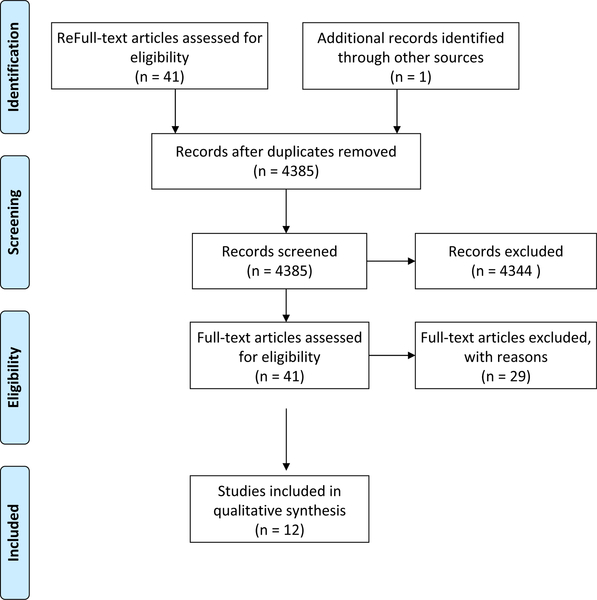
After searching 3 databases, 4,596 manuscripts were identified and one additional record was added by hand searching references of included articles. After removal of duplicates and elimination of articles based on title and abstract review, 41 full-text articles were reviewed. Finally, 12 articles were selected for inclusion in this scoping review (Fig. 2). These were the most common reasons for exclusion: (1) the study was not based in an acute-care setting; (2) the study did not include at least 1 of our selected MDROs; (3) the study took place during an epidemic or outbreak; (4) the study did not incorporate modern molecular techniques to assure same-strain transmission; (5) the article did not present original research; and (6) the type of manuscript/analysis was defined as part of the exclusion criteria (ie, letter to the editor, mathematical models, and case reports).
Fig. 2.
PRISMA flow diagram for literature review on transmission routes.
Transmission from patient to HCW.
The probable primary source of the MDRO is the colonized or infected patient, and the patient may contaminate the HCW. Several high-quality papers have shown the transmission of MDROs from patients to HCWs, and in this review, we highlight 4 such prospective studies (Table 1). All of these studies demonstrated transmission of the same strain from the patient to HCW hands, gloves, and/or gowns using PFGE.8,9,16,17 The main limitation of these studies is their ability to separate the effect of patient transmission to HCW from the effect of environmental transmission to HCW. However, the study published by Morgan et al in 2012 directly connected patient strains (instead of environmental strains) to the isolated strains in gowns and gloves in 82% of the analyzed cases.8 In summary, based on the studies identified in our review, existing evidence demonstrating transmission from patient to HCW is highly consistent across available research even though variability in the amount of transmission was observed across microorganisms as expected.
Table 1.
Summary of Best Quality Evidence by transmission Pathway
| First Author, Year |
Title | Study Design | Population | Relevant Objective | Related Molecular Analysis |
Related Finding |
|---|---|---|---|---|---|---|
| 1. Transmission from patient to HCW | ||||||
| Morgan DJ et al 20128 | Transfer of multidrug-resistant bacteria to HCW gloves and gowns after patient contact increases with environmental contamination. | Prospective cohort study | 27 HCW–patient interactions: 256 patient samples, 1,572 environmental sites, 131 pairs of HCW hands from the MICU of Rush University Medical Center | To estimate the level of hand or glove contamination with VRE among HCWs when interacting with the patient or environment | PFGE | Of 103 HCWs whose hand samples were negative for VRE when they entered the room, 70% contaminated their hands/gloves after interacting with the patient and the environment. |
| Morgan DJ et al 20109 | Frequent MDR A. baumannii contamination of gloves, gowns, and hands of HCWs | Prospective cohort study | 199 patient–HCW interactions in 48 ICUs at University of Maryland Medical Center | To determine the incidence of transmission of MDR A. baumannii and P. aeruginosa from patients to HCWs during routine care | PFGE | 39% of patient–HCW interactions resulted in HCW G&G contamination with A. baumannii. 9 (5%) episodes resulted in hand contamination after G&G removal; isolates were identical to the G&G strain. 8% of patient–HCW interactions resulted in HCW G&G contamination with MDR P. aeuroginosa (imipenem resistant). |
| Hayden, M.K. et al 200816 | Risk of hand or glove contamination after contact with patients colonized with VRE or the colonized patients’ environment | Prospective cohort study | 603 patient–HCW interactions in 6 different ICUs at University of Maryland Medical Center | To assess the role of environmental contamination in the transmission of MDROs to HCW clothing | PFGE | 33% of P-HCW interactions led to transmission of MDR A. baumannii to HCW G&G. Carbapenem-resistant P. aeruginosa was transmitted 17% of the time. MRSA and VRE were transmitted 14% of time. Of 22 cases (selected for PFGE), 82% of HCW had a strain related to the patient. |
| Schweizer M et al 201217 | The epidemiology of MRSA on a burn trauma unit | Prospective cohort study | 144 patients, 67 HCWs and 50 environmental samples from the burn trauma unit at a midwestern academic medical center | To determine whether HCW, environment, or admitted patients could be reservoirs for MRSA | PFGE | The USA100 subtype was shared by 3 patients and 1 HCW. In these cases, at least 1 patient was positive before the HCW was. |
| 2. Transmission from patient to environment | ||||||
| Munoz-Price LS et al 201311 | A. baumannii: association between environmental contamination of patient rooms and occupant status | Prospective cohort study | 186 air samples and 82 clinical specimens from Turgut Ozal Medical Center | To understand the dynamics of Acinetobacter spread in air | PCR-typing (DiversLab) PFGE | The Acinetobacter concentration was the highest in bedside sampling areas of infected patients. Air isolates were clonally related to clinical strains. |
| Thom KA et al 201112 | Environmental contamination because of multidrug-resistant A. baumannii surrounding colonized or infected patients. | Randomized sequential sampling of bed areas | 2,436 environmental samples around 114 patients; 349 samples from HCWs hands and phones from ICUs from 2 London teaching hospitals | To assess the degree of environmental contamination with MRSA in critical care and the likelihood of subsequent new patient acquisition | Phage typing | Of 52 patients colonized with MRSA, 34 (65%) had similar strains found consequently in their environment. |
| Yakupogullari Y et al 200618 | Is airborne transmission of A. baumannii possible? A prospective molecular epidemiologic study in a tertiary-care hospital | Prospective cohort study | 479 environmental samples from 50 unique patient rooms in 4 ICUs at University of Maryland Medical Center | To determine how frequently the environment surrounding the patient becomes contaminated | PGFE | 48% (24/50) of the rooms were positive for MDR A. baumannii. In 17 of 20 instances (85%) (among patients with MDR A. baumannii history), environmental isolates were genetically similar to the patient isolate. |
| Wilson AP et al 200719 | Importance of the environment for patient acquisition of MRSA in the ICU: a baseline study | Prospective cohort study | 628 ICU rooms at a county teaching hospital | To determine the association between room contamination and the status of the occupants | PFGE | 39% of rooms with A. baumannii positive patients were contaminated. 10% of rooms with A. baumannii–negative patients were contaminated as well. 6 of 7 instances (86%), environmental isolates were genetically similar to the patient isolate. |
| 3. Transmission from HCW to patient | ||||||
| Wilson AP et al 200719 | Importance of the environment for patient acquisition of MRSA in the ICU: a baseline study | Randomized sequential sampling of bed areas | 2,436 environmental samples around 114 patients, 349 samples from HCWs hands and phones from ICUs from 2 London teaching hospitals | To assess the degree of environmental contamination with MRSA in critical care and the likelihood of subsequent new patient acquisition | Phage typing | 5 (45%) patients became colonized but with different MRSA strains than the ones in their environment. 25 out 31 (81%) HCW hands were positive for MRSA; however, in no instances were these strains subsequently acquired by patients. |
| Ben-David D et al 200820 | MRSA transmission: the possible importance of unrecognized HCW carriage | Prospective cohort study | 19 burn patients, 133 HCWs from the TICU in Rhode Island Hospital | To detect MRSA acquisition and its source | PFGE | 7 patients and 4 HCWs harbored the same clone A. 2 patients and 1 HCW harbored clone B. Once the HCWs were successfully decolonized, a sustained reduction in MRSA infections occurred. |
| Loftus RW et al 201521 | The epidemiology of S. aureus transmission in the anesthesia work area | Prospective cohort study | 2,170 environmental sites, 2,640 HCW hand cultures, 1,087 patient skin samples from 274 case pairs across 3 major US academic medical centers. | To characterize the epidemiology of bacterial transmission events in the anesthesia work area | PFGE | 2 main phenotypes were identified (P and H). Strong evidence that patient-derived strains were transmitted to subsequent patients who had procedures on the same day. Their primary routes were the environment (66%) and HCW hands (80%). |
| 4. Transmission from environment to patient | ||||||
| Yakupogullari Y et al 200618 | Is airborne transmission of A. baumannii possible? A prospective molecular epidemiologic study in a tertiary-care hospital | Prospective cohort study | 186 air samples and 82 clinical specimens from Turgut Ozal Medical Center | To understand the dynamics of Acinetobacter spread in air | PCR-typing (DiversLab) PFGE | Epidemiological links were found between strains isolated in the air and strains isolated from clinical samples taken from patients discharged weeks earlier and patients who were hospitalized >3 mo later. |
| Wilson AP et al 200719 | Importance of the environment for patient acquisition of MRSA in the ICU: a baseline study | Randomized sequential sampling of bed areas | 2,436 environmental samples around 114 patients, 349 samples from HCWs hands and phones from ICUs from 2 London teaching hospitals | To assess the degree of environmental contamination with MRSA in critical care and the likelihood of subsequent new patient acquisition | Phage typing | 1 patient (2%) subsequently acquired the MRSA in the environment. 5 other patients (11%) became colonized but with different MRSA strains than those in their environment. |
| Loftus RW et al 201521 | The epidemiology of S. aureus transmission in the anesthesia work area | Prospective cohort study | 939 screened patients and 1252 environmental samples in a tertiary acute-care hospital | To investigate the possible routes of transmission of MRSA between MRSA positive patients | spa dru PFGE | Based on epidemiological investigation, 44 cross-transmission cases were identified. Cross-transmission was confirmed molecularly 25% of the time (11/44). In the 11 confirmed cases, patients were the source in 6 instances (55%), patient and the environment in three cases (27%), and only the environment on two instances (18%). |
| Creamer E et al 201222 | Transmission of endemic ST22MRSA-IV on four acute hospital wards investigated using a combination of spa, dru and PFGE typing | Prospective cohort study | 2,170 environmental sites, 2,640 HCW hand cultures, 1,087 patient skin samples from 274 case-pairs across 3 major US academic medical centers | To characterize the epidemiology of bacterial transmission events in the anesthesia work area | PFGE | 2 main phenotypes were identified (P and H). Strong evidence that patient-derived strains were transmitted to subsequent patients who had procedures on the same day. Their primary routes were the environment (66%) and HCW hands (80%). |
| 5. Transmission from environment to HCW | ||||||
| Morgan D et al 20128 | Transfer of multidrug-resistant bacteria to HCWs’ gloves and gowns after patient contact increases with environmental contamination | Prospective cohort study | 603 P-HCW interactions in 6 different ICUs at the University of Maryland Medical Center | To assess the role of environmental contamination in the transmission of MDROs to HCW clothing | PFGE | 33% of P-HCW interactions led to transmission of MDR A. baumannii to HCW G&G. Carbapenem-resistant P. aeruginosa was transmitted 17% of the time. MRSA and VRE were transmitted 14% of time. Of 22 cases, 91% had a strain related to the environment. |
| Hayden MK et al 200816 | Risk of hand or glove contamination after contact with patients colonized with VRE or the colonized patients’ environment | Structured observational study | 27 HCW–patient interactions: 256 patient samples, 1,572 environmental sites, 131 pairs of HCW hands from the MICU of Rush University Medical Center | To estimate the level of hand or glove contamination with VRE among HCW when interacting with the patient or environment | PFGE | From 103 HCWs whose hand samples were negative for VRE when they entered the room, 52% contaminated their hands/gloves after touching the environment, and 70% contaminated their hands/gloves after interacting with the patient and the environment. |
| Barbolla RE et al 200823 | Molecular epidemiology of A. baumannii spread in an adult ICU under an endemic setting | Prospective cohort study | 65 newly admitted patients, 378 environmental samples and 93 HCW hands from the adult ICU at the Sanatorio de la Trinidad-Mitre | To assess the prevalence of carbapenem-resistant clones and their way of spread | PFGE | Frequently touched surfaces close to contaminated patients seemed to play a role in transmissibility perhaps through enhancing staff hand carriage. The decrease of clone if hand carriage correlated with discharge of the last patient with this clone but also its decrease in the environment. The role of personnel hand carriage in environmental contamination was also evident. |
Note. G&G, gowns and gloves; HCW, healthcare worker; ICU, intensive care unit; MDR, multidrug resistant; MDRO, multidrug-resistant organisms; MICU, medical intensive care unit; MRSA, methicillin-resistant S. aureus; MSSA, methicillin-susceptible S. aureus; PFGE, Pulse-field gel electrophoresis; P-HCW, patient to HCW; spa, Staphylococcus protein A; TICU, trauma intensive care unit; VRE, vancomycin-resistant Enterococcus.
Transmission from patient to environment.
In addition to transmitting the MDRO to HCW, a colonized or infected patient may contaminate the surrounding hospital environment. This review highlights 4 studies providing strong evidence to support this pathway (Table 1). Yakupogullari et al18 described a positive correlation between levels of MDR A. baumannii isolated in air samples and proximity to an MDRO-positive patient. Thom et al12 and Munoz-Price et al11 reported that among rooms of patients with recent or current A. baumannii infection, the environmental strain was identical to the patient’s isolate ~85% of the time. Likewise, Wilson et al19 reported that 65% of patients recently colonized with MRSA had subsequently similar strains of MRSA isolated from their environment. In summary, based on the studies identified in our review, existing evidence demonstrating transmission from patient to the environment is highly consistent across MDRO.
Transmission from HCW to patient.
Transmission from HCWs to patients is more common under epidemic conditions,24 which we purposely excluded from this review. Nevertheless, this review highlights 3 prospective studies supporting this transmission route even under endemic conditions. In 2 included studies, the same MRSA clones were isolated from patients and HCWs, which were different from the clones isolated from the environment.19,20 Furthermore, Loftus et al21 described HCW hands as the primary route of patient-to-patient S. aureus transmission across 3 major US academic medical centers. Consequently, decontamination of HCWs was also associated with a sustained reduction in transmission.20 In summary, based on the studies identified in our review, existing evidence demonstrating transmission from HCW to patient is highly consistent for MRSA. However, we were unable to identify eligible high-quality papers providing evidence for this step of transmission for other MDROs included in this review, which demonstrates the complexity of studying this transmission route in real-life hospital settings.
Transmission from environment to patient.
Another critical factor of MDRO transmission is the hospital environment. We identified 4 studies providing evidence supporting this transmission route. Yakupogullari et al18 demonstrated the presence of A. baumannii strains from previously infected patients in hospital air that subsequently infected hospitalized patients 3 months later. Other studies concluded that MRSA patient acquisition from the hospital environment occurred between 2% and 66% of the time.19,21,22 In summary, based on the studies identified in our review, existing evidence demonstrating transmission from environment to patient is moderately consistent; however, most of the available data are focused on MRSA.
Transmission from environment to HCW.
The available evidence also suggests that the hospital environment plays a role in transmission by contaminating the HCW, who may later contaminate another patient as highlighted by 3 prospective studies that utilized PFGE to link MDRO strains. Morgan et al8 showed that 91% of the time when the HCW gowns or gloves are contaminated after interacting with a patient and his/her surrounding environment, the isolated strain is related to the environment. Similarly, Hayden et al16 reported the same phenomenon 52% of the time. Based on the studies identified in our review, existing evidence demonstrating transmission from the environment to the HCW is moderately consistent across different studies and different MDRO.
Overall patient-to-patient transmission
The overall impact of MDRO transmission regardless of its transmission pathway is the transfer of these organisms from colonized or infected patients to other susceptible noncolonized or uninfected hospitalized patients. In this section, we summarize the existing evidence demonstrating the overall end result of hospital transmission of MDROs.
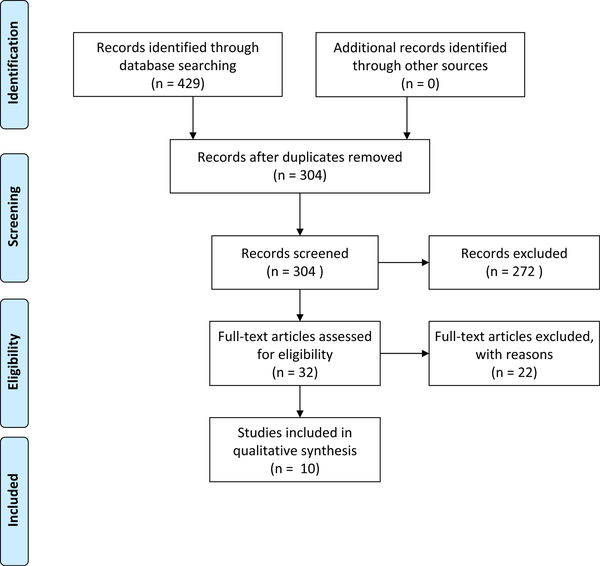
After searching 3 databases, 429 records were identified. After removal of duplicates and elimination of articles based on title and abstract review, 32 full-text articles were reviewed. Ten articles were selected for inclusion (Fig. 3). Exclusion criteria from the previous section were applied. All of the included manuscripts satisfactorily demonstrated the transmission of the same multidrug-resistant strain across patients using modern molecular laboratory techniques.
Fig. 3.
PRISMA flow diagram for literature review on overall patient-to-patient transmission regardless of transmission route.
Table 2 lists summaries of 8 cohort studies and 2 cross-sectional studies. Most of the 8 studies focused on MRSA transmission, whereas the rest investigated carbapenem-resistant A. baumannii (n = 1) and imipenem-resistant P. aeruginosa (IRPA; n = 1). The included studies specifically linked the strains isolated between patients using WGS (n = 4), PFGE (n = 4), multiple-locus variable-number tandem repeat analysis (MLVA; n = 1), and multilocus sequence typing (MLST; n = 1).
Table 2.
Summary of Best Quality Evidence of Overall Patient-to-Patient Transmission Regardless of Transmission Pathway in Acute-Care Settings
| First Author, Year | Title | Study Design | Population | Related Objective | Related Molecular Analysis |
Transmission Event Definition | Related Finding |
|---|---|---|---|---|---|---|---|
| Moore C et al 200825 | Risk factors for MRSA acquisition in roommate contacts of patients colonized or infected with MRSA in an acute-care hospital | Retrospective cohort study | 326 inpatients who shared a room with a patient who had unrecognized MRSA colonization; 198 completed follow-up | To identify risk factors for acquisition of MRSA in patients exposed to an MRSA colonized roommate | PFGE | Patient acquired a strain indistinguishable from the roommate | 25 (12.6%) patients acquired MRSA by 7–10 d after the exposure. |
| Bloemendaal AL et al 200926 | Acquisition and cross transmission of S. aureus in European ICUs | Multicenter cohort study | From 629 screened patients, 316 were at risk of acquiring S. aureus; 45 acquired S. aureus in 6 ICUs in 6 countries | To evaluate the cross-transmission rates of both MSSA and MRSA in different ICUs | MLVA Spa typing | When a patient acquired a strain that was carried by another patient within the 2 weeks preceding acquisition. Isolates were considered genetically highly related if MLVA and spa type were identical or if isolates differed only in a single locus or had a highly related spa repeat sequence. | In 8 of 18 cases (44%), MRSA cross-transmission was identified. |
| Johnson JK et al 200927 | The role of patient-topatient transmission in the acquisition of IRPA colonization in the ICU | Prospective cohort study | 7,071 patients admitted to the medical or surgical ICU; 151 were colonized and 149 acquired IRPA | To quantify the amount of patient-to-patient transmission versus endogenous acquisitions of IRPA | PFGE | Isolates were defined as similar on the basis of the PFGE type, and they were defined as epidemiologically related on the basis of any overlap in hospital length of stay. | 46 (31%) of IRPA acquisitions were defined as cases of patient-to-patient transmission and 28 (19%) were cases of acquisition by the patients’ endogenous flora. |
| Khandavilli S et al 200928 | Utility of spa typing for investigating the local epidemiology of MRSA on a UK ICU | Prospective clinical study | 115 MRSA isolates from ICU patients admitted during a 12-month period | To assess whether if spa typing can be used to investigate epidemiology of MRSA within a UK ICU, as well as the degree of spread between patients | MLST spa typing | For the patients with the same unusual spa type, epidemiological/clinical details were used to investigate if patients were present in the ICU concurrently or within 28 days. | 4 (9%) of 45 new MRSA isolates occurring within 28 days of isolation of an unusual spa type that could have been due to cross-transmission |
| El-Ageery SM et al 201129 | The role of HCWs and environment in transmission of MRSA among patients in a MICU in a Saudi hospital | Prospective cohort study | 117 MICU patients, 25 HCWs, and 12 environmental sites | To determine the clonal relationship and potential routes of transmission of MRSA isolates obtained from patients, HCWs and the environment | PFGE Antibiotyping | Isolates ≥80% similarity were considered to belong to the same pulsotype. Subtypes were assigned to isolates having ≤3 DNA band differences within the same pulsotype. | Several HCWs share isolates with the same PFGE patterns (pulsotype A3 and pulsotype C2) as those isolates from patients (P2, 3, 7), thereby establishing the transmission of MRSA between patients and HCWs. Similarly, MRSA isolates obtained from the environmental samples shared the same PFGE pattern (pulsotype A3) as that isolated from 2 patients (P2,7) and 2 HCWs (H1,9). |
| Irfan S et al 201130 | Molecular and epidemiological characterization of clinical isolates of CRAB from public and private sector intensive care units in Karachi, Pakistan | Cross-sectional study | 50 patients admitted to adult ICUs of a private-sector tertiary-care hospital and of a government hospital | To identify molecular and epidemiological characteristics of hospital-acquired CRAB | Sequence-based multiplex PCR PFGE VTNR | PFGE cluster shared identical or highly similar VNTR profiles | By PFGE, isolates fell into 8 distinct clusters suggesting cross-transmission. |
| Price JR et al 201431 | WGS shows that patient-to-patient transmission rarely accounts for acquisition of S. aureus in an ICU | Prospective cohort study | 680 patients admitted to ICU in a teaching hospital in England, 44 acquired S. aureus (7 were unable to be typed) | To investigate the role of colonized patients as the source of new S. aureus acquisitions | WGS spa typing | Using conventional criteria, acquisition of S. aureus with matching spa type and same susceptibility/resistance pattern of a strain cultured previously from a colonized patient with overlapping ICU stay. When, irrespective of overlapping stay, isolates had a 40 SNVs or less difference. | 7 of 37 (18.9%) acquisitions were transmissions from other colonized patients. |
| Long SW 201432 | Absence of patient-to-patient intrahospital transmission of S. aureus as determined by WGS | Cross-sectional study | Sterile-site S. aureus isolates identified in Houston Medical Clinical Microbiology | To identify patient-to-patient intrahospital transmission using high-resolution genetic analysis | WGS | When isolates pairs had a pairwise distance of 40 SNPs or less with a plausible transmission chain or other epidemiological linkage. | No evidence of transmission of S. aureus between patients with sterile-site infections were identified |
| Amissah NA et al 201533 | Molecular characterization S. aureus isolates transmitted between patients with Buruli ulcer (BU) | Prospective cohort study | 11 BU patients attending Pakro Health Center in the Eastern region of Ghana who were screened for S. aureus | To investigate possible patient-to-patient transmission events during wound care. | MLVF WGS | When the wound of a patient, previously not containing a particular S. aureus genotype, becomes colonized over time by an S. aureus with a genotype that is identical (same cluster type) with the genotype of an S. aureus isolate collected from the wound of another patient. | At least 2 events (18%) of transmission of MRSA between patients were confirmed. |
| Moore G et al 201534 | WGS in hierarchy with PFGE: the utility of this approach to establish possible sources of MRSA cross-transmission | Prospective cohort study | 40 potential transmission events were evaluated. | To explore the utility of WGS in a hierarchical approach with PFGE to help establish possible sources of MRSA cross-transmission in the intensive care setting | WGS PFGE | When donor and recipient isolates were indistinguishable using PFGE and GWS or just PFGE pattern in case of pulsotypes non-dominant in the United Kingdom. | A probable source was identified for 14 of 40 events (35%); PFGE supported links between patients occupying the same bay, the same bed space, adjacent isolation rooms, and different wards. |
Note. CRAB, carbapenem-resistant Acinetobacter baumannii, ICU, intensive care unit; IRPA, imipenem-resistant Pseudomonas aeruginosa; MICU, medical intensive care unit; MLVA, multilocus variable number of tandem repeats analysis; MLVF, multilocus variable number (MLVN) tandem repeat fingerprinting; MRSA, methicillin-resistant S. aureus; MSSA, methicillin-susceptible S. aureus; PFGE, pulse-field gel electrophoresis; SNP, single-nucleotide polymorphism; spa, Staphylococcus protein A; VREfm, Enterococcus faecium; VTNR, variable number tandem repeat; WG, whole-genome sequence.
The overall impact of patient-to-patient transmission reported by these studies varied. For MRSA, the most studied MDRO included in our review, the overall impact of cross-transmission was highly variable (0–44%).
Discussion
Although it would be logistically burdensome to follow the whole transmission process of MDRO from colonized or infected patients to other susceptible patients in real-life healthcare settings, we have illustrated that existing high-quality studies demonstrate each of the 5 components of the complete transmission pathway.
Nevertheless, the published and eligible evidence demonstrating transmission of MDRO from contaminated patients to the environment or to the HCW is more available and consistent than the evidence demonstrating the transmission in the contrary direction (from environment to patient or HCW to patient). However, if we had loosen our eligibility criteria (ie, removing the molecular technique requirement), additional evidence supporting the transmission route from the environment to a subsequent patient could have been identified. Prior room occupancy studies have demonstrated that admission to a room previously occupied by an MRSA-positive patient or a VRE-positive patient significantly increases the odds of the newly admitted patient to acquire these MDROs.35,36 Studying these 2 transmission routes in real-life healthcare settings is indeed even more complicated and burdensome than studying the rest of the potential transmission routes. Nevertheless, more research directly focused on the importance and impact of these 2 specific routes of transmission would greatly inform infection prevention efforts in the acute-care setting. Furthermore, most manuscripts we identified through this scoping review focused on MRSA. More studies are needed on the transmission pathways of other MDROs, particularly recently emergent ones such as CRE.
Additionally, the intercorrelation between transmission routes complicated our ability to measure the individual contributions from each vector of transmission (ie, HCW or healthcare environment). Likewise, the tendency of infection prevention and control efforts to bundle multiple interventions (eg, environmental cleaning plus contact precautions) restricted our ability to separate their effectiveness to prevent patient-to-patient MDRO transmission in hospitals. We need to incorporate new methods in our epidemiological toolkit such as mathematical modeling or benign surrogate markers to segregate these effects and identify interactions between them. For example, Barnes et al37 developed an agent-based model of patient-to-patient transmission to better understand the relative importance of hand hygiene and environmental cleaning. Likewise, Donskey et al38 explored the use of cauliflower mosaic virus DNA as a surrogate marker for study pathogen dissemination, which exemplifies how multiple unique markers can be introduced and followed. New tools such as these could allow us to better inform infection control measures and bundles to focus our efforts only on those measures that are highly effective. However, for these tools to be useful, these models must use parameters and data from high-quality field research based in real-life healthcare settings.
Finally, this scoping review emphasizes the meaningful impact of MDRO patient-to-patient transmission in healthcare settings. For MRSA, the most studied microorganism included in our review, patient-to-patient transmission was observed as much as 44% of the time. Moreover, a large study across multiple ICUs in the United States demonstrated that on average, 21 VRE or MRSA new acquisitions per 1,000 patient days occurred.4 Furthermore, our review is limited only to acute-care settings. Post-acute care and long-term settings may potentially present even higher rates of MDRO transmission. These high levels of transmission are concerning; ~20% of patients who acquire an MDRO go on to develop an infection with this organism post acquisition.27,39,40 Efforts to minimize MDRO acquisition events are critical and should be prioritized. It is essential to further strengthen infection prevention and control efforts in healthcare settings to assure that all patients receive the highest possible quality of care.
Acknowledgments
Financial support. This study was supported by the National Institutes of Health (NIH grant no. K24AI079040 to A. D. Harris) and by the Banting Postdoctoral Fellowship Program administered by the Government of Canada (L. O’Hara).
Footnotes
Conflict of interest. All authors report no conflicts of interest relevant to this article.
References
- 1.Blanco N, Perencevich E, Li SS, et al. Effect of meteorological factors and geographic location on methicillin-resistant staphylococcus aureus and vancomycin-resistant Enterococci colonization in the US. PLoS One 2017;12:e0178254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Corbella X, Pujol M, Ayats J, et al. Relevance of digestive tract colonization in the epidemiology of nosocomial infections due to multiresistant Acinetobacter baumannii. Clin Infect Dis 1996;23:329–334. [DOI] [PubMed] [Google Scholar]
- 3.Bratu S, Landman D, Haag R, et al. Rapid spread of carbapenem-resistant Klebsiella pneumoniae in New York City: a new threat to our antibiotic armamentarium. Arch Intern Med 2005;165:1430–1435. [DOI] [PubMed] [Google Scholar]
- 4.Harris AD, Pineles L, Belton B, et al. Universal glove and gown use and acquisition of antibiotic-resistant bacteria in the ICU: a randomized trial. JAMA 2013;310:1571–1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Causes of antimicrobial (drug) resistance. National Institutes of Allergy and Infectious Diseases website. https://www.niaid.nih.gov/research/antimicrobialresistance-causes. Published December 21, 2011. Accessed September 21, 2018.
- 6.Visalachy S, Palraj KK, Kopula SS, Sekar U. Carriage of multidrug resistant bacteria on frequently contacted surfaces and hands of health care workers. J Clin Diagn Res 2016;10:DC18–DC20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eveillard M, Martin Y, Hidri N, Boussougant Y, Joly-Guillou ML. Carriage of methicillin-resistant Staphylococcus aureus among hospital employees: prevalence, duration, and transmission to households. Infect Control Hosp Epidemiol 2004;25:114–120. [DOI] [PubMed] [Google Scholar]
- 8.Morgan DJ, Rogawski E, Thom KA, et al. Transfer of multidrug-resistant bacteria to healthcare workers’ gloves and gowns after patient contact increases with environmental contamination. Crit Care Med 2012;40: 1045–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morgan DJ, Liang SY, Smith CL, et al. Frequent multidrug-resistant Acinetobacter baumannii contamination of gloves, gowns, and hands of healthcare workers. Infect Control Hosp Epidemiol 2010;31:716–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duckro AN, Blom DW, Lyle EA, Weinstein RA, Hayden MK. Transfer of vancomycin-resistant Enterococci via health care worker hands. Arch Intern Med 2005;165:302–307. [DOI] [PubMed] [Google Scholar]
- 11.Munoz-Price LS, Namias N, Cleary T, et al. Acinetobacter baumannii: association between environmental contamination of patient rooms and occupant status. Infect Control Hosp Epidemiol 2013;34:517–520. [DOI] [PubMed] [Google Scholar]
- 12.Thom KA, Johnson JK, Lee MS, Harris AD. Environmental contamination because of multidrug-resistant Acinetobacter baumannii surrounding colonized or infected patients. Am J Infect Control 2011;39:711–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mitchell A, Spencer M, Edmiston C Jr. Role of healthcare apparel and other health care textiles in the transmission of pathogens: a review of the literature. J Hosp Infect 2015;90:285–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pham MT, Rajić A, Greig JD, Sargeant JM, Papadopoulos A, McEwen SA. A scoping review of scoping reviews: advancing the approach and enhancing the consistency. Res Synth Methods 2014;5:371–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Colquhoun HL, Levac D, O’Brien KK, et al. Scoping reviews: time for clarity in definition, methods, and reporting. J Clin Epidemiol 2014;67: 1291–1294. [DOI] [PubMed] [Google Scholar]
- 16.Hayden MK, Blom DW, Lyle EA, Moore CG, Weinstein RA. Risk of hand or glove contamination after contact with patients colonized with vancomycin-resistant Enterococcus or the colonized patients’ environment. Infect Control Hosp Epidemiol 2008;29:149–154. [DOI] [PubMed] [Google Scholar]
- 17.Schweizer M, Ward M, Cobb S, et al. The epidemiology of methicillin-resistant Staphylococcus aureus on a burn trauma unit. Infect Control Hosp Epidemiol 2012;33:1118–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yakupogullari Y, Otlu B, Ersoy Y, et al. Is airborne transmission of Acinetobacter baumannii possible: a prospective molecular epidemiologic study in a tertiary care hospital. Am J Infect Control 2016;44: 1595–1599. [DOI] [PubMed] [Google Scholar]
- 19.Wilson AP, Hayman S, Whitehouse T, et al. Importance of the environment for patient acquisition of methicillin-resistant staphylococcus aureus in the intensive care unit: a baseline study. Crit Care Med 2007;35:2275–2279. [DOI] [PubMed] [Google Scholar]
- 20.Ben-David D, Mermel LA, Parenteau S. Methicillin-resistant Staphylococcus aureus transmission: the possible importance of unrecognized health care worker carriage. Am J Infect Control 2008;36:93–97. [DOI] [PubMed] [Google Scholar]
- 21.Loftus RW, Koff MD, Brown JR, et al. The epidemiology of Staphylococcus aureus transmission in the anesthesia work area. Anesth Analg 2015; 120:807–818. [DOI] [PubMed] [Google Scholar]
- 22.Creamer E, Shore AC, Rossney AS, et al. Transmission of endemic ST22MRSA-IV on four acute hospital wards investigated using a combination of spa, dru and pulsed-field gel electrophoresis typing. Eur J Clin Microbiol Infect Dis 2012;31:3151–3161. [DOI] [PubMed] [Google Scholar]
- 23.Barbolla RE, Centron D, Maimone S, et al. Molecular epidemiology of Acinetobacter baumannii spread in an adult intensive care unit under an endemic setting. Am J Infect Control 2008;36:444–452. [DOI] [PubMed] [Google Scholar]
- 24.Danzmann L, Gastmeier P, Schwab F, Vonberg RP. Health care workers causing large nosocomial outbreaks: a systematic review. BMC Infect Dis 2013;13:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moore C, Dhaliwal J, Tong A, et al. Risk factors for methicillin-resistant Staphylococcus aureus (MRSA) acquisition in roommate contacts of patients colonized or infected with MRSA in an acute-care hospital. Infect Control Hosp Epidemiol 2008;29:600–606. [DOI] [PubMed] [Google Scholar]
- 26.Bloemendaal AL, Fluit AC, Jansen WM, et al. Acquisition and cross-transmission of Staphylococcus aureus in European intensive care units. Infect Control Hosp Epidemiol 2009;30:117–124. [DOI] [PubMed] [Google Scholar]
- 27.Johnson JK, Smith G, Lee MS, et al. The role of patient-to-patient transmission in the acquisition of imipenem-resistant pseudomonas aeruginosa colonization in the intensive care unit. J Infect Dis 2009;200:900–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khandavilli S, Wilson P, Cookson B, Cepeda J, Bellingan G, Brown J. Utility of spa typing for investigating the local epidemiology of MRSA on a UK intensive care ward. J Hosp Infect 2009;71:29–35. [DOI] [PubMed] [Google Scholar]
- 29.El-Ageery SM, Abo-Shadi MA, Elgendy AM, Alghaithy AA, Kandeel AY. The role of health care workers and environment on transmission of methicillin–resistant Staphylococcus aureus among patients in a medical intensive care unit in a Saudi hospital. J Pure Appl Microbiol 2011;5:1–8. [Google Scholar]
- 30.Irfan S, Turton JF, Mehraj J, et al. Molecular and epidemiological characterisation of clinical isolates of carbapenem-resistant Acinetobacter baumannii from public and private sector intensive care units in Karachi, Pakistan. J Hosp Infect 2011;78:143–148. [DOI] [PubMed] [Google Scholar]
- 31.Price JR, Golubchik T, Cole K, et al. Whole-genome sequencing shows that patient-to-patient transmission rarely accounts for acquisition of Staphylococcus aureus in an intensive care unit. Clin Infect Dis 2014;58: 609–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Long SW, Beres SB, Olsen RJ, Musser JM. Absence of patient-to-patient intrahospital transmission of Staphylococcus aureus as determined by whole-genome sequencing. MBio 2014;5:e01692–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Amissah NA, Chlebowicz MA, Ablordey A, et al. Molecular characterization of staphylococcus aureus isolates transmitted between patients with Buruli ulcer. PLoS Negl Trop Dis 2015;9:e0004049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moore G, Cookson B, Gordon NC, et al. Whole-genome sequencing in hierarchy with pulsed-field gel electrophoresis: the utility of this approach to establish possible sources of MRSA cross-transmission. J Hosp Infect 2015;90:38–45. [DOI] [PubMed] [Google Scholar]
- 35.Huang SS, Datta R, Platt R. Risk of acquiring antibiotic-resistant bacteria from prior room occupants. Arch Intern Med 2006;166:1945–1951. [DOI] [PubMed] [Google Scholar]
- 36.Mitchell B, Digney W, Ferguson J. Prior room occupancy increases risk of methicillin-resistant Staphylococcus aureus acquisition. Healthc Infect 2014;19:135–140. [Google Scholar]
- 37.Barnes SL, Morgan DJ, Harris AD, Carling PC, Thom KA. Preventing the transmission of multidrug-resistant organisms: modeling the relative importance of hand hygiene and environmental cleaning interventions. Infect Control Hosp Epidemiol 2014;35:1156–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Alhmidi H, John A, Mana TC, et al. Evaluation of viral surrogate markers for study of pathogen dissemination during simulations of patient care. Open Forum Infect Dis 2017;4:ofx128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Harris AD, Furuno JP, Roghmann MC, et al. Targeted surveillance of methicillin-resistant Staphylococcus aureus and its potential use to guide empiric antibiotic therapy. Antimicrob Agents Chemother 2010;54:3143–3148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huang SS, Platt R. Risk of methicillin-resistant Staphylococcus aureus infection after previous infection or colonization. Clin Infect Dis 2003;36: 281–285. [DOI] [PubMed] [Google Scholar]