Abstract
There is substantial research being conducted on the relationships between the gut microbiome, the immune response and health and disease. Environmental temperature and heat stress are known to modify the gut microbiome. Changes in core temperature have been linked, in multiple phyla, to altered microbiome composition and function. This raises the question of whether local/regional or whole body thermal therapies which targettumors in the abdomen, peritoneal cavity, or pelvis influence the gut microbiome. To date, there is little information on whether thermal therapy exerts positive or negative effects on the microbiome. This is an intriguing question since there is growing interest in the immunological impact of various thermal therapies.
The goal of this brief review is to highlight research on how environmental conditions, particularly temperature (internal as well as external temperatures) influences the gut microbiome. Given the potential for temperature shifts to modulate gut microbe function and composition, it is likely that various forms of thermal therapy, including hyperthermic intraperitoneal chemotherapy (HIPEC), deep regional, and whole body hyperthermia influence the microbiome in ways that are currently not appreciated. More research is needed to determine whether thermal therapy induced changes in the microbiome occur, and whether they are beneficial or detrimental to the host. Currently, although approaches to microbiome modification such as dietary intervention, fecal transfer, probiotics and prebiotics are being developed, the potential of temperature manipulation has, as yet, not been explored. Therefore, new research could reveal whether perturbations of the microbiome composition that have negative health consequences (dysbiosis) could be an important target for treatment by thermal medicine.
Keywords: Microbiome, Thermal Therapy, Temperature, Immune Response
Introduction:
Thermal medicine has evolved to include a variety of technologies that are linked by the goal of using temperature manipulation in tissues or fluids to reduce the severity pathological conditions, especially cancer. Substantial pre-clinical and clinical evidence exists that demonstrate that thermal manipulation of tissues containing tumors can result in long-lasting suppression of tumor growth when used alone or in combination with other therapies1-5. Whole body thermal therapy is being tested for the treatment of cancer6, inflammatory diseases such as rheumatoid arthritis7, and even psychological conditions such as depression8 . Importantly, in several of these conditions, there is the potential for the temperature of large regions of the GI tract (including cecum and rectum), located in areas directly heated or in close contact with the major bolus of heat delivered to a tumor, to experience significant hyperthermia. As outlined below, the gut microbial content (the microbiota and its genome, often referred to as just the microbiome) has recently been discovered to have a major impact on host biology, including the immune system, and, conversely, the host and its physiological responses to environmental factors are now recognized as major determinants of the microbiome. Indeed, the microbiome is exquisitely sensitive to a variety of external factors. Studies are accumulating showing that an important determinant of microbiome composition and function in plants and animals ranging from kelp, to insects, to amphibians, to poultry are environmental and core body temperatures 9-12. We suggest here the possibility that various thermal therapies being used world-wide have the potential for altering microbiome composition, an effect which could have either positive or negative consequences for health and response to therapies. Therefore learning more about how thermal therapies being used for cancer and other diseases influence the gut microbiome is an important area for future research.
Substantial recent evidence demonstrates critical interactions between the immune system, the gut microbiome and cancer.
That the microbiota and the immune systems, which co-evolved, have regulatory interactions is well established13, however, we are just beginning to appreciate how this relationship plays out in cancer development and response to therapies. Recent observations have supported the conclusion that the overall response to both radiotherapy 14-21 and chemotherapy 22-28 depend on induction of an anti-tumor immune response. Additionally, immunotherapies are inherently dependent on the quality of the immune response29. Therefore, in light of the mutual interaction of the immune system and the microbiome as well as the recognized importance of the anti-tumor immune response in tumor control, it is important to consider how changes in the microbiome affect the anti-tumor immune response and ultimately may regulate response of tumors to therapies, especially immunotherapies.
One of the first groups to look at the role of the microbiome in response to anti-cancer therapies found that the anti-tumor effect of cyclophosphamide was dependent on the gut microbiome and was lost in antibiotic treated or germ-free/specific pathogen free mice; furthermore, they identified an accumulation of a specific population of TH17 cells in the spleens of treated mice30. Another group found that responses to both platinum-based chemotherapy and CpG-oligonucleotide based immunotherapy were significantly reduced following antibiotic depletion of the microbiome in C57BL/6 mice and this loss was dependent on changes in the inflammatory cells in the tumor microenvironment31. In nude mice, engraftment of a rat xenograft tumor altered the microbiome, and treatment (by gavage) with a plant-extract Saponin (GpS) inhibited tumor growth and changed the gut microbiome, such that the microbiome of the treated tumor-bearing mice differed from that of tumor-bearing control mice32. Interestingly, GpS did not alter the microbiome of non-tumor bearing mice over the 10 day treatment period32. These authors concluded that further investigations were warranted to determine whether the effects of GpS on the microbiome (in addition to direct effects on the tumor) are a component of the anti-cancer effect of this treatment. These data support the conclusion that the microbiome does play a role in the response of tumors to chemotherapy and we can expect that in the future the differential roles of direct effects on tumor cells vs. immune effects will become better understood. To date, similar studies in the context of radiotherapy remain to be done33.
Given that the microbiome plays such a role in modulating the immune response, it is important to consider how the microbiome (and factors which may potentially perturb the normal microbiome) may affect responses to current, exciting immunotherapies such as checkpoint inhibitors, which currently have a response rate of about 20%29. Within the last 2-3 years, it has become clear that not only are the microbiomes of responders and non-responders different, but that fecal transfer can actually convey the associated phenotype to recipient mice. It has been reported that the microbiome influences the response to checkpoint inhibitors in mice and patients: anti-CTLA434, anti-PD-1 in melanoma35,36 and other epithelial tumors37. Zitvogel’s group examined a large patient cohort (with lung, renal, and urethral solid tumors) and found that patients who received antibiotics at the time of anti-PD-1 administration had faster relapses and lower overall survival than other patients who did not receive antibiotics and they identified certain species of bacteria associated with this result that when administered to mice, conveyed sensitivity to anti-PD-137. Wargo’s group conducted similar studies on melanoma patients, identified a favorable bacterial profile in responders, and showed that this was associated with a more favorable immune contexture in the tumors of patients and recipient mice35. At the same time, Gajewski’s group reported similar findings in a different group of melanoma patients, also finding that a favorable microbiome profile in responders was associated with enhanced T-cell responses and better tumor control that could be conveyed to a mouse model of melanoma36. Interestingly, each group identified different “favorable” bacteria, indicating that certain, unidentified, mechanisms may be shared by different bacterial species, but that the response to anti-PD-1 is clearly related to the status of the microbiome in both mice and humans. Because of this clear relationship between the status of the microbiome and the anti-tumor immune response and tumor immune contexture and response to therapy, it is important to consider factors that can perturb or sculpt the microbiome. These data could point to ways that manipulation of the microbiome could be developed as a therapeutic approach to improve responses to cancer therapies38-40.
The composition of the gut microbiome is influenced by many environmental variables
The composition and relative proportions of the microbiome are easily perturbed and can be altered by a variety of factors. One of the earliest factors to be identified was diet. Given the known contributions of the microbiota to digestion, it is not surprising that differences in the microbiome of lean vs. obese mice and human volunteers were identified and that transfer of microbiota from either lean or obese mice to recipient mice phenocopied the donor weight gains, indicating that the microbiota of obese mice were better able to harvest energy from the diet41,42. Analysis of the microbiota revealed a difference in the relative proportions of Firmicutes and Bacteroidetes with obese individuals having a higher proportion of Firmicutes 41,42. The role of the microbiota in obesity continues to be a major topic of investigation.
Recently, there has been a growing awareness of how environmental factors influence the outcomes of studies using pre-clinical mouse models. The choices for these parameters (including density, lighting, noise, room temperature) are mandated by The Guide for Care and Use of Laboratory Animals43 and are usually not reported in the literature. However, it is clear that many of these choices have the potential to affect the microbiota in these mice. For instance, a comparison of deer mice captured from the wild vs. deer mice raised in captivity showed that their microbiomes differed significantly, with the wild mice having higher diversity in their gut microbiome communities. When captured mice were let go into the wild and the recaptured a few weeks later, their microbiome had rapidly changed to become like that of mice in the wild demonstrating how sensitive the microbiome is to changes in the environment44. In another study, even transfer to a different, cross-campus facility altered the microbiome45. As concerns have arisen over the reproducibility of experiments, both in the same lab over time and between labs, the potential role of the microbiome in experimental reproducibility has been raised46. It was found that the same strain of mice obtained from different sources47 had different microbiomes and this is an independent source of variability between experiments48. One approach to this problem is development of protocols for standardizing the microbiome of control and experimental mice49 . On the other hand, differences in experimental outcomes which depend on the composition of the microbiome, can tell us a lot about how the microbiome impacts disease processes and accompanying immune responses. For instance, Parker et al50 point out that this could affect conclusions drawn about associations between certain disease phenotypes and the microbiome and furthermore, these authors suggest that experiments be repeated at different facilities with records of environmental factors and microbiome analyses. In an example of a compelling study demonstrating this effect, one group looked at severity of malarial infection in C57Bl/6 mice purchased from different vendors and found that not only did the microbiome differ, but mice varied widely in susceptibility to plasmodium infection and subsequent mortality; interestingly, given the known involvement of the microbiome in immunity, resistant mice had an increased humoral immune response51. This group further demonstrated that treatment with certain bacterial species could confer some degree of protection, suggesting manipulation of the microbiome as a therapeutic approach.
In addition to diet, several other environmental factors can alter the microbiome, including but not limited to water, bedding, light (type and circadian effects), temperature, caging type and how often they are changed, humidity and even the people that come into contact with the mice. The impact of two different bedding materials and four methods of water purification (8 combinations of commonly used choices) on gut microbiome were recently investigated52; this compared caecal and fecal microbiota and found that these two factors could alter the microbiota (primarily in caecal samples) and should be taken into consideration. In a study of the effects of chronic noise, Cui et al found a significant effect on the microbiome including an increase in the Firmicutes: Bacteroidetes ratio, and, in turn, an acceleration of aging related changes in a mouse model of Alzeheimer’s disease53. Another environmental variable that is subject to alterations is light exposure. Routinely, animal facilities maintain mice in 12 hrs of light/ 12 hours of darkness and this is associated with a circadian oscillation in the microbiome which is lost when animals are kept in D/D, and the D/D animals also show an alteration in microbial composition, with an elevation in Clostridia in the small intestine54. This points out that there is likely a time-of-day effect when sampling the microbiome55. Different types of light exposure can have an effect; mice exposed to UV irradiation showed a skewing of the microbiome towards increased Firmicutes: Bacteroidetes ratio56. Furthermore, Circadian disruption can alter the gut microbiome57 and sensitivity to anti-tumor therapies58.
In addition to these environment factors, the methods of collection and storage can also impact the bacterial taxa that are detected. This is particularly relevant to experiments in which the samples will be stored and processed at a later time, such as when samples are collected during field work or longitudinally over the duration of an experiment. Recently published studies have highlighted the biased profiles that the result from different storage methods when compared to results from analysis of freshly obtained samples; for instance, storage in EDTA reduces α-diversity and storage under hypoxic conditions alters the relative abundance of all detected phyla59. These authors found that although each condition causes some change, storage at −80˚C results in the fewest59. A second study tested methods for storage of field specimens (including freeze/thaw, −20˚C, 4˚C, ambient temperature, and several preservatives and identified three with alternation no higher than those found in technical replicates (95% ethanol, Whatman FTA cards, and OMNIgene Gut).60 From these studies it is clear that the methods of collection and storage should be thoughtfully selected and reported.
Evidence that temperature can influence the composition and function of microbiome
In terms of housing choices, there have been a handful of studies which demonstrate that the room temperature in which laboratory mice are housed has the potential to alter gut microbiome. One of these studies examined changes resulting from imposed cold stress (such as housing mice at 4˚C for several days) and preliminary results of this study showed that this exposure can altered the microbiome of research mice61. Our lab has previously reported that the mildly cool temperatures of subthermoneutral housing is sufficient to cause suppression of the anti-tumor immune response and a study by Giles et al62 is, to date, the only study which has reported that housing mice at 22˚C increased the Firmicutes: Bacteroidetes ratio compared to when mice are housed at 30˚C. Worthman and colleagues recently reported that cool housing leads to increased production of bile acids which in turn alter the microbiome to promote thermogenesis in lab mice63. Neither of these papers looked at tumor bearing mice. This is an important area that needs more research to clarify the relationships between housing temperature choice, microbiome composition, immune system development, immune responses, and and related effects of other stressors in preclinical mouse models, all of which have potential to significantly effect outcomes of pre-clinical disease research.
There has been considerable numbers of studies demonstrating that environmental temperature can affect the composition of the bacterial microbiome in a large variety of organisms. This includes land dwelling animals (including arachnids such as deer ticks64) , insects and vertebrates (including amphibians) as well as rodents and humans. Additionally, there have been studies on the impact of ocean temperatures on the microbiome functionality of members of diverse phyla including fish and sea urchins65, and even plants such as kelp12. These studies have revealed that the gut microbiome is very sensitive to ambient and internal temperature. These studies are generating increasing recent interest because of the importance of being able to predict the impact of both short term weather changes and more long-term climate shifts on the health of these species, and their relationship to humans. One very recent study reveals that tropical fish are able to adapt better to changes in their thermal environment by a coordinated adaptation process involving not only the host fish, but also the microbiome, and this interaction is thought to help fish and other organisms better survive temperature extremes66 (see also commentary in ScienceDaily67).
In addition to microbiome composition itself, multiple aspects of gut function, including digestive status, in vertebrates are temperature dependent and it is likely that temperature induced alterations to the gut may directly influence the microbiota which may, in turn, be influenced by, the relationship between digestion and environmental temperature. For example, a recent study9 shows that gut microbiota mediate the relationship between temperature and digestive efficiency and energy assimilation, gut passage time and metabolic response to feeding in ectotherms. Even a biobehavioral response such as “huddling” to conserve body heat is sufficient to alter microbial composition in small mammals and studies have revealed a “co-evolutionary mechanism between gut microbiota and host behavior during the cold to save energy during the winter in endotherms. Huddling is a long conserved interactive behavioral strategy that many mammals use to maximize their survival in harsh environments. Zhang et al. hypothesized that the ability of huddling to alter energy usage and thermoregulation could shape caecal microbiota in small mammals, such as voles68. In their study, voles were maintained either in a group where they could huddle or as separate individuals and exposed to warm (23 ± 1°C) and cold (4 ± 1°C) air temperature. Their research revealed that remodeling of gut microbiota was associated not only with host core temperature but also huddling activity which ultimately serves to orchestrate overall host metabolic and thermal homeostasis; most intriguingly, huddling remodels gut microbiota to reduce energy requirements in a small mammal species during cold exposure.
What are mechanisms by which temperature affects intestinal microbes? A major focus is on the sensitivity of the gut intestinal barrier to temperature, but the mechanisms by which heat stress alters intestinal permeability are not fully understood. Just a single layer of epithelial cells (enterocytes) connected by tight junctions forms the intestinal barrier that controls transport of molecules from the luminal compartment (containing microbial populations) to the lamina propria and the blood vessels which supply the epithelial barrier. Important factors seem to be inflammation and hypoxia, each of which can regulate intestinal “tight junction” (TJ) proteins (such as occludin and claudins) along with proteins such as heat shock proteins, hypoxia-inducible factor (HIF)69,70.
It is important to note that in the face of increased body temperature in response to environmental heat that mammals direct blood to the surface of the body to maximize radiant heat loss, and this change in directed blood flow is accompanied by vasoconstriction of the GI tract71. This may exert tensional stress on tight junctions which enhance leakiness but also may change the conformation of transport proteins. In one study using growing pigs, heat stress reduces the intestinal barrier integrity and favors intestinal glucose transport72. Heat stress (generated by exposure to an environmental temperature of 35 °C for 24 hours was associated with an increased rectal temperature of ~1.6 °C and increased respiration rates of 2 fold72. Numerous other studies have shown that heat stress is associated with an increase in intestinal permeability, not only in whole animal studies, but also from using isolated intestinal segments, which show that heat stress results in increased permeability to endotoxin or dextran (reviewed in Dokladny et al73. In mice using running wheels in a warm environment, body temperature was elevated to ~ 39.5˚C) and exhibited elevated intestinal permeability compared with control animals at a 4 hour time point74. Thus, exercise in the heat is sufficient to alter intestinal epithelial barrier function and tight junction proteins. However, while Doklady et al73 state that there is evidence that prolonged exercise or heat stress can produce an increase in gut permeability, more research is needed on this topic, and using different animal models.
In humans, due to ethical reasons, there is little direct experimental evidence under controlled environments regarding the onset of intestinal permeability classic (i.e., non-exertional) hyperthermic conditions. Instead, most data are available from studies of patients being treated for heat stroke. Among these individuals, frequently a core temperature of 42 °C is reached. Plasma endotoxin, which is used as an indirect measure of intestinal permeability, has been observed to be elevated75. Plasma endotoxin levels were observed to decrease after cooling for approximately an hour but remained higher than individuals who have been at thermal neutral conditions.
The potential for thermal therapies currently in place for the treatment of cancer and other diseases to influence the gut microbiome may be high.
Together these data related to the impact of elevated temperature on gut function and microbial compositions raise intriguing questions regarding the impact of thermal therapies for cancer. For example, 42˚C is often within the target temperature for local or regional hyperthermia treatments to the abdomen. Moreover, in Hyperthermic intraperitoneal chemotherapy (HIPEC) applications, the intestines may be directly bathed in heated chemotherapeutic fluids for an hour or more. Whole body hyperthermia protocols routinely keep mice at a higher core temperature for several hours7,76,77. To date, there have been no analyses of gut microbiome even in experimental tumor bearing mice treated with hyperthermia protocols. And, the opportunity to conduct such studies is growing each year.
A variety of thermal therapies are being tested not only for cancer treatment, but also for the treatment of inflammatory diseases, and even psychological conditions such as depression8. Thermal therapies include external and internal heating devices that have been used for several decades for the treatment of several different types of cancers, both superficial in location (i.e., melanoma, chest wall breast cancer recurrences) and tumors of deeper occurrence, as bladder, cervix, abdominal tumors, sarcomas and other cancer have been developed, and now adding to a growing base of correlative information about the properties of heating. Many of these treatments have been delivered to non-extremity tumors, which are located in the abdomen, or retro-abdominal positions and as such, come very close to regions of the small and large intestine. Careful thermal dosimetry has been conducted in these trials which provide a wealth of information on the characteristics of deep heating. For example, Juang et al78 measured thermal dose not only to the region of the tumor, which had a mean thermal dose of 21.3 +- 16.5 CEM43, but they also measured the thermal dose to the rectum which was 1.6 +- 1.2 CEM43 (CEM43 is a unit of standardization of thermal dose and indicates the “cumulative equivalent minutes at 43˚C” , it is a measure of thermal exposure plus damage79). These data indicate that while most of the thermal dose can be steered toward the tumor, there is also a temperature shift in the regions surrounding the tumor, including in the GI tract. In addition to regional direct heating, temperature of the surrounding tissues also increases due to the passage of normothermic blood through the tumors as its being heated, with the heat being deposited in adjacent cooler tissues. As a result, it is very likely that protocols that are aiming to treat cancer have already been affecting the microbiome.
Another major series of deep hyperthermia for cancer involves cervical cancer as a target. In another example, Lee et al. have measured the effect of modulated electro-hyperthermia on the temperature and blood flow in human cervical cancer80. Their data that regional heating of the pelvic volume not only increased the tumor and peritumoral temperature (measured directly with a temperature probe inserted into the cervical os, but it also increased blood flow into and out of the heating region as measured using 3D color Doppler ultrasound by determining peak systolic velocity/end-diastolic velocity ration (S/D ration) and the resistance index (RI) within blood vessels. Using this heating protocol, all patients exhibited an increased peritumoral temperature of at least 1-2 degrees C, while 3 patients had an increase of approximately 3.5 degrees C, which was maintained for at least 30 minutes.
Conclusions and Future Studies:
The gut microbiome exerts multiple levels of control on the immune system, inflammation, metabolism and energy usage and more, all of which influences the homeostatic balance. Disruption in the interactions between the microbiome and the host can lead to increased risk of several diseases, including cancer, autoimmunity, diabetes and other diseases. Environmental factors have a major impact on the homeostatic control of the microbiome and temperature shifts in core body emerge as a major factor which can impact microbiome function, in part, because of the sensitivity of the gut epithelium to temperature.
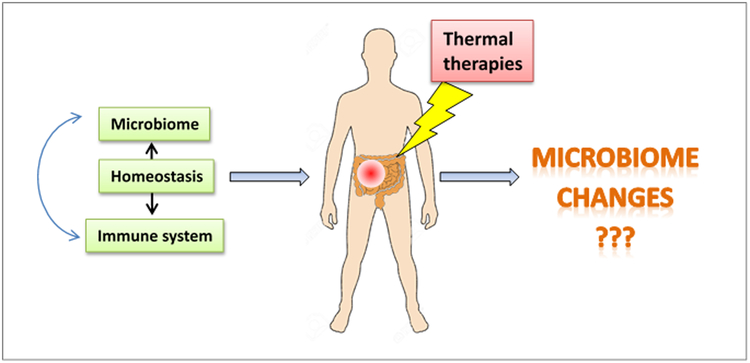
Thermal medicine applications, particularly those treatments which deliver local/regional heating to pelvic, abdominal, or cervical cavities, could be impacting the gut microbiome and thus present important opportunities to assess the impact of temperature on the human gut microbiome composition and function (Fig. 1). Thermal dosimetry or blood flow measurements amply demonstrate the potential for heated blood to warm regions of the GI tract. Similarly, in mouse studies, whole body hyperthermia as well as local heating procedures is likely creating heat stress or mild heat stroke-like conditions which could be affecting the gut microbiome.
Figure 1.
Under normal conditions, the microbiome and the immune system interact to regulate each other and maintain a homeostatic balance. The composition of the microbiome is sensitive to environmental factors such as diet and a variety of other stressors (including temperature) and dysbiosis is associated with several disease states. It has been reported that the composition of the microbiome directly regulates response to anti-cancer chemotherapy and immunotherapy. However, the effect of thermal therapy of the microbiome and anti-tumor immune response is unknown.
At the current time, we do not know whether thermal medicine applications can alter the microbiome and this is an important question for future research, not only in patients, but also in animal models, particularly mice, that are used to study thermal applications for diseases such as cancer, arthritis, diabetes and other diseases. Conversely, many humans suffer from conditions related to a dysfunctional microbiome. It could be very important to test whether heat treatments are able to modulate the microbiome and help re-establish a more “healthy” condition. Without a doubt, new research focusing on cellular and molecular mechanisms by which hyperthermia influences the microbiome of the gut is needed. At the present time, thermal therapy applications offer one of the only opportunities to obtain bodily fluids or fecal samples from patients undergoing hyperthermia to areas containing segments of the gut. This type of study may reveal significant clinical and therapeutic implications.
Acknowledgments:
This research was supported by NIH grants CA205246 and CA099326, The Roswell Park Alliance Foundation, and used Shared Resources supported by the Roswell Park Cancer Institute’s Comprehensive Cancer Center Support Grant CA016056.
Footnotes
Disclosure of Interest: The authors report no conflict of interest.
Contributor Information
B.L. Hylander, Dept. of Immunology, Roswell Park Comprehensive Cancer Center, Elm and Carlton St, Buffalo, NY, 14263.
E.A Repasky, Dept. of Immunology, Roswell Park Comprehensive Cancer Center, Elm and Carlton St, Buffalo, NY, 14263.
REFERENCES:
- 1.van der Zee J & van Rhoon GC Cervical cancer: radiotherapy and hyperthermia. Int J Hyperthermia 22, 229–234 (2006). [DOI] [PubMed] [Google Scholar]
- 2.Issels RD, Lindner LH, Verweij J, Wessalowski R, Reichardt P, Wust P, Ghadjar P, Hohenberger P, Angele M, Salat C, Vujaskovic Z, Daugaard S, Mella O, Mansmann U, Durr HR, Knosel T, Abdel-Rahman S, Schmidt M, Hiddemann W, Jauch KW, Belka C & Gronchi A Effect of Neoadjuvant Chemotherapy Plus Regional Hyperthermia on Long-term Outcomes Among Patients With Localized High-Risk Soft Tissue Sarcoma: The EORTC 62961-ESHO 95 Randomized Clinical Trial. JAMA Oncol 4, 483–492 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mauri G, Nicosia L, Xu Z, Di Pietro S, Monfardini L, Bonomo G, Varano GM, Prada F, Della Vigna P & Orsi F Focused ultrasound: tumour ablation and its potential to enhance immunological therapy to cancer. Br J Radiol 91, 20170641 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jones EL, Oleson JR, Prosnitz LR, Samulski TV, Vujaskovic Z, Yu D, Sanders LL & Dewhirst MW Randomized trial of hyperthermia and radiation for superficial tumors. J Clin Oncol 23, 3079–3085 (2005). [DOI] [PubMed] [Google Scholar]
- 5.Pavlov MJ, Ceranic MS, Latincic SM, Sabljak PV, Kecmanovic DM & Sugarbaker PH Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for the treatment of advanced epithelial and recurrent ovarian carcinoma: a single center experience. Int J Hyperthermia 34, 564–569 (2018). [DOI] [PubMed] [Google Scholar]
- 6.Bull JM, Scott GL, Strebel FR, Nagle VL, Oliver D, Redwine M, Rowe RW, Ahn CW & Koch SM Fever-range whole-body thermal therapy combined with cisplatin, gemcitabine, and daily interferon-alpha: a description of a phase I-II protocol. Int J Hyperthermia 24, 649–662 (2008). [DOI] [PubMed] [Google Scholar]
- 7.Lee CT, Kokolus KM, Leigh ND, Capitano M, Hylander BL & Repasky EA Defining immunological impact and therapeutic benefit of mild heating in a murine model of arthritis. PLoS One 10, e0120327 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Janssen CW, Lowry CA, Mehl MR, Allen JJ, Kelly KL, Gartner DE, Medrano A, Begay TK, Rentscher K, White JJ, Fridman A, Roberts LJ, Robbins ML, Hanusch KU, Cole SP & Raison CL Whole-Body Hyperthermia for the Treatment of Major Depressive Disorder: A Randomized Clinical Trial. JAMA Psychiatry 73, 789–795 (2016). [DOI] [PubMed] [Google Scholar]
- 9.Fontaine SS, Novarro AJ & Kohl KD Environmental temperature alters the digestive performance and gut microbiota of a terrestrial amphibian. J Exp Biol 221(2018). [DOI] [PubMed] [Google Scholar]
- 10.Sohail MU, Hume ME, Byrd JA, Nisbet DJ, Shabbir MZ, Ijaz A & Rehman H Molecular analysis of the caecal and tracheal microbiome of heat-stressed broilers supplemented with prebiotic and probiotic. Avian Pathol 44, 67–74 (2015). [DOI] [PubMed] [Google Scholar]
- 11.Thapa S, Zhang Y & Allen MS Effects of temperature on bacterial microbiome composition in Ixodes scapularis ticks. Microbiologyopen 8, e00719 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Minich JJ, Morris MM, Brown M, Doane M, Edwards MS, Michael TP & Dinsdale EA Elevated temperature drives kelp microbiome dysbiosis, while elevated carbon dioxide induces water microbiome disruption. PLoS One 13, e0192772 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hooper LV, Littman DR & Macpherson AJ Interactions between the microbiota and the immune system. Science 336, 1268–1273 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Demaria S, Ng B, Devitt ML, Babb JS, Kawashima N, Liebes L & Formenti SC Ionizing radiation inhibition of distant untreated tumors (abscopal effect) is immune mediated. Int J Radiat Oncol Biol Phys 58, 862–870 (2004). [DOI] [PubMed] [Google Scholar]
- 15.Sharp HJ, Wansley EK, Garnett CT, Chakraborty M, Camphausen K, Schlom J & Hodge JW Synergistic antitumor activity of immune strategies combined with radiation. Front Biosci 12, 4900–4910 (2007). [DOI] [PubMed] [Google Scholar]
- 16.Shiao SL & Coussens LM The tumor-immune microenvironment and response to radiation therapy. J Mammary Gland Biol Neoplasia 15, 411–421 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Demaria S & Formenti SC Role of T lymphocytes in tumor response to radiotherapy. Front Oncol 2, 95 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ludgate CM Optimizing cancer treatments to induce an acute immune response: radiation Abscopal effects, PAMPs, and DAMPs. Clin Cancer Res 18, 4522–4525 (2012). [DOI] [PubMed] [Google Scholar]
- 19.Zeng J, Harris TJ, Lim M, Drake CG & Tran PT Immune modulation and stereotactic radiation: improving local and abscopal responses. Biomed Res Int 2013, 658126 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zitvogel L, Galluzzi L, Smyth MJ & Kroemer G Mechanism of action of conventional and targeted anticancer therapies: reinstating immunosurveillance. Immunity 39, 74–88 (2013). [DOI] [PubMed] [Google Scholar]
- 21.Gerber SA, Lim JY, Connolly KA, Sedlacek AL, Barlow ML, Murphy SP, Egilmez NK & Lord EM Radio-responsive tumors exhibit greater intratumoral immune activity than nonresponsive tumors. Int J Cancer 134, 2383–2392 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Apetoh L, Ghiringhelli F, Tesniere A, Obeid M, Ortiz C, Criollo A, Mignot G, Maiuri MC, Ullrich E, Saulnier P, Yang H, Amigorena S, Ryffel B, Barrat FJ, Saftig P, Levi F, Lidereau R, Nogues C, Mira JP, Chompret A, Joulin V, Clavel-Chapelon F, Bourhis J, Andre F, Delaloge S, Tursz T, Kroemer G & Zitvogel L Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med 13, 1050–1059 (2007). [DOI] [PubMed] [Google Scholar]
- 23.Mattarollo SR, Loi S, Duret H, Ma Y, Zitvogel L & Smyth MJ Pivotal role of innate and adaptive immunity in anthracycline chemotherapy of established tumors. Cancer Res 71, 4809–4820 (2011). [DOI] [PubMed] [Google Scholar]
- 24.Bracci L, Schiavoni G, Sistigu A & Belardelli F Immune-based mechanisms of cytotoxic chemotherapy: implications for the design of novel and rationale-based combined treatments against cancer. Cell Death Differ 21, 15–25 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.de Biasi AR, Villena-Vargas J & Adusumilli PS Cisplatin-induced antitumor immunomodulation: a review of preclinical and clinical evidence. Clin Cancer Res 20, 5384–5391 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Emens LA & Middleton G The interplay of immunotherapy and chemotherapy: harnessing potential synergies. Cancer Immunol Res 3, 436–443 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Galluzzi L, Buque A, Kepp O, Zitvogel L & Kroemer G Immunological Effects of Conventional Chemotherapy and Targeted Anticancer Agents. Cancer Cell 28, 690–714 (2015). [DOI] [PubMed] [Google Scholar]
- 28.Zitvogel L, Kepp O & Kroemer G Immune parameters affecting the efficacy of chemotherapeutic regimens. Nat Rev Clin Oncol 8, 151–160 (2011). [DOI] [PubMed] [Google Scholar]
- 29.Galluzzi L, Chan TA, Kroemer G, Wolchok JD & Lopez-Soto A The hallmarks of successful anticancer immunotherapy. Sci Transl Med 10(2018). [DOI] [PubMed] [Google Scholar]
- 30.Viaud S, Saccheri F, Mignot G, Yamazaki T, Daillere R, Hannani D, Enot DP, Pfirschke C, Engblom C, Pittet MJ, Schlitzer A, Ginhoux F, Apetoh L, Chachaty E, Woerther PL, Eberl G, Berard M, Ecobichon C, Clermont D, Bizet C, Gaboriau-Routhiau V, Cerf-Bensussan N, Opolon P, Yessaad N, Vivier E, Ryffel B, Elson CO, Dore J, Kroemer G, Lepage P, Boneca IG, Ghiringhelli F & Zitvogel L The intestinal microbiota modulates the anticancer immune effects of cyclophosphamide. Science 342, 971–976 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Iida N, Dzutsev A, Stewart CA, Smith L, Bouladoux N, Weingarten RA, Molina DA, Salcedo R, Back T, Cramer S, Dai RM, Kiu H, Cardone M, Naik S, Patri AK, Wang E, Marincola FM, Frank KM, Belkaid Y, Trinchieri G & Goldszmid RS Commensal bacteria control cancer response to therapy by modulating the tumor microenvironment. Science 342, 967–970 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen L, Tai WC, Brar MS, Leung FC & Hsiao WL Tumor grafting induces changes of gut microbiota in athymic nude mice in the presence and absence of medicinal Gynostemma saponins. PLoS One 10, e0126807 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Al-Qadami G, Van Sebille Y, Le H & Bowen J Gut microbiota: implications for radiotherapy response and radiotherapy-induced mucositis. Expert Rev Gastroenterol Hepatol 13, 485–496 (2019). [DOI] [PubMed] [Google Scholar]
- 34.Vetizou M, Pitt JM, Daillere R, Lepage P, Waldschmitt N, Flament C, Rusakiewicz S, Routy B, Roberti MP, Duong CP, Poirier-Colame V, Roux A, Becharef S, Formenti S, Golden E, Cording S, Eberl G, Schlitzer A, Ginhoux F, Mani S, Yamazaki T, Jacquelot N, Enot DP, Berard M, Nigou J, Opolon P, Eggermont A, Woerther PL, Chachaty E, Chaput N, Robert C, Mateus C, Kroemer G, Raoult D, Boneca IG, Carbonnel F, Chamaillard M & Zitvogel L Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science 350, 1079–1084 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gopalakrishnan V, Spencer CN, Nezi L, Reuben A, Andrews MC, Karpinets TV, Prieto PA, Vicente D, Hoffman K, Wei SC, Cogdill AP, Zhao L, Hudgens CW, Hutchinson DS, Manzo T, Petaccia de Macedo M, Cotechini T, Kumar T, Chen WS, Reddy SM, Szczepaniak Sloane R, Galloway-Pena J, Jiang H, Chen PL, Shpall EJ, Rezvani K, Alousi AM, Chemaly RF, Shelburne S, Vence LM, Okhuysen PC, Jensen VB, Swennes AG, McAllister F, Marcelo Riquelme Sanchez E, Zhang Y, Le Chatelier E, Zitvogel L, Pons N, Austin-Breneman JL, Haydu LE, Burton EM, Gardner JM, Sirmans E, Hu J, Lazar AJ, Tsujikawa T, Diab A, Tawbi H, Glitza IC, Hwu WJ, Patel SP, Woodman SE, Amaria RN, Davies MA, Gershenwald JE, Hwu P, Lee JE, Zhang J, Coussens LM, Cooper ZA, Futreal PA, Daniel CR, Ajami NJ, Petrosino JF, Tetzlaff MT, Sharma P, Allison JP, Jenq RR & Wargo JA Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science 359, 97–103 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Matson V, Fessler J, Bao R, Chongsuwat T, Zha Y, Alegre ML, Luke JJ & Gajewski TF The commensal microbiome is associated with anti-PD-1 efficacy in metastatic melanoma patients. Science 359, 104–108 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Routy B, Le Chatelier E, Derosa L, Duong CPM, Alou MT, Daillere R, Fluckiger A, Messaoudene M, Rauber C, Roberti MP, Fidelle M, Flament C, Poirier-Colame V, Opolon P, Klein C, Iribarren K, Mondragon L, Jacquelot N, Qu B, Ferrere G, Clemenson C, Mezquita L, Masip JR, Naltet C, Brosseau S, Kaderbhai C, Richard C, Rizvi H, Levenez F, Galleron N, Quinquis B, Pons N, Ryffel B, Minard-Colin V, Gonin P, Soria JC, Deutsch E, Loriot Y, Ghiringhelli F, Zalcman G, Goldwasser F, Escudier B, Hellmann MD, Eggermont A, Raoult D, Albiges L, Kroemer G & Zitvogel L Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science 359, 91–97 (2018). [DOI] [PubMed] [Google Scholar]
- 38.McQuade JL, Daniel CR, Helmink BA & Wargo JA Modulating the microbiome to improve therapeutic response in cancer. Lancet Oncol 20, e77–e91 (2019). [DOI] [PubMed] [Google Scholar]
- 39.Fessler JL & Gajewski TF The Microbiota: A New Variable Impacting Cancer Treatment Outcomes. Clin Cancer Res 23, 3229–3231 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fessler J, Matson V & Gajewski TF Exploring the emerging role of the microbiome in cancer immunotherapy. J Immunother Cancer 7, 108 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ley RE, Turnbaugh PJ, Klein S & Gordon JI Microbial ecology: human gut microbes associated with obesity. Nature 444, 1022–1023 (2006). [DOI] [PubMed] [Google Scholar]
- 42.Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER & Gordon JI An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 444, 1027–1031 (2006). [DOI] [PubMed] [Google Scholar]
- 43.Animals., C.f.t.U.o.t.G.f.t.C.a.U.o.L. Guide for the Cared and Use of Laboratory Animals. 8th ed., (The National Academies Press, Washington, DC, 2012). [Google Scholar]
- 44.Schmidt E, Mykytczuk N & Schulte-Hostedde AI Effects of the captive and wild environment on diversity of the gut microbiome of deer mice (Peromyscus maniculatus). Isme j 13, 1293–1305 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ma BW, Bokulich NA, Castillo PA, Kananurak A, Underwood MA, Mills DA & Bevins CL Routine habitat change: a source of unrecognized transient alteration of intestinal microbiota in laboratory mice. PLoS One 7, e47416 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Franklin CL & Ericsson AC Microbiota and reproducibility of rodent models. Lab Anim (NY) 46, 114–122 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hufeldt MR, Nielsen DS, Vogensen FK, Midtvedt T & Hansen AK Variation in the gut microbiota of laboratory mice is related to both genetic and environmental factors. Comp Med 60, 336–347 (2010). [PMC free article] [PubMed] [Google Scholar]
- 48.Servick K Of mice and microbes. Science 353, 741–743 (2016). [DOI] [PubMed] [Google Scholar]
- 49.McCoy KD, Geuking MB & Ronchi F Gut Microbiome Standardization in Control and Experimental Mice. Curr Protoc Immunol 117, 23.21.21–23.21.13 (2017). [DOI] [PubMed] [Google Scholar]
- 50.Parker KD, Albeke SE, Gigley JP, Goldstein AM & Ward NL Microbiome Composition in Both Wild-Type and Disease Model Mice Is Heavily Influenced by Mouse Facility. Front Microbiol 9, 1598 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Villarino NF, LeCleir GR, Denny JE, Dearth SP, Harding CL, Sloan SS, Gribble JL, Campagna SR, Wilhelm SW & Schmidt NW Composition of the gut microbiota modulates the severity of malaria. Proc Natl Acad Sci U S A 113, 2235–2240 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bidot WA, Ericsson AC & Franklin CL Effects of water decontamination methods and bedding material on the gut microbiota. PLoS One 13, e0198305 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cui B, Su D, Li W, She X, Zhang M, Wang R & Zhai Q Effects of chronic noise exposure on the microbiome-gut-brain axis in senescence-accelerated prone mice: implications for Alzheimer's disease. J Neuroinflammation 15, 190 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wu G, Tang W, He Y, Hu J, Gong S, He Z, Wei G, Lv L, Jiang Y, Zhou H & Chen P Light exposure influences the diurnal oscillation of gut microbiota in mice. Biochem Biophys Res Commun 501, 16–23 (2018). [DOI] [PubMed] [Google Scholar]
- 55.Turner PV The role of the gut microbiota on animal model reproducibility. Animal Model Exp Med 1, 109–115 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ghaly S, Kaakoush NO, Lloyd F, Gordon L, Forest C, Lawrance IC & Hart PH Ultraviolet Irradiation of Skin Alters the Faecal Microbiome Independently of Vitamin D in Mice. Nutrients 10(2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Deaver JA, Eum SY & Toborek M Circadian Disruption Changes Gut Microbiome Taxa and Functional Gene Composition. Front Microbiol 9, 737 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Antoch MP, Kondratov RV & Takahashi JS Circadian clock genes as modulators of sensitivity to genotoxic stress. Cell Cycle 4, 901–907 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jenkins SV, Vang KB, Gies A, Griffin RJ, Jun SR, Nookaew I & Dings RPM Sample storage conditions induce post-collection biases in microbiome profiles. BMC Microbiol 18, 227 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Song SJ, Amir A, Metcalf JL, Amato KR, Xu ZZ, Humphrey G & Knight R Preservation Methods Differ in Fecal Microbiome Stability, Affecting Suitability for Field Studies. mSystems 1(2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nicholls HT, Krisko TI, LeClair KB, Banks AS & Cohen DE Regulation of Adaptive Thermogenesis by the Gut Microbiome. FASEB journal 30, Abstract #854.852 (2016). [Google Scholar]
- 62.GilGiles DA, Moreno-Fernandez ME, Stankiewicz TE, Graspeuntner S, Cappelletti M, Wu D, Mukherjee R, Chan CC, Lawson MJ, Klarquist J, Sunderhauf A, Softic S, Kahn CR, Stemmer K, Iwakura Y, Aronow BJ, Karns R, Steinbrecher KA, Karp CL, Sheridan R, Shanmukhappa SK, Reynaud D, Haslam DB, Sina C, Rupp J, Hogan SP & Divanovic S Thermoneutral housing exacerbates nonalcoholic fatty liver disease in mice and allows for sex-independent disease modeling. Nat Med 23, 829–838 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Worthmann A, John C, Ruhlemann MC, Baguhl M, Heinsen FA, Schaltenberg N, Heine M, Schlein C, Evangelakos I, Mineo C, Fischer M, Dandri M, Kremoser C, Scheja L, Franke A, Shaul PW & Heeren J Cold-induced conversion of cholesterol to bile acids in mice shapes the gut microbiome and promotes adaptive thermogenesis. Nat Med 23, 839–849 (2017). [DOI] [PubMed] [Google Scholar]
- 64.Thapa S, Zhang Y & Allen MS Effects of temperature on bacterial microbiome composition in Ixodes scapularis ticks. Microbiologyopen, e00719 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Brothers CJ, Van Der Pol WJ, Morrow CD, Hakim JA, Koo H & McClintock JB Ocean warming alters predicted microbiome functionality in a common sea urchin. Proc Biol Sci 285(2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kokou F, Sasson G, Nitzan T, Doron-Faigenboim A, Harpaz S, Cnaani A & Mizrahi I Host genetic selection for cold tolerance shapes microbiome composition and modulates its response to temperature. Elife 7(2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Commentary. Tropical fish adapt to cold temperatures in coordination with their microbiome. ScienceDaily November 20(2018). [Google Scholar]
- 68.Zhang XY, Sukhchuluun G, Bo TB, Chi QS, Yang JJ, Chen B, Zhang L & Wang DH Huddling remodels gut microbiota to reduce energy requirements in a small mammal species during cold exposure. Microbiome 6, 103 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Qi H, Wang P, Liu C, Li M, Wang S, Huang Y & Wang F Involvement of HIF-1alpha in MLCK-dependent endothelial barrier dysfunction in hypoxia. Cell Physiol Biochem 27, 251–262 (2011). [DOI] [PubMed] [Google Scholar]
- 70.Yamagata K, Tagami M, Takenaga F, Yamori Y & Itoh S Hypoxia-induced changes in tight junction permeability of brain capillary endothelial cells are associated with IL-1beta and nitric oxide. Neurobiol Dis 17, 491–499 (2004). [DOI] [PubMed] [Google Scholar]
- 71.Lambert GP Stress-induced gastrointestinal barrier dysfunction and its inflammatory effects. J Anim Sci 87, E101–108 (2009). [DOI] [PubMed] [Google Scholar]
- 72.Pearce SC, Mani V, Boddicker RL, Johnson JS, Weber TE, Ross JW, Rhoads RP, Baumgard LH & Gabler NK Heat stress reduces intestinal barrier integrity and favors intestinal glucose transport in growing pigs. PLoS One 8, e70215 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dokladny K, Zuhl MN & Moseley PL Intestinal epithelial barrier function and tight junction proteins with heat and exercise. J Appl Physiol (1985) 120, 692–701 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Costa KA, Soares AD, Wanner SP, Santos R, Fernandes SO, Martins Fdos S, Nicoli JR, Coimbra CC & Cardoso VN L-arginine supplementation prevents increases in intestinal permeability and bacterial translocation in male Swiss mice subjected to physical exercise under environmental heat stress. J Nutr 144, 218–223 (2014). [DOI] [PubMed] [Google Scholar]
- 75.Bouchama A, Parhar RS, el-Yazigi A, Sheth K & al-Sedairy S Endotoxemia and release of tumor necrosis factor and interleukin 1 alpha in acute heatstroke. J Appl Physiol (1985) 70, 2640–2644 (1991). [DOI] [PubMed] [Google Scholar]
- 76.Appenheimer MM, Girard RA, Chen Q, Wang WC, Bankert KC, Hardison J, Bain MD, Ridgley F, Sarcione EJ, Buitrago S, Kothlow S, Kaspers B, Robert J, Rose-John S, Baumann H & Evans SS Conservation of IL-6 trans-signaling mechanisms controlling L-selectin adhesion by fever-range thermal stress. Eur J Immunol 37, 2856–2867 (2007). [DOI] [PubMed] [Google Scholar]
- 77.Sakaguchi Y, Makino M, Kaneko T, Stephens LC, Strebel FR, Danhauser LL, Jenkins GN & Bull JM Therapeutic efficacy of long duration-low temperature whole body hyperthermia when combined with tumor necrosis factor and carboplatin in rats. Cancer Res 54, 2223–2227 (1994). [PubMed] [Google Scholar]
- 78.Juang T, Stauffer PR, Craciunescu OA, Maccarini PF, Yuan Y, Das SK, Dewhirst MW, Inman BA & Vujaskovic Z Thermal dosimetry characteristics of deep regional heating of non-muscle invasive bladder cancer. Int J Hyperthermia 30, 176–183 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sapareto SA & Dewey WC Thermal dose determination in cancer therapy. Int J Radiat Oncol Biol Phys 10, 787–800 (1984). [DOI] [PubMed] [Google Scholar]
- 80.Lee SY, Kim JH, Han YH & Cho DH The effect of modulated electro-hyperthermia on temperature and blood flow in human cervical carcinoma. Int J Hyperthermia 34, 953–960 (2018). [DOI] [PubMed] [Google Scholar]