Abstract
Cryoablation (CA) is unique as the singular energy deprivation therapy that impacts all cellular processes. CA is independent of cell cycle stage and degree of cellular stemness. Importantly, CA is typically applied as a non-repetitive (single session) treatment that does not support adaptative mutagenesis as do many repetitive therapies. CA is characterized by the launch of multiple forms of cell death including a) ice-related physical damage, b) initiation of cellular stress responses (kill switch activation) and launch of necrosis and apoptosis, c) vascular stasis and d) likely activation of ablative immune responses. CA is not without limitation related to the thermal gradient formed between cryoprobe surface (~−185 °C) and the distal surface of the freeze zone (~ 0 °C) requiring freeze margin extension beyond the tumor boundary (up to ~ 1 cm). This limitation is mitigated in part by commonly applied dual freeze thaw cycles and the use of freeze sensitizing adjuvants. This review will (1) identify the cascade of damaging effects of the freeze – thaw process, its physical and molecular-based relationships, (2) a likely immunological involvement (abscopic effect), and (3) explore the use of freeze-sensitizing adjuvants necessary to limit freezing beyond the tumor margin.
Keywords: cryoablation, cryotherapy, thermal therapy, cryo-immunology, adjuvants, cancer, freezing
Introduction
The use of combinatorial treatment paradigms is emerging as new generation “gold standards” of cancer therapy as long applied monotherapies have not often provided a curative response. Combinatorial treatments both within and between radiotherapy and chemotherapy have emerged as standards of care despite concern over further mutagenesis common to repetitive dosing. A unique feature of cryoablation (CA) is that the chill-freeze process disrupts all cellular processes through heat extraction which causes the initiation of diverse cell death processes while, importantly, not providing time for defensive mutation in cancer cells [1].
The impact of the freeze-thaw process on a targeted tissue is well recognized for the physical damage due to ice formation both intra-and extra-cellularly. Freezing, however, does not provide uniform damage across the targeted tumor. Absolute cell destruction is associated with proximity to the cryoprobe but less so in the distal regions of the freeze zone. While a second freeze cycle extends the zone of absolute destruction, in practice, a positive (excess) freeze margin beyond the tumor’s distal boundary is necessary to assure maximum lethality within the targeted tumor region [2]. With this practice some damage to normal tissue can be anticipated.
How then can damage to the freeze zone periphery be limited thereby enabling a greater degree of precision to CA? A series of post-freeze observations revealed that at milder subfreezing temperatures, typically associated within the outer 1cm of a frozen mass, gene regulated cell death or apoptosis was initiated [3]. Subsequent studies demonstrated that at these temperatures the intrinsic (mitochondrial-based) apoptotic pathway was activated, and at lower temperatures the extrinsic (membrane-based) apoptotic pathway was apparent [4,5]. These observations led to the realization that cell death could be improved in the freeze zone periphery with apoptosis-inducing adjuvants [6-9]. Hence, greater precision may be possible even with a reduced positive freeze margin and elevated nadir temperatures.
It was reported that secondary tumors would transitionally regress following freezing of the primary tumor [10-12]. This observation led to the belief that a systemic cryo-immunological response (CIR) was possible [13]. When cancer cells are ruptured due to intracellular freezing or the necrosis that follows, numerous cell components with antigenic potential are released including chemokines and cytokines, DNA, RNAs, proteins and membrane fragments [14]. Many of these components are proinflammatory attracting various white blood cells (macrophages, dendritic cells, T-cells, etc.) and may result in the launch of an innate immune response in some but not all cancers studied. Reports indicate that CIR can be enhanced with combinatorial agents such as cyclosphosamide or heat-cold sequencing [15]. However, due to the uncertainty of both the types (qualitative) and levels (quantitative) of danger signaling elements released during variable freeze-thaw protocols, the mechanisms of the immune-stimulating process has been difficult to identify and even observe with consistency.
Cryoablation provides more complex, sequenced effects on targeted cells than initially recognized [1]. Initial freeze rupture occurs during the freeze process and is observable immediately post-thaw. Partially damaged cells will undergo necrosis post-thaw due to unrepairable damage to cell membranes and tumor hypoxia following damage to tumor capillaries. Apoptosis occurs primarily in the frozen periphery within the first 24-hours post-thaw and may transition to secondary necrosis with increased hypoxia.
This review will detail the mechanisms of cell death following CA, review the scope of potential adjuvants that support a combinatorial treatment paradigm and identify the putative immunological consequence of CA.
Mechanisms of Cell Death following Cryoablation
Events of the freeze-thaw cycle
The freezing process is initiated by the activation of one or more cryoprobes appropriately placed to treat a tissue mass of certain geometry often with intraoperative ultrasound visualization. CT and MRI guidance can also be utilized. Probe positioning is accomplished, when possible, to assure that the −40 °C isotherm reaches the tumor margin. After the planned freeze volume is attained, the cryogen flow is terminated and the frozen mass of tissue is allowed to thaw passively. Slow thawing is more damaging than a rapid thaw [16]. Many cryoprobes have heating capability which may serve one of two functions. The first and most common is to loosen the cryoprobes from the frozen mass to end the procedure. The second supports the repositioning of the probes. Using the heat function to assist or speed tumor thawing may be counterproductive as tissue adjacent to the cryoprobe has already received the maximal destructive consequences of the highest freeze rate. Further, active probe heating will not significantly affect the tumor margin where tumor cell survival may be possible due to limited freezing. Thawing of the freeze zone periphery is accomplished by heat flux from surrounding tissue.
A second freeze interval is normal as it enhances cell death in the targeted tissue. The second freeze typically exposes the tissue to a more rapid freeze rate and moves the desired nadir temperature (i.e. −40 °C) closer to the periphery as the second freeze is initiated in colder tissue in which the vasculature has been damaged [1].
CA is typically thought of as a simple, physically destructive process dependent on ice migration through the targeted tumor mass. A number of observations have stimulated an in-depth analysis of the ablative process. It was first recognized that post-thaw inflammation and the infiltration of diverse leucocyte populations including macrophages, dendritic cells and T-cells occurred within 24-hours and maintained a presence for weeks to months depending on tumor mass [17]. A second observation, regression of some but not all secondary tumors (metastatic) originating from the primary lesion correlated with elevated antibody titers in patients, was thought to provide a putative immunostimulatory potential linked to the release of inflammatory debris. Third, a series of discoveries revealed that gene regulated cell death (apoptosis) occurred at mild sub-freezing temperatures common to the freeze zone periphery [3]. Hence a cascade of distinct modes of cell death occurs and understanding the “cascade” might provide a changed paradigm for CA optimization with beneficial adjuvants.
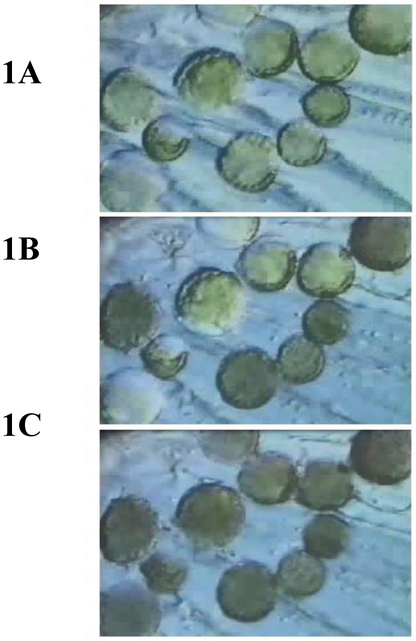
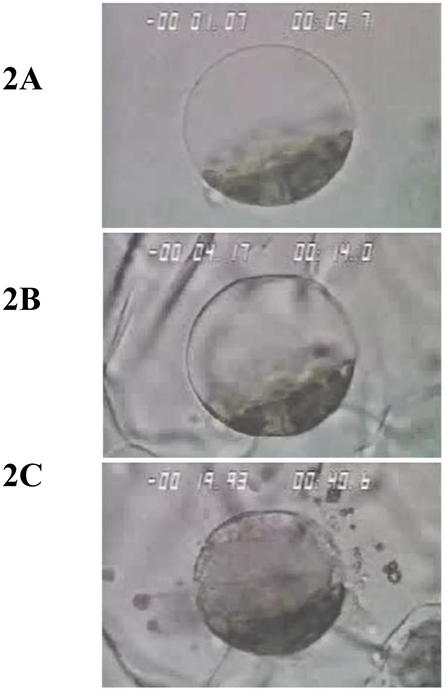
CA is a combinatorial therapy as it exposes targeted cells to numerous stressors that launch diverse cell death cascades. Table 1 provides an overview of the numerous physical and molecular-based stressors that contribute to targeted cell death. The CA process begins with chilling (hypothermia) which is not lethal when applied for short periods. It is, however, a condition that lasts throughout the CA period (~20–30 min.). Hypothermia causes disruption/uncoupling of most metabolic pathways which leads to depleted energy stores (i.e. ATP), ionic imbalances, cellular acidosis and free radical generation. The cell membranes including those of organelles (mitochondria, lysosomes, etc.) change fluidity and cytoskeletal structures disassemble [18]. As temperature is lowered further, nucleation of the aqueous extracellular fluid occurs resulting in the initiation of extracellular ice formation typically around −2 °C (Figure 1A and 2B). The presence of extracellular ice is not a major lethal event, but when sustained, results in severe hyperosmolality. Ice forms as a pure solid excluding both organic and inorganic solutes. Water diffuses from the cell to the extracellular environment supporting further ice growth followed by cell shrinkage and select macromolecule denaturation. With further lowering of the temperature the probability of lethal intracellular ice formation increases typically near −15 °C (Figure 1B and 2C). As ice growth is accretive (water is drawn from ahead of the advancing ice front), physical damage is limited to rigid tissue structures unable to “move” with the increased volume of ice (i.e. critically, capillaries lose their endothelial lining and become impatent after thawing). As temperature is lowered to ~ −40 °C, freezable water both intra- and extra-cellularly solidifies (Figure 1C) rendering the tumor mass hyperemic and stagnant due to interrupted blood flow, a state often referred to as coagulative necrosis.
Table 1.
Cellular Stress Events During Cryoablation
| Thermal Zone | Cellular Response | Target |
|---|---|---|
| Hypothermia (Cooling to Nadir Temperature) | Metabolic Uncoupling | Molecular |
| ATP Depletion | Molecular | |
| Ionic Imbalance | Molecular | |
| ROS Generation | Molecular | |
| Cellular Acidosis | Molecular | |
| Membrane Fluidity Changes | Molecular – Structural | |
| Cytoskeletal Disassembly | Molecular – Structural | |
| Waste Accumulation | Molecular | |
| Freezing | Extracellular Ice Formation | Molecular – Structural |
| Dehydration Induced Cell Shrinkage | Molecular – Structural | |
| Macromolecule Denaturation | Molecular | |
| Hyperosmolarity | Molecular – Structural | |
| Intracellular ice Formation | Structural | |
| Tissue Shearing | Structural | |
| Thawing & Recovery | “Local” Hypo-osmolarity (transient) | Molecular – Structural |
| Cell Swelling | Structural | |
| Ionic Imbalance | Molecular | |
| ROS Generation | Molecular | |
| Activation of Cell Death Cascades | Molecular – Structural | |
| Release of “Danger Signals” | Molecular | |
| Dendritic Cell Activation | Molecular | |
| Vascular Stasis | Molecular | |
| Chronic Inflammation | Molecular |
Figure 1.
Illustrates the progression of the freezing process in a population of cells. Fig. 1A shows the progression of extracellular ice formation during the initial freezing process. In Fig. 1B extracellular ice has completely surrounded the cells with approximately half of the cells experiencing intracellular ice. In Fig. 1C all cells are frozen intracellularly.
Figure 2.
Illustrates the freeze progression of single cell. In each figure temperature in degrees Celsius appears in the upper left and time in seconds appears in the upper right. In Fig. 2A cooling has proceeded to −1 °C without ice formation. In Fig. 2B extracellular has surrounded the cell without damage. Fig 2C illustrates the consequences of intracellular ice formation with extensive membrane rupture and expulsion of cellular content.
This first freezing cycle is terminated and thawing initiated. Passive thawing to the level of the −40 °C isotherm is recommended before applying a second freeze which will progress the −40 °C isotherm further in the tumor periphery due to a) the damaged vascular tree and attendant disruption of blood flow with diminished heat flow and b) re-initiating freezing in tissue previously rendered hypothermic.
Non-mutagenic benefits of CA
An essential benefit of CA is that it targets the entire energy profile of all cells within the tumor microenvironment. The majority of today’s CA treatments are single session which together with energy deprivation does not provide the opportunity for cancer cells to launch their armamentarium of defensive strategies. Table 2 provides an overview of cancer cell’s adaptative responses that may follow repetitive therapies thereby compromising curative potential. It is the combination of structural damage with necrosis, initiation of apoptosis in highly stressed cells, tumor hypoxia caused by destruction of tumor microvasculature and activation of both local and systemic cryoimmunologic effects that prevents adaptative responses [2].
Table 2.
Defensive Strategies of Cancer Cells
| Sustained Proliferative Signaling |
| Evasion of Growth Suppressors |
| Resisting Programmed Cell Death - apoptosis |
| Induction of Vasculogenesis and Angiogenesis |
| Overcoming Immune System Defenses |
| Reprogramming Cellular Energetics |
| Cellular Immortality |
| Mobilization and Metastasis |
| Create Tumor Microenvironment through Recruitment of Stromal Cells |
Cryo-immunological Responses
The control of metastatic disease has been a long-term goal of cancer therapy. Throughout the past half-century the prospect of activating the patient’s immune system in association with thermal therapies had been an intriguing prospect. It had been observed since the late 1960’s that distant secondary tumors show complete or partial ablative effects following CA of the primary lesion in both animal studies and select patient response [10-14]. This cryoimmunologic effect is not always observed, is transient, has demonstrated distinct chronologies and has not proved curative. Numerous studies in the decades that followed added (and continue to add) further knowledge and confirmation that cryoablation generates an anti-tumor immune response [15, 19, 20]. Transferring this knowledge to the clinic has no doubt been challenged by the diversity of animal species and cancer types tested along with limited single center retrospective analyses which together limits the ability to define manageable mechanisms of a cryo-immune response. While the margin of the ablated tumor is readily approximated by either thermography, ultrasound or other visualization modalities, the nature of cellular damage will likely vary with tumor size and therefore extent and timing of apoptosis, necrosis, freeze rupture and potentially autophagy. The full extent of the release of inflammatory agents and hence uniformity of a local and certainly a systemic immune response may be difficult to quantify and even repeat. Cellular breakdown of the tumor mass can occur over days to weeks such that proinflammatory cytokines, DNA, RNA and HSP levels can provide a stimulatory effect but in a non-quantifiable chronology. Hence, macrophage, NK cells, dendritic cells, granulocytes and other relevant immune cells (i.e. T-cells) infiltration and ultimate activation of an immune response and its transfer to metastatic sites through the draining lymphatics is problematic to study.
A Case for Cryo-adjuvants
Tissue sensitization
It is widely recognized that no single monotherapy provides assurance of cure especially with repetitive therapies. Absent a complete clinical response, radiotherapy provides “curative outcomes” of 5–10% for metastatic malignancies and 60–80% for organ confined disease [21]. Patient outcomes are similar following chemotherapy, hormonal ablation and diverse thermal therapies.
With improved understanding of the adaptative hallmarks of cancer and the survival functionality of the tumor environment, there is now a clear recognition that combinatorial treatment strategies are essential to the concept of cure. Treatments are challenged by the diverse and extensive survival strategies employed by cancer cells resident to a complex and heterogeneous tumor mass [22-25]. To elicit a tumor response a treatment strategy must involve the destruction of resistant cancer stem cells, recruited non-cancerous stromal cells (i.e. fibroblasts) that provide a cancer support system and tumor-associated immune cell population that functions to defeat the surveillance functions of the body’s innate immune system. Additionally, activating mutations down regulate tumor suppressor genes (i.e. p53), upregulate VEGF to support new blood vessel growth, enhance cancer cell immortality through telomerase activation and telomere production, upregulate anti-apoptotic mechanisms (i.e. Bcl2 upregulation), re-program metabolism to support both ATP and building blocks of cell growth production. To defeat these and other tumor defense mechanisms characteristic of cancer multiple (combinatorial) treatment paradigms are in development. Today, chemotherapy may include up to five distinct cytotoxic agents that attack different cellular defenses. Other therapy strategies employ chemotherapy – radiotherapy sequencing. Unfortunately, these approaches, while improving disease suppression, fail to kill cancer stem cells and indolent cancer cells (i.e. cell cycle G0 stage) due in part to the production of protective proteins by tumor-associated fibroblasts such as Wnt16b and upregulation of ABC cell transporter proteins that minimize the cytotoxicity [24, 26].
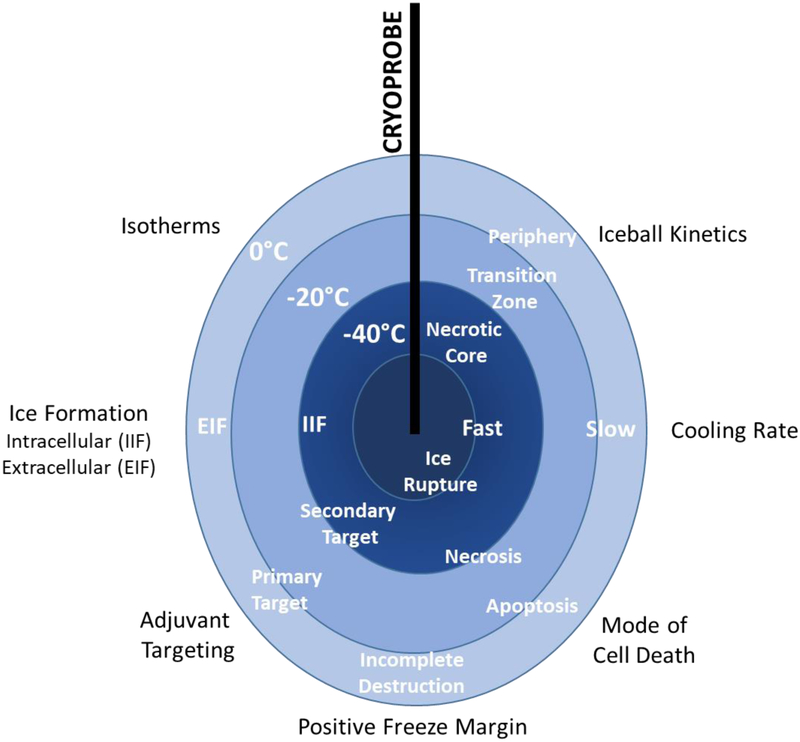
Cryoablation, like any therapy, has its “Achilles Heel.” The thermal gradient that is a function of the physics of freezing assures that the full morphology of the tumor will not experience a lethal nadir temperature unless a sufficient positive freeze margin (“over freeze”) is included in the procedure. The inclusion of an “over freeze” is often problematic as the volume of this treatment zone may exceed the volume of the cancerous target thereby damaging benign tissue (Figure 3). There are two possible solutions to this problem. The first entails the utilization of a cryogen capable of “driving” the nadir isotherm further toward the distal boundary of the freeze zone margin. Only one cryogen, supercritical nitrogen (SCN), has demonstrated this capability, but still is unable to provide nadir ablation temperatures at the tumor margin. To this end, studies suggest that through the use of SCN as the cryogen the −20 °C and −40 °C isotherms can be driven towards the iceball periphery resulting in a significant increase in the lethal volume [27]. For instance, tissue engineered prostate cancer tumor studies have shown that following a dual 5 minute freeze with a 5 min thaw in between freezes using SCN a 28.9 cm3 frozen mass with >60% being completely lethal, whereas when using an Argon driven device <50% was found to be lethal [27,28]. A similar level of improvement was found using a renal cancer tissue engineered model [27,28]. Unfortunately, SCN remains in the developmental stage.
Figure 3.
A schematic representation of the dynamics and events of the freeze zone.
The second solution relies on the use of cryosensitizing agents that would either enhance the physical effects of the freezing process and/or activate multiple cell death cascades that overwhelm and prevent the launch of cancer cells defensive strategies. Activation of multiple cell stress pathways and inhibition of cell repair mechanisms common to repetitive treatment strategies will assure an increased tumor ablation capacity [4, 29-35].
The optimal goal of a cryosensitization strategy is to render all cells within the tumor microenvironment sensitive to freeze temperatures near the edge of the freeze zone (approx. −1 to −2 °C). When first proposed in prostate cancer model studies using 5-flurouracil [4], lethal nadir temperatures were elevated from ~−40 °C to ~−20 °C, an improvement of nearly 20 °C. Subsequent studies using diverse cell stress activators including TNF-α and TRAIL revealed similar levels of sensitization [29,33,34]. The extent of developmental efforts to identify ideal cryosensitizers now includes three strategic approaches: (1) thermo-physical adjuvants, (2) chemotherapeutic and pro-inflammatory agents, (3) and nutraceuticals.
Thermophysical Adjuvants and Processes
The dual cycle or repetitive freeze-thaw practice provides an inherent sensitization observed during the second freeze [1,9,16,32]. During the first freeze, the targeted nadir temperature (−40 °C) is attained at a point distal from the cryoprobe. Exposure to above nadir temperatures occurs closer to the freeze margin. Cells in this region are stressed, some are partially damaged and may repair and survive. However, when cells in this region experience a second freeze-thaw excursion, increased cell death is observed. Efforts to enhance ice structure lethality have relied on the addition of high concentrations of glycine, salts and antifreeze proteins but may provide a challenge to clinical translation.
Chemotherapeutic Agents
Ikekawa et al. [36] first proposed the use of adriamycin and peplomycin with cryoablation in a mouse model. Freezing was thought to concentrate the cytotoxic agents in the tumor target thereby providing targeted enhancement of cell death. With the discovery of apoptotic cell death in the freeze zone periphery [3], the concept of initiating multiple cell death cascades specific to the chemotherapeutic agent and to the freezing process developed. The combinatorial actions of the dual stressors would conceptually lead to apoptotic enhancement and elevation of the cancer cell’s lethal nadir temperature. Diverse chemotherapeutic agents have been investigated so that known, select death cascades could be activated. The pro-inflammatory cytokine TNF-α in combination with freezing elevates the lethal temperature to −0.5 °C [33]. Similarly, RF ablation [37], radiation with cryoablation [38] and heat plus freezing [37] also enhance tumor cell death. One benefit reported on the use of CA in combination with chemotherapy is the potential to use lower, sub-lethal, doses of chemotherapeutic agents in combination with CA to obtain the desired outcome [4,31,32].
Nutraceuticals
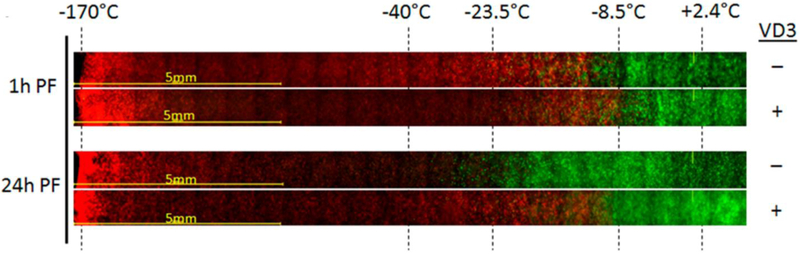
While the use of cytotoxic agents applied systemically followed by targeted freezing accomplishes the goal of enhanced cancer cell death, it also results in the co-morbidities common to chemotherapy. To use a chemo-cryo linked ablation process without co-morbidities, the use of natural cancer suppressor agents (nutraceuticals) has been explored. Vitamin D3 is a natural anti-proliferative agent for many cancers. When its active metabolite, calcitriol, is used with freezing of prostate cancer cells, freeze sensitivity increased to nadir temperatures as high as −5 °C to −15°C regardless of the androgen sensitivity or rate of division (Ki67) of the prostate cancer [40-43]. This response is like other cancer types which carry the VD3 receptor such as breast [44-46], lung [47], colon [48,49] and pancreas [50] in addition to prostate (Figure 4) [40-43]. The use of calcitriol as an adjunct as well as the VD3 receptor hypothesis is currently being tested in other cancers which do not contain the VD3 receptor, including bladder and liver cancer. These data indicate that the combination of calcitriol and CA in these cancers has minimal combinatorial benefit. While less effective, more importantly no negative impact was observed. While preliminary, these ongoing studies suggest there may be a universal benefit of VD3/CA combination which may be further enhanced with additional combinatorial agents. Other nutraceuticals such a resveratrol have shown positive results in renal cancers [51].
Figure 4.
Prostate Cancer TEM model results of a 5 minute cryosurgical procedure with a Supercritical Nitrogen device. For VD3 + samples, pTEMs were pre-treated for 24 hours with 50nM calcitriol prior to the freeze exposure. TEM samples were frozen, allowed to thaw and assessed for viability 1hr or 24hrs post freeze using Calcein-AM (green, live cells) and Propidium Iodide (red, dead cells). Images depict the center of the freeze zone (left side) to the iceball edge and non-frozen section (right side). Temperature during freezing were monitored with embedded thermocouples during the freeze and end point temperatures at their respective position in the samples are indicated. At 1hr post-freeze, both freeze only and VD3/freeze samples reveal cellular damage out to ~−10 °C. At 24hrs, in the freeze only samples most of the cells in the −10 °C to −25 °C region recovered revealing complete cell death below ~−30 °C. In VD3/freeze samples, cellular recovery was significantly reduced resulting in complete cell destruction below ~−10 °C.
Conclusion
Cryoablation, when applied as a monotherapy, is a long-proven cancer suppressive therapy with patient outcomes equivalent current “gold standard” radiation and chemotherapies [52-54]. As an energy deprivation treatment, its ablative actions are diverse ranging between thermophysical damage to the activation of numerous cell stressor pathways that lead directly to cell death cascade activation. Current trends to improve the ablative precision of freezing rely on patterned combinatorial additions of secondary stressors including chemotherapeutics and anti-proliferative nutraceuticals that function to enhance sensitivity of cancer cell’s molecular pathways to elevated subfreezing exposures. This sensitization results in changed ablative temperatures such that the goal of rendering exposure at the freeze zone periphery is lethal, thereby minimizing the “over-freeze” currently used to bring a nadir lethal temperature to the entire tumor mass. Immune system activation that has cryoablation dependencies is now being actively investigated in a series of clinical trials [55-58].
Acknowledgments
A portion of the research presented in the manuscript was supported in part by NIH grant 2R44CA183265–02A1
Abbreviations:
- CA
cryoablation
- CIR
cryo-immunologic response
- VEGF
vascular endothelial growth factor
- ATP
adenosine triphosphate
- US
ultrasound
- CT
computed tomography
- MRI
magnetic resonance imaging
- TNF-α
tumor necrosis factor alpha
- TRAIL
tumor necrosis factor-related apoptosis-inducing ligand
- LP
low passage
- HP
high passage
- AR
androgen receptor
- VD3
vitamin D3
- VDR
vitamin D3 receptor
Footnotes
Disclosure of interest
In accordance with T&F disclosure policy, the authors report that KKS, KLS, ATR, RVB and JMB are all employees of CPSI Biotech. JGB reports no potential conflicts of interest.
Contributor Information
John G. Baust, State University of New York, Binghamton, NY
Kristi K. Snyder, CPSI Biotech, Owego, NY
Kimberly L. Santucci, CPSI Biotech, Owego, NY
Anthony T. Robilotto, CPSI Biotech, Owego, NY
Robert G. Van Buskirk, State University of New York, Binghamton, NY, CPSI Biotech, Owego, NY
John M. Baust, CPSI Biotech, Owego, NY
References
- 1.Baust JG, Bischof J, Jiang-Hughes J, Polascik T, Rukstalis D, Corwin W, Gage AA, Baust JM. Re-purposing Thermal Ablative Therapies. Nature’s Prostate Cancer & Prostatic Diseases. 2015. 18(2): 87–95. [DOI] [PubMed] [Google Scholar]
- 2.Gage AA and Baust JG. Mechanisms of tissue injury in cryosurgery. Cryobiology. 1998. 37(3):171–186. [DOI] [PubMed] [Google Scholar]
- 3.Hollister WR, Mathew AJ, Baust JG, Van Buskirk RG. Effects of freezing on cell viability and mechanisms of cell death in a human prostate cancer cell line. Molecular Urology. 1998. 2(1):13–8. [Google Scholar]
- 4.Clarke D, Baust JM, Van Buskirk RG, Baust JG. Addition of Anti-cancer Agents Enhances Freezing-induced Prostate Cancer Cell Death: Implication of Mitochondrial Involvement. Cryobiology. 2004. 49(1):45–61. [DOI] [PubMed] [Google Scholar]
- 5.Robilotto AT, Baust JM, Van Buskirk RG, Gage AA, Baust JG. Temperature Dependent Apoptotic Pathway Activation During Cryoablation. Prostate Cancer & Prostatic Diseases 2013. 16: 41–49. [DOI] [PubMed] [Google Scholar]
- 6.Le Pivert P, Haddad RS, Aller A, Titus K, Doulat J, Renard M, et al. Ultrasound guided combined cryoablation and microencapsulated 5-Fluorouracil inhibits growth of human prostate tumors in xenogenic mouse model assessed by luminescence imaging. Technol Cancer Res Treat. 2004. April;3(2):135–42. [DOI] [PubMed] [Google Scholar]
- 7.Forest V, Peoc’h M, Campos L, Guyotat D, Vergnon JM. Effects of cryotherapy or chemotherapy on apoptosis in a non-small-cell lung cancer xenografted into SCID mice. Cryobiology. 2005. February;50(1):29–37. [DOI] [PubMed] [Google Scholar]
- 8.Forest V, Peoc’h M, Campos L, Guyotat D, Vergnon JM. Benefit of a combined treatment of cryotherapy and chemotherapy on tumour growth and late cryo-induced angiogenesis in a non-small-cell lung cancer model. Lung Cancer. 2006. October;54(1):79–86. [DOI] [PubMed] [Google Scholar]
- 9.Baust JG, Gage AA, Robilottto AT, Baust JM. The pathophysiology of thermoablation: optimizing cryoablation. Curr Opin Urol. 2009. March;19(2):127–32. [DOI] [PubMed] [Google Scholar]
- 10.Yantoro C, Soanes WA, Gonder MJ, Shulman S The production of antibodies to urogenital tissue in consequence of freezing treatment. Immunology. 1967. 12: 395–410. [PMC free article] [PubMed] [Google Scholar]
- 11.Shulman S, Brandt EJ, Yantoto C. Studies in cryoimmunology II. Tissue and species speificity of auto-antibody response and comparison with iso-immunization. Immunology. 1968. 14:149–158. [PMC free article] [PubMed] [Google Scholar]
- 12.Soanes WA, Ablin RJ, Gonder MJ Remission of metastatic lesions following cryosurgery in prostatic cancder. J. Urology. 1970. 104:154–159. [DOI] [PubMed] [Google Scholar]
- 13.Soanes WA, Gonder MJ, Ablin RJ. A possible immuno-cryothermic response in prostatic cancer. Clin. Radiol. 1970. 21:253–255. [DOI] [PubMed] [Google Scholar]
- 14.Veenstra JJ, Gibson HM, Freytag S, Littrup PJ, Wei WZ In situ immunization via non-surgical ablation to prevent local and distant tumor recurrence. OncoImmunology. 2015. 4(3):e989762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sidana A, Rodriguez R Urologic applications of cryo-immunology. Kor. J. Urology. 2009. July;50(7):629–634. [Google Scholar]
- 16.Baust JG, Gage AA, Klossner D, Clarke D, Miller R, Cohen J, Katz A, Polascik T, Clarke H, Baust JM. Issues Critical to the Successful Application of Cryosurgical Ablation of the Prostate. Technol. Cancer Res. & Treatment. 2007. 6: 97–110. [DOI] [PubMed] [Google Scholar]
- 17.Chu KF, Dupuy DE. Thermal ablation of tumors: biological mechanisms and advances in therapy. Nature Reviews: Cancer. 2014. March;14:199–208. [DOI] [PubMed] [Google Scholar]
- 18.Baust JG, Gage AA, Bjerklund Johansen TE, Baust JM. Mechanisms of Cryoablation: Clinical Consequences on Malignant Tumors. Cryobiology. 2014. 68: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sabel MS, Arora A, Su G, Chang AE. Adoptive immunotherapy of breast cancer with lymph node cells primed by cryoablation of the primary tumor. Cryobiology. 2006. December;53(3):360–6. [DOI] [PubMed] [Google Scholar]
- 20.Sabel MS. Cryo-immunology: a review of the literature and proposed mechanisms for stimulatory versus suppressive immune responses. Cryobiology. 2009. February;58(1):1–11. [DOI] [PubMed] [Google Scholar]
- 21.Coventry BJ, Ashdown ML. Complete clinical responses to cancer therapy caused by multiple divergent approaches: a repeating theme lost in translation. Cancer Manag Res. 2012;4:137–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li Y, Zou L, Li Q, Haibe-Kains B, Tian R, Desmedt C, et al. Amplification of LAPTM4B and YWHAZ contributes to chemotherapy resistance and recurrence of breast cancer. Nat Med. 2010. February;16(2):214–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Balko JM, Cook RS, Vaught DB, Kuba MG, Miller TW, Bhola NE, et al. Profiling of residual breast cancers after neoadjuvant chemotherapy identifies DUSP4 deficiency as a mechanism of drug resistance. Nat Med. 2012. July;18(7):1052–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sun Y, Campisi J, Higano C, Beer TM, Porter P, Coleman I, et al. Treatment-induced damage to the tumor microenvironment promotes prostate cancer therapy resistance through WNT16 B. Nat Med. 2012. September;18(9):1359–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gupta A, Yang Q, Pandita RK, Hunt CR, Xiang T, Misri S, et al. Cell cycle checkpoint defects contribute to genomic instability in PTEN deficient cells independent of DNA DSB repair. Cell Cycle. 2009. July 15;8(14):2198–210. [DOI] [PubMed] [Google Scholar]
- 26.Ghisolfi L, Keates AC, Hu X, Lee DK, Li CJ. Ionizing radiation induces stemness in cancer cells. PLoS One. 2012;7(8):e43628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baust JM, Snyder KK, Santucci KL, Robilotto AT, Smith J, McKain JF, Sahay A, Baust JG Assessment of SCN and argon cryoablation devices in an in vivo-like 3-D tissue engineered prostate and renal cancer model, Cryobiology: 69(1); 198 2014 [Google Scholar]
- 28.Robilotto AT, Baust JM, Santucci KL, Snyder KK, Van Buskirk RG and Baust JG. Assessment of a Novel Supercritical Nitrogen Cryosurgical Device Using Prostate and Renal Cancer Tissue Engineered Models. Sage Open Medicine, Submitted [Google Scholar]
- 29.Clarke DM, Robilotto AT, VanBuskirk RG, Baust JG, Gage AA, Baust JM. Targeted induction of apoptosis via TRAIL and cryoablation: a novel strategy for the treatment of prostate cancer. Prostate Cancer Prostatic Dis. 2007;10(2):175–84. [DOI] [PubMed] [Google Scholar]
- 30.Clarke DM, Baust JM, Van Buskirk RG, Baust JG. Addition of anticancer agents enhances freezing-induced prostate cancer cell death: implications of mitochondrial involvement. Cryobiology. 2004 2004. August;49(1):45–61. [DOI] [PubMed] [Google Scholar]
- 31.Clarke DM, Baust JM, Van Buskirk RG, Baust JG. Chemo-cryo combination therapy: an adjunctive model for the treatment of prostate cancer. Cryobiology. 2001 2001. June;42(4):274–85. [DOI] [PubMed] [Google Scholar]
- 32.Goel R, Anderson K, Slaton J, Schmidlin F, Vercellotti G, Belcher J, et al. Adjuvant approaches to enhance cryosurgery. J Biomech Eng. 2009. July;131(7):074003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Han B, Swanlund DJ, Bischof JC. Cryoinjury of MCF-7 human breast cancer cells and inhibition of post-thaw recovery using TNF-alpha. Technol Cancer Res Treat. 2007. December;6(6):625–34. [DOI] [PubMed] [Google Scholar]
- 34.Jiang J, Goel R, Iftekhar MA, Visaria R, Belcher JD, Vercellotti GM, et al. Tumor necrosis factor-alpha-induced accentuation in cryoinjury: mechanisms in vitro and in vivo. Mol Cancer Ther. 2008. August;7(8):2547–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Han B, Iftekhar A, Bischof JC. Improved cryosurgery by use of thermophysical and inflammatory adjuvants. Technol Cancer Res Treat. 2004. April;3(2):103–11. [DOI] [PubMed] [Google Scholar]
- 36.Ikekawa S, Ishihara K, Tanaka S, Ikeda S Basic studies of cryochemotherapy in a murine tumor system. Cryobiology. 1985. 22(5):477–483. [DOI] [PubMed] [Google Scholar]
- 37.den Brok MH, Sutmuller RP, Niekens S, Bennink EJ, Frielink C, Tooen LW et al. Efficient loading of dendritic cells following cryo and radiofrequency ablation combination with immune modulation induces anti-tumor immunity. Br. J. Cancer. 2006. 95:896–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mobley WC, Loening SA, Narayana AS Combination perineal cryosurgery and external radiation therapy for adenocarcinoma of prostate. Urology. 198424(1):11–14. [DOI] [PubMed] [Google Scholar]
- 39.Baumann KW, Baust JM, Snyder KK, Baust JG, Van Buskirk RG. Characterization of Pancreatic Cancer Cell Thermal Response to Heat Ablation or Cryoablation. Technology in Cancer Research & Treatment. 2017. 16(4): 393–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Santucci KL, Snyder KK, Baust JM, Van Buskirk RG, Mouraviev V, Polascik TJ, et al. The use of 1,25a dihydroxyvitamin D3 as a cryosensitizing agent in a murine prostate cancer model. Prostate Cancer Prostatic Dis. [Original]. 2011 June 2011;14(2):97–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Baust JM, Klossner DP, Robilotto A, Van Buskirk RG, Gage AA, Mouraviev V, et al. Vitamin D(3) cryosensitization increases prostate cancer susceptibility to cryoablation via mitochondrial-mediated apoptosis and necrosis. BJU Int. 2012. March;109(6):949–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kimura M, Rabbani Z, Mouraviev V, Tsivian M, Caso J, Satoh T, et al. Role of vitamin D(3) as a sensitizer to cryoablation in a murine prostate cancer model: preliminary in vivo study. Urology. 2010. September;76(3):764 e14–20. [DOI] [PubMed] [Google Scholar]
- 43.Santucci KL, Baust JM, Snyder KK, Van Buskirk RG, Baust JG Dose Escalation of Vitamin D3 Yields Similar Cryosurgical Outcome to Single Dose Exposure in a Prostate Cancer Model. Cancer Control. 2018. Jan-Mar;25(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Garcia-Becerra R, Diaz L, Camacho J, Barrera D, Ordaz-Rosado D, Morales A, et al. Calcitriol inhibits Ether-a go-go potassium channel expression and cell proliferation in human breast cancer cells. Exp Cell Res. 2010. February 1;316(3):433–42. [DOI] [PubMed] [Google Scholar]
- 45.Ferronato MJ, Obiol DJ, Fermento ME, Gandini NA, Alonso EN, Salomon DG, et al. The alkynylphosphonate analogue of calcitriol EM1 has potent anti-metastatic effects in breast cancer. J Steroid Biochem Mol Biol. 2015. November;154:285–93. [DOI] [PubMed] [Google Scholar]
- 46.Johnson AL, Zinser GM, Waltz SE. Vitamin D3-dependent VDR signaling delays ron-mediated breast tumorigenesis through suppression of beta-catenin activity. Oncotarget. 2015. June 30;6(18):16304–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Verone-Boyle AR, Shoemaker S, Attwood K, Morrison CD, Makowski AJ, Battaglia S, et al. Diet-derived 25-hydroxyvitamin D3 activates vitamin D receptor target gene expression and suppresses EGFR mutant non-small cell lung cancer growth in vitro and in vivo. Oncotarget. 2016. January 5;7(1):995–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen S, Zhu J, Zuo S, Ma J, Zhang J, Chen G, et al. 1,25(OH)2D3 attenuates TGF-beta1/beta2-induced increased migration and invasion via inhibiting epithelial-mesenchymal transition in colon cancer cells. Biochem Biophys Res Commun. 2015. December 4-11;468(1–2):130–5. [DOI] [PubMed] [Google Scholar]
- 49.Wierzbicka JM, Binek A, Ahrends T, Nowacka JD, Szydlowska A, Turczyk L, et al. Differential antitumor effects of vitamin D analogues on colorectal carcinoma in culture. Int J Oncol. 2015. September;47(3):1084–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kanemaru M, Maehara N, Chijiiwa K. Antiproliferative effect of 1alpha,25-dihydroxyvitamin D3 involves upregulation of cyclin-dependent kinase inhibitor p21 in human pancreatic cancer cells. Hepatogastroenterology. 2013. Jul-Aug;60(125):1199–205. [DOI] [PubMed] [Google Scholar]
- 51.Clarke DM, Robilotto AT, Rhee E, VanBuskirk RG, Baust JG, Gage AA, et al. Cryoablation of renal cancer: variables involved in freezing-induced cell death. Technol Cancer Res Treat. 2007. April;6(2):69–79. [DOI] [PubMed] [Google Scholar]
- 52.Babaian RJ, Donnelly B, Bahn D, Baust JG, Dineen M, Ellis D, et al. Best practice statement on cryosurgery for the treatment of localized prostate cancer. J Urol. 2008. 2008 November;180(5):1993–2004. [DOI] [PubMed] [Google Scholar]
- 53.Baust JG, Gage AA, Bjerklund Johansen TE, Baust JM. Mechanisms of cryoablation: clinical consequences on malignant tumors. Cryobiology. 2014. February;68(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cohen JK, Miller RJJ, Ahmed S, Lotz MJ, Baust J. Ten-year biochemical disease control for patients with prostate cancer treated with cryosurgery as primary therapy. Urology. 2008. March;71(3):515–8. [DOI] [PubMed] [Google Scholar]
- 55.ClinicalTrials.gov [Internet]. A Study of Pre-Operative Treatment With Cryoablation and Immune Therapy in Early Stage Breast Cancer. 2018. [cited 2018 May 2]. Available from: https://clinicaltrials.gov/ct2/show/NCT02833233
- 56.ClinicalTrials.gov [Internet]. Phase I Clinical Trial of Cryoimmunotherapy Against Prostate Cancer (CryoIT). 2018. [cited 2018 May 2]. Available from: https://clinicaltrials.gov/ct2/show/NCT02423928
- 57.ClinicalTrials.gov [Internet]. A Study of Pre-Operative Treatment With Cryoablation and Immune Therapy [Norway]
- 58.ClinicalTrials.gov [Internet]. Dendritic Cell Therapy After Cryosurgery in Combination With Pembrolizumab in Treating Patients With Stage III-IV Melanoma That Cannot Be Remove by Surgery. 2018. [cited 2018 May 2]. Available from: https://clinicaltrials.gov/ct2/show/NCT03325101