Abstract
Purpose of Review
This review elucidates the concept of frailty in relationship to reserve and resilience, the relationships and shared pathophysiology between physical frailty and cognitive impairment, the theoretical underpinnings of three integrated phenotypes of physical and cognitive impairments, and the potential of incorporating biomarkers into phenotype refinement and validation.
Recent Findings
The fact that frailty and cognitive impairment are associated and often coexist in older adults has led to the popular view of expanding the definition of frailty to include cognitive impairment. However, there is great variability in approaches to and assumptions regarding the integrated phenotypes of physical frailty and cognitive impairment.
Summary
The development of integrated frailty and cognitive phenotypes should explicate the types of frailty and cognitive impairment they intend to capture and prioritize the incorporation of biological theories that help determine shared and distinct pathways in the progression to physical and cognitive impairments.
Keywords: Aging Phenotypes, Cognitive Frailty, Geriatric Syndrome, Reserve, Resilience, Stressors
Introduction
One of the biggest healthcare challenges worldwide is the medical and economic burden of caring for dependent older adults ravaged by physical and cognitive impairments. Two of the most common geriatric conditions, frailty and cognitive impairment, are known to predict poor health outcomes [1, 2]. An estimated 15% of the older US non-nursing home population aged 65+ is frail [3], 22% of older US adults aged 70+ have mild cognitive impairment [4] and 14% have dementia [5]. Frailty and cognitive impairments often coexist. One study found that 53% of the frail had cognitive impairment [6]. Many of the aging processes underlying frailty may also impact brain aging and cognitive decline [7], but the mechanisms behind the association are unknown.
With increased interest in the frailty syndrome and its relationship with brain aging, recent consensus papers have suggested expanding the definition of frailty to include cognition [8, 9]. Approximately 50% of the frailty instruments in the literature include a measure of cognition [10], although the type of measure varies across studies from global measures (e.g., MMSE [11]) to clinical diagnosis (e.g., dementia [12]). Such inclusion primarily aims to improve the predictive accuracy of frailty for future adverse outcomes [11]. Meanwhile, increasing epidemiological evidence suggests that cognition is separable from physical functioning [13], supported by the finding that 22% of people with Alzheimer’s disease (AD) had no physical indicators of frailty [14]. We therefore advocate caution when creating integrated phenotypes by combining physical and cognitive impairments that may or may not share common etiologies and pathways.
This chapter seeks to clarify the concept of frailty in relationship to reserve and resilience, review bidirectional relationships and shared pathophysiology between physical frailty and cognitive impairment, and elucidate the importance of developing and using integrated phenotypes of physical and cognitive impairments for their intended purposes. To this end, the chapter is organized into five sections. Section 1 reviews the theoretical conceptualization of frailty and its closely related concepts of reserve and resilience. Section 2 provides a brief summary on the bidirectional relationships between frailty and cognitive dysfunction. Section 3 discusses pathophysiological pathways linking frailty and cognitive impairment, focusing on the stress-response systems. Section 4 offers examples of integrated physical and cognitive phenotypes that vary in suppositions about common pathophysiologic pathways. These phenotypes include: frailty index [15, 16], motoric cognitive risk syndrome [17], and cognitive frailty [18]. Section 5 outlines future directions, followed by concluding remarks.
Reserve, Resilience, and Frailty
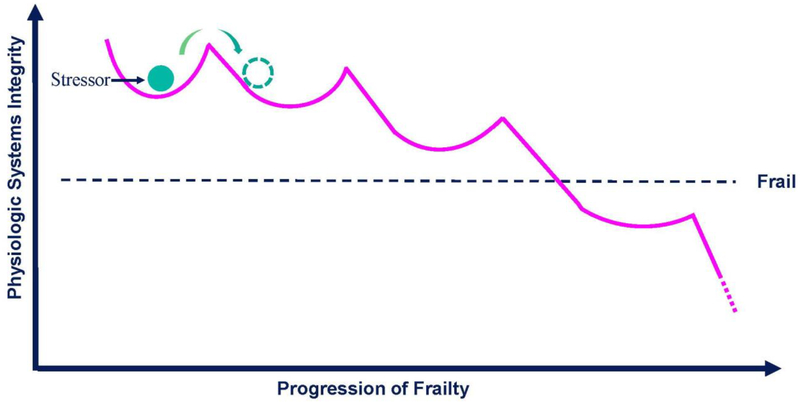
Frailty is theoretically defined as a clinically recognizable state of increased vulnerability resulting from aging-associated declines in reserve and function across multiple physiologic systems, such that one’s ability to withstand, compensate and overcome stressors is compromised [1]. We further theorize that the frailty state manifests as a medical syndrome, operationally defined by the physical frailty phenotype consisting of muscle weakness, weight loss, slow gait, exhaustion and low activity [19]. Frailty is a clinical entity that both overlaps and is distinct from disability and multimorbidity [20]. Frailty could be viewed as an emergent phenomenon defined as an emergent aggregation of multiple frailty manifestations caused by depletion of reserve and compensatory mechanisms, such that any new deficit leads to failure of the whole organism [21]. As depicted by the schematic diagram in the Figure, we hypothesize that the development of frailty is the result of complex interactions among reserve, resiliency, and type/magnitude of the stressor. Specifically, the progression of frailty may consist of a series of transitions between states of dynamic equilibrium of decreasing integrity before reaching a critical threshold beyond which frailty emerges.
Figure 1.
Hypothesized evolution of degradation of physiologic integrity underlying the progression of frailty. Physiologic integrity of the system is defined by the maintenance of equilibrium in the face of stressors. The level of integrity is theorized to be a function of physiological reserves represented by both the depth of each convex curve and the degree of its curvature, with greater depth and curvature representing greater reserve. Both episodic (e.g. stroke, fall) and chronic insults (e.g. chronic inflammation) are hypothesized to degrade return to equilibrium and decrease integrity of the system to respond to a subsequent stressor. Progression of frailty consists of a series of downward transitions between states of equilibrium of decreasing integrity; and at some critical tipping point, the system becomes overwhelmed and can no longer harness the resources needed to maintain integrity, leading to frailty.
Before addressing connections between frailty and cognition in the following sections, we first aim to crystalize the concepts of vulnerability, reserve and resiliency as they relate to frailty. In the context of frailty, we define vulnerability as lack of ability to resist functional impairment in the presence of intrinsic (e.g., disease pathology) or extrinsic (e.g., air pollution) insults. We posit that vulnerability results from insufficient reserves that impede individual’s ability to withstand stressors, where reserves denote “the means that are available to recover from idiosyncratic adverse events, stressors or nonnormative transitory periods during the life course [22].” In other words, chronic depletion of reserves creates vulnerability.
Studies of physical health have largely confined the discussion of reserve to organ systems or physiological systems. For example, cardiac reserve is an important indicator of “the potential capacity of the heart to function well beyond its basal level, in response to alterations in physiological demands [23].” Its application has since extended beyond diagnosis of coronary artery disease to include determination of post-surgical risks [24]. Recent work has linked energy reserve, another example that is essential to the study of aging processes, to the varying degree of gait slowing with age – a strong and robust predictor of disability and mortality [25, 26]. It is hypothesized that this slowing is the body’s natural adaptive response to shrinking energy reserve as a greater proportion of energy is recruited to combat disease pathologies or declining efficiency in energy utilization [27]. The all-encompassing concept, of “physiologic reserve” is used to characterize “the potential capacity of a cell, tissue, or organ system to function beyond its basal level in response to alterations in physiologic demands [28].”
Closely related to reserve is the concept of resilience, a construct most commonly studied in the field of neuropsychology to characterize individual’s capacity to cope, adapt, or even thrive in the face of adversity in life such as a tragedy, family estrangement, and sudden health problems [29]. The term resilience has extended beyond psychological domain to include emotional, social, physiological, and physical resilience. For example, physical resilience has been defined as “the ability to recover or optimize function in the face of age-related losses or disease,” and may influence or be influenced by other types of resilience [30]. If vulnerability is about susceptibility to stressors and reserve refers to the resource-dependent capacity to cope with stressors, the focus of resilience is on the state of the recovery process itself following a stressor.
Upon reviewing these closely related concepts, we report evidence that vulnerability, reserve, and resilience have permeated the thinking in frailty-related research without making direct reference to them. For example, in the field of immunology, the concept of immunological reserves pertains to “the ability of the immune system to respond to a challenge with greatly increased activity compared to its state of relative rest [31].” Adequate innate and adaptive immunity may be viewed as indicators of immunological reserves. Interestingly, one difference between innate vs. adaptive immunity bears strong resemblance to the concept of brain reserve vs. cognitive reserve [32]: both innate immunity and brain reserve are essentially hardwired, that is - passively acquired through genetic factors and modified by early-life experiences; whereas adaptive immunity, like cognitive reserve, is dynamic and actively acquired throughout the life course. Frailty has been associated with dysregulated innate [33] and adaptive immunity [34], which could explain the blunted antibody response to influenza vaccination among frail older adults, therefore rendering them more susceptible to influenza and its complications [35]. The relationship between frailty and resilience is exemplified by the data showing that glucose and insulin responses to glucose challenge were more exaggerated and prolonged in frail versus nonfrail or prefrail women [36]. At the level of whole-body function, epidemiological data have linked frailty to poor recovery from incident disability [37] or hospitalization-related functional decline [38]. Taken together, these findings support the applicability of the reserve-resilience paradigm in frailty research. Progress made in studies of reserve and resilience in neurocognitive science may therefore facilitate cross-fertilization between disciplines that is necessary to guide future studies of frailty.
Bidirectional relationships between cognitive function and physical frailty
In order to better understand the development of frailty and possible hierarchies and intersecting pathways with cognitive impairments, researchers have studied relationships between frailty (by the physical frailty phenotype [39]) and cognition – both global cognition and domain-specific cognitive status [40]. Cross-sectional studies have reported significant associations between greater prevalence of frailty and increased prevalence of poor global cognition in older adults [40–45]. However, several studies found non-significant associations between global cognition and pre-frailty [43, 46] or frailty [47]. A recent longitudinal study reported that non-frail, cognitively-impaired older adults at baseline had a significantly higher risk for pre-frailty/frailty onset over 4 years when compared to non-frail, cognitively intact participants [48]. In addition, older adults with baseline frailty showed significantly greater risk for onset or worsening of global cognitive impairment over 2–3 years when compared to those without baseline frailty [44, 49]. These results are consistent with previous research, including studies where baseline frailty was associated with incident dementia [7].
Studies of community-dwelling older adults have also observed associations between the physical frailty phenotype and specific cognitive domains, including perceptual-motor function, language, learning and memory, executive function, and complex attention [41, 42, 45, 50]. However, only certain domains – perceptual motor function, and specific measures of language and memory – were consistently associated with frailty across studies. Longitudinal studies of physical frailty and cognitive domains have shown varied associations. In a longitudinal study of processing speed, executive function, and immediate-and-delayed word recall among 331 older women, executive function (measured by Trail Making Test B) was the only cognitive domain whose annual rate of change over time was significantly associated with risk of frailty onset [51]. Another longitudinal study reported that baseline frailty status was not associated with linear change over time in cognitive domains of processing speed, verbal fluency, reaction time/variability, even after adjusting for possible dementia [52]. Overall, the paucity of longitudinal studies on frailty and domains of cognitive function is a notable research gap. We recommend additional longitudinal studies of the natural progression of incident cognitive and physical impairments to improve our understanding of the bi-directional relationships between frailty and cognition.
Physiological Pathways Linking Physical Frailty and Cognitive Impairment
Given that impaired stress response is a hallmark of frailty [53], and cellular energetics is central to stress adaption, we hypothesize that decline in mitochondrial function with age may be the initiator of a pathophysiological cascade, contributing to the organism’s maladaptive multisystemic response to stress through dysregulated energetics, excessive oxidative stress, and overstimulated inflammatory response. Consistent with this hypothesis, a recent study showed that mice with mitochondrial dysfunctions, compared to wild type, had concurrently altered neuroendocrine, inflammatory, metabolic, and transcriptional responses when encountering acute psychological stress [54]. Studies of humans have also generated evidence linking mitochondrial function to three stress-response systems: the innate immune system [55], the hypothalamic-pituitary-adrenal (HPA) axis [56], and the sympathetic nervous system [57, 58]. Mounting evidence implicates these stress-response systems in the pathogenesis of frailty [59]. Chronic activation of these systems and the mutual exacerbation through interactive feedback loops are posited to be the primary driver of the downward spiral of degradation of systems integrity, which at a later stage engenders clinical manifestations and the overt frailty syndrome [53]. Dysregulation of the same stress-response systems has also been associated with decline in cognitive function [60–62]. Of these systems, the innate immune system, and chronic, low-grade inflammation (also termed inflamm-aging [63]) in particular has attracted a great deal of attention in studies of biology of aging. Chronic inflammation marked by both overproduction of pro-inflammatory cytokines (e.g., interleukin-6) and dysregulated balance between pro-inflammatory and anti-inflammatory cytokines (e.g. interleukin-10) may be a key mechanism linking physical frailty and cognitive impairment [7, 64].
Examples of Integrated Physical and Cognitive Phenotypes
There has been a recent surge in the creation of combined physical and cognitive phenotypes likely motivated by the desire to improve: predictive utility [11], early risk detection by exploiting their shared biological and physiological underpinnings [17], or diagnostic accuracy via pathway-specific subtyping of a clinical entity with multifaceted etiology such as cognitive impairment [18]. Having reviewed the shared physiology and bidirectional relationships between frailty and cognitive impairment, we introduce three examples of integrated phenotypes and discuss the scientific rationale, the intended clinical contexts and purposes of use.
Frailty Index
A risk index combines multiple related or unrelated risk factors into a unidimensional measure that is agnostic with regard to the temporal order and underlying etiology of physical and cognitive impairments. A corresponding overall risk score is created by summing the individual scores assigned to the risk factors in the composite according to a set of consistent rules, with a higher risk score indicating a higher level of risk. For example, the Frailty Index (also termed Deficit Accumulation Index) operationalizes frailty by counting the number of deficits accumulated over time, including disability, diseases, physical and cognitive impairments, psychosocial risk factors, and geriatric syndromes (e.g. falls, delirium, and urinary incontinence) [15, 16]. The resulting integrated phenotype constitutes a comprehensive risk index and is viewed as an estimate of biological age [65]. The Frailty Index has proven useful for risk stratification in the general population [66], as well as predicting postoperative outcomes in surgical populations [67]. Its strength in aggregating risk across outcomes poses challenges to identifying and targeting underlying physiologic processes in preclinical and early clinical stages of frailty.
Motoric Cognitive Risk Syndrome
In comparison to the frailty index, Verghese and colleagues coined the term “Motoric Cognitive Risk (MCR) Syndrome” [17] to represent “a transitional state between normal aging and dementia” [68]. The MCR is operationally defined as the presence of both objective slow gait and subjective cognitive complaints in the absence of dementia and difficulty with activities of daily living [17]. The specific criteria used to define slow gait and cognitive complaints however varied across studies [68–70]. This combined phenotype was motivated by the hypothesis of shared brain substrates and pathologies between gait and cognition [71], and the finding that gait slowing preceded decline in objective cognitive performance and subsequent diagnosis of Mild Cognitive Impairment (MCI) a decade later [72]. MCR was shown to be predictive of incident cognitive impairment [69] and dementia onset [17, 69]. In addition, MCR was found to independently predict disability [73], falls [70], and mortality [74]. These findings coupled with the ease, affordability, and noninvasiveness of its assessment make MCR an appealing early marker of dementia applicable to both research and clinical settings.
There are a couple of points worth noting. First, MCR as an early sign of cognitive decline preceding MCI is built on the premise that shared pathophysiology underlying decline in motor function and cognitive function manifests hierarchically over time with slowing gait occurring first. However, given that slow gait may result from non-neurological as well as neurological diseases [75], gait-related immobility may also be causally related to dementia independent of neuropathology, for example, via sedentariness, which has been linked to increased risk of dementia [76], possibly through vascular pathways. The latter could help explain findings that the association between MCR and dementia remained significant after accounting for MCI [69]. The distinction between a marker and a cause has important implications in intervention selection. Interventions targeting gait impairment without addressing the shared pathology would have no impact on dementia if slow gait were merely a risk marker. Second, given the finding of a stronger tie between MCR and vascular dementia [17], it begs the question of whether the relationship between MCR and dementia is specific to certain types of dementia and whether parameters of gait performance other than velocity may also play a role [68].
Cognitive frailty
The third integrated phenotype differs from the preceding two examples by making physical frailty the core phenotype to which cognitive impairment is added to increase predictive utility. Cognitive frailty is a relatively new expansion upon frailty, representing a premorbid cognitive state caused by physical frailty rather than neurodegenerative disorders [18]. The operational definition includes the concurrent presence of physical frailty and cognitive impairment in the absence of clinical diagnosis of dementia [18]. Given the multifaceted etiology of cognitive impairment and the lack of effective treatment strategies beyond short-term symptomatic relief for many neurodegenerative diseases such as AD, it is important to identify a subset of a heterogeneous population for whom preventive or rehabilitative interventions may hold the greatest potential. By focusing on a specific cause of cognitive impairment, i.e., physical frailty, this construct enables a more targeted approach to delay or ameliorate cognitive decline through, for example, behavioral interventions addressing negative consequences of frailty including sedentariness and social isolation. Recent studies have shown that cognitive frailty, or the addition of global cognitive impairment to physical frailty was associated with risks of incident dementia [77–79], functional disability [80, 81], poor quality of life [81], and mortality [78, 81].
Overall, these three phenotypes combine physical and cognitive risk but systematically vary in the focus of interest in and articulation of common neurobiological pathways that may explain progression and inform treatment options.
Future Directions
Twenty-five years of research on frailty across multiple epidemiologic and clinical patient studies demonstrates the value and utility of the concept. However, a critical question facing the field of frailty research remains: whether frailty is ready for widespread clinical use? According to a recent editorial, the answer is no, arguing that while “frailty remains a powerful predictor of patient-centered outcomes but is not yet ready for a role as a full-fledged outcome measure in geriatrics research [82].” Here we propose that addressing this question depends on answering to the fundamental question of what is frailty, and how knowing this will help us advance to answer the more important question of – What we can do about frailty? In our view, knowing that frailty, variously defined, is a strong predictor of adverse outcomes brings little comfort to those whose primary goal is to develop intervention strategies targeting frailty progression. Similarly, combining different types of frailty across physical, cognitive, psychological, social, and environmental domains may be counterproductive or even misleading if the intended goal for such use is not clearly specified [83]. The lasting controversy surrounding the measurement of frailty is rooted in the fact that the public as well as the academics often interpret the word “frailty” in the most generalized sense as vulnerability of all kinds. Overemphasis on predictive validity has also fed into the pursuit of power of prediction without appropriate consideration of construct validity, i.e., the degree to which an index or scale measures what it purports to be measuring [84]. Therefore, we conclude by highlighting three areas of research that are urgently needed in order to bridge the gap between frailty theory and frailty measurement and guide the development and validation of integrated frailty and cognitive phenotypes.
Theory
Although reserve and resilience have been integral parts of the theoretical definition of frailty [85], the relationships between reserve, resilience, and frailty are not well defined. Beginning with reserve and resilience, we find it useful to view the distinction between the two analogousto the difference between hardware and software. For example, in neurocognitive science, reserve is indicative of innate capacity present early in life and is thought to be a function of both brain reserve measured by anatomic parameters (e.g., brain size, neuronal count) and neuronal reserve (i.e., the efficiency of task-related cognitive processing) [32]. As such, reserve can be viewed as the “hardware” in the absence of brain pathology. In contrast, resilience has to do with how the brain processes cognitive tasks and maintain function in the face of brain pathology; therefore, resilience is more in line with the concept of “software”. If frailty is a clinical state of reduced resilience, stress-response experiments arguably give us the best chance to identify such state [86]; and to operationally define frailty, including dynamic measures of the body’s response to a stressor such as glucose challenge is critical to diagnostic accuracy. The challenge then becomes how to select dynamic measures that can capture multisystem physiological dysregulation, which is at the core of frailty biology [53]. On the other hand, if frailty is meant to denote a threshold of reserve, or tipping point (see Figure) below which the ability to mount an effective response to a stressor is greatly diminished, high priority should be placed on identifying that threshold over time, regardless of stressors, in the context of frailty and developing and validating measures accordingly.
Measurement
In order to meaningfully advance frailty measurement, it is critical to distinguish primary from secondary frailty [87, 88]. We consider primary frailty a unique clinical entity in itself and its underlying pathophysiology is separable from other disease-specific processes. By comparison, secondary frailty is clinically in conjunction with signs and symptoms of a preexisting disease (e.g., congestive heart failure) or a direct consequence of the preexisting disease or an acute health event (e.g., hip fracture). The distinction is important for several reasons. First, despite the emerging consensus that frailty is distinct from disability and comorbidity [89], many frailty instruments, such as the Frailty Index, include comorbidity as a key assessment component thereby combining primary and secondary frailty [10]. Second, with declining reserve and resilience being hallmarks of frailty, the validation of frailty requires that observed differences in functional trajectories between the frail and the non-frail are more than a reflection of severity of disease-specific pathology. This is in line with the work on reserves in neuroscience in attempt to explain the mismatch between brain pathology and its clinical manifestations, as evidenced by empirical data including the work by Esiri et al. [90] showing that about 25% of older adults with normal neuropsychological testing scores prior to death meet full pathologic criteria for AD. In the absence of direct measures of reserve, quantifying and accounting for disease pathology become necessary for ascertaining the true meaning and impact of frailty in the presence of comorbidity.
Natural Progression of Frailty and Cognitive Impairment
The cognitive frailty phenotype represents an ongoing effort to understand the heterogeneity in the pathogenesis of cognitive impairment by focusing specifically on MCI caused by physical frailty in the absence of neurodegenerative pathology. However, the stated causal relationship underlying this new clinical entity has not been validated and operational definitions of cognitive frailty so far are exclusively based on cross-sectional data [91]. In order to improve specificity in diagnosis, studies of natural progression of physical frailty and cognitive impairment are urgently needed. Only then can we begin to identify biological and clinical markers that distinguish patterns of coevolution, which may generate insights into the mechanistic pathway(s) implicated in cognitive frailty. A recent study from a US nationally representative sample of 7,439 community-dwelling older adults examined the temporal ordering in frailty and cognitive impairment onset, and found that participants with incident dementia during the 5-year follow-up were at increased risk of developing cognitive impairment first, or frailty and cognitive impairment concurrently. In contrast, dementia onset was associated with reduced risk of physical frailty onset before cognitive impairment [92]. These findings suggest that dementia-related pathology is less likely to be the cause of cognitive impairment if preceded by physical frailty, therefore providing support for the current definition of “cognitive frailty.”
Biomarkers
The term “biomarker” here refers to “objective indications of medical state observed from outside the patient – which can be measured accurately and reproducibly [93].” Biomarkers can help doctors evaluate a patient’s susceptibility to a disease, diagnose a disorder and its severity, predict its likely progression and future outcomes, determine optimal intervention strategies and monitor response to treatment. In the field of preclinical AD detection, cognitive testing remains the primary means to measure preclinical-to-clinical progression to AD; but the last 10 years has seen a considerable increase in the use of biomarkers to predict and track pre-clinical cognitive declines [94]. For example, structural imaging of hippocampal regions related to memory has proved useful in tracking transitions from healthy to MCI to AD. Blood biomarkers (e.g., lipids, inflammatory markers, and hormone levels) are also frequently collected as possible explanatory variables that inform cognitive declines [95–102] and merit similar concurrent study for their utility in predicting transitions to frailty.
Frailty research on biomarkers has focused more on disease-nonspecific mechanisms of the biology of aging. As in the field of AD detection, interest in frailty-related biomarker discovery is expected to intensify rapidly. In addition to disease staging as in the case of detection of AD-related cognitive decline, biomarkers can be helpful for distinguishing etiologies and pathways underlying physical frailty and cognitive impairment. For example, in order to improve diagnostic specificity of cognitive frailty, biomarkers of brain Aβ plaque deposition (e.g., cerebrospinal fluid Aβ42), apolipoprotein Eε4 genotype, as well as advanced neuroimaging techniques can serve to exclude neurodegenerative disease-related pathologies in case-finding of cognitive frailty [18]. While biomarker research holds great promise for a wide range of research and clinical applications, biomarker assays can be cost-prohibitive for large-scale epidemiological surveys and of limited value for population screening. In the case of cognitive frailty, a cost-effective alternative is through proactive health monitoring of older adults as they become frail and flag them at the first sign of cognitive impairment following frailty onset; biomarkers of neurodegenerative pathologies can then be used for case confirmation. From a data collection perspective, as the new generation of electronic medical record systems is increasingly being implemented to allow integrated record keeping and individual-level tracking, monitoring of health change over time is now an attainable goal in the near future.
Conclusions
In this chapter, we summarized the current variability in approaches to and assumptions regarding the integrated relationships between physical frailty and cognitive impairment, the definitions and potential roles of reserve and resilience in buffering or precipitating physical and cognitive impairments (e.g., Figure), and the importance of developing measures that are specific in the types of frailty (e.g., primary vs. secondary) and cognitive impairment (e.g., global vs. domain-specific) they intend to capture. We have prioritized the incorporation of biological theories that help determine shared and distinct pathways in the progression to physical and cognitive impairments (e.g., inflammation) and associated biomarkers that may then be incorporated into phenotype refinement and validation. Importance of bridging the gap between frailty theory and frailty measurement, such that science concerning the biology of frailty can be meaningfully advanced and preventive and treatment strategies can be developed and tested, persists.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version. Terms of use and reuse: academic research for non-commercial purposes, see here for full terms.
Conflict of Interest
Qian-Li Xue, Brian Buta, Lina Ma, Meiling Ge, and Michelle Carlson declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
- 1.Xue QL. The frailty syndrome: definition and natural history. Clin Geriatr Med. 2011;27(1):1–15. doi: 10.1016/j.cger.2010.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Todd S, Barr S, Roberts M, Passmore AP. Survival in dementia and predictors of mortality: a review. Int J Geriatr Psychiatr. 2013;28(11):1109–24. doi: 10.1002/gps.3946. [DOI] [PubMed] [Google Scholar]
- 3.Bandeen-Roche K, Seplaki CL, Huang J, Buta B, Kalyani RR, Varadhan R et al. Frailty in Older Adults: A Nationally Representative Profile in the United States. J Gerontol A Biol Sci Med Sci 2015. November;70(11):1427–34. doi: 10.1093/gerona/glv133. [DOI] [PMC free article] [PubMed] [Google Scholar]; * Provides most up-to-date US population representative estimates of frailty prevalence by demographic characteristics.
- 4.Katz MJ, Lipton RB, Hall CB, Zimmerman ME, Sanders AE, Verghese J et al. Age-specific and Sex-specific Prevalence and Incidence of Mild Cognitive Impairment, Dementia, and Alzheimer Dementia in Blacks and Whites A Report From the Einstein Aging Study. Alz Dis Assoc Dis. 2012;26(4):335–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Plassman BL, Langa KM, Fisher GG, Heeringa SG, Weir DR, Ofstedal MB et al. Prevalence of dementia in the united states: The aging, demographics, and memory study. Neuroepidemiology. 2007;29(1–2):125–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Subra J, Gillette-Guyonnet S, Cesari M, Oustric S, Vellas B, Platform T. The integration of frailty into clinical practice: preliminary results from the Gerontopole. J Nutr Health Aging. 2012;16(8):714–20. doi: 10.1007/s12603-012-0391-7. [DOI] [PubMed] [Google Scholar]
- 7.Robertson DA, Savva GM, Kenny RA. Frailty and cognitive impairment--a review of the evidence and causal mechanisms. Ageing Res Rev. 2013;12(4):840–51. doi: 10.1016/j.arr.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 8.Gobbens RJJ, Luijkx KG, Wijnen-Sponselee MT, Schols JMGA. Towards an integral conceptual model of frailty. J Nutr Health Aging. 2010;14(3):175–81. [DOI] [PubMed] [Google Scholar]
- 9.Rodriguez-Manas L, Feart C, Mann G, Vina J, Chatterji S, Chodzko-Zajko W et al. Searching for an Operational Definition of Frailty: A Delphi Method Based Consensus Statement. The Frailty Operative Definition-Consensus Conference Project. J Gerontol A Biol Sci Med Sci. 2013;68(1):62–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sternberg SA, Wershof Schwartz A, Karunananthan S, Bergman H, Mark Clarfield A. The identification of frailty: a systematic literature review. J Am Geriatr Soc. 2011;59(11):2129–38. doi: 10.1111/j.1532-5415.2011.03597.x. [DOI] [PubMed] [Google Scholar]
- 11.Avila-Funes JA, Amieva H, Barberger-Gateau P, Le Goff M, Raoux N, Ritchie K et al. Cognitive impairment improves the predictive validity of the phenotype of frailty for adverse health outcomes: the three-city study. J Am Geriatr Soc. 2009;57(3):453–61. doi: 10.1111/j.1532-5415.2008.02136.x. [DOI] [PubMed] [Google Scholar]
- 12.Rockwood K, Howlett SE, MacKnight C, Beattie BL, Bergman H, Hebert R et al. Prevalence, attributes, and outcomes of fitness and frailty in community-dwelling older adults: Report from the Canadian Study of Health and Aging. J Gerontol A Biol Sci Med Sci. 2004;59(12):1310–7. [DOI] [PubMed] [Google Scholar]
- 13.Sourial N, Wolfson C, Bergman H, Zhu B, Karunananthan S, Quail J et al. A correspondence analysis revealed frailty deficits aggregate and are multidimensional. J Clin Epidemiol. 2010;63(6):647–54. doi: 10.1016/j.jclinepi.2009.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bilotta C, Bergamaschini L, Nicolini P, Case A, Pina G, Rossi SV et al. Frailty syndrome diagnosed according to the Study of Osteoporotic Fractures criteria and mortality in older outpatients suffering from Alzheimer’s disease: A one-year prospective cohort study. Aging Ment Health. 2012;16(3):273–80. [DOI] [PubMed] [Google Scholar]
- 15.Rockwood K, Mitnitski A. Frailty in relation to the accumulation of deficits. J Gerontol A Biol Sci Med Sci.. 2007;62(7):722–7. [DOI] [PubMed] [Google Scholar]
- 16.Mitnitski AB, Mogilner AJ, Rockwood K. Accumulation of Deficits as a Proxy Measure of Aging. The Scientific World. 2001;1 323–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Verghese J, Wang CL, Lipton RB, Holtzer R. Motoric Cognitive Risk Syndrome and the Risk of Dementia. J Gerontol A Biol Sci Med Sci. 2013;68(4):412–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kelaiditi E, Cesari M, Canevelli M, van Kan GA, Ousset PJ, Gillette-Guyonnet S et al. Cognitive frailty: rational and definition from an (I.A.N.A./I.A.G.G.) international consensus group. J Nutr Health Aging. 2013;17(9):726–34. doi: 10.1007/s12603-013-0367-2. [DOI] [PubMed] [Google Scholar]
- 19.Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J et al. Frailty in older adults: evidence for a phenotype. The journals of gerontology. 2001;56(3):M146–56. [DOI] [PubMed] [Google Scholar]
- 20.Fried LP, Ferrucci L, Darer J, Williamson JD, Anderson G. Untangling the concepts of disability, frailty, and comorbidity: implications for improved targeting and care. J Gerontol A Biol Sci Med Sci. 2004;59(3):255–63. [DOI] [PubMed] [Google Scholar]
- 21.Xue QL, Bandeen-Roche K, Varadhan R, Zhou J, Fried LP. Initial manifestations of frailty criteria and the development of frailty phenotype in the Women’s Health and Aging Study II. J Gerontol A Biol Sci Med Sci. 2008;63(9):984–90. [DOI] [PubMed] [Google Scholar]
- 22.Cullati S, Kliegel M, Widmer E. Development of reserves over the life course and onset of vulnerability in later life. Nat Hum Behav. 2018;2(8):551–8. doi: 10.1038/s41562-018-0395-3. [DOI] [PubMed] [Google Scholar]; * Provides a theoretical framework for the development and onset of vulnerability in later life based on the concept of reserves.
- 23.Mosby’s medical dictionary. 9th ed. St. Louis, MO: Mosby Elsevier; 2013. [Google Scholar]
- 24.Sniecinski RM, Skubas NJ, London MJ. Testing cardiac reserve: then and now. 1923. Anesth Analg. 2012;115(5):991–2. doi: 10.1213/ANE.0b013e31825d2c09. [DOI] [PubMed] [Google Scholar]
- 25.Guralnik JM, Ferrucci L, Pieper CF, Leveille SG, Markides KS, Ostir GV et al. Lower extremity function and subsequent disability: consistency across studies, predictive models, and value of gait speed alone compared with the short physical performance battery. J Gerontol A Biol Sci Med Sci. 2000;55(4):M221–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Studenski S, Perera S, Patel K, Rosano C, Faulkner K, Inzitari M et al. Gait speed and survival in older adults. JAMA. 2011;305(1):50–8. doi: 10.1001/jama.2010.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schrack JA, Simonsick EM, Ferrucci L. The energetic pathway to mobility loss: an emerging new framework for longitudinal studies on aging. J Am Geriatr Soc. 2010;58 Suppl 2:S329–36. doi: 10.1111/j.1532-5415.2010.02913.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Whitson HE, Duan-Porter W, Schmader KE, Morey MC, Cohen HJ, Colon-Emeric CS. Physical Resilience in Older Adults: Systematic Review and Development of an Emerging Construct. J Gerontol A Biol Sci Med Sci. 2016;71(4):489–95. doi: 10.1093/gerona/glv202. [DOI] [PMC free article] [PubMed] [Google Scholar]; * Characterizes the emerging construct of resilience as it pertains to physical health in older adults.
- 29.Luthar SS, Cicchetti D, Becker B. The construct of resilience: a critical evaluation and guidelines for future work. Child Dev. 2000;71(3):543–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Resnick B, Galik E, Dorsey S, Scheve A, Gutkin S. Reliability and validity testing of the physical resilience measure. Gerontologist. 2011;51(5):643–52. doi: 10.1093/geront/gnr016. [DOI] [PubMed] [Google Scholar]
- 31.Pershin BB, Kuz’Min SN, Suzdal’Nitskii RS, Levando VA. Reserve potential of immunity. Sports Medicine, Training and Rehabilitation. 1988;1(1):53–60. doi: 10.1080/15438628809511845. [DOI] [Google Scholar]
- 32.Stern Y Cognitive reserve. Neuropsychologia. 2009;47(10):2015–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leng SX, Yang H, Walston JD. Decreased cell proliferation and altered cytokine production in frail older adults. Aging Clin Exp Res. 2004;16(3):249–52. [DOI] [PubMed] [Google Scholar]
- 34.Semba RD, Margolick JB, Leng S, Walston J, Ricks MO, Fried LP. T cell subsets and mortality in older community-dwelling women. Expl Gerontol. 2005;40(1–2):81–7. doi: 10.1016/j.exger.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 35.Yao X, Hamilton RG, Weng NP, Xue QL, Bream JH, Li H et al. Frailty is associated with impairment of vaccine-induced antibody response and increase in post-vaccination influenza infection in community-dwelling older adults. Vaccine. 2011;29(31):5015–21. doi: 10.1016/j.vaccine.2011.04.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kalyani RR, Varadhan R, Weiss CO, Fried LP, Cappola AR. Frailty Status and Altered Glucose-Insulin Dynamics. J Gerontol A Biol Sci Med Sci. 2012;67(12):1300–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu C, Kim DH, Xue QL, Lee DSH, Varadhan R, Odden MC. Association of Frailty with Recovery from Disability among Community-Dwelling Older Adults: Results from Two Large U.S. Cohorts. J Gerontol A Biol Sci Med Sci. 2018. doi: 10.1093/gerona/gly080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Boyd CM, Ricks M, Fried LP, Guralnik JM, Xue QL, Xia J et al. Functional decline and recovery of activities of daily living in hospitalized, disabled older women: the Women’s Health and Aging Study I. J Am Geriatr Soc. 2009;57(10):1757–66. doi: 10.1111/j.1532-5415.2009.02455.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146–M56. [DOI] [PubMed] [Google Scholar]
- 40.Furtado GE, Caldo A, Rieping T, Filaire E, Hogervorst E, Teixeira AMB et al. Physical frailty and cognitive status over-60 age populations: A systematic review with meta-analysis. Arch Gerontol Geriatr. 2018;78:240–8. [DOI] [PubMed] [Google Scholar]
- 41.Sleight C, Holtzer R. Differential associations of functional and cognitive health outcomes with pre-frailty and frailty states in community-dwelling older adults. J Health Psychol. 2017:1359105317745964. doi: 10.1177/1359105317745964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Robertson DA, Savva GM, Coen RF, Kenny RA. Cognitive function in the prefrailty and frailty syndrome. J Am Geriatr Soc. 2014;62(11):2118–24. doi: 10.1111/jgs.13111. [DOI] [PubMed] [Google Scholar]
- 43.Chen S, Honda T, Narazaki K, Chen T, Nofuji Y, Kumagai S. Global cognitive performance and frailty in non-demented community-dwelling older adults: Findings from the Sasaguri Genkimon Study. Geriatr Gerontol Int. 2016;16(6):729–36. doi: 10.1111/ggi.12546. [DOI] [PubMed] [Google Scholar]
- 44.Feng L, Nyunt MS, Gao Q, Feng L, Lee TS, Tsoi T et al. Physical Frailty, Cognitive Impairment, and the Risk of Neurocognitive Disorder in the Singapore Longitudinal Ageing Studies. J Gerontol A Biol Sci Med Sci. 2017;72(3):369–75. doi: 10.1093/gerona/glw050. [DOI] [PubMed] [Google Scholar]
- 45.Rosado-Artalejo C, Carnicero JA, Losa-Reyna J, Guadalupe-Grau A, Castillo-Gallego C, Gutierrez-Avila G et al. Cognitive Performance across 3 Frailty Phenotypes: Toledo Study for Healthy Aging. J Am Med Dir Assoc. 2017;18(9):785–90. doi: 10.1016/j.jamda.2017.04.008. [DOI] [PubMed] [Google Scholar]
- 46.Nishiguchi S, Yamada M, Fukutani N, Adachi D, Tashiro Y, Hotta T et al. Differential association of frailty with cognitive decline and sarcopenia in community-dwelling older adults. J Am Med Dir Assoc. 2015;16(2):120–4. doi: 10.1016/j.jamda.2014.07.010. [DOI] [PubMed] [Google Scholar]
- 47.Tay L, Lim WS, Chan M, Ye RJ, Chong MS. The Independent Role of Inflammation in Physical Frailty among Older Adults with Mild Cognitive Impairment and Mild-to-Moderate Alzheimer’s Disease. J Nutr Health Aging. 2016;20(3):288–99. doi: 10.1007/s12603-015-0617-6. [DOI] [PubMed] [Google Scholar]
- 48.Yu R, Morley JE, Kwok T, Leung J, Cheung O, Woo J. The Effects of Combinations of Cognitive Impairment and Pre-frailty on Adverse Outcomes from a Prospective Community-Based Cohort Study of Older Chinese People. Front Med (Lausanne). 2018;5:50. doi: 10.3389/fmed.2018.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen S, Honda T, Narazaki K, Chen T, Kishimoto H, Haeuchi Y et al. Physical Frailty Is Associated with Longitudinal Decline in Global Cognitive Function in Non-Demented Older Adults: A Prospective Study. J Nutr Health Aging. 2018;22(1):82–8. doi: 10.1007/s12603-017-0924-1. [DOI] [PubMed] [Google Scholar]
- 50.Wu YH, Liu LK, Chen WT, Lee WJ, Peng LN, Wang PN et al. Cognitive Function in Individuals With Physical Frailty but Without Dementia or Cognitive Complaints: Results From the I-Lan Longitudinal Aging Study. J Am Med Dir Assoc. 2015;16(10):899 e9–16. doi: 10.1016/j.jamda.2015.07.013. [DOI] [PubMed] [Google Scholar]
- 51.Gross AL, Xue QL, Bandeen-Roche K, Fried LP, Varadhan R, McAdams-DeMarco MA et al. Declines and Impairment in Executive Function Predict Onset of Physical Frailty. J Gerontol A Biol Sci Med Sci. 2016;71(12):1624–30. doi: 10.1093/gerona/glw067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bunce D, Batterham PJ, Mackinnon AJ. Long-term Associations Between Physical Frailty and Performance in Specific Cognitive Domains. J Gerontol B Psychol Sci Soc Sci. 2018. doi: 10.1093/geronb/gbx177. [DOI] [PubMed] [Google Scholar]
- 53.Fried LP, Hadley EC, Walston J, Newman AB, Guralnik JM, Studenski S et al. From bedside to bench: research agenda for frailty. Sci Aging Knowledge Environ. 2005;2005 (31):24. [DOI] [PubMed] [Google Scholar]
- 54.Picard M, McManus MJ, Gray JD, Nasca C, Moffat C, Kopinski PK et al. Mitochondrial functions modulate neuroendocrine, metabolic, inflammatory, and transcriptional responses to acute psychological stress. Proc Natl Acad Sci U S A. 2015;112(48):E6614–E23. doi: 10.1073/pnas.1515733112. [DOI] [PMC free article] [PubMed] [Google Scholar]; * Provides experimental data supporting the associations between mitochondrial dysfunctions and impaired multisystem stress-response.
- 55.Green DR, Galluzzi L, Kroemer G. Mitochondria and the autophagy-inflammation-cell death axis in organismal aging. Science (New York, NY. 2011;333(6046):1109–12. doi: 10.1126/science.1201940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Picard M, McEwen BS, Epel ES, Sandi C. An energetic view of stress: Focus on mitochondria. Front Neuroendocrinol. 2018;49:72–85. doi: 10.1016/j.yfrne.2018.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Di Leo R, Musumeci O, de Gregorio C, Recupero A, Grimaldi P, Messina C et al. Evidence of cardiovascular autonomic impairment in mitochondrial disorders. J Neurol. 2007;254(11):1498–503. doi: 10.1007/s00415-007-0536-5. [DOI] [PubMed] [Google Scholar]
- 58.Parikh S, Gupta A. Autonomic Dysfunction in Epilepsy and Mitochondrial Diseases. Semin Pediatr Neurol. 2013;20(1):31–4. doi: 10.1016/j.spen.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 59.Walston JD. Connecting Age-Related Biological Decline to Frailty and Late-Life Vulnerability. Nestle Nutr Inst Workshop Ser. 2015;83:1–10. doi: 10.1159/000382052. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** Provides an overview of biological underpinnings of frailty, with the focus on physiological stress-response systems.
- 60.Critchley HD, Eccles J, Garfinkel SN. Interaction between cognition, emotion, and the autonomic nervous system. Handb Clin Neurol. 2013;117:59–77. doi: 10.1016/B978-0-444-53491-0.00006-7. [DOI] [PubMed] [Google Scholar]
- 61.Sartori AC, Vance DE, Slater LZ, Crowe M. The impact of inflammation on cognitive function in older adults: implications for healthcare practice and research. J Neurosci Nurs. 2012;44(4):206–17. doi: 10.1097/JNN.0b013e3182527690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ferrari E, Magri F. Role of neuroendocrine pathways in cognitive decline during aging. Ageing Res Rev. 2008;7(3):225–33. doi: 10.1016/j.arr.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 63.Franceschi C, Bonafe M, Valensin S, Olivieri F, De Luca M, Ottaviani E et al. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann N Y Acad Sci. 2000;908:244–54. [DOI] [PubMed] [Google Scholar]
- 64.Halil M, Kizilarslanoglu MC, Kuyumcu ME, Yesil Y, Jentoft AJC. Cognitive aspects of frailty: Mechanisms behind the link between frailty and cognitive impairment. J Nutr Health Aging. 2015;19(3):276–83. [DOI] [PubMed] [Google Scholar]
- 65.Rockwood K, Mitnitski A. Frailty defined by deficit accumulation and geriatric medicine defined by frailty. Clin Geriatr Med. 2011;27(1):17–26. doi: 10.1016/j.cger.2010.08.008. [DOI] [PubMed] [Google Scholar]
- 66.Rockwood K, Mitnitski A, Song X, Steen B, Skoog I. Long-term risks of death and institutionalization of elderly people in relation to deficit accumulation at age 70. J Am Geriatr Soc. 2006;54(6):975–9. doi: 10.1111/j.1532-5415.2006.00738.x. [DOI] [PubMed] [Google Scholar]
- 67.Imaoka Y, Kawano T, Hashiguchi A, Fujimoto K, Yamamoto K, Nishi T et al. Modified frailty index predicts postoperative outcomes of spontaneous intracerebral hemorrhage. Clin Neurol Neurosurg. 2018;175:137–43. doi: 10.1016/j.clineuro.2018.11.004. [DOI] [PubMed] [Google Scholar]
- 68.Allali G, Ayers EI, Verghese J. Motoric Cognitive Risk Syndrome Subtypes and Cognitive Profiles. J Gerontol A Biol Sci Med Sci. 2016;71(3):378–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Verghese J, Annweiler C, Ayers E, Barzilai N, Beauchet O, Bennett DA et al. Motoric cognitive risk syndrome Multicountry prevalence and dementia risk. Neurology. 2014;83(8):718–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Callisaya ML, Ayers E, Barzilai N, Ferrucci L, Guralnik JM, Lipton RB et al. Motoric Cognitive Risk Syndrome and Falls Risk: A Multi-Center Study. J Alzheimers Dis. 2016;53(3):1043–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Parihar R, Mahoney JR, Verghese J. Relationship of Gait and Cognition in the Elderly. Curr Transl Geriatr Exp Gerontol Rep. 2013;2(3). doi: 10.1007/s13670-013-0052-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Buracchio T, Dodge HH, Howieson D, Wasserman D, Kaye J. The Trajectory of Gait Speed Preceding Mild Cognitive Impairment. Arch Neurol. 2010;67(8):980–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Doi T, Shimada H, Makizako H, Tsutsumimoto K, Verghese J, Suzuki T. Motoric Cognitive Risk Syndrome: Association with Incident Dementia and Disability. J Alzheimers Dis. 2017;59(1):77–84. [DOI] [PubMed] [Google Scholar]
- 74.Ayers E, Verghese J. Motoric cognitive risk syndrome and risk of mortality in older adults. Alzheimers Dement. 2016;12(5):556–64. [DOI] [PubMed] [Google Scholar]
- 75.Verghese J, LeValley A, Hall CB, Katz MJ, Ambrose AF, Lipton RB. Epidemiology of gait disorders in community-residing older adults. J Am Geriatr Soc. 2006;54(2):255–61. doi: 10.1111/j.1532-5415.2005.00580.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rovio S, Kareholt I, Helkala EL, Viitanen M, Winblad B, Tuomilehto J et al. Leisure-time physical activity at midlife and the risk of dementia and Alzheimer’s disease. Lancet Neurol. 2005;4(11):705–11. doi: 10.1016/S1474-4422(05)70198-8. [DOI] [PubMed] [Google Scholar]
- 77.Montero-Odasso MM, Barnes B, Speechley M, Muir-Hunter S, Doherty T, Duque G et al. Physical Frailty, Cognitive Frailty, and the Risk of Dementia in the Gait & Brain Study. J Am Geriatr Soc. 2016;64:S129–S. [Google Scholar]
- 78.Solfrizzi V, Scafato E, Seripa D, Lozupone M, Imbimbo BP, D’Amato A et al. Reversible Cognitive Frailty, Dementia, and All-Cause Mortality. The Italian Longitudinal Study on Aging. J Am Med Dir Assoc. 2017;18(1). [DOI] [PubMed] [Google Scholar]
- 79.Shimada H, Doi T, Lee S, Makizako H, Chen LK, Arai H. Cognitive Frailty Predicts Incident Dementia among Community-Dwelling Older People. J Clin Med. 2018;7(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Roppolo M, Mulasso A, Rabaglietti E. Cognitive frailty in Italian community-dwelling older adults: Prevalence rate and its association with disability. J Nutr Health Aging. 2017;21(6):631–6. [DOI] [PubMed] [Google Scholar]
- 81.Feng L, Nyunt MSZ, Gao Q, Feng L, Yap KB, Ng TP. Cognitive Frailty and Adverse Health Outcomes: Findings From the Singapore Longitudinal Ageing Studies (SLAS). J Am Med Dir Assoc. 2017;18(3):252–8. [DOI] [PubMed] [Google Scholar]
- 82.Brown RT, Covinsky KE. Frailty as an Outcome in Geriatrics Research: Not Ready for Prime Time? Ann Internal Med. 2018;168(5):361–+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Buta BJ, Walston JD, Godino JG, Park M, Kalyani RR, Xue QL et al. Frailty assessment instruments: Systematic characterization of the uses and contexts of highly-cited instruments. Ageing Res Rev. 2016;26:53–61. doi: 10.1016/j.arr.2015.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** Illustrates the importance of selecting frailty instruments to match their intended contexts and purposes of use.
- 84.Cronbach LJ, Meehl PE. Construct Validity in Psychological Tests. Psychol Bull. 1955;52(4):281–302. [DOI] [PubMed] [Google Scholar]
- 85.Fried LP, Walston J, Hazzard WR, Blass JP, Ettinger WH Jr., Halter JB et al. Frailty and failre to thrive Principles of Geriatric Medicine and Gerontology. New York: McGraw Hill; 1998. p. 1387–402. [Google Scholar]
- 86.Varadhan R, Seplaki CL, Xue QL, Bandeen-Roche K, Fried LP. Stimulus-response paradigm for characterizing the loss of resilience in homeostatic regulation associated with frailty. Mech Ageing Dev. 2008;129(11):666–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Xue QL, Buta B, Varadhan R, Szanton SL, Chaves P, Walston JD et al. , editors. Frailty and Geriatric Syndromes. Aging, Place and Health: A Global Perspective. Burlington, MA: Jones & Bartlett Learning; 2017. [Google Scholar]
- 88.Bergman H, Ferrucci L, Guralnik J, Hogan DB, Hummel S, Karunananthan S et al. Frailty: An emerging research and clinical paradigm - Issues and controversies. J Gerontol A Biol Sci Med Sci. 2007;62(7):731–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Morley JE, Vellas B, van Kan GA, Anker SD, Bauer JM, Bernabei R et al. Frailty consensus: a call to action. J Am Med Dir Assoc. 2013;14(6):392–7. doi: 10.1016/j.jamda.2013.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Esiri MM, Matthews F, Brayne C, Ince PG, Matthews FE, Xuereb JH et al. Pathological correlates of late-onset dementia in a multicentre, community-based population in England and Wales. Lancet. 2001;357(9251):169–75. [DOI] [PubMed] [Google Scholar]
- 91.Dartigues JF, Amieva H. Cognitive Frailty: Rational and Definition from an (I.A.N.A./I.A.G.G.) International Consensus Group. J Nutr Health Aging. 2014;18(1):95–. [DOI] [PubMed] [Google Scholar]
- 92.Chu N, Tian J, Gross AL, Bandeen-Roche K, Carlson MC, Xue Q. Hierarchical development of physical frailty and cognitive impairment: clues into etiological pathways. Innov Aging. 2018;2(suppl_1):23-. doi: 10.1093/geroni/igy023.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Strimbu K, Tavel JA. What are biomarkers? Curr Opin HIV AIDS. 2010;5(6):463–6. doi: 10.1097/COH.0b013e32833ed177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Jack CR Jr., Knopman DS, Jagust WJ, Petersen RC, Weiner MW, Aisen PS et al. Tracking pathophysiological processes in Alzheimer’s disease: an updated hypothetical model of dynamic biomarkers. Lancet Neurol. 2013;12(2):207–16. doi: 10.1016/S1474-4422(12)70291-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mielke MM, Xue QL, Zhou J, Chaves PH, Fried LP, Carlson MC. Baseline serum cholesterol is selectively associated with motor speed and not rates of cognitive decline: the Women’s Health and Aging Study II. J Gerontol A Biol Sci Med Sci. 2008;63(6):619–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mielke MM, Bandaru VV, Haughey NJ, Xia J, Fried LP, Yasar S et al. Serum ceramides increase the risk of Alzheimer disease: the Women’s Health and Aging Study II. Neurology. 2012;79(7):633–41. doi: 10.1212/WNL.0b013e318264e380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bernick C, Katz R, Smith NL, Rapp S, Bhadelia R, Carlson M et al. Statins and cognitive function in the elderly: the Cardiovascular Health Study. Neurology. 2005;65(9):1388–94. doi: 10.1212/01.wnl.0000182897.18229.ec. [DOI] [PubMed] [Google Scholar]
- 98.Grady D, Yaffe K, Kristof M, Lin F, Richards C, Barrett-Connor E. Effect of postmenopausal hormone therapy on cognitive function: the Heart and Estrogen/progestin Replacement Study. Am J Med. 2002;113(7):543–8. [DOI] [PubMed] [Google Scholar]
- 99.Holden KF, Lindquist K, Tylavsky FA, Rosano C, Harris TB, Yaffe K et al. Serum leptin level and cognition in the elderly: Findings from the Health ABC Study. Neurobiol Aging. 2009;30(9):1483–9. doi: 10.1016/j.neurobiolaging.2007.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Metti AL, Yaffe K, Boudreau RM, Ganguli M, Lopez OL, Stone KL et al. Change in inflammatory markers and cognitive status in the oldest-old women from the Study of Osteoporotic Fractures. J Am Geriatr Soc. 2014;62(4):662–6. doi: 10.1111/jgs.12739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Akrivos J, Ravona-Springer R, Schmeidler J, LeRoith D, Heymann A, Preiss R et al. Glycemic control, inflammation, and cognitive function in older patients with type 2 diabetes. Int J Geriatr Psychiatry. 2015;30(10):1093–100. doi: 10.1002/gps.4267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lai KSP, Liu CS, Rau A, Lanctot KL, Kohler CA, Pakosh M et al. Peripheral inflammatory markers in Alzheimer’s disease: a systematic review and meta-analysis of 175 studies. J Neurol Neurosurg Psychiatry. 2017;88(10):876–82. doi: 10.1136/jnnp-2017-316201. [DOI] [PubMed] [Google Scholar]