Abstract
Early life, including prenatal development and childhood, is a period of sensitivity, with potential for developmental programming under conditions of adversity. The intergenerational effects of early adversity have received attention, most often studied in relation to fetal development according to maternal exposures. Less often considered but critically important is the effect of early adversity on future prenatal risk (e.g., risk for preeclampsia, preterm birth), which threatens the health of mother and infant. The body’s ability to turn collections of genes “on” or “off” across a range of tissues via receptor-driven transcription factors and epigenetic mechanisms (i.e., chemical modifications to the genome) in response to the perceived environment may help to explain such associations. This review aims to summarize discoveries surrounding the effects of early adversity on gene expression, emphasizing prenatal populations. First, we review findings from gene expression studies examining the effects of early adversity on various tissues known to contribute to prenatal health in adulthood. Next, we review several gene regulatory mechanisms thought to underlie differences in gene expression. Finally, we discuss potential implications for prenatal risk among early adversity-exposed mothers according to our current understanding of the biology that contributes to the development of prenatal syndromes.
Keywords: Adverse childhood experiences, epigenetics, gene expression, preeclampsia, pregnancy, premature birth
Introduction
Early life, including prenatal development and childhood, is a period of particular sensitivity, with the potential for developmental programming of biological systems under conditions of adversity. First studied among individuals exposed to physiologic stressors during development (e.g., gross nutrient deprivation [1–3]), it has become clear that early adversity imposed in the form of psychosocial stressors can also have lasting effects on health. This has now been shown to be true across a broad range of early adversity exposures, including abuse, neglect, and various forms of interpersonal or financial loss or instability, particularly among women [4–9]. While a dose-response effect of early adversity on health parameters appears to be at play [10–12*], it is noteworthy that a single instance of adversity in early life shows associations with notable differences in health into adulthood [13]. As such, it is particularly concerning that more than 10% of children experience abuse or neglect of a severity capable of substantiation by protective services and more than 50% of children report exposure to some form of early adversity [14,15].
The potential for intergenerational effects of early adversity has also received increasing attention, most often studied as it relates to the development of a fetus according to maternal exposures during pregnancy (e.g., maternal socioeconomic status [16], prenatal psychosocial stress [17,18]). Less often considered but also critically important to intergenerational health is the effect of early adversity on prenatal risk among expectant mothers who were exposed to adverse experiences during their own childhood. Indeed, though the biological pathways remain unclear, there is a growing body of literature linking early adversity to major complications of pregnancy, including preeclampsia [19**] and preterm birth [19**–21*]. As such, exposed women and their children face a heightened risk for perinatal, neonatal, and infant mortality and both generations of survivors bear a disproportionate burden of lifelong morbidity [22–24].
Since realizing that time-limited exposures in early life are capable of affecting adult health trajectories, the scientific community has pursued the discovery of lasting “biological marks” or persistent physiological alterations that convey early adversity-associated disease burden, including in the context of pregnancy. Early research in this area focused largely on gross differences in neurobiology (e.g., regional volume, fiber tract integrity [25–27]), as the developing brain has long been known to display enormous plasticity. With the discovery that collections of genes can be “turned on” or “turned off” via receptor-mediated alterations in transcription factor activity and via epigenetic mechanisms (i.e., chemical modifications to the genome) in response to one’s perception of their environment, a literature focused on what has been coined “social genomics” is swiftly developing and beginning to provide insight into the potential pathways by which early adversity affects disease risk across a wide range of biological systems [28–30*].
The purpose of the current review is to summarize recent discoveries surrounding the effects of early adversity on gene expression, with special emphasis on implications for prenatal health among early adversity-exposed expectant mothers. Indeed, pregnancy may be an ideal time to disrupt the intergenerational cycle of childhood maltreatment by targeting the molecular underpinnings of impaired prenatal health that establish the foundation for subsequent development. First, we review findings from gene expression studies examining the effects of early adversity on various tissues known to contribute to prenatal health in adulthood. Next, we review several gene regulatory mechanisms that could potentially mediate early adversity-associated differences in gene expression. Finally, we discuss potential implications for prenatal risk among early adversity-exposed mothers according to our current understanding of the biology that contributes to the development of prenatal syndromes.
Early adversity and gene expression
Gene expression is a cell-specific parameter. In fact, our ability to generate tissues with diverse functions despite an identical genetic code is reliant upon our capacity to refine which genes are readily expressed dependent upon cellular conditions. Therefore, similar to the structural neuroplasticity exhibited during critical periods of development, our cells appear to exhibit “genomic plasticity” in the expression of genes. As the literature base grows, patterns in gene expression have emerged across cell types that lend biologic plausibility to the associations witnessed among early adversity and various health sequelae into adulthood.
Many studies have focused on neuroregulatory gene expression, with investigators often turning to animal models of early adversity (e.g., maternal separation, social isolation) delivered in the context of carefully controlled conditions. Effects of such exposures have been witnessed among model organisms (e.g., rodents, rhesus macaques) on the expression of genes related to, for example, synaptic transmission within sensory processing brain regions [31], stress resilience within the amygdala [32], stress-related neuronal dysfunction within the hippocampus [33], synaptic plasticity within the prefrontal cortex [34], and dopaminergic signaling within the nucleus accumbens [35*], with some follow-up periods extending into adulthood. Corresponding differences in behavior (e.g., sensory gating, anxiousness, reward seeking) have also been noted. Thus, social genomic mechanisms may contribute to lasting early adversity-associated differences in both neural anatomy and fundamental cell signaling pathways critical to processing sensory inputs, threats, emotions, and reward.
The effect of early adversity on gene expression within the hypothalamus, which serves as the bridge between the brain and endocrine system, has also received particular attention. Multiple studies now show that genes involved in hypothalamic-pituitary-adrenal (HPA) axis activation (e.g., CRH [36,37], AVP [36,38], EGR1 [39*,40]) show heightened expression under static conditions as a function of adverse early life conditions. The ready expression of stress-responsive genes within the hypothalamus may contribute to the disturbances in basal levels of glucocorticoids and the blunted HPA-mediated stress response witnessed among adults with a history of maltreatment, a common finding [41–43] with recent extension to pregnant adults [12*,39*]. Morrison et al., for example, recently revealed associations among preadolescent chronic variable stress and the expression of 24 genes within the hypothalamic paraventricular nucleus during murine pregnancy [39*]. This group went on to show that the expected rise in glucocorticoid levels during maternal-offspring separation was blunted among postpartum adolescently-stressed mice and adverse childhood experience-exposed women [39*].
Inflammatory parameters also appear to be particularly susceptible to regulation via social genomic mechanisms. For example, Zajdel et al. report that maternally-separated, bacterially-challenged C57Bl/6 mice show augmented expression of interleukin(IL)-6, IL-1β, and tumor necrosis factor(TNF)-α within the hypothalamus [44]. Similar findings have been reported in examining the brains of maternally-separated rodents under basal and acute stress conditions (hypothalamus [45], hippocampus [46]), with neuroinflammation thought to contribute to differences in social behavior [47]. In relation to psychosocial stress, immune cells themselves also show gene expression patterns consistent with enhanced proinflammatory and dampened anti-inflammatory activity (e.g., [48**–52]). In fact, the prevalence of this pattern has led to its coining as the “conserved transcriptional response to adversity” (CTRA) [28]. While the CTRA-related literature has focused primarily on the effects of chronic psychosocial stress conditions in adulthood, several animal and human studies have now linked early adversity with similar patterns of differential gene expression that appear to persist into adulthood [41,53**,54]. Such studies are sorely lacking among pregnant populations, though there is evidence to suggest that early adversity is associated with peripheral inflammation [55,56*] and up-regulation of pro-inflammatory genes [57] during pregnancy. Studies of placental tissue also suggest that psychosocial conditions during pregnancy can affect fetal tissue development [58,59].
Potential biological mechanisms
As discussed, despite a stable genetic code, cells display a remarkable ability to turn genes “on” or “off” dependent upon their physiologic needs. This feat is accomplished through a combination of gene regulatory processes that include receptor-mediated activation of transcription factors that stimulate transcription of specific genes as well as epigenetic mechanisms that gate the access of transcription factors to the DNA genome. These processes work in concert to establish and transmit patterns of gene expression by dynamically regulating transcription of our DNA-encoded genes into messenger RNAs that subsequently guide the production of proteins that give rise to cellular structure and function [60–62]. Gene regulation by transcription factors represents the primary biological pathway through which extracellular processes induce acute effects on gene expression profiles. By contrast, epigenetic mechanisms are more often endogenously regulated as part of basic cellular development and differentiation programs, and as such their effects tend to be more durable over time. Non-Mendelian inheritance and environmental exposures shape the epigenome and some epigenetic marks are capable of perpetuation across cycles of cell replication and even during meiosis, providing a foundation for highly persistent effects on gene expression within an individual and across generations [63,64]. The joint effects of acutely responsive transcription factor activation (and feedback circuits that can propagate such effects over development) and more protracted epigenetic modifications allow social conditions early in life to affect the physiological underpinnings of disease process that manifest clinically much later in life.
DNA methylation, described as the classic epigenetic mark, involves the addition or removal of methyl groups to phosphate-linked cytosine-guanine (CpG) dinucleotides, which tend to be concentrated in promotor regions of genes [65]. Typically, loss of CpG methylation promotes gene expression by allowing transcription factors to access DNA. Gain of CpG methylation promotes gene silencing by hindering the binding ability of transcription factors. However, DNA methylation across varying genomic regions can also have transcriptional implications via alternative mechanisms [60,66]. DNA methylation has been increasingly examined as a function of early adversity, with differential methylation of DNA now witnessed in a number of epigenomic regions approximating with genes critical to navigating the social world (e.g., CRFR2 [cortocotrophin-releasing factor 2] [67], NR3C1 [glucocorticoid receptor] [68–70], FKBP5 [affects glucocorticoid receptor sensitivity] [71], BDNF [nerve growth factor] [72]). Moreover, DNA methylation measured in peripheral tissues (e.g., leukocytes) has been linked to, for example, a blunted HPA response following challenge [68], suggesting that, at least under some circumstances, peripheral epigenomes may provide a glimpse into the epigenetic regulation of central neuroendocrine processes. DNA methylation has received attention for its potential role in conveying risk to the developing fetus via prenatal exposures to their mother (e.g., by examining cord blood [73,74]), though the effects of early adversity on future gene expression among gravid adults via the methylome remains to be determined.
Noncoding RNAs (e.g., microRNA [miRNA]) are transcribed, untranslated RNA molecules with the ability to affect gene expression through pre- and post-transcriptional mechanisms [60,75]. Several studies now link adverse early life experiences to differences in miRNA expression and/or activity [76,77]. miRNA expression has also been shown to target genes active in brain regions critical to threat perception, emotional processing, reward circuitry, and stress responsiveness (e.g., amygdala, hippocampus, prefrontal cortex, nucleus accumbens, hypothalamus) [78]. The regulatory role of miRNAs in immune adaptation is relatively well established, with the deleterious effects of aberrations in miRNA expression more recently realized (e.g., potentiated pro-inflammatory nuclear factor κ B signaling) [79]. During murine pregnancy, gene expression appears to be susceptible to psychosocial stress paradigms via miRNA-based mechanisms [80*]; though, human studies are lacking.
Implications for Prenatal Health
The aforementioned biological effects of early adversity have potential implications for prenatal health in adulthood among exposed mothers, with hypothesized pathways depicted in Figure 1. First, a robust literature links early adversity to numerous behavioral and psychiatric sequelae during non-pregnant and pregnant adulthood, including smoking, alcohol use, drug use, depression, and anxiety [81,82]. For example, compared to their counterparts, pregnant women with a history of childhood physical abuse are approximately 1.4 times more likely to smoke during pregnancy and at 2.8 times the odds of experiencing persistent, significant perinatal depressive symptoms [83,84]. Maternal tobacco use and prenatal depression, in turn, increase the odds of preterm birth by roughly 3.9-fold and 1.6-fold, respectively [85,86]. Prenatal depression also increases the odds of preeclampsia by 1.5-fold [87]. Such findings suggest that early adversity is associated with future prenatal complications, at least in part, through its effects on behavior and mental health during pregnancy. However, associations among early adversity and prenatal complications persist despite exertion of statistical control over such covariates, suggesting the potential for direct effects.
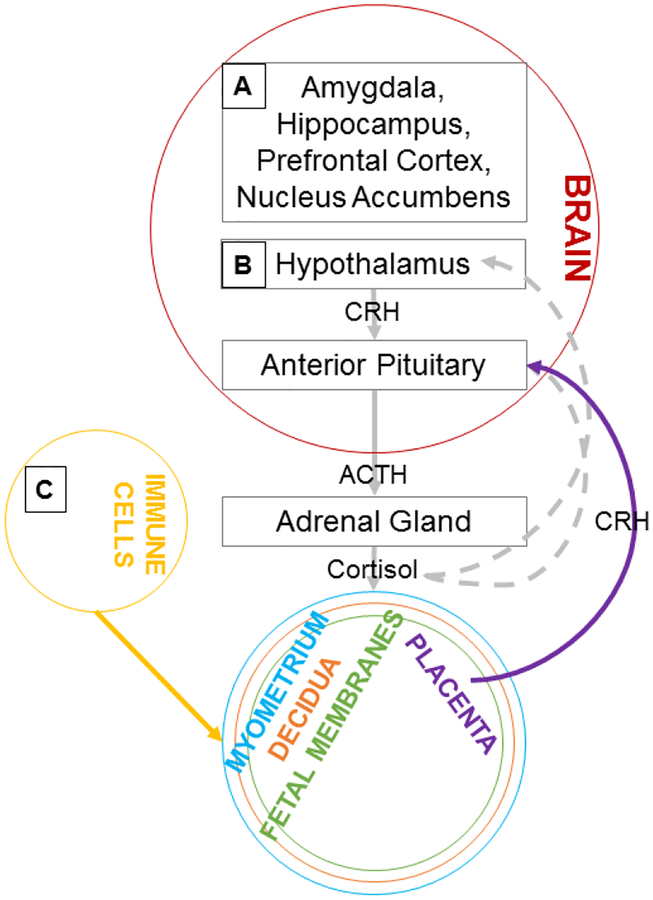
Figure 1. Proposed Pathways Linking Early Adversity to Prenatal Health.
Adversity during prenatal development and childhood is hypothesized to affect gene expression among the future expectant mother within: A) regions of the brain critical to processing sensory inputs, threats, emotions, and reward, increasing risk for behaviors and perturbations in mental health linked to risk for complications of pregnancy; B) the hypothalamus, ultimately dysregulating cortisol and placental CRH production, posited to contribute to preeclampsia and preterm birth; C) immune cells, enhancing pro-inflammatory and dampening anti-inflammatory activity, which is thought to contribute to preeclampsia and preterm birth. CRH = corticotropin-releasing hormone; ACTH = adrenocorticotropin-releasing hormone; solid lines = positive feedback; dashed lines = negative feedback
In this regard, neuroendocrine perturbations have received particular attention for their potential etiologic contributions to complications of pregnancy. Specifically, the theory of the accelerated “placental clock” has been put forward to explain the heightened risk for preterm birth witnessed in the context of psychosocial stress, as over-activity of the HPA axis is thought to accelerate the rise in circulating glucocorticoid levels expected and well described during pregnancy [88]. In response, the placenta (i.e., the organ responsible for fetal oxygenation and nutrient exchange) produces increasing amounts of corticotropin-releasing hormone, which promotes early labor by, for example, disrupting the integrity of the baby’s bag of water and exciting the mother’s uterine smooth muscle to encourage contractions [89,90]. Heightened expression of corticotropin-releasing hormone has also been witnessed in the blood and placental extracts of preeclamptic versus normotensive women [91,92], which may be related to the effects of corticotropin-releasing hormone on, for example, placental apoptosis (e.g., by activating Fas ligand-positive macrophages [93]) or function (e.g., by promoting greater umbilical artery resistance [94]). While studies of the mediational role of neuroendocrine disruption in early adversity-associated complications of pregnancy are rare, some data suggests lasting effects of the exposure on prenatal neuroendocrine biology [95], including when psychosocial stress in adulthood is held constant [12*].
Decades of research also support that inflammation (of sterile or infectious origin; at the systemic or local level) promotes several processes involved in the premature initiation of labor (i.e., dilation of the uterine cervix, contraction of the uterine smooth muscle, rupture of the baby’s bag of water) [96]. Here, activated leukocytes infiltrate maternal and fetal tissues and produce large amounts of pro-inflammatory cytokines, driving labor through a unique feed-forward cascade [97–99]. However, factors promoting or deterring initiation of this cascade remain elusive and interventions such as antibiotics fail to prevent preterm birth [100,101], positioning the inflammatory origins of preterm birth as a continued topic of inquiry. Similarly, preeclampsia is marked by maternal inflammation both in examining placental tissues and in interrogating the maternal circulation [102,103]. Though, it remains unclear whether aberrations associated with preeclampsia such as placental ischemia drive processes known to promote inflammation (e.g., reduced T regulatory and enhanced CD8(+) T cell composition [104], upregulation of leukocyte nuclear factor κ B signaling [105]) or inflammatory processes play an etiologic role in the onset of the syndrome. As above, formal tests of mediation are sorely lacking. Though, associations among childhood maltreatment, peripheral inflammation, and preterm birth have been witnessed [56*] and Miller and colleagues [19**] recently reported that, among 744 U.S. women, economic hardship during childhood was associated with greater risk for preterm birth, with both elevated plasma interleukin-6 and heightened risk for preeclampsia identified as significant mediators in the association.
Conclusions
We have reviewed data suggesting that early adversity exerts lasting effects on the neural regulation of behavior and emotion, the neuroendocrine response to psychosocial stress, and inflammatory signaling across cells and tissues. While the structural neuroplasticity of fetal development and childhood has long been a topic of interest, the scientific community has only begun to appreciate the potential for “genomic plasticity” during critical periods of development and the implications of such plasticity across a broad range of disease processes in adulthood. Pregnancy may represent a time of particular vulnerability to early adversity-associated differences in gene expression, as prenatal health is clearly susceptible to the indirect effects of behavioral and mental health sequelae of early adversity and potentially susceptible to the direct effects of early adversity via neuroendocrine and immune pathways. Prenatal syndromes such as preeclampsia and preterm birth, in turn, threaten the health of mother and baby, perpetuating early adversity-associated risk across generations. Though considerable work remains in the elucidation of mechanistic pathways linking early adversity to poor prenatal health, recognition of the potential for “social genomic” underpinnings represents a significant advancement in the field.
Highlights.
Early life is a period of sensitivity with potential for developmental programming.
Early adversity has been linked to greater risk for preeclampsia and preterm birth.
Associations may be mediated by effects on gene expression.
Early adversity predicts future neuroregulatory and inflammatory gene expression.
Behavioral, mental, neuroendocrine, and immune health are key to prenatal health.
Acknowledgements and Disclosures
Preparation of this manuscript was supported by the National Institutes of Health, National Institute of Nursing Research (K23NR017902, SLG). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The funding agency had no role in the writing of the report and in the decision to submit the report for publication. The authors report no conflicts of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interest: None.
References
- 1.Roseboom TJ, van der Meulen JH, Osmond C et al. : Coronary heart disease after prenatal exposure to the Dutch famine, 1944–45. Heart 84(6), 595–598 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roseboom TJ, van der Meulen JH, van Montfrans GA et al. : Maternal nutrition during gestation and blood pressure in later life. J. Hypertens 19(1), 29–34 (2001). [DOI] [PubMed] [Google Scholar]
- 3.Roseboom TJ, van der Meulen JH, Osmond C, Barker DJ, Ravelli AC, Bleker OP: Plasma lipid profiles in adults after prenatal exposure to the Dutch famine. Am. J. Clin. Nutr 72(5), 1101–1106 (2000). [DOI] [PubMed] [Google Scholar]
- 4.Gallo EAG, Munhoz TN, Loret de Mola C, Murray J: Gender differences in the effects of childhood maltreatment on adult depression and anxiety: A systematic review and meta-analysis. Child Abuse Negl. 79, 107–114 (2018). [DOI] [PubMed] [Google Scholar]
- 5.Holman DM, Ports KA, Buchanan ND et al. : The Association Between Adverse Childhood Experiences and Risk of Cancer in Adulthood: A Systematic Review of the Literature. Pediatrics 138(Suppl 1), S81–S91 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weber S, Jud A, Landolt MA: Quality of life in maltreated children and adult survivors of child maltreatment: a systematic review. Qual. Life Res 25(2), 237–255 (2016). [DOI] [PubMed] [Google Scholar]
- 7.Carr CP, Martins CM, Stingel AM, Lemgruber VB, Juruena MF: The role of early life stress in adult psychiatric disorders: a systematic review according to childhood trauma subtypes. J. Nerv. Ment. Dis 201(12), 1007–1020 (2013). [DOI] [PubMed] [Google Scholar]
- 8.Danese A, Tan M: Childhood maltreatment and obesity: systematic review and meta-analysis. Mol. Psychiatry 19(5), 544–554 (2014). [DOI] [PubMed] [Google Scholar]
- 9.Lee TK, Wickrama KAS, O’Neal CW: Early socioeconomic adversity and cardiometabolic risk in young adults: mediating roles of risky health lifestyle and depressive symptoms. J. Behav. Med (2018). [DOI] [PubMed] [Google Scholar]
- 10.Croft J, Heron J, Teufel C et al. : Association of Trauma Type, Age of Exposure, and Frequency in Childhood and Adolescence With Psychotic Experiences in Early Adulthood. JAMA Psychiatry. (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Monteleone AM, Monteleone P, Volpe U et al. : Impaired cortisol awakening response in eating disorder women with childhood trauma exposure: evidence for a dose-dependent effect of the traumatic load. Psychol. Med 48(6), 952–960 (2018). [DOI] [PubMed] [Google Scholar]
- 12*.Gillespie SL, Christian LM, Alston AD, Salsberry PJ: Childhood stress and birth timing among African American women: Cortisol as biological mediator. Psychoneuroendocrinology 84, 32–41 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]; The authors report that, among 89 women prospectively followed during pregnancy, dose of early adversity was associated with earlier birth timing in adulthood as mediated by maternal cortisol levels during pregnancy. Associations were found to be driven by early adversity marked by interpersonal loss and physical danger.
- 13.Rehan W, Antfolk J, Johansson A, Santtila P: Do Single Experiences of Childhood Abuse Increase Psychopathology Symptoms in Adulthood? J. Interpers. Violence (2016). [DOI] [PubMed] [Google Scholar]
- 14.Brown MJ, Masho SW, Perera RA, Mezuk B, Cohen SA: Sex and sexual orientation disparities in adverse childhood experiences and early age at sexual debut in the United States: results from a nationally representative sample. Child Abuse Negl. 46, 89–102 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blosnich JR, Dichter ME, Cerulli C, Batten SV, Bossarte RM: Disparities in adverse childhood experiences among individuals with a history of military service. JAMA Psychiatry. 71(9), 1041–1048 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alfano R, Guida F, Galobardes B et al. : Socioeconomic position during pregnancy and DNA methylation signatures at three stages across early life: epigenome-wide association studies in the ALSPAC birth cohort. Int. J. Epidemiol (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marchetto NM, Glynn RA, Ferry ML et al. : Prenatal stress and newborn telomere length. Am. J. Obstet. Gynecol 215(1), 94.e1–94.e8 (2016). [DOI] [PubMed] [Google Scholar]
- 18.Entringer S, Epel ES, Lin J et al. : Maternal psychosocial stress during pregnancy is associated with newborn leukocyte telomere length. Am. J. Obstet. Gynecol 208(2), 134.e1–134.e7 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19**.Miller GE, Culhane J, Grobman W et al. : Mothers’ childhood hardship forecasts adverse pregnancy outcomes: Role of inflammatory, lifestyle, and psychosocial pathways. Brain Behav. Immun 65, 11–19 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]; The authors report that, among a prospectively followed sample of 744 pregnant women, childhood economic hardship was associated with earlier birth and preterm birth among the mothers and lower birth weights, small for gestational age determinations, longer hospital stays, and more special care nursery admissions among the infant. Maternal circulating interleukin-6, lower educational attainment, greater pre-pregnancy body mass index, and obstetrical risk were supported as mediators linking childhood economic hardship to assessed outcomes.
- 20.Selk SC, Rich-Edwards JW, Koenen K, Kubzansky LD: An observational study of type, timing, and severity of childhood maltreatment and preterm birth. J. Epidemiol. Community Health 70(6), 589–595 (2016). [DOI] [PubMed] [Google Scholar]
- 21*.Nesari M, Olson JK, Vandermeer B, Slater L, Olson DM: Does a maternal history of abuse before pregnancy affect pregnancy outcomes? A systematic review with meta-analysis. BMC Pregnancy Childbirth 18(1), 404–018–2030–8 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]; The authors systematically reviewed the literature examining associations among pre-pregnancy abuse, preterm birth, and low birth weight birth. Sixteen articles were included in a meta-analysis, with maternal pre-pregnancy abuse found to be associated with greater odds of both outcomes. Abuse during childhood was noted as particularly salient in the prediction of low birth weight birth.
- 22.Pinheiro TV, Brunetto S, Ramos JG, Bernardi JR, Goldani MZ: Hypertensive disorders during pregnancy and health outcomes in the offspring: a systematic review. J. Dev. Orig Health. Dis 7(4), 391–407 (2016). [DOI] [PubMed] [Google Scholar]
- 23.Wu P, Haththotuwa R, Kwok CS et al. : Preeclampsia and Future Cardiovascular Health: A Systematic Review and Meta-Analysis. Circ. Cardiovasc. Qual. Outcomes 10(2), 10.1161/CIRCOUTCOMES.116.003497. Epub 2017 Feb 22 (2017). [DOI] [PubMed] [Google Scholar]
- 24.Matthews TJ, MacDorman MF, Thoma ME: Infant Mortality Statistics From the 2013 Period Linked Birth/Infant Death Data Set. Natl. Vital Stat. Rep 64(9), 1–30 (2015). [PubMed] [Google Scholar]
- 25.Heany SJ, Groenewold NA, Uhlmann A, Dalvie S, Stein DJ, Brooks SJ: The neural correlates of Childhood Trauma Questionnaire scores in adults: A meta-analysis and review of functional magnetic resonance imaging studies. Dev. Psychopathol 30(4), 1475–1485 (2018). [DOI] [PubMed] [Google Scholar]
- 26.Teicher MH, Samson JA, Anderson CM, Ohashi K: The effects of childhood maltreatment on brain structure, function and connectivity. Nat. Rev. Neurosci 17(10), 652–666 (2016). [DOI] [PubMed] [Google Scholar]
- 27.Callaghan BL, Sullivan RM, Howell B, Tottenham N: The international society for developmental psychobiology Sackler symposium: early adversity and the maturation of emotion circuits--a cross-species analysis. Dev. Psychobiol 56(8), 1635–1650 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cole SW: Social regulation of human gene expression. Curr. Dir. Psychol. Sci 18(3), 132–137 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29*.Cole SW: Social regulation of human gene expression: mechanisms and implications for public health. Am. J. Public Health 103 Suppl 1, S84–92 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]; Dr. Steven W. Cole describes an emerging field that he has coined “social genomics,” which recognizes that patterns of gene expression are sensitive to one’s perceptions of the social world. He describes the mutual dependency of genes and environment in shaping behaviors and structuring cellular signaling and reviews potential implications of these new discoveries to public health.
- 30.Slavich GM, Cole SW: The Emerging Field of Human Social Genomics . Clin. Psychol. Sci 1(3), 331–348 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Katsouli S, Stamatakis A, Giompres P, Kouvelas ED, Stylianopoulou F, Mitsacos A: Sexually dimorphic long-term effects of an early life experience on AMPA receptor subunit expression in rat brain. Neuroscience 257, 49–64 (2014). [DOI] [PubMed] [Google Scholar]
- 32.Sachs BD, Tran HL, Folse E, Caron MG: Brain-region-specific Molecular Responses to Maternal Separation and Social Defeat Stress in Mice. Neuroscience 373, 122–136 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kiser DP, Popp S, Schmitt-Bohrer AG et al. : Early-life stress impairs developmental programming in Cadherin 13 (CDH13)-deficient mice. Prog. Neuropsychopharmacol. Biol. Psychiatry 89, 158–168 (2019). [DOI] [PubMed] [Google Scholar]
- 34.Turnock-Jones JJ, Jennings CA, Robbins MJ et al. : Increased expression of the NR2A NMDA receptor subunit in the prefrontal cortex of rats reared in isolation. Synapse 63(10), 836–846 (2009). [DOI] [PubMed] [Google Scholar]
- 35*.Sasagawa T, Horii-Hayashi N, Okuda A, Hashimoto T, Azuma C, Nishi M: Long-term effects of maternal separation coupled with social isolation on reward seeking and changes in dopamine D1 receptor expression in the nucleus accumbens via DNA methylation in mice. Neurosci. Lett 641, 33–39 (2017). [DOI] [PubMed] [Google Scholar]; The authors report an association between maternal separation coupled with social isolation and dopamine D1 receptor mRNA and protein expression in the nucleus accumbens using a murine model. Female mice exposed to early adversity concomitantly exhibited decreased exploration time in a chocolate-paired compartment.
- 36.de Almeida Magalhaes T, Correia D, de Carvalho LM, Damasceno S, Brunialti Godard AL: Maternal separation affects expression of stress response genes and increases vulnerability to ethanol consumption. Brain Behav. 8(1), e00841 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alcantara-Alonso V, Amaya MI, Matamoros-Trejo G, de Gortari P: Altered functionality of the corticotrophin-releasing hormone receptor-2 in the hypothalamic paraventricular nucleus of hyperphagic maternally separated rats. Neuropeptides 63, 75–82 (2017). [DOI] [PubMed] [Google Scholar]
- 38.Zhang L, Hernandez VS, Liu B et al. : Hypothalamic vasopressin system regulation by maternal separation: its impact on anxiety in rats. Neuroscience 215, 135–148 (2012). [DOI] [PubMed] [Google Scholar]
- 39*.Morrison KE, Epperson CN, Sammel MD et al. : Preadolescent Adversity Programs a Disrupted Maternal Stress Reactivity in Humans and Mice. Biol. Psychiatry 81(8), 693–701 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]; The authors report associations among chronic stress during pre-adolescence and the expression of 24 genes within the paraventricular nucleus of the hypothalamus during murine pregnancy. The authors also report that stressed, gravid mice exhibited a blunted corticosterone response to acute restraint stress and pup separation and early adversity-exposed women exhibited a blunted cortisol response to infant separation.
- 40.Sood A, Pati S, Bhattacharya A, Chaudhari K, Vaidya VA: Early emergence of altered 5-HT2A receptor-evoked behavior, neural activation and gene expression following maternal separation. Int. J. Dev. Neurosci 65, 21–28 (2018). [DOI] [PubMed] [Google Scholar]
- 41.Schwaiger M, Grinberg M, Moser D et al. : Altered Stress-Induced Regulation of Genes in Monocytes in Adults with a History of Childhood Adversity. Neuropsychopharmacology 41(10), 2530–2540 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moran-Santa Maria MM, McRae-Clark AL, Back SE et al. : Influence of cocaine dependence and early life stress on pituitary-adrenal axis responses to CRH and the Trier social stressor. Psychoneuroendocrinology 35(10), 1492–1500 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Carpenter LL, Carvalho JP, Tyrka AR et al. : Decreased adrenocorticotropic hormone and cortisol responses to stress in healthy adults reporting significant childhood maltreatment. Biol. Psychiatry 62(10), 1080–1087 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zajdel J, Zager A, Blomqvist A, Engblom D, Shionoya K: Acute maternal separation potentiates the gene expression and corticosterone response induced by inflammation. Brain Behav. Immun (2018). [DOI] [PubMed] [Google Scholar]
- 45.Roque A, Ochoa-Zarzosa A, Torner L: Maternal separation activates microglial cells and induces an inflammatory response in the hippocampus of male rat pups, independently of hypothalamic and peripheral cytokine levels. Brain Behav. Immun 55, 39–48 (2016). [DOI] [PubMed] [Google Scholar]
- 46.Amini-Khoei H, Haghani-Samani E, Beigi M et al. : On the role of corticosterone in behavioral disorders, microbiota composition alteration and neuroimmune response in adult male mice subjected to maternal separation stress. Int. Immunopharmacol 66, 242–250 (2019). [DOI] [PubMed] [Google Scholar]
- 47.Inagaki TK, Muscatell KA, Irwin MR, Cole SW, Eisenberger NI: Inflammation selectively enhances amygdala activity to socially threatening images. Neuroimage 59(4), 3222–3226 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48**.Cole SW, Hawkley LC, Arevalo JM, Sung CY, Rose RM, Cacioppo JT: Social regulation of gene expression in human leukocytes. Genome Biol. 8(9), R189 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]; In examining peripherally-sampled leukocytes, the authors report differential expression of 209 genes among 14 individuals categorized as integrated (i.e., low-lonely) versus isolated (i.e., high-lonely). Patterns of gene expression suggested that isolated individuals are subject to enhanced immune activation and pro-inflammatory signaling in concert with reduced mature B lymphocyte function and innate antiviral responsiveness.
- 49.Powell ND, Sloan EK, Bailey MT et al. : Social stress up-regulates inflammatory gene expression in the leukocyte transcriptome via beta-adrenergic induction of myelopoiesis. Proc. Natl. Acad. Sci. U. S. A 110(41), 16574–16579 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Miller GE, Rohleder N, Cole SW: Chronic interpersonal stress predicts activation of pro- and anti-inflammatory signaling pathways 6 months later. Psychosom. Med 71(1), 57–62 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Miller GE, Chen E, Sze J et al. : A functional genomic fingerprint of chronic stress in humans: blunted glucocorticoid and increased NF-kappaB signaling. Biol. Psychiatry 64(4), 266–272 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cole SW, Capitanio JP, Chun K, Arevalo JM, Ma J, Cacioppo JT: Myeloid differentiation architecture of leukocyte transcriptome dynamics in perceived social isolation. Proc. Natl. Acad. Sci. U. S. A 112(49), 15142–15147 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53**.Cole SW, Conti G, Arevalo JM, Ruggiero AM, Heckman JJ, Suomi SJ: Transcriptional modulation of the developing immune system by early life social adversity. Proc. Natl. Acad. Sci. U. S. A 109(50), 20578–20583 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]; In examining peripherally-sampled leukocytes, the authors report differential expression of 249 and 256 genes in comparing maternally-reared to surrogate/peer-reared and peer-reared rhesus macaques, respectively. Patterns of gene expression suggested that macaques exposed to early adversity are subject to enhanced pro-inflammatory signaling and T-cell activation in concert with reduced innate antimicrobial defense.
- 54.Spindola LM, Pan PM, Moretti PN et al. : Gene expression in blood of children and adolescents: Mediation between childhood maltreatment and major depressive disorder. J. Psychiatr. Res 92, 24–30 (2017). [DOI] [PubMed] [Google Scholar]
- 55.Finy MS, Christian LM: Pathways linking childhood abuse history and current socioeconomic status to inflammation during pregnancy. Brain Behav. Immun 74, 231–240 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56*.Bublitz M, De La Monte S, Martin S, Larson L, Bourjeily G: Childhood maltreatment and inflammation among pregnant women with gestational diabetes mellitus: A pilot study. Obstet. Med 10(3), 120–124 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]; The authors, in a pilot study of women with gestational diabetes (n = 24), associations among childhood maltreatment (i.e., physical or sexual abuse), circulating levels of interleukin-15 at a median 30 weeks of pregnancy, and marginally increased risk for preterm birth during the current pregnancy.
- 57.Ross KM, Cole SW, Carroll JE, Dunkel Schetter C: Elevated pro-inflammatory gene expression in the third trimester of pregnancy in mothers who experienced stressful life events. Brain Behav. Immun (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Keenan-Devlin LS, Ernst LM, Ross KM et al. : Maternal Income during Pregnancy is Associated with Chronic Placental Inflammation at Birth. Am. J. Perinatol 34(10), 1003–1010 (2017). [DOI] [PubMed] [Google Scholar]
- 59.Miller GE, Borders AE, Crockett AH et al. : Maternal socioeconomic disadvantage is associated with transcriptional indications of greater immune activation and slower tissue maturation in placental biopsies and newborn cord blood. Brain Behav. Immun 64, 276–284 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Allis CD, Jenuwein T, Caparros M, Reinberg D, Lachner M, Epigenetics, 2nd ed., Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, 2015,. [Google Scholar]
- 61.Carey MF, Peterson CL, Smale ST: Transcriptional Regulation in Eukaryotes: Concepts, Strategies, and Techniques Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY: (2008). [Google Scholar]
- 62.Alberts B, Johnson A, Lewis J et al. : Molecular Biology of the Cell. Garland Science, New York, NY: (2015). [Google Scholar]
- 63.Lyko F: The DNA methyltransferase family: a versatile toolkit for epigenetic regulation. Nat. Rev. Genet 19(2), 81–92 (2018). [DOI] [PubMed] [Google Scholar]
- 64.Skvortsova K, Iovino N, Bogdanovic O: Functions and mechanisms of epigenetic inheritance in animals. Nat. Rev. Mol. Cell Biol 19(12), 774–790 (2018). [DOI] [PubMed] [Google Scholar]
- 65.Schubeler D: Function and information content of DNA methylation. Nature 517(7534), 321–326 (2015). [DOI] [PubMed] [Google Scholar]
- 66.Chen F, Zhang Q, Deng X et al. : Conflicts of CpG density and DNA methylation are proximally and distally involved in gene regulation in human and mouse tissues. Epigenetics 13(7), 721–741 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Franklin TB, Russig H, Weiss IC et al. : Epigenetic transmission of the impact of early stress across generations. Biol. Psychiatry 68(5), 408–415 (2010). [DOI] [PubMed] [Google Scholar]
- 68.Tyrka AR, Price LH, Marsit C, Walters OC, Carpenter LL: Childhood adversity and epigenetic modulation of the leukocyte glucocorticoid receptor: preliminary findings in healthy adults. PLoS One 7(1), e30148 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bockmuhl Y, Patchev AV, Madejska A et al. : Methylation at the CpG island shore region upregulates Nr3c1 promoter activity after early-life stress. Epigenetics 10(3), 247–257 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Romens SE, McDonald J, Svaren J, Pollak SD: Associations between early life stress and gene methylation in children. Child Dev. 86(1), 303–309 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Harms MB, Birn R, Provencal N et al. : Early life stress, FK506 binding protein 5 gene (FKBP5) methylation, and inhibition-related prefrontal function: A prospective longitudinal study. Dev. Psychopathol 29(5), 1895–1903 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Roth TL, Lubin FD, Funk AJ, Sweatt JD: Lasting epigenetic influence of early-life adversity on the BDNF gene. Biol. Psychiatry 65(9), 760–769 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Unternaehrer E, Bolten M, Nast I et al. : Maternal adversities during pregnancy and cord blood oxytocin receptor (OXTR) DNA methylation. Soc. Cogn. Affect. Neurosci 11(9), 1460–1470 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Viuff AC, Sharp GC, Rai D et al. : Maternal depression during pregnancy and cord blood DNA methylation: findings from the Avon Longitudinal Study of Parents and Children. Transl. Psychiatry 8(1), 244–018–0286–4 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dominissini D, Rechavi G: Epitranscriptome regulation. Nat. Struct. Mol. Biol (2018). [DOI] [PubMed] [Google Scholar]
- 76.Liu Y, Liu D, Xu J, Jiang H, Pan F: Early adolescent stress-induced changes in prefrontal cortex miRNA-135a and hippocampal miRNA-16 in male rats. Dev. Psychobiol 59(8), 958–969 (2017). [DOI] [PubMed] [Google Scholar]
- 77.Cattane N, Mora C, Lopizzo N et al. : Identification of a miRNAs signature associated with exposure to stress early in life and enhanced vulnerability for schizophrenia: New insights for the key role of miR-125b-1–3p in neurodevelopmental processes. Schizophr. Res (2018). [DOI] [PubMed] [Google Scholar]
- 78.Issler O, Chen A: Determining the role of microRNAs in psychiatric disorders. Nat. Rev. Neurosci 16(4), 201–212 (2015). [DOI] [PubMed] [Google Scholar]
- 79.Mehta A, Baltimore D: MicroRNAs as regulatory elements in immune system logic. Nat. Rev. Immunol 16(5), 279–294 (2016). [DOI] [PubMed] [Google Scholar]
- 80*.Miao Z, Mao F, Liang J, Szyf M, Wang Y, Sun ZS: Anxiety-Related Behaviours Associated with microRNA-206–3p and BDNF Expression in Pregnant Female Mice Following Psychological Social Stress. Mol. Neurobiol 55(2), 1097–1111 (2018). [DOI] [PubMed] [Google Scholar]; The authors report associations among witnessed social defeat of male partner during murine pregnancy, decreased expression of miR-206–3p, and increased expression of BDNF in the hippocampus, medial prefrontal cortex, and amygdala. Decreased preference for sucrose, time in the open arms of the elevated plus maze, and time in the light chamber of the light/dark box during pregnancy and lactation among stressed mice are also reported.
- 81.Hughes K, Bellis MA, Hardcastle KA et al. : The effect of multiple adverse childhood experiences on health: a systematic review and meta-analysis. Lancet Public. Health 2(8), e356–e366 (2017). [DOI] [PubMed] [Google Scholar]
- 82.Norman RE, Byambaa M, De R, Butchart A, Scott J, Vos T: The long-term health consequences of child physical abuse, emotional abuse, and neglect: a systematic review and meta-analysis. PLoS Med. 9(11), e1001349 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pear VA, Petito LC, Abrams B: The Role of Maternal Adverse Childhood Experiences and Race in Intergenerational High-Risk Smoking Behaviors. Nicotine Tob. Res 19(5), 623–630 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Giallo R, Pilkington P, McDonald E, Gartland D, Woolhouse H, Brown S: Physical, sexual and social health factors associated with the trajectories of maternal depressive symptoms from pregnancy to 4 years postpartum. Soc. Psychiatry Psychiatr. Epidemiol 52(7), 815–828 (2017). [DOI] [PubMed] [Google Scholar]
- 85.Ko TJ, Tsai LY, Chu LC et al. : Parental smoking during pregnancy and its association with low birth weight, small for gestational age, and preterm birth offspring: a birth cohort study. Pediatr. Neonatol 55(1), 20–27 (2014). [DOI] [PubMed] [Google Scholar]
- 86.Jarde A, Morais M, Kingston D et al. : Neonatal Outcomes in Women With Untreated Antenatal Depression Compared With Women Without Depression: A Systematic Review and Meta-analysis. JAMA Psychiatry. 73(8), 826–837 (2016). [DOI] [PubMed] [Google Scholar]
- 87.Hu R, Li Y, Zhang Z, Yan W: Antenatal depressive symptoms and the risk of preeclampsia or operative deliveries: a meta-analysis. PLoS One 10(3), e0119018 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sandman CA, Glynn L, Schetter CD et al. : Elevated maternal cortisol early in pregnancy predicts third trimester levels of placental corticotropin releasing hormone (CRH): priming the placental clock. Peptides 27(6), 1457–1463 (2006). [DOI] [PubMed] [Google Scholar]
- 89.You X, Liu J, Xu C et al. : Corticotropin-releasing hormone (CRH) promotes inflammation in human pregnant myometrium: the evidence of CRH initiating parturition? J. Clin. Endocrinol. Metab 99(2), E199–208 (2014). [DOI] [PubMed] [Google Scholar]
- 90.Li W, Challis JR: Corticotropin-releasing hormone and urocortin induce secretion of matrix metalloproteinase-9 (MMP-9) without change in tissue inhibitors of MMP-1 by cultured cells from human placenta and fetal membranes. J. Clin. Endocrinol. Metab 90(12), 6569–6574 (2005). [DOI] [PubMed] [Google Scholar]
- 91.Mayor-Lynn K, Toloubeydokhti T, Cruz AC, Chegini N: Expression profile of microRNAs and mRNAs in human placentas from pregnancies complicated by preeclampsia and preterm labor. Reprod. Sci 18(1), 46–56 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Paiva P, Whitehead C, Saglam B, Palmer K, Tong S: Measurement of mRNA transcripts of very high placental expression in maternal blood as biomarkers of preeclampsia. J. Clin. Endocrinol. Metab 96(11), E1807–15 (2011). [DOI] [PubMed] [Google Scholar]
- 93.Petsas G, Jeschke U, Richter DU et al. : Aberrant expression of corticotropin-releasing hormone in pre-eclampsia induces expression of FasL in maternal macrophages and extravillous trophoblast apoptosis. Mol. Hum. Reprod 18(11), 535–545 (2012). [DOI] [PubMed] [Google Scholar]
- 94.Harville EW, Savitz DA, Dole N, Herring AH, Thorp JM, Light KC: Stress and placental resistance measured by Doppler ultrasound in early and mid-pregnancy. Ultrasound Obstet. Gynecol 32(1), 23–30 (2008). [DOI] [PubMed] [Google Scholar]
- 95.Moog NK, Buss C, Entringer S et al. : Maternal Exposure to Childhood Trauma Is Associated During Pregnancy With Placental-Fetal Stress Physiology. Biol. Psychiatry 79(10), 831–839 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Cappelletti M, Della Bella S, Ferrazzi E, Mavilio D, Divanovic S: Inflammation and preterm birth. J. Leukoc. Biol 99(1), 67–78 (2016). [DOI] [PubMed] [Google Scholar]
- 97.Yuan M, Jordan F, McInnes IB, Harnett MM, Norman JE: Leukocytes are primed in peripheral blood for activation during term and preterm labour. Mol. Hum. Reprod 15(11), 713–724 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Thomson AJ, Telfer JF, Young A et al. : Leukocytes infiltrate the myometrium during human parturition: further evidence that labour is an inflammatory process. Hum. Reprod 14(1), 229–236 (1999). [PubMed] [Google Scholar]
- 99.Osman I, Young A, Ledingham MA et al. : Leukocyte density and pro-inflammatory cytokine expression in human fetal membranes, decidua, cervix and myometrium before and during labour at term. Mol. Hum. Reprod 9(1), 41–45 (2003). [DOI] [PubMed] [Google Scholar]
- 100.Brocklehurst P, Gordon A, Heatley E, Milan SJ: Antibiotics for treating bacterial vaginosis in pregnancy. Cochrane Database Syst. Rev (1):CD000262 doi(1), CD000262 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Thinkhamrop J, Hofmeyr GJ, Adetoro O, Lumbiganon P, Ota E: Antibiotic prophylaxis during the second and third trimester to reduce adverse pregnancy outcomes and morbidity. Cochrane Database Syst. Rev (6):CD002250 doi(6), CD002250 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Black KD, Horowitz JA: Inflammatory Markers and Preeclampsia: A Systematic Review. Nurs. Res 67(3), 242–251 (2018). [DOI] [PubMed] [Google Scholar]
- 103.Valencia-Ortega J, Zarate A, Saucedo R, Hernandez-Valencia M, Cruz JG, Puello E: Placental Proinflammatory State and Maternal Endothelial Dysfunction in Preeclampsia. Gynecol. Obstet. Invest, 1–8 (2018). [DOI] [PubMed] [Google Scholar]
- 104.Quinn KH, Lacoursiere DY, Cui L, Bui J, Parast MM: The unique pathophysiology of early-onset severe preeclampsia: role of decidual T regulatory cells. J. Reprod. Immunol 91(1–2), 76–82 (2011). [DOI] [PubMed] [Google Scholar]
- 105.Vaughan JE, Walsh SW: Activation of NF-kappaB in placentas of women with preeclampsia. Hypertens. Pregnancy 31(2), 243–251 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]