Abstract
Hexavalent chromium [Cr(VI)] is a known occupational and environmental contaminant and carcinogen, but new mechanisms of Cr(VI)-induced carcinogenesis remain to be elucidated. In this study, we found that expression of miR-143 is decreased, whereas that of Interleukin 6 (IL-6) is increased in blood samples of Cr(VI)-exposing workers compared with corresponding unexposed workers. In addition, IL-6 was increased in human bronchial epithelial cells (BEAS-Cr) exposed to Cr(VI) compared with unexposed BEAS-2B cells. To further investigate the mechanisms by which Cr(VI) promotes these changes, we assessed the effects of miR-143 on gene expression and found that miR-143 suppressed expression of IL-6, HIF-1α and NF-κB p65, and that inhibiting miR-143 promoted expression of IL-6, HIF-1α and NF-κB p65. Interestingly, IL-6 regulated expression of HIF-1α, and HIF-1α transcriptionally regulated expression of IL-6. Experiments in animals showed that miR-143 inhibited tumor growth and angiogenesis by regulating IL-6/HIF-1α and downstream signaling pathways in vivo. These outcomes support the hypothesis that the miR-143/IL-6/HIF-1α pathway functions to regulate Cr(VI)-induced carcinogenesis.
Keywords: Cr (VI), IL-6, miR-143, Tumor growth, Toxicology
1. Introduction
Lung cancer has the highest incidence and mortality of all malignant tumors in the world (Torre et al., 2015). Multiple factors have been suggested as important causes of lung cancer, including metal exposure, air pollution, and cigarette smoke, but the underlying molecular mechanisms for metal exposure-induced carcinogenesis are still not fully defined (Pratheeshkumar et al., 2016). Hexavalent chromium [Cr(VI)] compounds have been recognized as Class I human carcinogens by the IARC (International Agency for Research on Cancer) (Kim et al., 2016). Several epidemiological studies have shown that long-time Cr(VI) exposure is associated with a higher risk of lung cancer (Wilbur et al., 2012). Recent studies showed that Cr(VI) alters microRNA expression (He et al., 2013; Pratheeshkumar et al., 2017), DNA methylation (Lou et al., 2013) and histone modifications (Xia et al., 2014), which may contribute to Cr(VI)-induced carcinogenesis (Rodrigues et al., 2009). However, the precise molecular mechanisms of Cr(VI)-induced lung cancer are poorly understood.
MicroRNAs are small noncoding regulatory RNA molecules composed of 18–24 nucleotides (~22 nt) (Calin and Croce, 2006; Liu et al., 2011; Humphries et al., 2017). They have been recently implicated in the regulation of tumorigenesis by inhibiting major signaling pathways (Pasquinelli, 2012; Xu et al., 2013; Jiang et al., 2018). Some major signaling pathways have been reported to be influenced by different microRNAs in various diseases, including the PI3K/AKT pathway, MAPK/ERK pathway, the mTOR /p70S6K1 pathway, and the TGFβ signaling pathway (Xu et al., 2012; He et al., 2013; Wang et al., 2014; Niu et al., 2017; Jin et al., 2018; Lam et al., 2018). miR-143 has been demonstrated as a tumor suppressor. However, the expression and role of miR-143 in Cr(VI)-exposed human samples are not known yet.
Interleukin 6 (IL-6) is a pleiotropic cytokine that functions to regulate cell differentiation and proliferation, and plays various roles in modulating the cellular activities of tumor and immune cells (Mantovani et al., 2008). IL-6signals through the common gp130 receptor and the specific IL-6Rα co-receptor to activate the Janus kinase (JAK)-signal transducer and activator of transcription (STAT) signaling pathway and downstream signaling pathways that are involved in tumorigenesis (Heinrich et al., 2003; Heikkila et al., 2008; Pan et al., 2018).
In this study, we addressed the following questions: (a) what are the expression levels of IL-6 and miR-143 in Cr(VI)-exposed human blood samples; (b) what downstream molecule(s) of miR-143 is/are inhibited in human blood samples and Cr(VI)-transformed cells; (c) what signaling molecules are important for miR-143-inhibition of IL-6 expression; (d) what are the roles of miR-143, IL-6 and other related signaling molecules in tumor growth and angiogenesis.
2. Materials and methods
2.1. Human blood samples
Human blood samples were obtained from the tissue bank of Zhengzhou University. These samples have been prepared for cases in the Zhengzhou University biorepository, and clinical annotation is available through a database using caBIG. Cases were classified and selected based on diagnosis using the CoPath Anatomic Pathology system as well as caBIG. Among the effects that are known and reported, there is no racial difference regarding Cr(VI)-induced toxicity. Samples were collected for several years from an electroplating factory in China for chromium exposure, with a standard questionnaire performed to obtain information about the age, gender, occupational history, medical history, and lifestyle factors (alcohol drinking and smoking) via person-to-person interviews. Workers with a history of more than three years of chromium exposure and nonsmokers were selected for the study. The nonsmoking control subjects were recruited from a machinery factory without chromium exposure. For this study, no information regulated by HIPPA was included. The protocols were approved by the Institutional Review Committees of Zhengzhou University. Plasma was isolated from the blood by centrifugation at 3500 rpm for 10 min; subsequently, a volume of 300 μL of plasma and 900 μL of Trizol were thoroughly mixed and C. elegans miR-39 (cel-miR39) was added to a final concentration of 10−5 nM as a spiked-in control. RNAs were extracted and analyzed by qRT-PCR by following the manufacturer’s instructions(Invitrogen, USA).
2.2. Cell culture, antibodies, and reagents
Human bronchial epithelial BEAS-2B cells (purchased from ATCC) were cultured in Dulbecco’s Modified Eagle’s medium (DMEM; Invitrogen, CA) supplemented with 10% fetal bovine serum (FBS). BEAS-2B cells were maintained with DMEM containing 0.5 μM Cr(VI) for 6 months to select Cr(VI) resistant cells (BEAS-Cr cells). Parallel cultures grown in Cr(VI)–free medium acted as passage-matched controls. After 6 months of Cr(VI) exposure, BEAS-Cr cells had the ability to transform and grow tumors, as previously described (He et al., 2013). Sodium dichromate (Na2Cr2O7·H2O) was obtained from Sigma (St Louis, USA). Antibodies against insulin receptor substrate-1 (IRS1), NF-κB p65, IL-6, and Ki67 were purchased from Proteintech (Wuhan, China); antibodies against ribosomal protein S6 kinase B1 (p70S6K1) were from Cell Signaling Technology (Beverly, MA, USA). Antibodies against hypoxia-induced factor-1α (HIF-1α) were from BD Biosciences (Franklin Lakes, USA).
2.3. Immunoblotting
RIPA lysis and extraction buffer were used to lyse cells on ice for 30 min as previously described (Niu et al., 2017). Samples were separated by SDS-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to membranes. Membranes were blocked with 5% nonfat milk and incubated with primary antibodies at 4 °C overnight. Protein bands were detected by Chemiluminescent Kit (Thermo Scientific, USA).
2.4. RT-qPCR analysis
Total RNAs were extracted using Trizol (Life Technologies, CA). The cDNA synthesis was performed using a reverse transcription (RT) kit (Vazyme, China), and qPCR was performed to detect mRNA levels using the SYBR Green PCR Master Mix (Vazyme, China). Primer sequences for RT-PCR are shown in Supplemental Table 1.
2.5. miRNA transfection and preparation
The negative control miRNA (miR-NC) and miR-143 mimic, as well as anti-miR-NC inhibitor and anti-miR-143 inhibitor were obtained from RIBOBIO (Guangzhou, China). Cells were cultured in a six-well plate and transfected using a miR-143 mimic or miR-NC at 50 nM (inhibitors at 150 nM) using Lipofectamine 3000 (Invitrogen, USA) according to the manufacturer’s instructions. Total protein and RNA were prepared from the cells 48–72 h after the transfection, and were used for subsequent analysis.
2.6. Luciferase activity assay
Cells were cultured in 24-well plates and co-transfected with luciferase reporter plasmids (NF-κB p65 or VEGF), Renilla luciferase reporter plasmid (internal control), and equal amounts of mimic or inhibitor using Lipofectamine 3000 (Invitrogen, USA). Firefly and Renilla luciferase activities were measured by a dual-luciferase assay kit 24–48 h after transfection (Promega, WI, USA).
2.7. Animal experiments
Female BALB/cA-nu nude mice (4–5 weeks old) were purchased from Shanghai Experimental Animal Center (Chinese Academy of Sciences, Shanghai, China) and maintained in pathogen-free conditions. BEAS-Cr cells stably expressing miR-143 or miR-NC were injected into the flanks of nude mice (5 × 106 cells in 150 μl). Tumor volumes were calculated according to the formula (width2 × length)/2. The mice were euthanized 29 days after cell injection, and tumors were weighed and analyzed.
2.8. Immunohistochemistry
Tumor tissues were sliced and fixed with Bouin’s solution for 24–48 h, and processed by the conventional paraffin-embedded method. Dako Envision two-step method of immunohistochemistry was used to analyze IL-6, Ki67, p70S6K1, and CD31 expression in xenograft tumor sections according to the manufacturer’s instructions.
2.9. Statistical analysis
All results were obtained from at least three independent experiments. Results were presented as mean ± SD from independent experiments, unless otherwise stated. Differences between the groups were considered significant at the value p < .05.
3. Results
3.1. miR-143 expression levels are significantly lower in blood samples of Cr(VI)-exposed workers, whereas IL-6 expression levels are higher
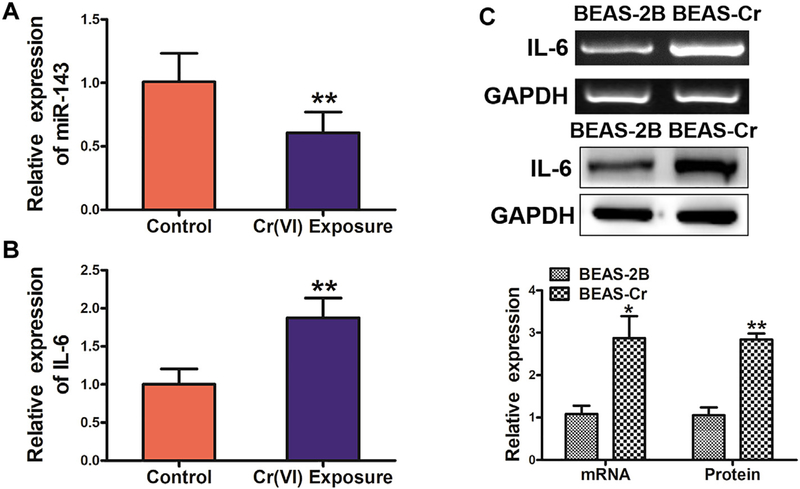
To investigate the role of miR-143 in Cr(VI)-exposed workers, we first tested expression levels of miR-143 in blood samples of Cr(VI)-exposed workers by qRT-PCR (Fig. 1A). The results showed that expression of miR-143 was significantly downregulated in human samples with Cr(VI) exposure, whereas IL-6 expression levels were significantly higher when compared to those from workers not exposed to Cr(VI) (Fig. 1B). We then tested expression of IL-6 in BEAS-Cr cells, and showed that IL-6 mRNA and protein levels were greatly upregulated in BEAS-Cr cells compared to BEAS-2B cells (Fig. 1C). Consistent with the above study, miR-143 levels were much lower in BEAS-Cr cells compared to BEAS-2B cells (data not shown).
Fig. 1.
miR-143 expression levels are significantly lower in blood samples of Cr(VI)-exposed workers, whereas IL-6 expression levels are increased. (A) miR-143 expression is significantly downregulated in human blood samples obtained from workers with Cr(VI)-exposure (n = 40) compared with human blood samples obtained from workers without Cr(VI)-exposure (n = 20). (B) IL-6 mRNA expression is significantly upregulated in human samples from workers exposed to Cr(VI). (C)IL-6 mRNA and protein expression is upregulated in BEAS-Cr cells compared to BEAS-2B cells. Analysis of IL-6 mRNA and protein expression. Data are presented as mean ± SD from 3 replicates. * indicates significant difference at P < .05; ** indicates significant difference at P < .01.
3.2. miR-143 is involved in inhibiting IL-6 and other related signaling molecules
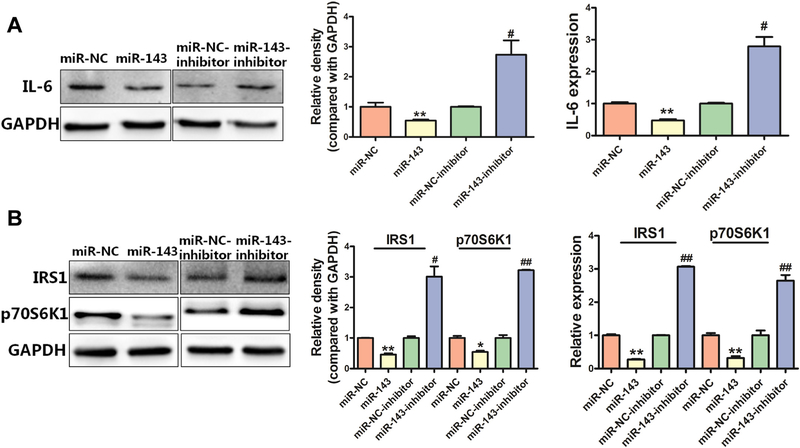
IL-6 is known to regulate cell differentiation and cell proliferation by balancing pro-inflammatory and anti-inflammatory signals. To test whether miR-143 directly affects IL-6 expression, we manipulated miR-143 expression and assessed the effects on mRNA and protein expression of IL-6. We found that overexpression of miR-143 decreased mRNA and protein levels of IL-6, while an anti-miR-143 inhibitor increased IL-6 expression (Fig. 2A). We then assessed the effect of altering miR143 expression on IL-6-related signaling molecules and we found that overexpression of miR-143 decreased mRNA and protein expression levels of both IRS1 and p70S6K1; in contrast, an anti-miR-143 inhibitor increased the expression levels of IRS1 and p70S6K1 (Fig. 2B). These results showed that expression of miR-143 is sufficient to inhibit expression levels of IL-6, as well as the IGFIR/IRS1 and mTOR/p70S6K1 pathways in BEAS-Cr cells.
Fig. 2.
miR-143 is involved in inhibiting IL-6 and other related signaling molecules. (A)miR-143 overexpression decreased IL-6 protein (left) and IL-6 mRNA (right) expression. (B)miR-143 overexpression greatly decreased protein expression of IRS1 and p70S6K1, whereas an anti-miR-143 inhibitor induced expression of IRS1 and p70S6K1. Data are presented as mean ± SD from 3 replicates. * indicates significant difference at P < .05, ** indicates significant difference at P < .01, * and ** indicate P value when miR-143 is compared with miR-NC; # indicates significant difference at P < .05, ## indicates significant difference at P < .01, # and ## indicate P value when an anti-miR-143 inhibitor is compared with a miR-NC inhibitor.
3.3. miR-143 inhibits expression of NF-κB p65
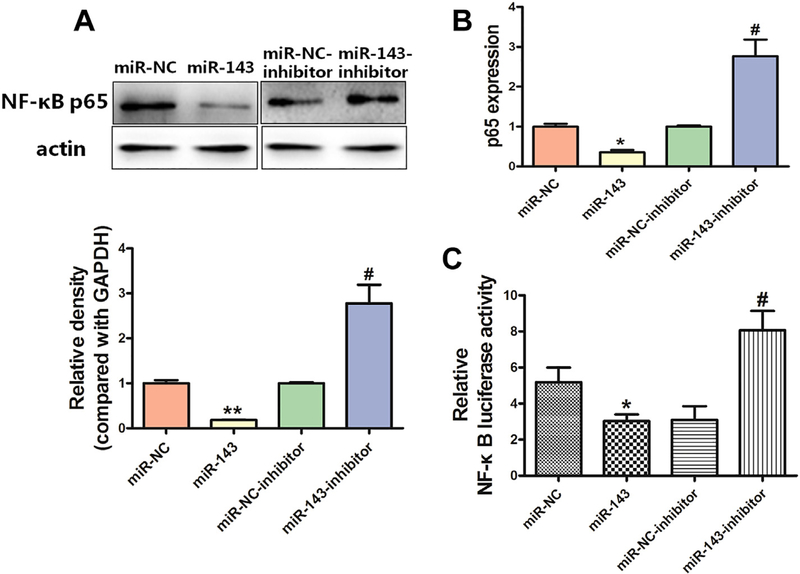
Constitutive overactivation of NF-κB signaling is a common event in human cancers and acts as a key factor in cancer development and progression (Rayet and Gelinas, 1999; Ghosh and Karin, 2002; Chen and Greene, 2004; Perkins, 2007; Naugler and Karin, 2008). We examined whether miR-143 affects NF-κB expression in BEAS-Cr cells, and found that overexpression of miR-143 decreased NF-κB p65 expression at both the protein and mRNA levels (Fig. 3A–B); in contrast, an antimiR-143 inhibitor induced expression of NF-κB p65 in the cells. Consistent with this, miR-143 decreased the luciferase reporter activities of NF-κB, indicating that the transcriptional activation of NF-κB p65 was suppressed (Fig. 3C).
Fig. 3.
miR-143 inhibits expression of NF-κB p65. (A-B)Overexpression of miR-143 decreased NF-κB p65 expression at the protein (A) and RNA (B) levels, whereas an anti-miR-143 inhibitor induced NF-κB p65 protein and RNA expression. (C)Overexpression of miR-143 decreased NF-κB transcriptional activation via lower luciferase reporter activities, whereas an anti-miR-143 inhibitor increased luciferase activities. Data are presented as mean ± SD from 3 replicates. * indicates a significant difference at P < .05, ** indicates a significant difference at P < .01, * and ** indicate P values when miR-143 is compared with miR-NC; # indicates significant difference at P < .05, # indicates P value when antimiR-143 inhibitor is compared with miR-NC inhibitor.
3.4. miR-143 inhibits HIF-1α expression, whereas IL-6 induces HIF-1α expression
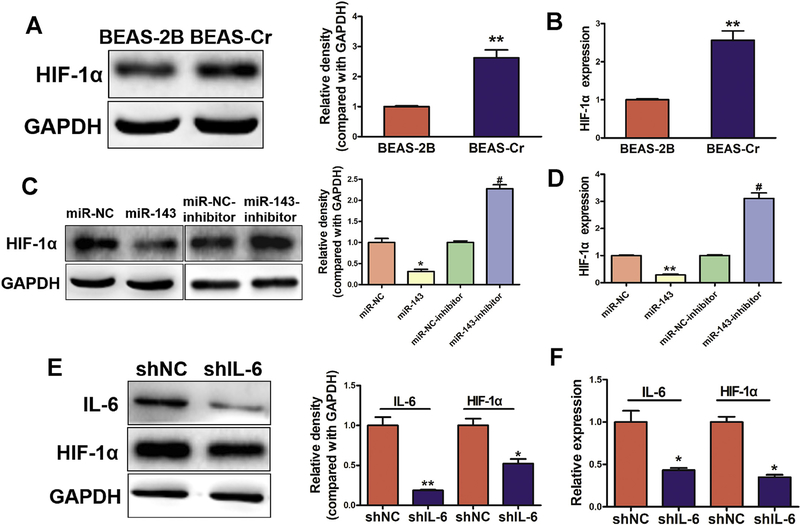
Given the important role of HIF-1α in regulating tumor growth and angiogenesis, we investigated whether miR-143 affected HIF-1α expression in BEAS-Cr cells, and found that overexpression of miR-143 greatly decreased HIF-1α mRNA and protein expression (Fig. 4A–D). We also found that knockdown of IL-6 was sufficient to decrease HIF-1α mRNA and protein expression in the cells (Fig. 4E–F), suggesting that miR-143 inhibits HIF-1α expression through the IL-6 pathway.
Fig. 4.
miR-143 inhibits HIF-1α expression, whereas IL-6 induces HIF-1α expression. (A-B)HIF-1α is upregulated in BEAS-Cr cells compared with BEAS-2B cells. Analysis of HIF-1α mRNA and protein expression. Data are presented as mean ± SD. of 3 replicates. ** indicates significant difference at P < .01. (C-D)miR-143 decreases HIF-1α expression at the RNA and protein levels, whereas HIF-1α was induced when miR-143 was inhibited. Data are presented as mean ± SD from 3 replicates. * indicates significant difference at P < .05, ** indicates significant difference at P < .01, * and ** indicates P value when miR-143 is compared with miR-NC; # indicates significant difference at P < .05, # indicates P value when an anti-miR-143 inhibitor is compared with an miR-NC inhibitor. (E-F)Loss of expression of IL-6 decreased HIF-1α at the mRNA and protein level. Data are presented as mean ± SD from 3 replicates. * indicates significant difference at P < .05, ** indicates significant difference at P < .01.
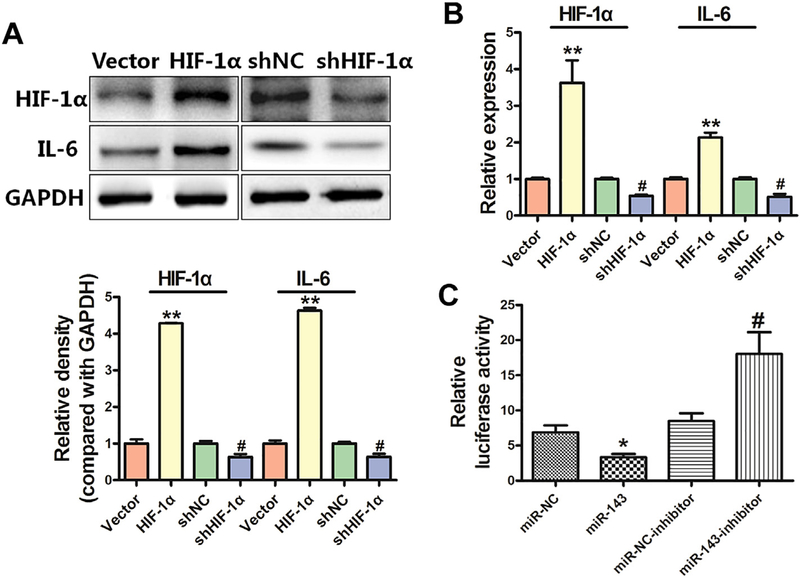
3.5. HIF-1α forms a positive feedback loop to induce IL-6 expression and VEGF levels via suppression of miR-143
HIF-1α is known to induce gene expression in human cancers. Therefore, we tested whether HIF-1α affects IL-6 expression, and found that overexpression of HIF-1α cDNA induced IL-6 levels, whereas knockdown of HIF-1α reduced expression of both IL-6 mRNA and protein (Fig. 5A–B). Our previous study showed that HIF-1α played an important role in regulating VEGF expression at the transcriptional level and in tumor angiogenesis (Forsythe et al., 1996; Jiang et al., 1997). To determine whether miR-143 inhibited VEGF expression through HIF-1α binding to the VEGF promoter, we analyzed the effects of miR-143 on reporters of the VEGF promoter that contained the HIF-1α binding site. miR-143 inhibited VEGF transcriptional activation as indicated by the decrease in luciferase reporter activities in BEAS-Cr cells, whereas an anti-miR-143 inhibitor significantly induced VEGF transcriptional activation (Fig. 5C), suggesting miR-143/HIF-1α/VEGF may be involved in tumor growth.
Fig. 5.
HIF-1α forms a positive feedback loop to induce IL-6 expression and VEGF levels via miR-143 suppression. (A)Overexpression of HIF-1α induced activity of IL-6, whereas downregulation of HIF-1α reduced expression of IL-6 at the protein level. (B) Overexpression of HIF-1α induced activity of IL-6, whereas downregulation of HIF-1α reduced expression of IL-6 at the mRNA level. Data are presented as mean ± SD from 3 replicates. ** indicates significant difference at P < .01, ** indicates P value when HIF-1α is compared with vector; # indicates significant difference at P < .05, # indicates P value when shHIF-1α is compared with shNC·(C)miR-143 decreased the luciferase activity of HIF-1α, whereas an anti-miR-143 inhibitor increased the luciferase activity. Data are presented as mean ± SD from 3 replicates. * indicates significant difference at P < .05, * indicates P value when miR-143 is compared with miR-NC; # indicates significant difference at P < .05, # indicates P value when an anti-miR-143 inhibitor is compared with an miR-NC inhibitor.
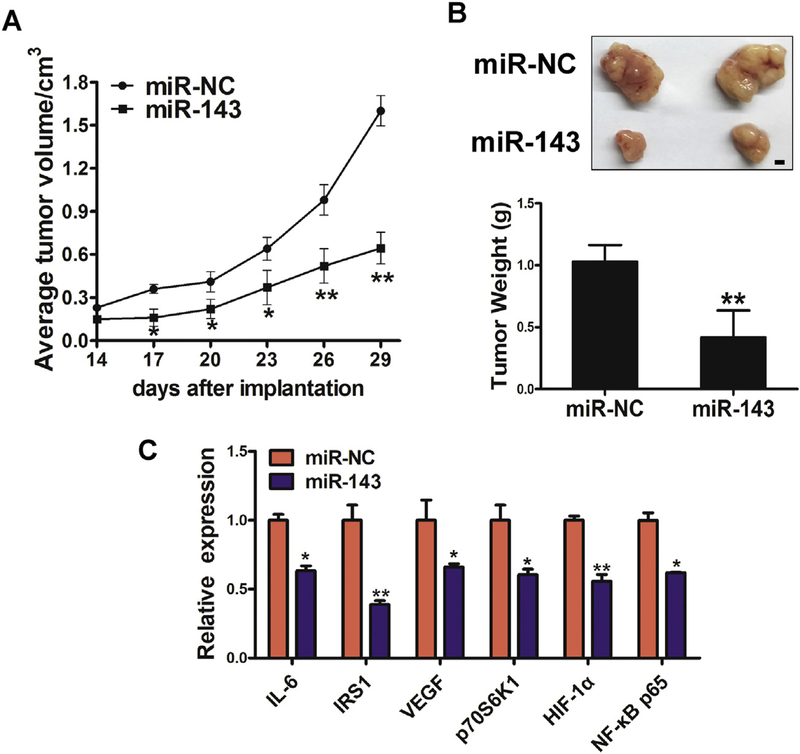
3.6. miR-143 inhibits tumor growth and decreases expression of IL-6-related pathways and HIF-1α/VEGF signaling molecules
In order to test whether overexpression of miR-143 attenuates tumor growth, we established BEAS-Cr cells that stably express miR-NC or miR-143, and these cells were subsequently implanted into both posterior flanks of immunodeficient mice. Tumor sizes were measured six times at 3-day intervals starting 2 weeks after cell injection until day 29. On day 29, mice were sacrificed and tumors were dissected and analyzed. Overexpression of miR-143 significantly inhibited tumor growth (Fig. 6A). Representative tumors and tumor weight are shown in Fig. 6B. The mRNA expression levels of IL-6, IRS1, VEGF, p70S6K1, HIF-1α and NF-κB p65 were analyzed by qRT-PCR in tumor tissues (Fig. 6C). Protein levels of these molecules in the tumors were measured by immunoblotting (Fig. 6D). These results showed that miR-143 inhibited expression of IL-6, HIF-1α, and downstream signaling molecules in vivo. IHC staining revealed that the expression of Ki67, IL-6, CD31 and p70S6K1 were significantly decreased by overexpression of miR-143, which indicated that miR-143 inhibited tumor growth and angiogenesis in vivo (Fig. 6E).
Fig. 6.
miR-143 inhibits tumor growth and decreases expression of IL-6-related pathways and HIF-1α/VEGF signaling molecules. (A)Tumor growth assay in nude mice. (B) Representative pictures and weights of xenograft tumors. (C) mRNA expression of IL-6, IRS1, VEGF, p70S6K1, HIF-1α and NF-κB p65 was analyzed in tumor tissues by qRT-PCR. (D) Protein levels of IRS1, HIF-1α, p70S6K1, NF-κB p65 and IL-6 in xenograft tumors. (E)IHC images of Ki67, IL-6, CD31 and p70S6K1 in miR-NC and miR-143 groups. Data are presented as mean ± SD from 3 replicates. * indicates significant difference at P < .05; ** indicates significant difference at P < .01.
4. Discussion
Hexavalent chromium [Cr(VI)] compounds are well-known carcinogens and are associated with a higher incidence of lung cancer (Abreu et al., 2014). Short-term environmental exposure to r(VI) causes toxicity, with long-term exposure leading to carcinogenesis (Freeman et al., 1997). We assessed changes in serum miRNAs and proteins in Cr(VI)-exposed workers and found that IL-6 levels were significantly higher, whereas miR-143 levels were significantly lower, in blood samples of Cr(VI)-exposed workers.
We found that IL-6 was an important downstream molecule of miR-143, suggesting a potential molecular mechanism of Cr(VI)-induced carcinogenesis. IL-6 is a pleiotropic inflammatory cytokine first discovered as a B-cell growth factor that is synthesized by T-cells, macrophages and stromal cells in response to stimulation from tumor necrosis factor-alpha (TNF-a) and interleukin-1 (IL-1) (Hodge et al., 2005; Song and Kellum, 2005). High circulating concentrations of IL-6are associated with many diseases, including cardiovascular disease, type 2 diabetes, and cancer (Ridker et al., 2000; Pradhan et al., 2001; Lu et al., 2006). In our study, we discovered that IL-6 is overexpressed in blood samples of Cr(VI)-exposed workers and in BEAS-Cr cells compared with BEAS-2B cells. miR-143 decreased IL-6 expression at the RNA and protein level, whereas IL-6 was induced when miR-143 was inhibited. Some important signaling pathways, such as the IGFIR/IRS1 and mTOR/p70S6K1 pathways, may be influenced by miR-143. In this study, we found that expression of p70S6K1 was influenced by miR-143. A previous study showed that p70S6K1 is involved in various cellular functions such as cell proliferation, cell apoptosis and regulation of cell cycle. Overexpression and/or activation of the p70S6K1 protein is important in cancer. This is the first study to show that p70S6K1 is negatively regulated by miR-143 in BEAS-Cr cells, necessitating further study to determine the precise molecular mechanisms by which Cr(VI) induces lung cancer. Our findings not only provide evidence supporting the role of IL-6 and related signaling pathways in Cr(VI)-induced carcinogenesis, but also suggest a tumor suppressive role of miR-143.
Since angiogenesis is a key characteristic of malignant tumors, we also investigated the effects of miR-143/IL-6/HIF-1α on this process. In this study, we found that HIF-1α is upregulated by IL-6, and could form a positive feedback loop to induce IL-6 expression and increase VEGF levels by suppressing miR-143. With animal experiments, we found that miR-143 inhibited tumor growth and angiogenesis by decreasing IL-6/HIF-1α and downstream signaling pathways. These findings from our study and other recent studies strongly support an anti-angiogenesis approach to cancer treatment.
In summary, our findings are the first demonstrate that IL-6 is overexpressed in blood samples of Cr(VI)-exposed workers and in BEAS-Cr cells compared to human bronchial epithelial BEAS-2B cells, and that this is inhibited by miR-143. Our data suggest that the miR-143/IL-6/HIF-1α signaling axis plays an indispensable role in Cr(VI)-induced cell transformation and carcinogenesis. This information will likely be useful for developing new prevention or/and treatment options in the future.
Supplementary Material
Acknowledgements
This work was supported in part by the National Natural Science Foundation of China (81472944, 81803197), by the National Institutes of Health (R01ES020868, R01ES024151 and R01CA232587), by the American Cancer Society Research Scholar NEC-129306, by Key Scientific Research Projects of Universities in Henan Province (No. 18A310010), and by Medical Science and Technology in Henan Province (201702298).
Footnotes
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.taap.2019.114603.
Conflicts of interests
The authors declare that they have no competing interests.
References
- Abreu PL, Ferreira LM, Alpoim MC, Urbano AM, 2014. Impact of hexavalent chromium on mammalian cell bioenergetics: phenotypic changes, molecular basis and potential relevance to chromate-induced lung cancer. Biometals 27, 409–443. [DOI] [PubMed] [Google Scholar]
- Calin GA, Croce CM, 2006. MicroRNA signatures in human cancers. Nat. Rev. Cancer 6, 857–866. [DOI] [PubMed] [Google Scholar]
- Chen LF, Greene WC, 2004. Shaping the nuclear action of NF-kappaB. Nat. Rev. Mol. Cell Biol. 5, 392–401. [DOI] [PubMed] [Google Scholar]
- Forsythe JA, Jiang BH, Iyer NV, Agani F, Leung SW, Koos RD, Semenza GL, 1996. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol. Cell. Biol. 16, 4604–4613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman NC, Stern AH, Lioy PJ, 1997. Exposure to chromium dust from homes in a Chromium Surveillance Project. Arch. Environ. Health 52, 213–219. [DOI] [PubMed] [Google Scholar]
- Ghosh S, Karin M, 2002. Missing pieces in the NF-kappaB puzzle. Cell 109 (Suppl), S81–S96. [DOI] [PubMed] [Google Scholar]
- He J, Qian X, Carpenter R, Xu Q, Wang L, Qi Y, Wang ZX, Liu LZ, Jiang BH, 2013. Repression of miR-143 mediates Cr (VI)-induced tumor angiogenesis via IGF-IR/IRS1/ERK/IL-8 pathway. Toxicol. Sci. 134, 26–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heikkila K, Ebrahim S, Lawlor DA, 2008. Systematic review of the association between circulating interleukin-6 (IL-6) and cancer. Eur. J. Cancer 44, 937–945. [DOI] [PubMed] [Google Scholar]
- Heinrich PC, Behrmann I, Haan S, Hermanns HM, Muller-Newen G, Schaper F, 2003. Principles of interleukin (IL)-6-type cytokine signalling and its regulation. Biochem. J. 374, 1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodge DR, Hurt EM, Farrar WL, 2005. The role of IL-6and STAT3 in inflammation and cancer. Eur. J. Cancer 41, 2502–2512. [DOI] [PubMed] [Google Scholar]
- Humphries B, Wang Z, Li Y, Jhan JR, Jiang Y, Yang C, 2017. ARHGAP18 downregulation by miR-200b suppresses metastasis of triple-negative breast cancer by enhancing activation of RhoA. Cancer Res. 77, 4051–4064. [DOI] [PubMed] [Google Scholar]
- Jiang BH, Agani F, Passaniti A, Semenza GL, 1997. V-SRC induces expression of hypoxia-inducible factor 1 (HIF-1) and transcription of genes encoding vascular endothelial growth factor and enolase 1: involvement of HIF-1 in tumor progression. Cancer Res. 57, 5328–5335. [PubMed] [Google Scholar]
- Jiang CF, Shi ZM, Li DM, Qian YC, Ren Y, Bai XM, Xie YX, Wang L, Ge X, Liu WT, Zhen LL, Liu LZ, Jiang BH, 2018. Estrogen-induced miR-196a elevation promotes tumor growth and metastasis via targeting SPRED1 in breast cancer. Mol. Cancer 17, 83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin YP, Hu YP, Wu XS, Wu YS, Ye YY, Li HF, Liu YC, Jiang L, Liu FT, Zhang YJ, Hao YJ, Liu XY, Liu YB, 2018. miR-143–3p targeting of ITGA6 suppresses tumour growth and angiogenesis by downregulating PLGF expression via the PI3K/AKT pathway in gallbladder carcinoma. Cell Death Dis. 9, 182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D, Dai J, Park YH, Fai LY, Wang L, Pratheeshkumar P, Son YO, Kondo K, Xu M, Luo J, Shi X, Zhang Z, 2016. Activation of epidermal growth factor receptor/p38/hypoxia-inducible factor-1alpha is pivotal for angiogenesis and tumorigenesis of malignantly transformed cells induced by hexavalent chromium. J. Biol. Chem. 291, 16271–16281. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Lam J, van den Bosch M, Wegrzyn J, Parker J, Ibrahim R, Slowski K, Chang L, Martinez-Hoyer S, Condorelli G, Boldin M, Deng Y, Umlandt P, Fuller M, Karsan A, 2018. miR-143/145 differentially regulate hematopoietic stem and progenitor activity through suppression of canonical TGFbeta signaling. Nat. Commun. 9, 2418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu LZ, Li C, Chen Q, Jing Y, Carpenter R, Jiang Y, Kung HF, Lai L, Jiang BH, 2011. MiR-21 induced angiogenesis through AKT and ERK activation and HIF-1alpha expression. PLoS One 6, e19139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lou J, Wang Y, Yao C, Jin L, Wang X, Xiao Y, Wu N, Song P, Song Y, Tan Y, Gao M, Liu K, Zhang X, 2013. Role of DNA methylation in cell cycle arrest induced by Cr (VI) in two cell lines. PLoS One 8, e71031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H, Ouyang W, Huang C, 2006. Inflammation, a key event in cancer development. Mol. Cancer Res. 4, 221–233. [DOI] [PubMed] [Google Scholar]
- Mantovani A, Allavena P, Sica A, Balkwill F, 2008. Cancer-related inflammation. Nature 454, 436–444. [DOI] [PubMed] [Google Scholar]
- Naugler WE, Karin M, 2008. NF-kappaB and cancer-identifying targets and mechanisms. Curr. Opin. Genet. Dev. 18, 19–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu XB, Fu GB, Wang L, Ge X, Liu WT, Wen YY, Sun HR, Liu LZ, Wang ZJ, Jiang BH, 2017. Insulin-like growth factor-I induces chemoresistence to docetaxel by inhibiting miR-143 in human prostate cancer. Oncotarget 8, 107157–107166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan L, Xiao H, Liao R, Chen Q, Peng C, Zhang Y, Mu T, Wu Z, 2018. Fatty acid binding protein 5 promotes tumor angiogenesis and activates the IL-6/STAT3/VEGFA pathway in hepatocellular carcinoma. Biomed. Pharmacother. 106, 68–76. [DOI] [PubMed] [Google Scholar]
- Pasquinelli AE, 2012. MicroRNAs and their targets: recognition, regulation and an emerging reciprocal relationship. Nat. Rev. Genet. 13, 271–282. [DOI] [PubMed] [Google Scholar]
- Perkins ND, 2007. Integrating cell-signalling pathways with NF-kappaB and IKK function. Nat. Rev. Mol. Cell Biol. 8, 49–62. [DOI] [PubMed] [Google Scholar]
- Pradhan AD, Manson JE, Rifai N, Buring JE, Ridker PM, 2001. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. Jama 286, 327–334. [DOI] [PubMed] [Google Scholar]
- Pratheeshkumar P, Son YO, Divya SP, Wang L, Zhang Z, Shi X, 2016. Oncogenic transformation of human lung bronchial epithelial cells induced by arsenic involves ROS-dependent activation of STAT3-miR-21-PDCD4 mechanism. Sci. Rep. 6, 37227. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Pratheeshkumar P, Son YO, Divya SP, Wang L, Turcios L, Roy RV, Hitron JA, Kim D, Dai J, Asha P, Zhang Z, Shi X, 2017. Quercetin inhibits Cr(VI)-induced malignant cell transformation by targeting miR-21-PDCD4 signaling pathway. Oncotarget 8, 52118–52131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayet B, Gelinas C, 1999. Aberrant rel/nfkb genes and activity in human cancer. Oncogene 18, 6938–6947. [DOI] [PubMed] [Google Scholar]
- Ridker PM, Hennekens CH, Buring JE, Rifai N, 2000. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N.Engl. J. Med. 342, 836–843. [DOI] [PubMed] [Google Scholar]
- Rodrigues CF, Urbano AM, Matoso E, Carreira I, Almeida A, Santos P, Botelho F, Carvalho L, Alves M, Monteiro C, Costa AN, Moreno V, Alpoim MC, 2009. Human bronchial epithelial cells malignantly transformed by hexavalent chromium exhibit an aneuploid phenotype but no microsatellite instability. Mutat. Res. 670, 42–52. [DOI] [PubMed] [Google Scholar]
- Song M, Kellum JA, 2005. Interleukin-6. Crit. Care Med. 33, S463–S465. [DOI] [PubMed] [Google Scholar]
- Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A, 2015. Global cancer statistics, 2012. CA Cancer J. Clin. 65, 87–108. [DOI] [PubMed] [Google Scholar]
- Wang L, Shi ZM, Jiang CF, Liu X, Chen QD, Qian X, Li DM, Ge X, Wang XF, Liu LZ, You YP, Liu N, Jiang BH, 2014. MiR-143 acts as a tumor suppressor by targeting N-RAS and enhances temozolomide-induced apoptosis in glioma. Oncotarget 5, 5416–5427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilbur S, Abadin H, Fay M, Yu D, Tencza B, Ingerman L, Klotzbach J, James S, 2012. Toxicological Profile for Chromium, Atlanta (GA). [PubMed] [Google Scholar]
- Xia B, Ren XH, Zhuang ZX, Yang LQ, Huang HY, Pang L, Wu DS, Luo J, Tan YL, Liu JJ, Zou F, 2014. Effect of hexavalent chromium on histone biotinylation in human bronchial epithelial cells. Toxicol. Lett. 228, 241–247. [DOI] [PubMed] [Google Scholar]
- Xu Q, Liu LZ, Qian X, Chen Q, Jiang Y, Li D, Lai L, Jiang BH, 2012. MiR-145 directly targets p70S6K1 in cancer cells to inhibit tumor growth and angiogenesis. Nucleic Acids Res. 40, 761–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Q, Jiang Y, Yin Y, Li Q, He J, Jing Y, Qi YT, Xu Q, Li W, Lu B, Peiper SS, Jiang BH, Liu LZ, 2013. A regulatory circuit of miR-148a/152 and DNMT1 in modulating cell transformation and tumor angiogenesis through IGF-IR and IRS1. J. Mol. Cell Biol. 5, 3–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.