Abstract
Background:
Prescription drug monitoring programs (PDMP), by reducing access to prescribed opioids (POs), may contribute to a policy environment in which some people with opioid dependence are at increased risk for transitioning from POs to heroin/other illegal opioids. This study examines how PDMP adoption and changes in the characteristics of PDMPs over time contribute to changes in fatal heroin poisoning in counties within states from 2002 to 2016.
Methods
Latent transition analysis to classify PDMPs into latent classes (Cooperative, Proactive, and Weak) for each state and year, across three intervals (1999-2004, 2005-2009, 2010-2016). We examined the association between probability of PDMP latent class membership and the rate of county-level heroin poisoning death.
Results:
After adjustment for potential county-level confounders and co-occurring policy changes, adoption of a PDMP was significantly associated with increased heroin poisoning rates (22% increase by third year post-adoption). Findings varied by PDMP type. From 2010-2016, states with Cooperative PDMPs (those more likely to share data with other states, to require more frequent reporting, and include more drug schedules) had 19% higher heroin poisoning rates than states with Weak PDMPs (adjusted rate ratio [ARR] = 1.19; 95% CI = 1.14, 1.25). States with Proactive PDMPs (those more likely to report outlying prescribing and dispensing and provide broader access to law enforcement) had 6% lower heroin poisoning rates than states with No/Weak PDMPs (ARR = 0.94; 95% CI = 0.90, 0.98).
Conclusion:
There is a consistent, positive association between state PDMP adoption and heroin poisoning mortality. However, this varies by PDMP type, with Proactive PDMPs associated with a small reduction in heroin poisoning deaths. This raises questions about the potential for PDMPs to support efforts to decrease heroin overdose risk, particularly by using proactive alerts to identify patients in need of treatment for opioid use disorder. Future research on mechanisms explaining the reduction in heroin poisonings after enactment of Proactive PDMPs is merited.
Keywords: Prescription drug monitoring programs, fatal heroin poisonings, latent classes, overdose
Introduction
Heroin poisonings in the United States have increased nearly 5-fold from 2010 to 2017, significantly contributing to the ongoing epidemic of opioid-related harm (NIDA, 2018). There are at least four potential explanations in the published literature for this increase: increased availability of heroin in regions with previously little access to the drug (Compton, Jones, & Baldwin, 2016; DEA, 2015; Jalal et al., 2018; NIDA, 2014), increases in heroin purity and decreases in price (Compton et al., 2016; ONDCP, 2014; Unick, Rosenblum, Mars, & Ciccarone, 2014), the emergence of heroin adulterated with illicitly manufactured fentanyl (Carroll, Marshall, Rich, & Green, 2017; Jalal et al., 2018; Mars, Ondocsin, & Ciccarone, 2018) and other analogs, and increased heroin use due to transitions from prescription opioid use, misuse, or dependence to heroin use (Banerjee et al., 2019; Jones, 2013; Kerridge et al., 2015; Kolodny et al., 2015; Mars et al., 2015; Martins, Sarvet, et al., 2017; Martins, Segura, et al., 2017).
Prescription drug monitoring programs (PDMPs) are state-level databases that collect information on controlled prescription medications dispensed in that state. The data PDMPs collect is made available only to approved health care providers, law enforcement officials, and sometimes other parties, depending on state law (Davis, Pierce, & Dasgupta, 2014). As of December 2017, all US states except Missouri had an operational PDMP (NAMSDL, 2018). Despite their stated goals, however, evidence on whether PDMPs reduce opioid-related harm, particularly harm related to prescription opioids (PO) (Fink et al., 2018), by reducing inappropriate and potentially dangerous opioid prescribing, is mixed. (Davis et al., 2014; Dowell, Zhang, Noonan, & Hockenberry, 2016; Haffajee, 2019; Pew Charitable Trusts, 2016; Smith et al., 2019). A recent systematic review (Fink et al., 2018) of 10 studies found weak evidence that PDMP implementation is associated with reductions in fatal opioid-related poisonings; however, three (Delcher et al., 2016; Kilby, 2015; Meinhofer, 2018) of six studies (Delcher et al., 2016; Dowell et al., 2016; Kilby, 2015; Meinhofer, 2018; Nam, Shea, Shi, & Moran, 2017; Radakrishnan, 2015) that measured that outcome found an increase in heroin poisonings after PDMP implementation.
While evidence is mixed on whether PDMPs are associated with overall changes in opioid prescribing, it is hypothesized that increased utilization of PDMPs and more restrictive PDMP features may result in reductions in opioids prescribed to patients on high dose opioid therapy, and may be one driver within the overall policy environment that may lead some of these patients to seek heroin on the illicit market as an alternative to prescription opioids (Bao et al., 2016; Beletsky, 2018; Deyo et al., 2018; Mars, Bourgois, Karandinos, Montero, & Ciccarone, 2014). A recent survey of 37 PDMP administrators, law enforcement officials and administrative agency employees in Florida, Kentucky, New Jersey and Ohio, showed that some believe that this substitution is currently occurring in some patients (Yuanhong Lai et al., 2019).
The few studies that have shown PDMP implementation to be associated with increasing rates of heroin poisoning have all treated PDMP as present or absent, and so did not take into account that PDMP characteristics vary greatly across states and over time, as each state enacts laws that define specific aspects of their program (Davis et al., 2014) and updates PDMP legislation as the opioid epidemic evolves (Cerda et al., In Press). In addition, one of these studies examined data from a single US state (Delcher et al., 2016), and another examined data only from a subset of 38 US states and D.C. (Kilby, 2015). Given evidence that specific PDMP operational features are associated with greater reductions in prescription opioid-related mortality rates (Fink et al., 2018), further research is needed to identify whether different combinations of PDMP features can explain the heterogeneous results in previous studies and, if so, which combinations of PDMP features have made the strongest contribution to changes in heroin poisoning. Finally, all prior studies that examine the relationship between PDMPs and fatal heroin poisoning have been conducted at the state level, and do not account for within-state heterogeneity in rates of change in heroin poisoning and in the distribution of key demographic covariates that may affect heroin poisoning, thus generating the potential for aggregation bias in published findings (Cerda et al., In Press; W. A. V. Clark & Avery, 1976).
To better understand which combinations of PDMP features are associated with changes in heroin poisoning, we took three steps. First, we used latent transition analysis (LTA) to reduce complex and frequently co-occurring PDMP features into simpler latent classes (i.e., combinations) of PDMP characteristics that are likely to be adopted together, and that may reflect distinct underlying “typologies” of PDMPs in three distinct PDMP program periods (1999-2004, 2005-2009, and 2010-2016) as defined in a prior paper (Smith et al., 2019). Second, we examined two types of associations: (1) the association between year of electronic PDMP implementation and the rate of heroin poisoning; and (2) the association between transitions across types of PDMP latent classes and changes in fatal heroin poisoning in counties within states from 2002 to 2016. This approach allowed us to identify the combinations of PDMP features that were associated with the greatest change in relative rates of heroin poisoning fatalities over time across the United States. Third, we adopted a geospatial approach to examine the impact of state-level PDMPs on county-level fatal heroin poisonings, thus accounting for within-state variation in the level and rate of growth of heroin poisonings, and for spatial autocorrelation in heroin poisonings across counties and states. Hence, our main aim in this study was to examine how transitions across types of PDMP classes over time contributed to changes in fatal heroin poisoning in counties within states from 2002 to 2016.
Methods
Outcomes
We used the National Vital Statistics System to extract data on heroin poisoning deaths ([dataset] NCHS, 2017). Using the International Classification of Disease, Tenth Revision (ICD-10) underlying cause-of-death codes, we identified drug-poisoning deaths, defined as unintentional poisoning (X40-44), suicide by drug self-poisoning (X60-64), homicide by drug poisoning (X85), and undetermined intent (Y10-14). Among these deaths, we restricted our analyses to deaths involving the ICD-10 multiple cause-of-death code for heroin poisoning (T40.1), these include deaths that had codes for other drug poisoning together with heroin. We computed annual county-level counts of heroin poisoning for 3,109 counties in 49 US states and Washington D.C. in 2002-2016. Alaska was not included due to frequent changes in county boundaries during the study period (U.S. Census Bureau, 2019a, 2019b). We classified poisonings by county of death, rather than county of residence, since county of death likely more closely represents the place where the poisoning occurred (overlap between county of residence and county of death across years was of 81.5%-85.2%).
Exposures
We used latent transition analysis (LTA) to identify typologies of PDMPs, or “classes,” described in detail elsewhere (Smith et al., 2019). Briefly, we obtained dates of electronic PDMP access from the National Alliance for Model State Drug Laws (NAMSDL) and state PDMP administrators, and we compiled PDMP characteristics from the Prescription Drug Abuse Policy System (PDAPS) database of legal provisions ([dataset] PDAPS, 2017). Next, we considered PDMP characteristics that have been identified by prescription opioid policy experts as potentially important determinants of prescribing practices and heroin poisoning events (see supplemental Figure 1) (Davis et al., 2014; Pew Charitable Trusts, 2016). These included: a) state authorizes prescribers to access PDMP data; b) state authorizes in-state law enforcement to access PDMP data; c) state permits or requires PDMP to proactively identify suspicious or statistically outlying prescribing, dispensing, or purchasing activity; d) timeframe in which dispensers are required to report data to the PDMP; e) number of drug schedules state requires to be reported to the PDMP; f) state requires prescribers to check the PDMP before prescribing controlled substances; and g) law permits the PDMP to share data with other state PDMPs (Smith et al., 2019).
We used LTA (Duncan et al., 1997; Lanza, Dziak, Huang, Wagner, & Collins, 2015; Muthén, 2004; Muthen & Muthen, 2000; “PROC LCA & PROC LTA (Version 1.3.2) ”, 2015) to identify groups of states with similar combinations of PDMP characteristics for each year of the study and identified latent classes for three separate intervals: 1999-2004, 2005-2009, and 2010-2016. The methods used to create these classes and the results from this analysis have been described elsewhere (Smith et al., 2019); results are also presented in the online Supplement. The three intervals represent different historical periods in the opioid poisoning epidemic and the evolution of PDMPs including: (1) 1999-2004, when PDMPs first started to transmit data electronically; (2) 2005-2009, when federal funding for PDMPs from the Bureau of Justice Assistance and SAMHSA, among others, increased; and (3) 2010-2016, when PDMP capacity expanded (T. Clark, Eadie, Kreiner, & Strickler, 2012; Gugelmann, Perrone, & Nelson, 2012; Hedegaard, Warner, & Minino, 2017; Rudd, Aleshire, Zibbell, & Gladden, 2016).
In the final LTA models, three distinct classes of PDMPs were identified for each time period. Since the pattern of PDMP characteristics within each class was most comparable in the first two intervals, we used the same labels for classes in 1999-2004 and 2005-2009: No/Weak PDMP, Reactive PDMP, and Proactive PDMP. The key distinguishing features of the three latent classes were: (1) the No/Weak PDMP class represented states with no operational or limited PDMP; (2) the Reactive PDMP class represented states with no requirements to proactively report outlying patterns to law enforcement, licensing bodies and prescribers/dispensers, limited data access for law enforcement, and less frequent reporting requirements for dispensers; and (3), the Proactive PDMP class represented states that tend to permit and/or require proactive reporting of outlying patterns to law enforcement, licensing bodies and prescribers/dispensers, to provide access to PDMP data to law enforcement without requiring a warrant, subpoena, or active investigation, and to require dispensers to report data to the PDMP on a more frequent basis. In the last interval (2010-2016), two additional variables not available in earlier years were included, so we used different class names to reflect the evolving nature of the PDMPs: Weak PDMP, Cooperative PDMP, and Proactive PDMP. In this interval, the Weak PDMP class represented states with fairly basic PDMPs; the Proactive PDMP class was similar to the Proactive PDMP class in the first 2 intervals, and the Cooperative PDMP class had a lower probability than states in the Proactive PDMP class of permitting/requiring reporting of outlying patterns to PDMP users, or providing broader access of PDMP data to law enforcement, but had a higher probability of allowing PDMP data to be shared with other states, requiring dispensers to report data to the PDMP on a more frequent basis, and reporting more federal drug schedules than states in the Proactive class (Cerda et al., In Press; Smith et al., 2019).
Covariates
Based on prior studies (Bohnert et al., 2011; Cerda et al., 2017; Paulozzi, Kilbourne, & Desai, 2011), we accounted for the following county-level demographic characteristics, obtained from annual Geolytics data ([dataset] Geolytics Estimates Premium, 2014): population density (thousands of people/square mile); age composition (% of the population aged 0-19, 20-44, 45-64, and >65 years); racial/ethnic composition (% non-Hispanic White, non-Hispanic Black, Hispanic); % male; and socioeconomic conditions (% of families in poverty, median household income, % unemployed). We also accounted for the overall mortality rate per 1,000 residents in the county. Finally, we accounted for co-occurring policy changes associated with opioid poisoning in prior studies that may confound the association between PDMP characteristics and heroin poisoning, including: medical marijuana legalization (Bachhuber, Saloner, Cunningham, & Barry, 2014; Shi, 2017), overdose Good Samaritan laws, and naloxone access laws (McClellan et al., 2018; Rees, Sabia, Argys, Latshaw, & Dave, 2017). We obtained annual, state-level information on these laws through PDAPS ([dataset] PDAPS, 2017; Smith et al., 2019). The research study protocol was reviewed and approved by Columbia University’s Institutional Review Board.
Analyses
We modeled the county-by-year mortality counts using hierarchical Bayesian Poisson models, with county population included as the offset (Bernardinelli et al., 1995; Besag, York, & Mollie, 1991; Carlin & Louis, 2000). Other covariates, including demographic characteristics and the overall mortality rate, were modeled as concurrent predictors of heroin poisoning deaths.
The models captured baseline differences between states in heroin poisoning rates using state dummy variables, while county-specific unit random effects allow for varying levels within each state. A linear fixed-effect time trend predicted constant proportional growth due to the log link function within Poisson models. In combination with county random trend effects, this accounts for heterogenous accelerated growth between counties over time (i.e., growth mixtures that could otherwise bias estimates of covariate effects). A conditional autoregressive spatial random effect allowed for greater similarity between adjacent counties than between distant ones (spatial autocorrelation), a lack of independence that could bias uncorrected models (Waller & Gotway, 2004). The model also incorporated a non-spatial county random effect which effectively controlled for over-dispersion (Lord, Washington, & Ivan, 2005). These Bayesian Poisson models were performed using the R-INLA package (Integrated Nested Laplace Approximation) (Blangiardo & Cameletti, 2015; Rue, Martino, & Chopin, 2009). The analytic approach used was similar to a difference-in-difference approach, as: it used each state as its own control (through the specification of state fixed effects); it assumed (by fitting a county-level random intercept) that each county was different at baseline; and it specified that counties and states experienced linear growth (by fitting a linear time trend common to all states). However, the approach also improved upon difference-in-difference models by specifying a separate growth parameter (i.e., random slope) for each county, thus allowing us to obtain unbiased estimates in the context of heterogeneous policy effects across counties within states, and avoiding biases due to over- and under-differencing.
First, we examined the association between the proportion of each year with electronic PDMP implementation and the rate of heroin poisoning death. Using linear distributed lags over three years, we assessed both the concurrent impact within the year of PDMP implementation and three subsequent years (Greene, 2012). Then, we examined the association between probability of PDMP latent class membership in each year and interval and the rate of heroin poisoning death. All models accounted for demographic characteristics, overall mortality rates, and distributed-lag specifications for co-occurring marijuana, Good Samaritan and naloxone laws.
Sensitivity Analyses
We conducted two sensitivity analyses to address sources of bias that may arise from relying on ICD coding of death certificate data, notably that specific drugs involved in drug poisoning are often not identified on death certificates, resulting in differential underestimation of drug-specific poisoning rates between states (Ruhm, 2017). We replicated our analyses excluding states that were found to have a >5% absolute difference in reported versus corrected heroin poisoning rates in a prior study that imputed county-level opioid poisoning rates when no drug was specified (i.e., Alabama, Indiana, Louisiana, and Pennsylvania) (Ruhm, 2017). Finally, we replicated our analyses using the county-level overall drug poisoning rate (thus not specific to any drugs) as the outcome, as this rate would not depend on state-level changes in coding of specific drugs.
Results
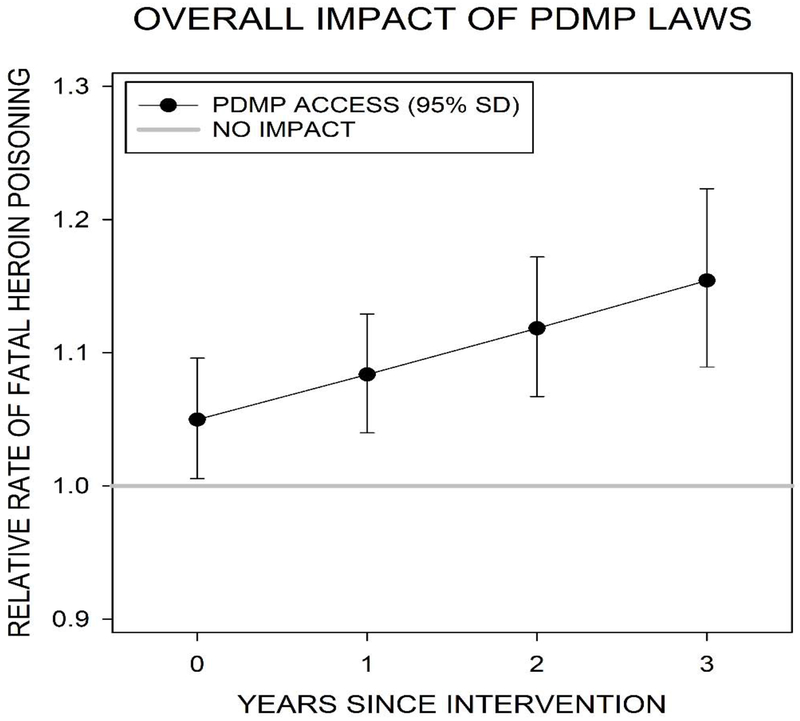
Figure 1 presents the overall impact of state adoption of an electronic PDMP on the log relative rate of heroin poisoning fatalities in the years since the electronic PDMP became operational. We observed a consistent, positive, and significant association between state adoption of an electronic PDMP that provides electronic access to data and heroin poisoning fatalities in all years, an effect which increased over time. By the third year of adoption of an electronic PDMP, there was a 22% increase in heroin poisoning fatalities (Adjusted Rate Ratio [ARR]=1.22; 95% Credible Interval [CI]=1.16,1.29), compared to no PDMP adoption.
Figure 1:
Linear distributed-lag model showing the overall impact of state adoption of an electronic PDMP on the relative rate of fatal heroin poisoning
Note: Results based on models that adjust for annual county-level age, race/ethnic, sex, and socioeconomic composition, population density, and the overall mortality rate; annual state-level medical marijuana laws, Good Samaritan laws, and naloxone poisoning prevention laws; calendar year; and state-level fixed effects.
Table 1 presents the association between each PDMP latent class and the relative rate of county-level heroin poisoning at each of the three intervals of study. In the first interval (1999-2004), states that had adopted Reactive PDMPs had 12% lower heroin poisoning fatality as compared to states with No/Weak PDMPs (ARR = 0.88; 95% CI = 0.82, 0.94). However, there were no differences in heroin poisoning fatality between states that adopted Proactive PDMPs as compared to states with No/Weak PDMPs, as well as between states that adopted Proactive PDMPs as compared to states that adopted Reactive PDMPs. In the second interval (2005-2009), states with Reactive PDMPs had 5% higher heroin poisoning fatality rates than states with No/Weak PDMPs (ARR = 1.05; 95% CI = 1.01, 1.09). There were no differences in rates of heroin poisonings between states with Proactive PDMPs and states with No/Weak PDMPs or between states with Proactive PDMPs and states with Reactive PDMPs. Finally, in the last interval (2010-2016), states with Cooperative PDMPs had 19% higher heroin poisoning rates than states with No/Weak PDMPs (ARR = 1.19; 95% CI = 1.14, 1.25), while states with Proactive PDMPs had 6% lower heroin poisoning rates than states with No/Weak PDMPs (ARR = 0.94; 95% CI = 0.90, 0.98). States with Proactive PDMPs also had 21% lower heroin poisoning rates than states with Cooperative PDMPs (ARR = 0.79; 95% CI = 0.74, 0.83).
Table 1.
Estimated relationship between PDMP latent class membership probability, and the county-level rate of heroin poisoning fatalities, 2002-2016, United States1
| PDMP latent classes | Full sample (49 states and D.C.) | After dropping 4 states with highest levels of underreporting for specific drugs | All drug poisonings as an outcome | |||
|---|---|---|---|---|---|---|
| Median RR | 95% CI | Median RR | 95% CI | Median RR | 95% CI | |
| Interval 1: 1999-2004 | ||||||
| Reactive PDMP relative to No/Weak PDMP | 0.88 | 0.82,0.94 | 0.77 | 0.72, 0.83 | 0.98 | 0.96, 0.99 |
| Proactive PDMP relative to No/Weak PDMP | 1.00 | 0.83,1.20 | 0.93 | 0.77,1.11 | 0.96 | 0.93, 1.00 |
| Proactive PDMP relative to Reactive PDMP | 1.14 | 0.95,1.37 | 1.20 | 1.00,1.44 | 0.99 | 0.95, 1.03 |
| Interval 2: 2005-2009 | ||||||
| Reactive PDMP relative to No/Weak PDMP | 1.05 | 1.01,1.09 | 1.01 | 0.97, 1.05 | 1.09 | 1.07, 1.09 |
| Proactive PDMP relative to No/Weak PDMP | 1.05 | 0.98,1.11 | 0.96 | 0.90,1.02 | 1.01 | 0.99, 1.03 |
| Proactive PDMP relative to Reactive PDMP | 1.00 | 0.94.1.06 | 0.95 | 0.89,1.01 | 0.94 | 0.92, 0.95 |
| Interval 3: 2010-2016 | ||||||
| Cooperative PDMP relative to Weak PDMP | 1.19 | 1.14,1.25 | 1.17 | 1.11,1.24 | 1.01 | 0.99, 1.03 |
| Proactive PDMP relative to Weak PDMP | 0.94 | 0.90,0.98 | 0.95 | 0.91,0.99 | 0.90 | 0.89, 0.92 |
| Proactive PDMP relative to Cooperative PDMP | 0.79 | 0.74,0.83 | 0.81 | 0.76,0.86 | 0.90 | 0.88, 0.91 |
Results based on models that adjust for annual county-level age, race/ethnic, sex, and socioeconomic composition, population density, and the overall mortality rate; annual state-level medical marijuana laws, Good Samaritan laws, and naloxone poisoning prevention laws; calendar year; and state-level fixed effects.
Note: estimates significant at p ≤ 0.05 are bolded.
Sensitivity analyses
Findings remained largely unchanged in the two sensitivity analyses: excluding those states with high levels of underreporting of specific drugs (Table 1), and focusing on the overall drug poisoning rate as the outcome (Table 1). In both cases, results yielded small differences in earlier years as compared to those of the main analysis.
Discussion
Our findings indicate that there is a consistent, positive, significant association between state adoption of electronic PDMPs and heroin poisoning mortality; however, the overall effect of PDMP implementation on heroin-related poisoning mortality depended on both the type of PDMP being implemented and the years over which the effects were assessed. In particular, in recent years, Proactive PDMPs, which had a higher probability than the other classes of permitting/require proactive reporting of outlying patterns to authorized PDMP users, as well as of providing broader access to PDMP data to law enforcement, have been associated with reductions in heroin poisoning mortality. On the other hand, Cooperative PDMPs, which had a higher probability of allowing PDMP data to be shared with other states, requiring dispensers to report data to the PDMP on a more frequent basis, and reporting more federal drug schedules than states in the Proactive class, were significantly associated with increases in heroin poisoning fatality.
There were mixed findings regarding Reactive/Cooperative PDMPs as compared to No/Weak PDMPs. From 2005 to 2016, Reactive/Cooperative PDMPs might have had unintended consequences, similar to what has been reported by some prior studies focusing on a binary PDMP classification (Delcher et al., 2016; Kilby, 2015; Meinhofer, 2018). In this period, Reactive and Cooperative PDMPs may have contributed to the transition of a subgroup of prescription opioid users moving to the illicit heroin market.
In the third time period (2010-2016), Proactive PDMPs showed as much as a 21% decrease in heroin poisoning fatalities as compared to Cooperative PDMPs. Findings associated with Proactive PDMP programs from the 2010-2016 period differ somewhat from those reported by prior studies, in which there were increases in fatal heroin poisonings or no effects after PDMP implementation (Smith et al., 2019). None of these prior studies examined specific characteristics of PDMP programs in the level of detail that we examined in this study, and most of them assumed PDMP to be absent or operational with no variation across time and across states, an assumption proven false by prior studies (Fink et al., 2018). Most importantly, proactive PDMPs might potentially reflect PDMPs that reduce non-evidence based prescribing practices. That is, PDMPs that provide feedback about potentially problematic dispensing and prescribing practices may help change inappropriate prescribing and help better identify patients in need of treatment for opioid use disorder secondary to prescribed opioid use, thus decreasing the potential probability of transition from prescription opioid into heroin use (Carlson, Nahhas, Martins, & Daniulaityte, 2016; Cerda, Santaella, Marshall, Kim, & Martins, 2015; Jalal et al., 2018; Martins, Sarvet, et al., 2017). In addition, our findings corroborate results from a previous study that show that operational PDMPs with robust features including sending unsolicited reports and requiring more frequent reporting from dispensers, can have a protective effect on those at risk of developing OUD (Pauly, Slavova, Delcher, Freeman, & Talbert, 2018).
To the best of our knowledge, this study is the first to identify specific classes of PDMP characteristics that are most strongly associated with changes in rates of fatal heroin poisonings. These results demonstrate that, while PDMPs overall are associated with an increase in fatal heroin poisonings, in more recent years PDMPs that were characterized as Proactive have shown a decrease in heroin poisonings. A better understanding of how specific characteristics of these programs (i.e., to require dispensers to report data to the PDMP on a more frequent basis) may contribute to a reduction in heroin poisonings is needed. In addition, it should be noted that certain features of Proactive PDMPs (in particular greater sharing of prescription information with law enforcement) may implicate privacy concerns and have the potential to perpetuate biases towards and reduce access to care for underserved and stigmatized populations (Beletsky, 2018). Thus, those authorized to access Proactive PDMP data should be trained to protect individual privacy and confidentiality and ensure that PDMP data is used only to improve care for the patient.
Our study’s findings should be considered in light of the following limitations. First, we relied on ICD coding of death certificate data, which may not reliably identify the drugs involved in fatal drug poisonings and may lead to differential underestimation of fatal heroin poisoning rates across states (Ruhm, 2017). However, our findings were robust to sensitivity analyses conducted to address this concern. Second, our study was not able to examine the mechanisms through which specific PDMP features influence the risk of heroin poisoning. Future research should identify whether specific PDMP typologies inadvertently influence inappropriate opioid prescribing behavior (e.g., abrupt discontinuation of opioid therapy without referral to follow-up care), which subsequently may cause a subset of patients to seek opioids through the illicit market. Future in-depth studies of single states could potentially provide more granular data and combine data from multiple sources to assist in elucidating causal pathways/mechanisms between PDMPs typologies and heroin poisonings. Our results may also be due to confounding, since states that adopted stricter PDMP models may also have been more likely to implement other policies or state-level initiatives aimed at reducing heroin poisoning rates that were not considered in the LTA. It should be noted that PDMPs are only one of a group of potential ecological determinants (i.e., other system efforts and measures of prescription opioid control; public health and treatment interventions for opioid disorders, changes in illicit market supply) that can contribute to changes in heroin use and heroin poisoning mortality. However, our model did adjust for several important state level policies (medical marijuana legalization, Good Samaritan Laws and naloxone access laws) and included state fixed effects, which addressed all time-fixed sources of confounding. Fourth, this study focused on the impact of PDMPs on heroin fatal poisoning only. Future studies should consider the potential unintended effects of PDMPs on other opioid-related outcomes (i.e., illicit fentanyl exposure) and on non-fatal heroin poisoning.
In conclusion, our study suggests that that, at least in recent years, Proactive PDMPs are associated with the greatest reductions in heroin poisoning fatalities. Unfortunately, both Reactive and Cooperative PDMPs were associated with increases in fatal heroin poisonings in different time periods. Future studies need to closely monitor the mid- to long-term effects different subtypes of PDMPs have on both fatal and non-fatal heroin poisonings. States with No/Weak and Cooperative PDMPs should consider implementing features of Proactive PDMPs. Future research is needed to identify how PDMP interface may be modified to support providers in not only identifying patients and providers with potentially problematic prescription histories but also linking patients who may benefit from specialized pain management or substance use disorder treatment to the most appropriate services and care for their conditions.
Supplementary Material
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interest: None
References:
- [dataset]Geolytics Estimates Premium. (2014). Geolytics, Inc. [Google Scholar]
- [dataset]NCHS. (2017). Mortality—all county micro-data, 2002-2016, for all states, as compiled from data provided by the 57 vital statistics jurisdictions through the Vital Statistics Cooperative Program. .
- [dataset]PDAPS. (2017). Prescription Drug Abuse Policy System. Retrieved August 14, 2017
- Bachhuber MA, Saloner B, Cunningham CO, & Barry CL (2014). Medical cannabis laws and opioid analgesic overdose mortality in the United States, 1999-2010. JAMA Intern Med, 174(10), 1668–1673. doi: 10.1001/jamainternmed.2014.4005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee G, Edelman EJ, Barry DT, Crystal S, Gordon KS, Gordon AJ, … Marshall BDL. (2019). High-dose prescribed opioids are associated with increased risk of heroin use among United States military veterans. Pain, 160(9), 2126–2135. doi: 10.1097/j.pain.0000000000001606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao Y, Pan Y, Taylor A, Radakrishnan S, Luo F, Pincus HA, & Schackman BR (2016). Prescription Drug Monitoring Programs Are Associated With Sustained Reductions In Opioid Prescribing By Physicians. Health Aff (Millwood), 35(6), 1045–1051. doi: 10.1377/hlthaff.2015.1673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beletsky L (2018). Deploying Prescription Drug Monitoring to Address the Overdose Crisis: Ideology Meets Reality. Indiana Health Law Review, 15(2), 139–187. [Google Scholar]
- Bernardinelli L, Clayton D, Pascutto C, Montomoli C, Ghislandi M, & Songini M (1995). Bayesian analysis of space-time variation in disease risk. Stat Med, 14(21-22), 2433–2443. doi: 10.1002/sim.4780142112 [DOI] [PubMed] [Google Scholar]
- Besag J, York JC, & Mollie A (1991). Bayesian image restoration, with two applications to spatial statistcs (with discussion). Annals of the Institute of Statistical Mathematics, 43, 1–59. [Google Scholar]
- Blangiardo M, & Cameletti M (2015). Spatial and Spatial-Temporal Bayesian Models with R-INLA. Chichester, UK: Wiley. [Google Scholar]
- Bohnert AS, Valenstein M, Bair MJ, Ganoczy D, McCarthy JF, Ilgen MA, & Blow FC (2011). Association between opioid prescribing patterns and opioid overdose-related deaths. JAMA, 305(13), 1315–1321. doi: 10.1001/jama.2011.370 [DOI] [PubMed] [Google Scholar]
- Carlin BP, & Louis TA (2000). Bayes and Empirical Bayes Methods for Data Analysis, 2nd ed New York: Chapman & Hall. [Google Scholar]
- Carlson RG, Nahhas RW, Martins SS, & Daniulaityte R (2016). Predictors of transition to heroin use among initially non-opioid dependent illicit pharmaceutical opioid users: A natural history study. Drug Alcohol Depend, 160, 127–134. doi: 10.1016/j.drugalcdep.2015.12.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll JJ, Marshall BDL, Rich JD, & Green TC (2017). Exposure to fentanyl-contaminated heroin and overdose risk among illicit opioid users in Rhode Island: A mixed methods study. Int J Drug Policy, 46, 136–145. doi: 10.1016/j.drugpo.2017.05.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerda M, Gaidus A, Keyes KM, Ponicki W, Martins S, Galea S, & Gruenewald P (2017). Prescription opioid poisoning across urban and rural areas: identifying vulnerable groups and geographic areas. Addiction, 112(1), 103–112. doi: 10.1111/add.13543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerda M, Ponicki W, Smith N, Rivera-Aguirre A, Davis C, Marshall BD, … Martins SS, (In Press). Measuring relationships between proactive reporting state-level prescription drug monitoring programs and county-level fatal prescription opioid overdoses. Epidemiology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerda M, Santaella J, Marshall BDL, Kim JH, & Martins SS (2015). Nonmedical Prescription Opioid Use in Childhood and Early Adolescence Predicts Transitions to Heroin Use in Young Adulthood: A National Study. Journal of Pediatrics, 167(3), 605–612. doi: 10.1016/j.jpeds.2015.04.071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark T, Eadie JL, Kreiner P, & Strickler G (2012). Prescription Drug Monitoring Programs: An Assessment of the Evidence for Best Practices. Retrieved from https://www.pewtrusts.org/-/media/assets/0001/pdmp_update_1312013.pdf
- Clark WAV, & Avery KL (1976). The Effects of Data Aggregation in Statistical Analysis. Geographical Analysis, 8(4), 428–438. [Google Scholar]
- Compton WM, Jones CM, & Baldwin GT (2016). Relationship between Nonmedical Prescription-Opioid Use and Heroin Use. N Engl J Med, 374(2), 154–163. doi: 10.1056/NEJMra1508490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis CS, Pierce M, & Dasgupta N (2014). Evolution and convergence of state laws governing controlled substance prescription monitoring programs, 1998-2011. Am J Public Health, 104(8), 1389–1395. doi: 10.2105/AJPH.2014.301923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEA. (2015). National drug threat assessment summary 2014. Retrieved from
- Delcher C, Wang Y, Wagenaar AC, Goldberger BA, Cook RL, & Maldonado-Molina MM. (2016). Prescription and Illicit Opioid Deaths and the Prescription Drug Monitoring Program in Florida. Am J Public Health, 106(6), e10–11. doi: 10.2105/AJPH.2016.303104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deyo RA, Hallvik SE, Hildebran C, Marino M, Springer R, Irvine JM, … McCarty D (2018). Association of Prescription Drug Monitoring Program Use With Opioid Prescribing and Health Outcomes: A Comparison of Program Users and Nonusers. Journal of Pain, 19(2), 166–177. doi: 10.1016/j.jpain.2017.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowell D, Zhang K, Noonan RK, & Hockenberry JM (2016). Mandatory Provider Review And Pain Clinic Laws Reduce The Amounts Of Opioids Prescribed And Overdose Death Rates. Health Aff (Millwood), 35(10), 1876–1883. doi: 10.1377/hlthaff.2016.0448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan TE, Duncan SC, Alpert A, Hops H, Stoolmiller M, & Muthen B (1997). Latent Variable Modeling of Longitudinal and Multilevel Substance Use Data. Multivariate Behav Res, 32(3), 275–318. doi: 10.1207/s15327906mbr3203_3 [DOI] [PubMed] [Google Scholar]
- Fink DS, Schleimer JP, Sarvet A, Grover KK, Delcher C, Castillo-Carniglia A, … Cerda M. (2018). Association Between Prescription Drug Monitoring Programs and Nonfatal and Fatal Drug Overdoses: A Systematic Review. Ann Intern Med, 168(11), 783–790. doi: 10.7326/M17-3074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene W (2012). Econometric Analysis, 7th edition New York, NY: Prentice-Hall. [Google Scholar]
- Gugelmann H, Perrone J, & Nelson L (2012). Windmills and pill mills: can PDMPs tilt the prescription drug epidemic? J Med Toxicol, 8(4), 378–386. doi: 10.1007/s13181-012-0273-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haffajee RL (2019). Prescription Drug Monitoring Programs - Friend or Folly in Addressing the Opioid-Overdose Crisis? N Engl J Med, 381(8), 699–701. doi: 10.1056/NEJMp1904714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedegaard H, Warner M, & Minino A (2017). Drug Overdose Deaths in the United States, 1999-2015. Retrieved from https://www.cdc.gov/nchs/products/databriefs/db273.htm [PubMed]
- Jalal H, Buchanich JM, Roberts MS, Balmert LC, Zhang K, & Burke DS (2018). Changing dynamics of the drug overdose epidemic in the United States from 1979 through 2016. Science, 361(6408). doi: 10.1126/science.aau1184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones CM (2013). Heroin use and heroin use risk behaviors among nonmedical users of prescription opioid pain relievers - United States, 2002-2004 and 2008-2010. Drug Alcohol Depend, 132(1-2), 95–100. doi: 10.1016/j.drugalcdep.2013.01.007 [DOI] [PubMed] [Google Scholar]
- Kerridge BT, Saha TD, Chou SP, Zhang H, Jung J, Ruan WJ, … Hasin DS (2015). Gender and nonmedical prescription opioid use and DSM-5 nonmedical prescription opioid use disorder: Results from the National Epidemiologic Survey on Alcohol and Related Conditions - III. Drug Alcohol Depend, 156, 47–56. doi: 10.1016/j.drugalcdep.2015.08.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilby AA (2015). Opioids for the masses: Welfare tradeoffs in the regulation of narcotic pain medications. Cambridge, MA: Massachusetts Institute of Technology. [Google Scholar]
- Kolodny A, Courtwright DT, Hwang CS, Kreiner P, Eadie JL, Clark TW, & Alexander GC (2015). The prescription opioid and heroin crisis: a public health approach to an epidemic of addiction. Annu Rev Public Health, 36, 559–574. doi: 10.1146/annurevpublhealth-031914-122957 [DOI] [PubMed] [Google Scholar]
- Lanza ST, Dziak JJ, Huang L, Wagner A, & Collins LM (2015). PROC LCA & PROC LTA users’ guide (Version 1.3.2). University Park: The Methodology Center, Penn State. [Google Scholar]
- Lord D, Washington SP, & Ivan JN (2005). Poisson, Poisson-gamma and zero-inflated regression models of motor vehicle crashes: balancing statistical fit and theory. Accid Anal Prev, 37(1), 35–46. doi: 10.1016/j.aap.2004.02.004 [DOI] [PubMed] [Google Scholar]
- Mars SG, Bourgois P, Karandinos G, Montero F, & Ciccarone D (2014). “Every ‘never’ I ever said came true”: transitions from opioid pills to heroin injecting. Int J Drug Policy, 25(2), 257–266. doi: 10.1016/j.drugpo.2013.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mars SG, Fessel JN, Bourgois P, Montero F, Karandinos G, & Ciccarone D (2015). Heroin-related overdose: The unexplored influences of markets, marketing and source-types in the United States. Soc Sci Med, 140, 44–53. doi: 10.1016/j.socscimed.2015.06.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mars SG, Ondocsin J, & Ciccarone D (2018). Sold as Heroin: Perceptions and Use of an Evolving Drug in Baltimore, MD. J Psychoactive Drugs, 50(2), 167–176. doi: 10.1080/02791072.2017.1394508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins SS, Sarvet A, Santaella-Tenorio J, Saha T, Grant BF, & Hasin DS (2017). Changes in US Lifetime Heroin Use and Heroin Use Disorder: Prevalence From the 2001-2002 to 2012-2013 National Epidemiologic Survey on Alcohol and Related Conditions. JAMA Psychiatry, 74(5), 445–455. doi: 10.1001/jamapsychiatry.2017.0113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins SS, Segura LE, Santaella-Tenorio J, Perlmutter A, Fenton MC, Cerda M, … Hasin DS. (2017). Prescription opioid use disorder and heroin use among 12-34 year-olds in the United States from 2002 to 2014. Addict Behav, 65, 236–241. doi: 10.1016/j.addbeh.2016.08.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClellan C, Lambdin BH, Ali MM, Mutter R, Davis CS, Wheeler E, … Kral AH. (2018). Opioid-overdose laws association with opioid use and overdose mortality. Addict Behav, 86, 90–95. doi: 10.1016/j.addbeh.2018.03.014 [DOI] [PubMed] [Google Scholar]
- Meinhofer A (2018). Prescription Drug Monitoring Programs: The Role of Asymmetric Information on Drug Availability and Abuse. American Journal of Health Economics, 4(4), 504–00000. doi: 10.1162/ajhe_a_00101 [DOI] [Google Scholar]
- Muthén B (2004). Latent Variable Analysis: Growth Mixture Modeling and Related Techniques for Longitudinal Data In Kaplan D (Ed.), The SAGE Handbook of Quantitative Methodology for the Social Sciences (pp. 346–369). Newbury Park, CA: Sage. [Google Scholar]
- Muthen B, & Muthen LK (2000). Integrating person-centered and variable-centered analyses: growth mixture modeling with latent trajectory classes. Alcohol Clin Exp Res, 24(6), 882–891. [PubMed] [Google Scholar]
- Nam YH, Shea DG, Shi Y, & Moran JR (2017). State prescription drug monitoring programs and fatal drug overdoses. Am J Manag Care, 23(5), 297–303. [PubMed] [Google Scholar]
- NAMSDL. (2018). Prescription Drug Monitoring Program (PDMP/PMP) Basics. Retrieved from Harrisburg, PA: http://namsdl.dynamicwebware.com/News%20Tab/Highlighted%20Issues/PDMP%201-Pager%20(FINAL).pdf [Google Scholar]
- NIDA. (2014). Epidemiologic trends in drug abuse: proceedings of the Community Epidemiology Work Group, highlights and executive summary, January 2014. Retrieved from Bethesda, MD: [Google Scholar]
- NIDA. (2018, August 2018). Overdose Death Rates. Trends and Statistics. Retrieved from https://www.drugabuse.gov/related-topics/trends-statistics/overdose-death-rates
- ONDCP. (2014). National drug control strategy: data supplement 2014. Retrieved from Washington, D.C: https://obamawhitehouse.archives.gov/sites/default/files/ondcp/policy-and-research/ndcs_data_supplement_2014.pdf [Google Scholar]
- Paulozzi LJ, Kilbourne EM, & Desai HA (2011). Prescription drug monitoring programs and death rates from drug overdose. Pain Med, 12(5), 747–754. doi: 10.1111/j.1526-4637.2011.01062.x [DOI] [PubMed] [Google Scholar]
- Pauly NJ, Slavova S, Delcher C, Freeman PR, & Talbert J (2018). Features of prescription drug monitoring programs associated with reduced rates of prescription opioid-related poisonings. Drug Alcohol Depend, 184, 26–32. doi: 10.1016/j.drugalcdep.2017.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pew Charitable Trusts. (2016). Prescription Drug Monitoring Programs: Evidence-based practices to optimize prescriber use. Retrieved from http://www.pewtrusts.org/~/media/assets/2016/12/prescription_drug_monitoring_programs.pdf
- PROC LCA & PROC LTA (Version 1.3.2) (2015). University Park: The Methodology Center, Penn State. [Google Scholar]
- Radakrishnan S (2015). Essays In The Economics Of Risky Health Behaviors. (Ph.D. of Economics), Cornell University, Ithaca, NY. [Google Scholar]
- Rees DI, Sabia J, Argys L, Latshaw J, & Dave D (2017). With a Little Help from My Friends: The Effects of Naloxone Access and Good Samaritan Laws on Opioid-Related Deaths. NBER Working Paper No. 23171. [Google Scholar]
- Rudd RA, Aleshire N, Zibbell JE, & Gladden RM (2016). Increases in Drug and Opioid Overdose Deaths--United States, 2000-2014. MMWR Morb Mortal Wkly Rep, 64(50-51), 1378–1382. doi: 10.15585/mmwr.mm6450a3 [DOI] [PubMed] [Google Scholar]
- Rue H, Martino S, & Chopin N (2009). Approximate Bayesian inference for latent Gaussian models using integrated nested Laplace approximations (with discussion). Journal of the Statistical Society, Series B, 71(2), 319–392. [Google Scholar]
- Ruhm CJ (2017). Geographic Variation in Opioid and Heroin Involved Drug Poisoning Mortality Rates. Am J Prev Med, 53(6), 745–753. doi: 10.1016/j.amepre.2017.06.009 [DOI] [PubMed] [Google Scholar]
- Shi Y (2017). Medical marijuana policies and hospitalizations related to marijuana and opioid pain reliever. Drug Alcohol Depend, 173, 144–150. doi: 10.1016/j.drugalcdep.2017.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith N, Martins SS, Kim J, Rivera-Aguirre A, Fink DS, Castillo-Carniglia A, … Cerda M. (2019). A typology of prescription drug monitoring programs: a latent transition analysis of the evolution of programs from 1999 to 2016. Addiction, 114(2), 248–258. doi: 10.1111/add.14440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Census Bureau. (2019a). Substantial Changes to Counties and County Equivalent Entities: 1970-Present. 2000 Retrieved from https://www.census.gov/programs-surveys/geography/technical-documentation/county-changes.2000.html
- U.S. Census Bureau. (2019b). Substantial Changes to Counties and County Equivalent Entities: 1970-Present. 2010 Retrieved from https://www.census.gov/programs-surveys/geography/technical-documentation/country-changes.2010.html
- Unick G, Rosenblum D, Mars S, & Ciccarone D (2014). The relationship between US heroin market dynamics and heroin-related overdose, 1992-2008. Addiction, 109(11), 1889–1898. doi: 10.1111/add.12664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waller L, & Gotway C (2004). Applied Spatial Statistics for Public Health Data: Wiley Publishers. [Google Scholar]
- Yuanhong Lai A, Smith KC, Vernick JS, Davis CS, Caleb Alexander G, & Rutkow L (2019). Perceived Unintended Consequences of Prescription Drug Monitoring Programs. Subst Use Misuse, 54(2), 345–349. doi: 10.1080/10826084.2018.1491052 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.