Abstract
Central nervous system (CNS) diseases are rapidly increasing globally. Currently used therapeutic agents to treat CNS diseases exhibit significant efficacy. However, the inability of these drugs to cross the blood–brain barrier (BBB) and invasiveness of the technologies to achieve localized drug delivery in disease-specific parts of the brain have thwarted pain-free and complete treatment of CNS diseases. Therefore, the safe, non-invasive, and targeted delivery of drugs to the brain using nanoparticles (NPs) is currently receiving considerable research attention. Here, we highlight advances in state-of-the-art personalized nanomedicine for the treatment of CNS diseases (with a focus on dementia), the related challenges, possible solutions, and prospects for nano-enabled personalized medicine.
CNS diseases: therapeutics challenges and solutions
Healthcare statistics confirm that the incidence of CNS diseases, including neuroinfections and neurocognitive disorders, is rapidly increasing across the globe [1–5], with a 6–8% global economic burdon resulting from neurological disorders [4]. The associated healthcare costs of these diseases (~US$600 billion–700 billion based on 2010 reports) are high, adding to their socioeconomic burden [5]. Advances in current medical therapies have had a significant role in the treatment and management of CNS diseases, resulting in increased survival rates, but complete cures are lacking for most CNS diseases, including Alzheimer’s disease (AD), Parkinson’s disease (PD), stroke, epilepsy, and neuroAIDS [1,6–8]. The BBB and its selective transport of drugs to the brain are the main hurdles to improving the efficacy of therapeutic agents. Other obstacles to treatment include the invasiveness of some medical therapies, such as deep brain stimulation (DBS) and injecting medication directly into the brain, and the expensive diagnostic and prognostic-related infrastructure.
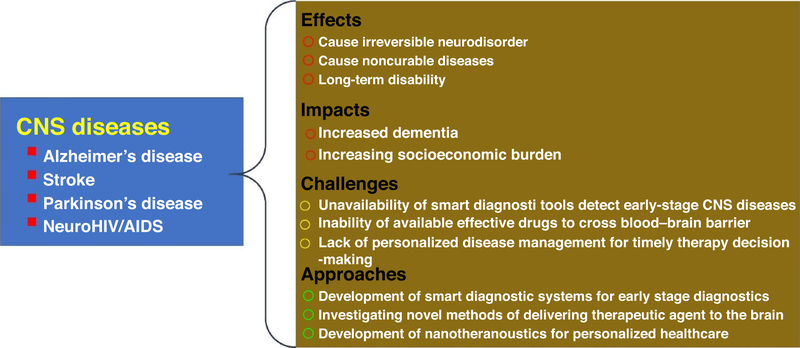
These challenges have motivated researchers from a variety of fields (e.g., medicinal chemistry, pharmacology, genetic engineering, etc.) to collaborate over the development of better strategies for the formulation of new drugs and drug delivery approaches with improved efficacy for the personalized treatment of CNS diseases. To overcome the issue of BBB transport and other physicochemical limitations of conventional drug delivery systems, nanocarriers (NCs) have been investigated as drug delivery vehicles for CNS therapeutics. NPs (100 nm or less) can be used and engineered to carry multiple therapeutic agents and imaging modalities for treatment and monitoring therapy. For example, magnetoelectric NPs (MENPs) can be used to achieve dual functions (non-invasive drug transport across the BBB and on-demand drug release without any thermal adverse effects) that are not otherwise technically possible with conventional magnetic NPs (MNPs). Applications of MENPs have been reported for the treatment of neuroAIDS and AD [1,6,7,9]. The amount of drug(s) needed to achieve the desired therapeutics effect varies among patients. Therefore, achieving the desired level of drug release (i.e., on-demand) for a particular patient would allow physicians to fine tune the therapeutic dose, resulting in personalized medicine [10–21]. Furthermore, on integration with longitudinal monitoring techniques to help neurosurgeons understand how well a patient is responding to treatment, remote control over the release of multiple drugs would allow physicians to individualize treatment protocols during each therapy session. The effects and impacts of CNS diseases, along with related challenges and possible approaches for their management or treatment, are illustrated in Fig. 1.
FIGURE 1.
Illustration of central nervous system (CNS) diseases: related impact, effects, challenges, and adopted approaches.
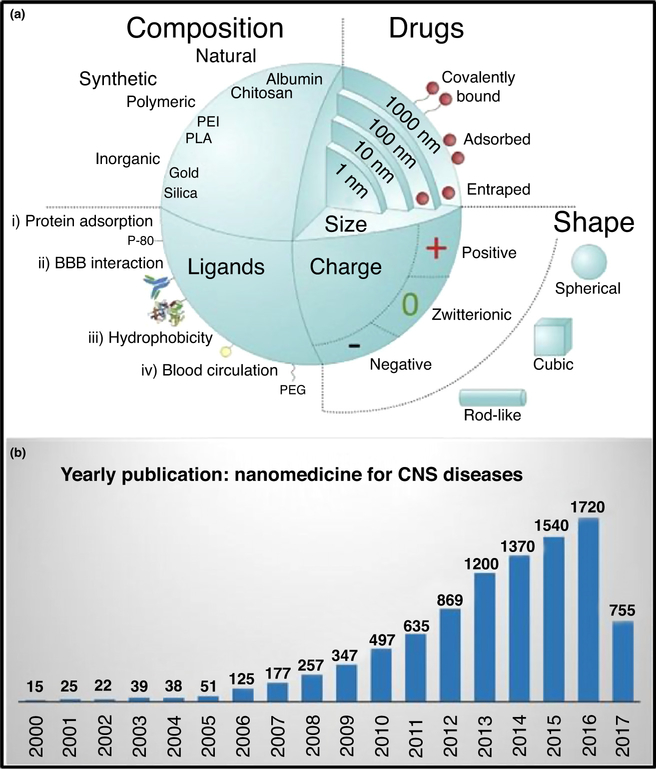
Figure 2a maps the advantages that NPs offer for drug delivery systems, including a high surface area (higher drug loading); range of biomaterial options; organic (natural or synthetic polymers) or inorganic (e.g., metals for photonanomedicine); presence of ionic surface charge (aids drug binding and other functionalizations; e.g., PEGylation); target specificity via ligand binding (e.g., antibodies tagging), and so on. Additionally, bioactive NPs have unique physicochemical properties; for example, MNPs and MENPs allow drug transport across the BBB on application of an external magnetic field. Transport across the BBB for drug delivery to the CNS is optimal with NPs that are 100–150 nm in size [7]. This approach is known as ‘nanotherapeutics’ because of the involvement of nanobiotechnology in therapeutic applications [6,21,22]. The research interest in nanotherapeutics for use against CNS diseases has grown continuously over the past decade, as evidenced by the increasing number of scientific publications year on year (Fig. 2b).
FIGURE 2.
Illustration of (a) nanomedicine formulations and (b) yearly publication rate of articles on nanomedicine from 2000 to June 2017. Reproduced from Ref. [69] (a) and based on data from Google Scholar (b). Abbreviations: BBB, blood–brain barrier; CNS, central nervous system.
Various types of NP (i.e., NCs, such as gold, silica, hydrogels, liposome, MNPs, MENPs, etc.) have been explored for CNS drug delivery applications [1,21]. However, formulating a nanomedicine with higher efficacy relies on the salient features of the selected NC (Fig. 2a). For example, the surface charge properties of NPs can be altered to achieve higher loading and sustained drug release. Electrostatically driven layer-by-layer assembly (LBL) of biopolymers and drugs on NPs have been explored for neuroHIV treatment. Electroactive polymer-based NFs that are responsive to pH and temperature can also be used for sustained or controlled drug delivery. As mentioned above, MENP-based NFs are a good choice for the non-invasive and image-guided personalized therapy of CNS diseases. This is because of their unique magneto-electric-actuation effect, which allows on-demand controlled release of drugs and longitudinal non-invasive monitoring of therapy using magnetic resonance imaging (MRI) [23]. Liposome-based NFs have shown potential because of their easy surface modification, facilitation of loading with hydrophilic and/or hydrophobic drugs, and the sustained release of those drugs once they reach the brain. Plasmonic NC-based NFs are recommended for targeted delivery and light-responsive drug release. Similarly, along with developing NFs of higher efficacy with no adverse effects, the investigation and selection of safe brain delivery methods will be crucial. The delivery of drug to the brain based on NF functionalization using proteins has been demonstrated, although challenged by the limited delivery because of the larger final size of the NFs. To overcome these, size-related issues, researchers have focused on opening the BBB by applying external stimuli. Focused ultrasound (FUS), ultrasound-assisted microbubble approaches, and external electromagnetic field-based methodologies have been used to temporarily open the BBB to deliver therapeutic agents to the brain. These methods could deliver a larger-sized NF or a higher therapeutic dose to the brain. However, the transient opening of the BBB limits their long-term application for use in the clinic [24] because of the risk of delivering unsolicited agents to the brain, with adverse effects [24]. Thus, developing a safe and non-invasive brain delivery method is a priority. Using a mouse model, Nair et al. reported the magnetically guided delivery of antiHIV drugs to the brain across the BBB to be safe and non-invasive, with no toxic or neurobehavioral alterations [1,6,24] T. However, most of the methods and approaches discussed above are still in preclinical stages of development and need further optimization before they can be used in the clinical setting.
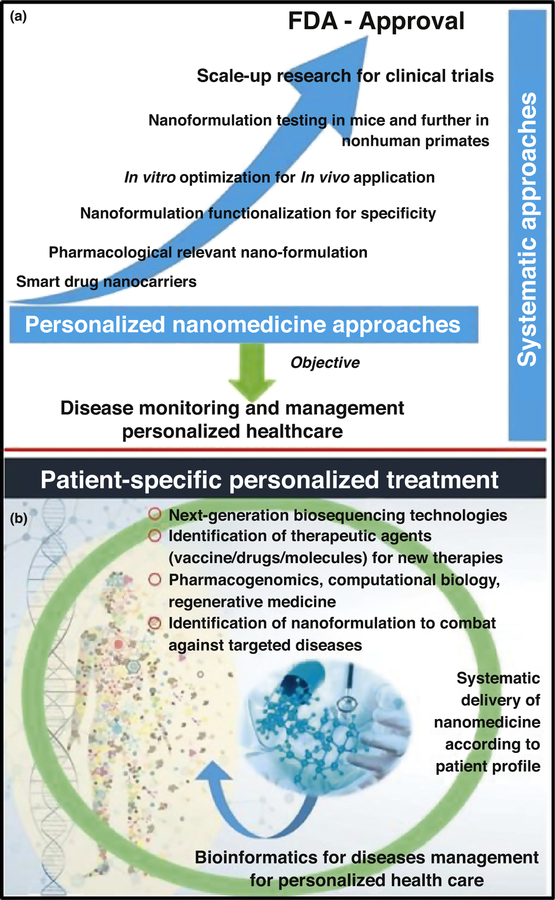
The US Food and Drug Administration (FDA) and National Institute of Health (NIH) are building a roadmap for personalized medicine that will revolutionize healthcare [25]. Thus, the successful preclinical use of CNS therapeutics as personalized nanomedicines [11–17,21,26] could lead to FDA approval and, therefore, would be more likely to be used in a clinical setting (Fig. 3a). Based on the disease profile of a patient, the design of personalized nanomedicine can be used to understand a disease pattern and generate bioinformatics to manage the disease via timely therapeutic decisions (Fig. 3b). The state of the art of nanomedicine for the treatment of CNS diseases is discussed below, with a focus on some of the most prevalent CNS diseases, including AD, stroke, PD, and HIV-associated neuroinfection in the brain leading to neuroAIDS.
FIGURE 3.
Systematic approach over time with the ultimate objective of personalized healthcare (a), and illustration of patient profile-specific personalized treatment approaches (b).
Nanomedicine for Alzheimer’s disease
AD is one of the most common neurological disorders among the older population, with a socioeconomic burden of more than US $600 billion [27]. Reports suggest that at least 17 million people worldwide are currently diagnosed with AD, and this figure is increasing, especially in the aging population; estimates suggest that there will be a threefold increase in patients with AD by 2050 [27]. AD is a brain disorder that results from the limited capacity of the brain to repair neurons, leading to memory loss, mood variations, depression, delusions, and anxiety [27]. There is currently a lack of AD-specific therapeutic agents and, therefore, it is impossible to cure, and difficult to manage, this disease. Efforts have been made to develop smart diagnostic sensing systems to monitor AD progression under therapy [27,28]. In addition to pharmacotherapy, DBS is also used in clinical practice to remove the amyloid-beta (Aβ) plaques characteristic of this disease. However, such an approach is not widely adopted in the clinic because of the invasiveness of this approach, as well as the expense and associated length of time needed for treatment. Currently, pharmacotherapies are used in the clinic include cholinesterase inhibitors, recombinant monoclonal antibodies, and neuroprotecting agents.
Various NCs have been investigated for the controlled and site-specific delivery of drugs for the treatment of AD. For example, carbon nanotubes (CNTs) have been used to achieve neuroregeneration and neuroprotection in the treatment of AD; however, the success rate of in vivo studies has been low, hindering the wide acceptance of these CNTs as NCs [29].
Other biocompatible NCs have also been investigated, such as chitosan-coated poly lactic-co-glycolic acid (PLGA) immuno-NPs (200 nm) for the delivery of a novel anti-Aβ antibody [30]. Such NFs need to be retained in the cerebral vasculature without entering the brain parenchyma. Chitosan has been used to provide colloidal stability, biocompatibility, and high levels of binding to immunoglobulin (Ig)-G for the selective targeting of intracellular Aβ antigens [30]. However, this NF-based approach still needs to be tested in animal models to provide preclinical proof of concept.
Curcumin, a water-insoluble natural compound with significant antioxidant properties, has been used as a potential anti-amyloid compound. Recently, various efforts have been made to deliver this compound to the brain using nanotechnology approaches for AD treatment. For example, Mathew et al. developed curcumin-loaded NPs conjugated with a Tet-1 peptide-based NF for the treatment of AD and showed that the NF crossed the BBB and was able to achieve good efficacy in reducing the amyloid aggregates because of its antioxidative properties [31]. Similarly, Lazar et al. developed and tested a liposome-based NF conjugated with fluorescent curcumin for the treatment of AD using brain tissue collected from patients with AD postmortem and APPxPS1 mice [32]. The authors stated that further efforts are required to functionalize this NF to naturally cross the BBB with high permeability [32]. In addition, Tiwari et al. explored curcumin encapsulated in PLGA-NPs capable of inducing neural stem cell (NSC) proliferation and neuronal differentiation via neurogenesis induced by targeting endogenous NSC. In vivo data showed that this approach significantly influenced the self-regenerative capacity of the brain and, thus, could be used as be a potential therapeutic approach to treat AD [33]. MNPs bound a curcumin NF (100 nm) were explored for the non-invasive therapeutic monitoring of AD using MRI [34]. In vivo (Tg2576 mouse) and MRI imaging (T2* weighted) was used to monitor the effects of this NF on Aβ plaques.
Neuroinflammation affects AD progression and, thus, its regulation is crucial for managing AD-related risks. Bernardi et al. developed an indomethacin-loaded lipid-core nanocapsule (IndOH-LNC) to control Aβ1–42-induced cell damage and neuroinflammation in hippocampal cultures [35]. The results suggested that this NF controlled motor coordination function and managed neuroinflammation [35]. Elnaggar et al. delivered piperine (PIP), a natural alkaloid that enhances memory, to the brain using Tween-integrated monoolein cubosomes (T-cubs) as bioactive drug NCs [36]. The T-cubs enhanced PIP cognitive function in mice, and exhibited anti-inflammatory and antiapoptosis activity, highlighting this NF as a potential therapeutic for AD management [36]. Multifunctional liposomes functionalized with two peptides (the apolipoprotein-E receptor for facilitating transmigration across the BBB and phosphatidic acid for amyloid binding) were designed to reduce brain amyloids and improve memory [37]. This bifunctional liposome was administrated in APP/presenilin-1 transgenic mice and successfully decreased total brain-insoluble amyloid1–42 by 33%. This NF also improved memory in these mice and, therefore, is a potential therapy for AD [37,38]. Another approach has been the development of smart nanoprobes for drug delivery and monitoring using imaging [38,39]. However, this strategy has off-target issues and unnecessary heat generation, resulting in neuroinflammation.
Nanomedicine for stroke
Strokes kills approximately 140 000 patients in the USA annually, equivalent to one out of every 20 deaths (www.cdc.gov/stroke/facts.htm). The main etiology of strokes is the blockage or rupture of blood vessels to the brain caused by a clot [40]. Preclinical studies have shown promising results using neuroprotective agents but have failed at the clinical trial stage because of safety concerns or low efficacy. Nanomedicine approaches have been investigated in combination with stem cell transplantation to repair injured areas [41,42]. Stroke treatment using nanomedicine has been successfully demonstrated in the brain [43]. NP approaches help to overcome issues associated current pharmacotherapies (e.g., tissue plasminogen activators), such as a short therapeutic window, selective efficacy, and hemorrhagic concerns [41–45]. Along with pharmacotherapy, stimuli-response approaches (e.g., ultrasound), have also been used to treat patients with ischemic stroke [46]. However, issues related to targeted stimulation, the target-specific brain delivery of NPs, and the design of safe and biocompatible NPs for the treatment of stroke remain challenging. Multiple approaches that have been used to develop nanomedicine-based approaches for the treatment of stroke are discussed below.
Amine-modified single-walled CNTs (SWCNTs) targeting brain tissue resulting in the improved recovery of motor coordination function in a stroke-induced rat model [40]. Higgins et al. showed that stroke-induced animals treated with NH2-CNTs exhibited significantly improved motor coordination and less neurological damage [47]. Moon et al. explored the ability of hydrophilic/hydrophobic CNTs to repair stroke-induced neural progenitor cells (SVZ NPCs) [48]. Outcomes of this research suggested that transplanted CNTs accumulated around the ischemic injury and were successful in improving stem cell differentiation to repair stroke-induced damage [48]. A simvastatin-loaded liposome was also developed as a therapeutic formulation to treat stroke [49]. Middle cerebral arterial occlusion (MCAOt) surgery and study of the intravenously administrated liposome (after 90 min) in the animals confirmed that the positively charged liposome exhibited good biodistribution. However, the therapeutic performance of this formulation needs to be explored further. Recently, mesenchymal stem cells (MSCs) were reported as a potential therapy to treat ischemic stroke, but their systematic targeted delivery remains challenging [50]. MSCs labeled with dextran-coated MNPs were distributed in the brain to areas of increased cerebral lesion risk, and showed improved functional recovery. Although the intravenous administration routes were safe, the amount of MSCs that crossed the BBB was limited [50].
Nanomedicine for Parkinson’s disease
PD is the second-most common age-related neurodegenerative disorder. This disease affects the motor or cognitive abilities of an individual because of the loss of dopaminergic neurons in the midbrain [51]. The lack of vaccines, drugs, or therapies against PD make this disorder challenging to manage. Over the past two decades, high-frequency DBS of the subthalamic nucleus has been developed and is now used in clinical practice to treat PD. However, the off-target effects and generation of additional neurocognitive effects caused by DBS are major issues [52]. To make DBS more effective, NPs were used for DBS applications. A simulation study confirmed that external ac-magnetic field stimulation on MENPs would produce an electric field capable for DBS to cure PD without any adverse effects [53]. However, this method is yet to be tested in an animal model. NP-based advances in the management of PD are discussed below.
Linder et al. developed an NF comprising resveratrol (RVT)-loaded polysorbate 80 (PS80)-coated poly(lactide)-NPs to improve neuroprotection in PD-C57BL/6 mice [54]. Neuroprotection was evaluated by studying the neurochemical and neurobehavioral characteristics of the mice. These RVT-loaded poly(lactide)-NPs exhibited significant therapeutic effects against PD [54]. A controlled release system was developed by Fernandez et al. for the delivery of rasagiline mesylate (RM) for the treatment of PD and tested using a rotenone-induced rat model [55]. The PLGA-RM microspheres, administrated via an intraperitoneal route, showed a significant reversal in catalepsy, akinesia, and swim tests conducted in rotenone-treated animals. Results confirmed the improved therapeutic efficacy of PLGA-RM compared with RM alone, suggesting the potential of this formulation to treat PD [55].
Haney et al. explored exosomes for the delivery of catalase, an antioxidant, for the treatment of symptoms of PD [56]. As natural lipid bilayer proteins, exosomes were selected as potential drug carriers because of their ability to interact with cellular membranes and to avoid uptake by mononuclear phagocytic immune cells. This approach exhibited significant neuroprotective effects in a mouse model [56]. Given the importance of delivering an antioxidant therapeutic agent to treat dopaminergic neurons, Zhao et al. developed the macrophage-assisted active delivery of glial cell line-derived neurotropic factor (GDNF) to the brains of PD mice [57]. The motor coordination-related study outcomes suggested that the release of GDNF via exosome-targeted neurons resulted in desired therapeutic effects [57]. Herran et al. developed poly (lactic-co-glycolic acid) nanosphere-loaded GDNF and vascular endothelial growth factor (VEGF) to explore the role of growth factors in the treatment of PD using a neuroprotection approach [58]. The in vivo studies confirmed that the growth factors released reduced amphetamine-induced rotations and enhancement in neurons. Therefore, these NFs promote a neuroreparative approach to treat PD [58].
Another nanotechnology-based approach [PEGylated immunoliposomes (PILs, 85 nm)] was explored for targeting the brain via gene-targeting technology to reverse the activity of tyrosine hydroxylase (TH) in the striatum 6-hydroxydopamine rat model of PD [59]. The receptor-mediated transcytosis- and endocytosis-based brain delivery of TfRMAb-PIL was demonstrated in the rats. The results of in vivo studies confirmed that the entire striatum of PD rats was immunoreactive for TH after intravenous gene therapy. The neurobehavioral performance of the treated rats was also improved significantly [59]. Resveratrol, a natural antioxidant, was encapsulated in a nanoemulsion by Pangeni et al. for delivery to the brain as a management approach to PD [60]. The NF (102 nm) prepared using a nanoemulsion and resveratrol (150 mg/ml) exhibited cumulative percentage drug release of 85.48 ± 1.34% within 24 h. The outcomes of the studies confirmed that the resveratrol released from the nanoemulsion reversed PD-induced degenerative changes [60]. Lopez et al. explored silica (tetraethyl orthosilicate)-dopamine NFs, prepared using a sol-gel method, as a reservoir device for the controlled release of dopamine in the striatum of rats with apomorphine-induced PD [61]. The results confirmed that the implanted device reversed the rotational asymmetry in the rats. However, effects on dyskinesias and other motor abnormalities were not observed in [61].
Overall, the above-mentioned studies have reported the significant therapeutic performance of various nanomedicine approaches; however, these methodologies need extensive preclinical studies in smaller and larger animal models, followed by clinical trials in humans, before they can be accepted for clinical use.
Challenges and alternative approaches
Significant efforts are currently underway to explore personalized nanomedicine approaches to treat or manage CNS diseases. However, many of the developments are still in the initial stages and require more-detailed preclinical testing in a range of animal models. Safety, efficacy, and regulatory issues are major challenges for the progression of personalized nanomedicine to treat CNS diseases in the clinic. The methods used to deliver drugs to the brain have been optimized by opening the BBB non-invasively on applying external stimulation (e.g., ultrasound, electromagnetic fields, etc.), but are known to result in various neurobehavioral or other related adverse effects [24]. Additionally, these stimulations can also change the intrinsic properties of the NCs, resulting in heat or changes in their surface properties. External stimulations are also known to affect the cell membrane potential, which can impact the efficacy of the therapeutics and cause off-target effects. Thus, the optimization of all operational parameters related to the brain delivery of therapeutics is essential [62]. The brain delivery methods selected for targeting CNS diseases must be completely optimized with respect to their safety, non-invasiveness, and biocompatibility. Among the brain delivery methods tested thus far, magnetically guided brain delivery methods (using MRI-assisted static magnetic fields) have shown promise in delivering NFs across the BBB, although have yet to be tested in larger animal models.
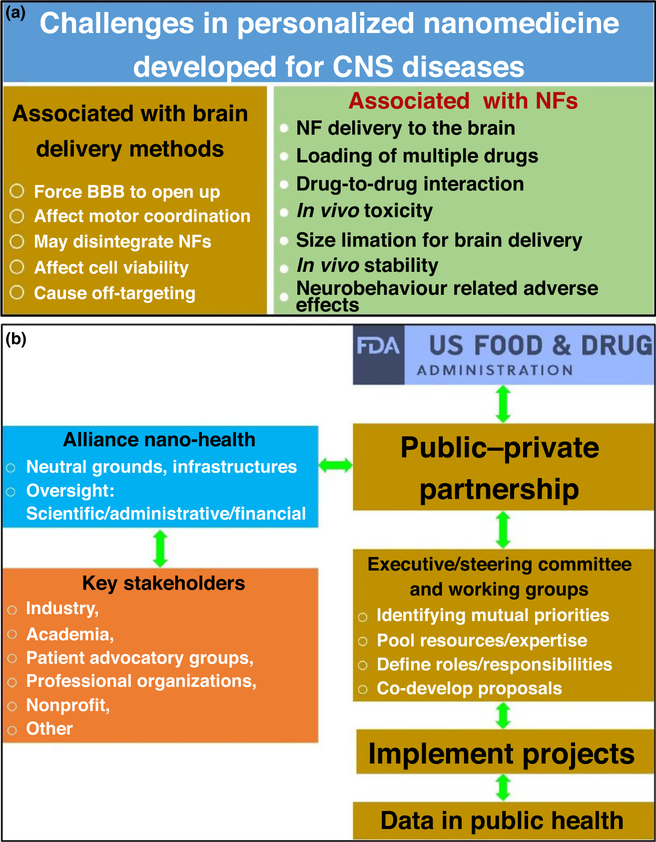
In addition to developing safe and non-invasive brain delivery methods, formulating nanomedicines with desired properties is also crucial, yet challenging (Fig. 4a). In combination with appropriate brain delivery methods, personalized nanomedicines have been designed to exhibit maximum efficacy with minimum possible adverse effects during the treatment of targeted CNS diseases. Thus, in 2006, the FDA declared this approach to be on the Critical Path Opportunities List [63]. The aim of this list was to protect and promote public health by developing new analytical tools to explore the safety and efficacy of biomedical devices and therapies. Sanhai et al. summarized the challenges highlighted by the FDA in relation to future nanomedicines as: (i) the determination and distribution of NCs in the body after systematic administration; (ii) ability to carry multiple drugs along with imaging payloads; (iii) mass transport across the BBB or compartmental boundaries; (iv) novel mathematical and computational models to categorize NCs and NFs according to risk assessment and benefit predictions; (vi) establishment of standards to evaluate properties of newly developed nanomedicines; and (vii) availability of analytical tool kits and facts sheets for nanopharmaceuticals to highlight their safety, procedures, and validation methods [64]. To address these challenges, experts believe that public–private partnerships involving the FDA and stakeholders would be one of the most-suitable approaches (Fig. 4b).
FIGURE 4.
Illustration of challenges in personalized nanomedicine developed to target central nervous system (CNS) diseases (a), and schematic presentation of public–private partnership involving key stakeholders and US Food and Drug Administration (FDA) to promote and protect public health (b). Reproduced from Ref. [64]. Abbreviations: BBB, blood–brain barrier; NF, nanoformulation.
The challenges to personalized nanomedicine can be managed by exploring nanobioengineering [65]. Biocompatibility in most of the NCs available is generated by the adoption of novel synthesis methods and surface functionalization. Thus, biopolymers, such as hydrogels and liposome-based NFs, are popular. Such NCs have been further modified using hybrid systems, such as organic-inorganic hybrid nanocomposite systems based on host-guest chemistry to achieve stability and labeling to trace the biodistribution of the nanomedicine in the body. The latter can be understood by tagging or developing light-responsive systems followed by monitoring using sensitive imaging systems. To make NFs more efficient, cocktails or multidrug payload-based nanomedicines are under development. At the laboratory level, such NFs were able to eradicate the targeted CNS diseases and other associated disorders. In addition, continuous efforts are being made to develop NCs that allow the sustained release of drug for longer time periods. Such NC-based nanomedicines would serve as longer-acting therapeutic agents to treat CNS diseases more efficiently [6]. Enabling nanomedicines to avoid barriers such as endosomal entrapment, enzymatic degradation, efflux pump, off-targeting, and so on, is possible by adopting appropriate surface functionalization, preservation of therapeutic agents, selecting the most-suitable strategy, and minimizing external stimulation [1,6].
Optimized nanomedicines have been administrated through various routes and their efficacy has been assessed using advanced assays and analytical methods [66]. The outcomes suggest that the efficient delivery and release of nanomedicine is crucial and also increase the demand for novel brain delivery methods [66]. Maintaining the appropriate level of therapeutics in the brain for a longer duration remains a challenge. A significant amount of nanomedicine can be delivered to the brain using stimulation-based approaches, but there are associated adverse effects. The operational parameters of FUS-based brain delivery methods were optimized and demonstrated in higher animals. However, magnetic NPs stand out from all other NCs as the delivery method of choice because of their multifunctional properties, including non-invasive and rapid drug delivery across the BBB, and MRI-guided longitudinal monitoring of therapy [67]. In addition to these magnetic properties, the approach of Nair et al., based on MENPs and magneto-electric-actuation, can result in the on-demand remote-controlled release of the drug and/or therapeutics agents, which could be an important step towards individualized treatment doses and protocols [24].
Besides the need to complete evaluations of risk assessments for nanomedicines on human health, another critical aspect is the effects of such advanced nanomedicine on the environment because of bioecotoxicity effects, their bioaccumulation, and environmental transformation [68]. Even though the exposure of nanomedicine to the environment is minimal, it should not be ignored [68]. The nanomedicine fabrication process in laboratories result in the mixing of drug residues that are then released into the environment, which could be reasons for serious health concerns. Environmentalists suggest that a well-documented risk assessment and regulatory systems for evaluating the effects of nanomedicine on the environment should be developed. The USA, via the FDA, has taken significant steps toward this by declaring an environmental assessment necessary before approval of a new drug [68]. Agencies have suggested environmental assessment at ppb level in the presence of nanoengineered particles and NFs. Therefore, there is a need to protect the environment by using highly sensitive analytical tools to detect the lowest possible amount of NPs and developing new training approaches along with safety procedures to reduce or prevent the interactions of nanomedicines with the environment [68]. Health agencies and regulatory bodies are making efforts to introduce such recommendations in the near future.
Concluding remarks
Here, we have discussed state-of-the-art efficient and targeted drug delivery across the BBB using nanotechnologies. In addition, we have focused on sustained or controlled ‘on-demand’ drug release in the brain, and the related challenges and solutions. We have also highlighted the prospects for nanotechnology in the development of treatment protocols according to individual needs (i.e., personalized nanomedicine). Based on recent results, magnetic NFs could herald the transformation of generalized medicine practice into personalized medicine, which appears to be best suited for CNS diseases. This approach will allow non-invasive drug delivery to the brain, remote control over drug release (therapeutic dose), and the simultaneous longitudinal monitoring of therapy using MRI.
Acknowledgments
We acknowledge funding from NIH grantsRO1DA042706, R01DA040537, RO1DA037838, and RO1DA034547. We also thank the Institute of NeuroImmune Pharmacology (INIP) and Advanced Materials Engineering Research Institute (AMERI) of Florida International University for support.
References
- 1.Nair M et al. (2016) Getting into the brain: potential of nanotechnology in the management of NeuroAIDS. Adv. Drug Deliv. Rev 103, 202–217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Albert SM (2007) Projecting neurologic disease burden: difficult but critical. Neurology 68, 322–323 [DOI] [PubMed] [Google Scholar]
- 3.Brew BJ and González-Scarano F (2007) HIV-associated dementia: an inconvenient truth. Neurology 68, 324–325 [DOI] [PubMed] [Google Scholar]
- 4.Hirtz D et al. (2007) How common are the ‘common’ neurologic disorders? Neurology 68, 326–337 [DOI] [PubMed] [Google Scholar]
- 5.WHO (2006) Neurological Disorders: Public Health Challenges. WHO [Google Scholar]
- 6.Kaushik A et al. (2016) Advancements in nano-enabled therapeutics for neuroHIV management. Int. J. Nanomed 11, 4317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaushik A et al. (2014) The potential of magneto-electric nanocarriers for drug delivery. Expert Opin. Drug Deliv. 11, 1635–1646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ruiz A et al. (2015) Recent update in NanoCure of NeuroAIDS. Sci. Lett. J 4, 172 [Google Scholar]
- 9.Nair M et al. (2013) Externally controlled on-demand release of anti-HIV drug using magneto-electric nanoparticles as carriers. Nat. Commun 4, 1707. [DOI] [PubMed] [Google Scholar]
- 10.Imaoka T et al. (1998) Significant behavioral recovery in Parkinson’s disease model by direct intracerebral gene transfer using continuous injection of a plasmid DNA–liposome complex. Hum. Gene Ther. 9, 1093–1102 [DOI] [PubMed] [Google Scholar]
- 11.Jain K (2009) Role of nanobiotechnology in the development of personalized medicine. Nanomedicine 4, 249–252 [DOI] [PubMed] [Google Scholar]
- 12.Jain KK (2008) The Handbook of Nanomedicine. Springer [Google Scholar]
- 13.Jain KK (2017) The Handbook of Nanomedicine (3rd edn.), Springer [Google Scholar]
- 14.Lammers T et al. (2012) Personalized nanomedicine. Clin. Cancer Res. 18, 4889–4894 [DOI] [PubMed] [Google Scholar]
- 15.Mura S and Couvreur P (2012) Nanotheranostics for personalized medicine. Adv. Drug Deliv. Rev 64, 1394–1416 [DOI] [PubMed] [Google Scholar]
- 16.Theek B et al. (2014) The theranostic path to personalized nanomedicine. Clin. Transl. Imaging 2, 66–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tietjen GT and Saltzman WM (2015) Nanomedicine gets personal. Sci. Transl. Med 7 314fs347 [DOI] [PubMed] [Google Scholar]
- 18.Vizirianakis IS (2011) Nanomedicine and personalized medicine toward the application of pharmacotyping in clinical practice to improve drug-delivery outcomes. Nanomedicine 7, 11–17 [DOI] [PubMed] [Google Scholar]
- 19.Janowski M et al. (2012) Personalized nanomedicine advancements for stem cell tracking. Adv. Drug Deliv. Rev 64, 1488–1507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yu D et al. (2017) Multiplexed RNAi therapy against brain tumor-initiating cells via lipopolymeric nanoparticle infusion delays glioblastoma progression. Proc. Natl. Acad. Sci. U. S. A 114, E6147–E6156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaushik A et al. (2017) Advances in Personalized Nanotherapeutics. Springer [Google Scholar]
- 22.Wong HL et al. (2012) Nanotechnological advances for the delivery of CNS therapeutics. Adv. Drug Deliv. Rev 64, 686–700 [DOI] [PubMed] [Google Scholar]
- 23.Stimphil E et al. (2017) Physics considerations in targeted anticancer drug delivery by magnetoelectric nanoparticles. Appl. Phys. Rev 4, 021101 [Google Scholar]
- 24.Kaushik A et al. (2016) Magnetically guided central nervous system delivery and toxicity evaluation of magneto-electric nanocarriers. Sci. Rep 6, 25309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hamburg MA and Collins FS (2010) The path to personalized medicine. N. Engl. J. Med 2010, 301–304 [DOI] [PubMed] [Google Scholar]
- 26.Fornaguera C and García-Celma MJ (2017) Personalized nanomedicine: a revolution at the nanoscale. J. Personal. Med 7, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaushik A et al. (2016) Nano-biosensors to detect beta-amyloid for Alzheimer’s disease management. Biosens. Bioelectron 80, 273–287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kaushik A et al. (2016) A label-free electrochemical immunosensor for beta–amyloid detection. Anal. Meth 8, 6115–6120 [Google Scholar]
- 29.Yang Z et al. (2010) Pharmacological and toxicological target organelles and safe use of single-walled carbon nanotubes as drug carriers in treating Alzheimer disease. Nanomedicine 6, 427–441 [DOI] [PubMed] [Google Scholar]
- 30.Jaruszewski KM et al. (2012) Chitosan enhances the stability and targeting of immuno-nanovehicles to cerebro-vascular deposits of Alzheimer’s disease amyloid protein. Nanomedicine 8, 250–260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mathew A et al. (2012) Curcumin loaded-PLGA nanoparticles conjugated with Tet-1 peptide for potential use in Alzheimer’s disease. PLoS One 7, e32616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lazar AN et al. (2013) Curcumin-conjugated nanoliposomes with high affinity for Aβ deposits: possible applications to Alzheimer disease. Nanomedicine 9, 712–721 [DOI] [PubMed] [Google Scholar]
- 33.Tiwari SK et al. (2014) Curcumin-loaded nanoparticles potently induce adult neurogenesis and reverse cognitive deficits in Alzheimer’s disease model via canonical Wnt/b-catenin pathway. ACS Nano 8, 76–103 [DOI] [PubMed] [Google Scholar]
- 34.Cheng KK et al. (2015) Curcumin-conjugated magnetic nanoparticles for detecting amyloid plaques in Alzheimer’s disease mice using magnetic resonance imaging (MRI). Biomaterials 44, 155–172 [DOI] [PubMed] [Google Scholar]
- 35.Bernardi A et al. (2012) Indomethacin-loaded lipid-core nanocapsules reduce the damage triggered by A(1–42 in Alzheimer’s disease models. Int. J. Nanomed 7, 4927–4942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Elnaggar YS et al. (2015) Novel piperine-loaded Tween-integrated monoolein cubosomes as brain-targeted oral nanomedicine in Alzheimer’s disease: pharmaceutical, biological, and toxicological studies. Int. J. Nanomed 10, 5459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Balducci C et al. (2014) Multifunctional liposomes reduce brain β-amyloid burden and ameliorate memory impairment in Alzheimer’s disease mouse models. J. Neurosci 34, 14022–14031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jaruszewski KM et al. (2014) Multimodal nanoprobes to target cerebrovascular amyloid in Alzheimer’s disease brain. Biomaterials 35, 1967–1976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li M et al. (2012) Using graphene oxide high near-infrared absorbance for photothermal treatment of Alzheimer’s disease. Adv. Mater 24, 1722–1728 [DOI] [PubMed] [Google Scholar]
- 40.Lee HJ et al. (2011) Amine-modified single-walled carbon nanotubes protect neurons from injury in a rat stroke model. Nat. Nanotechnol 6, 121–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Etheridge ML et al. (2013) The big picture on nanomedicine: the state of investigational and approved nanomedicine products. Nanomedicine 9, 1–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Santos T et al. (2016) Nanomedicine approaches to modulate neural stem cells in brain repair. Trends Biotechnol. 34, 437–439 [DOI] [PubMed] [Google Scholar]
- 43.Silva-Candal A et al. (2017) Vectorized nanodelivery systems for ischemic stroke: a concept and a need. J. Nanobiotechnol 15, 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shcharbina N et al. (2013) Nanomaterials in stroke treatment. Stroke 44, 2351–2355 [DOI] [PubMed] [Google Scholar]
- 45.Martín Giménez VM et al. (2017) Nanomedicine applied to cardiovascular diseases: latest developments. Ther. Adv. Cardiovasc. Dis 11, 133–142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Alexandrov AV et al. (2004) Ultrasound-enhanced systemic thrombolysis for acute ischemic stroke. N. Engl. J. Med 351, 2170–2178 [DOI] [PubMed] [Google Scholar]
- 47.Higgins P et al. (2011) Nanomedicine: nanotubes reduce stroke damage. Nat. Nanotechnol 6, 83–84 [DOI] [PubMed] [Google Scholar]
- 48.Moon SU et al. (2012) Carbon nanotubes impregnated with subventricular zone neural progenitor cells promotes recovery from stroke. Int. J. Nanomed 7, 2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Campos-Martorell M et al. (2016) Charge effect of a liposomal delivery system encapsulating simvastatin to treat experimental ischemic stroke in rats. Int. J. Nanomed 11, 3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Argibay B et al. (2017) Intraarterial route increases the risk of cerebral lesions after mesenchymal cell administration in animal model of ischemia. Sci. Rep 7, 40758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Newland B et al. (2016) Targeting delivery in Parkinson’s disease. Drug Discov. Today 21, 1313–1320 [DOI] [PubMed] [Google Scholar]
- 52.Benabid AL et al. (2009) Deep brain stimulation of the subthalamic nucleus for the treatment of Parkinson’s disease. Lancet Neurol. 8, 67–81 [DOI] [PubMed] [Google Scholar]
- 53.Yue K et al. (2012) Magneto-electric nano-particles for non-invasive brain stimulation. PLoS One 7, e44040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.da Rocha Lindner G et al. (2015) Improved neuroprotective effects of resveratrol-loaded polysorbate 80-coated poly (lactide) nanoparticles in MPTP-induced Parkinsonism. Nanomedicine 10, 1127–1138 [DOI] [PubMed] [Google Scholar]
- 55.Fernández M et al. (2011) An effective novel delivery strategy of rasagiline for Parkinson’s disease. Int. J. Pharm 419, 271–280 [DOI] [PubMed] [Google Scholar]
- 56.Haney MJ et al. (2015) Exosomes as drug delivery vehicles for Parkinson’s disease therapy. J. Control. Release 207, 18–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhao Y et al. (2014) GDNF-transfected macrophages produce potent neuroprotective effects in Parkinson’s disease mouse model. PLoS One 9, e106867. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 58.Herrán E et al. (2014) Increased antiparkinson efficacy of the combined administration of VEGF-and GDNF-loaded nanospheres in a partial lesion model of Parkinson’s disease. Int. J. Nanomed 9, 2677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang Y et al. (2003) Intravenous nonviral gene therapy causes normalization of striatal tyrosine hydroxylase and reversal of motor impairment in experimental parkinsonism. Hum. Gene Ther. 14, 1–12 [DOI] [PubMed] [Google Scholar]
- 60.Pangeni R et al. (2014) Vitamin E loaded resveratrol nanoemulsion for brain targeting for the treatment of Parkinson’s disease by reducing oxidative stress. Nanotechnology 25, 485102. [DOI] [PubMed] [Google Scholar]
- 61.López T et al. (2011) Treatment of Parkinson’s disease: nanostructured sol-gel silica-dopamine reservoirs for controlled drug release in the central nervous system. Int. J. Nanomed 6, 19–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kaushik A et al. (2017) Investigation of ac-magnetic field stimulated nanoelectroporation of magneto-electric nano-drug-carrier inside CNS cells. Sci. Rep 7, 45663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ferrari M et al. (2009) Nanomedicine and society. Clin. Pharmacol. Ther 85, 466–467 [DOI] [PubMed] [Google Scholar]
- 64.Sanhai WR et al. (2008) Seven challenges for nanomedicine. Nat. Nanotechnol 3, 242–244 [DOI] [PubMed] [Google Scholar]
- 65.Nordmann A and Rip A (2009) Mind the gap revisited. Nat. Nanotechnol 4, 273–274 [DOI] [PubMed] [Google Scholar]
- 66.Kranz C et al. (2011) Analytical Challenges in Nanomedicine. Springer; [DOI] [PubMed] [Google Scholar]
- 67.Muldoon L et al. (2006) Imaging and nanomedicine for diagnosis and therapy in the central nervous system: report of the eleventh annual Blood–Brain Barrier Disruption Consortium meeting. Am. J. Neuroradiol 27, 715–721 [PMC free article] [PubMed] [Google Scholar]
- 68.Baun A and Hansen SF (2008) Environmental challenges for nanomedicine. Nanomedicine 3, 605–608 [DOI] [PubMed] [Google Scholar]
- 69.Saraiva et al. (2016) Nanoparticle-mediated brain drug delivery: Overcome blood-brain-barrier to treat neurodenerative disease. J. Control. Release 235, 34–47 [DOI] [PubMed] [Google Scholar]