Abstract
Intranasal administration of oxytocin (OT) has been found to facilitate prosocial behaviors, emotion recognition and cooperation between individuals. Recent electroencephalography (EEG) investigations have reported enhanced mu rhythm (alpha: 8–13 Hz; beta: 15–25 Hz) desynchronization during the observation of biological motion and stimuli probing social synchrony after the administration of intranasal OT. This hormone may therefore target a network of cortical circuits involved in higher cognitive functions, including the mirror neuron system (MNS). Here, in a double-blind, placebo-controlled, between-subjects exploratory study, we investigated whether intranasal OT modulates the cortical activity from sensorimotor areas during the observation and the execution of social and non-social grasping actions. Participants underwent EEG testing after receiving a single dose (24 IU) of either intranasal OT or placebo. Results revealed an enhancement of alpha - but not beta - desynchronization during observation and execution of social grasps, especially over central and parietal electrodes, in participants who received OT. No differences between conditions were found in the control group (CTRL). Moreover, we found a significant difference over the central-parietal region between the OT and CTRL group only within the social condition. These results suggest a possible action of intranasal OT on sensorimotor circuits involved in social perception and action understanding, which might contribute to facilitate the prosocial effects typically reported by behavioral studies.
Keywords: Oxytocin, ERD; Mirror neuron system; Grasping actions; Electroencephalogram
1.1. Introduction
Over the past two decades, there has been a growing interest in the neuropeptide oxytocin (OT), which has been identified as a key modulator of human social behaviors. Following extensive research in animal models (Chang et al., 2012; Chang and Platt, 2014; Simpson et al., 2014), investigations involving human participants have demonstrated that, beyond the well known peripheral effects exerted by OT on reproduction-related behaviors, its central release in the brain, as a neuromodulator, has a critical role in anxiety and stress control as well as in the modulation of higher cognitive functions and prosocial behaviors (Guastella and MacLeod, 2012; Meyer-Lindenberg et al., 2011).
Intranasal administration or inhalation has been the most commonly used approach to test the effects of OT on social behaviors as it has been suggested that this neuropeptide, as others, is capable of reaching the central nervous system (CNS) through the nasal cavity, thus bypassing the blood-brain barrier (Born et al., 2002; Dal Monte et al., 2014b).
In both human and non-human primates, intranasal OT increases the time spent to gazing at others’ eye region (Dal Monte et al., 2014a; Guastella et al., 2008a; Parr et al., 2013; Simpson et al., 2014) and facilitates facial emotion recognition, especially when faces represent positive values (Guastella et al., 2008b). Moreover, intranasal OT promotes human trust (Kosfeld et al., 2005), empathic reactions (Domes et al., 2007b) and altruistic interactions (Zak et al., 2007), while it produces anxiolytic and anti-stress effects in adverse situations (Campbell, 2010).
This line of behavioral research has drawn attention to the potential implications of intranasal OT as a clinical treatment for social impairments associated with specific neuropsychiatric disorders, such as autism spectrum disorder (ASD), social anxiety or schizophrenia (Bakermans-Kranenburg and van I Jzendoorn, 2013). A growing body of research reports improved social abilities in individuals with ASD who received exogenous OT compared to placebo (Andari et al., 2010; Domes et al., 2013; Guastella and MacLeod, 2012). Similar results have been observed in people suffering from social anxiety (Neumann and Slattery, 2016), and converging evidence from both research and clinical studies suggests that single doses of intranasal OT can modulate both negative and positive symptoms in people with schizophrenia (Feifel et al., 2016).
Given the wide range of altered social behaviors observed after the administration of exogenous OT, on both healthy and clinical populations, several assumptions have been made about the possible mechanisms through which this neuropeptide would exert its effects in the brain. One hypothesis proposes that OT operates by enhancing affiliate and prosocial behaviors through brain circuits involved in social processing (Kosfeld et al., 2005). According to the ‘fear/stress hypothesis’, OT attenuates stress- and anxiety-related neural responses, thus facilitating the propensity to social engagement (Campbell, 2010). A third hypothesis argues, instead, that OT enhances perception selectivity and social salience of a given stimulus independently from its value (i.e., positive or negative) and, more importantly, in a context-dependent manner (Bartz et al., 2011).
All these mechanisms are likely not mutually exclusive and probably served by partially overlapping neural circuits. In fact, neuroimaging studies have shown that specific brain structures are influenced by the action of OT. In particular, the amygdala is one of its core targets (Bethlehem et al., 2013). Following the administration of acute OT, several functional neuroimaging (fMRI) investigations have reported attenuated amygdala activation for fearful and stressful stimuli and, in contrast, enhanced activation of this region in response to the presentation of stimuli with positive valence (Gamer et al., 2010; Labuschagne et al., 2010; Petrovic et al., 2008). However, a few studies have reported attenuated amygdala activation regardless of the value of the presented stimuli (Domes et al., 2007a). Altered activation of other brain areas, including the hippocampus, the insula, the anterior cingulate cortex (ACC), and the orbitofrontal cortex, has also been reported in response to the presentation of social stimuli with either positive or negative values, after acute intranasal OT administration (Labuschagne et al., 2012; Petrovic et al., 2008).
Recent studies have investigated the possible effect of OT on specific cortical rhythms, through the electroencephalogram (EEG) or magnetoencephalogram (MEG). Enhanced mu rhythm suppression has been reported in subjects who received a single dose of intranasal OT during perception of biological motions (Perry et al., 2010; Singh et al., 2016) and stimuli probing social synchrony (Levy et al., 2016). The mu rhythm is an EEG oscillation falling within the alpha (8–13 Hz) and beta (15–25 Hz) frequency bands, which is typically recorded over sensorimotor cortical regions (Hari, 2006; Pineda, 2005). It is maximally expressed during rest, while it is attenuated during movements or observation of movements performed by others. For this reason, it has been widely investigated as a marker of the mirror neuron system (MNS) activity (Fox et al., 2016; Pineda, 2005). The MNS, a cortical system initially discovered in the premotor and parietal cortices of the adult macaque (Bonini et al., 2010; di Pellegrino et al., 1992; Fogassi et al., 2005; Gallese et al., 1996), which activates during both the execution and the observation of goal directed actions, is thought to mediate higher cognitive functions such as action understanding and imitation (Rizzolatti et al., 2001). The fact that mu suppression can be enhanced following intranasal OT administration has thus raised the idea that, besides the neural substrates strictly involved in sociality, this hormone might also target the mirror neuron (MN) network.
The main objective of this exploratory study was to further test this hypothesis by means of a task conforming to the previous EEG research examining the modulation of the MNS in both monkeys and humans (Bimbi et al., 2018; Coudé et al., 2014; Fox et al., 2016). We therefore examined the possible effect of intranasal OT administration on the suppression of the mu rhythm, in both its alpha (8–13 Hz) and beta (15–25 Hz) band subcomponents, during both the execution and the observation of grasping actions directed to either a social (grasp an object to give it to another individual) or a non-social goal (grasp an object to place it into a container). Our main hypothesis was that OT, compared to placebo, would enhance mu suppression during both executed and observed goal-directed actions, particularly in electrodes located over sensorimotor cortical areas and especially when these occur within a social context. This would, in fact, confirm that fronto-parietal mirror networks involved in social perception and action understanding might be targeted by the action of OT and thus facilitate the prosocial effects typically reported by behavioral studies.
1.2. Materials and methods
1.2.1. Sample size and Participants
An a priori power analysis, using the G*power software (Faul et al., 2007), indicated that a sample of 34 participants would be needed for main effects and interactions within and between factors of interest, in order to detect medium size effects (f = .25), with 85% of power and the traditional .05 criterion of statistical significance. Forty-one healthy male volunteers participated to this study (MAge=20.5 years; SDAge=1.8 years). Only young adult male participants were recruited in order to avoid potential interaction of the OT with the female hormonal cycle, and sex- and age-related differences in response to the OT. All participants were students at the University of Maryland-College Park (UMCP), recruited through the PAID University of Maryland Psychology Research Sign-Up System (SONA) or through flyers at the main UMCP campus. Participants’ eligibility was determined through an online secure screening interview (Psychdata.com), during the recruitment phase, and the assessment of their vital signs at the laboratory right before the participation to the study. At their arrival to the laboratory, participants also completed a screening form regarding their physical and mental health. None of the participants reported a history of psychiatric or neurological disorders or drug and alcohol abuse. All participants had normal or corrected to normal visual acuity and all were right-handed, but one who was ambidextrous. Participants were randomly and equally assigned to one of two groups: Oxytocin group (OT group; N=20, MAge= 21.4 years, SDAge= 2.2 years) or Control group (CTRL group; N=21, MAge= 20.5 years, SDAge=1.3 years). The randomization process was performed prior to the beginning of the study using a randomized paired design.
Participants in the OT group identified themselves as Caucasian (40%), African-American/Black (20%), and Asian/Pacific Islander (40%). Participants in the CTRL group identified themselves as Caucasian (38.1%), African-American/Black (23.8%), Asian/Pacific Islander (33,3%), and Hispanic (4.8%). Age (t=1.536, p=0.133) and Race/Ethnicity (χ2= 4.912, p= 0.187) did not differ between the two groups. Six participants were excluded from final analyses due to technical problems during the time of testing (OT group, N=1; CTRL group, N=2), or because they were identified as statistical outliers (OT group=1, CTRL N=2) as described in the section “1.2.5 EEG acquisition and processing”. The final sample thus included 35 subjects (OTG, N=18; CTRL, N=17). The Institutional Review Board at the University of Maryland Baltimore (UMB) and the University of Maryland-College Park (UMCP) approved the study. All participants were over the age of 18 and provided written informed consent after study procedures had been fully explained and before participating to the study. Monetary compensation was provided to each participant for his participation to the study.
1.2.2. Procedure
A placebo-controlled, double-blind, between-subjects design was employed in this study and each subject participated in one experimental session lasting about 2.5 h. After signing the informed consent, an experimenter assessed the participant vital signs, which included the measurement of heart rate, body temperature and blood pressure. Subsequently, each participant received either 24 international units (IU) of intranasal oxytocin, OT (Syntocinon, Novartis Pharma Schweiz Inc., Switzerland), or the same dosage of placebo solution (PL), corresponding to the same solution in which the hormone was dissolved, but lacking the hormone itself. Both solutions were self-administered via intranasal spray under the supervision of an experimenter. While comfortably seating, participants were asked to tilt their head backwards and to gently spray the solution in their nostrils for a total of three puffs, each releasing 8 IU. Both the participant and the supervising experimenter were blinded to the content of the intranasal spray at the moment of the solution administration and throughout the entire visit at the laboratory. The pharmacy at the UMB and the lab manager at UMCP organized the randomization and blinding procedures. Participants underwent EEG testing 45 minutes after solution administration, which corresponds to the putative time at which the drug reaches a plateau in the central nervous system (Illum, 2000). During this period of time, participants were first asked to complete a demographic questionnaire and subsequently an experimenter started the EEG net and electrodes placement and instructed the participants regarding the EEG procedures and the experimental tasks. Participants were monitored onsite throughout their permanence to the laboratory and, at the end of the EEG procedures, their vital signs were re-assessed by an experimenter. No side effects were reported.
1.2.3. Visuo-motor experimental task
During EEG recordings, participants were comfortably seated in a chair located in a soundproof and electrically shielded room, in front of a puppet stage set up on a table (99 cm wide × 61 cm deep × 89 cm tall). A taupe curtain placed on the front of the stage could be manually raised and lowered by an experimenter hidden behind the stage and not visible to participants during EEG acquisition. Participants were instructed to refrain from any movements, but the ones required for the experimental task, and their behavior was monitored by a video-camera allocated in the room, on the participant’s side.
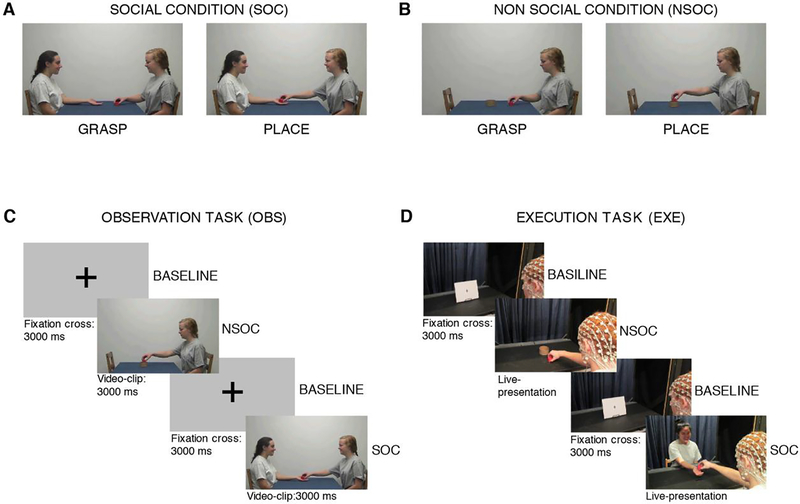
EEG data were acquired while each participant completed a visuo-motor task, which included a social and a non-social condition (see Figure 1 A–B). Both conditions started with the grasping of an object (a red cube, side: 3 cm) while they differed for the final goal of the action. In the social condition (SOC) the grasping action was aimed at giving the grasped object to another person (Figure 1A); while in the non-social condition (NSOC) the grasped object was placed into a container (diameter: 8.5 cm) (Figure 1B).
Figure 1: Experimental conditions and Design.
The upper part of the figure (A-B) depicts the two experimental conditions. A. Social grasping condition (SOC), B. Non-social grasping condition (NSOC). The bottom part of the figure (C-D) depicts the task design. C. Observation Task (OBS), D. Execution Task (EXE).
During the visual task (OBS), participants observed video-clips depicting the two types of actions performed by two actresses on a computer screen (placed on the tabletop). During the motor task (EXE), participants performed the social and non-social grasps themselves. A 10-minute break separated the motor and the visual task, and the order of the two tasks was counter-balanced across participants.
The task design is described in Figure 1 (C–D). In OBS, each trial started with a 3-second baseline, corresponding to the presentation of a fixation cross on the computer screen, which was followed by the presentation of a 3-second video-clip depicting an actress grasping the cube in order to either placing it into the container (NSOC) or giving it to a second actress (SOC) (see Figure 1C). During EXE, stimuli were the same used in the video clips, but live-presented. In each trial, the curtain was raised to reveal a black and white picture (28 × 23 cm) representing a fixation cross for 3 seconds and then it was lowered. This corresponded to the baseline period. The curtain was then raised again and the participant was presented with the red cube to be grasped and 1) placed into a container located on the table (NSOC) or 2) given to an experimenter sitting across from him and extending the hand in order to receive the object (SOC) (see Figure 1D). The duration of each action was about 3 seconds. OBS included 32 trials per condition while EXE included 25 trials per condition. Within both the EXE and OBS tasks, SOC and NSOC trials were presented in a randomized order.
1.2.4. Behavioral coding for EEG segmentation and behavioral analyses
Each EEG recording session was video-recorded and the video was synchronized to the EEG at a resolution of 640 × 480 pixels and at a frame rate of 30 Hz. Two independent coders viewed each video offline (100%) and identified the first frame in which the participant first moved his hand to reach the red cube to be grasped (GRASP START) and the first frame in which the participant first made contact with it (GRASP STOP). The inter–rater agreement within three frames (about 100 ms), was achieved on at least 95 % of the trials for each participant.
Video-clips presented during OBS, corresponding to 4 different videos for SOC and 4 for NSOC respectively, were also coded by two independent coders and synchronized to the EEG. The EEG data were segmented around the GRASP STOP event for both EXE and OBS trials. Trials in which participants were not attending to or moving during baseline or video-clips presentation were marked and excluded from analyses. Additionally, the same behavioral events were used to determine the duration of the grasping action in each EXE trial, by subtracting the timestamp of the GRASP STOP event from that of the GRASP START event. Trials from each experimental condition were then averaged to calculate the mean duration of grasping actions in SOC and NSOC, for each participant.
1.2.5. EEG acquisition and processing
EEG was continuously recorded at a sampling rate of 500 Hz from a 128 channel Hydrocel Geodesic Sensor Net (Electrical Geodesic Inc., Eugene, OR). Impedance for all electrodes was kept below the manufacturer recommended limit of 50 KΩ at the start of data acquisition of each experimental task (OBS, EXE). Signals were referenced to the vertex during recording.
EEG data pre-processing and analyses were carried out using MATLAB (R2013b; Mathworks, Natick, MA, USA). Continuous EEG data from each experimental task and for each participant were first baseline-corrected by removing the DC shift from the data mean, linear detrended using the Matlab’s detrend.m function, and average referenced. A set of channels from the net (channels 38, 43, 44, 48, 49, 113, 114, 119, 120, 121, and 125–128), which lie about the sides of the face and eyes, were excluded from the average reference, because they are heavily prone to net-displacement artifacts.
A threshold of 150 μV was used for editing and removing artifacts associated with gross movements and spurious noise. Continuous EEG data were sectioned into 250 ms epochs, and epochs in which more than five channels (as in Thorpe et al., 2016) exceeded this threshold were deemed bad and removed from the record. Further blinks/eye movements, net displacements or artifacts were also identified and rejected using the independent components analysis (ICA) (Hyvärinen, 1999) as in Thorpe et al. (2016). Independent components were identified for rejection using a twofold criterion. First, rejected components had to have greatest loading magnitude at one of a designated set of channels located over the most anterior part of the head (closest to the eyes). Specifically, these were channels 1, 2, 8, 9, 14,15, 21, 22, 25, 26, 32, and 122. Second, rejected components had to have peak spectral power outside a band of interest chosen as 4–16 Hz. This criterion ensured we only rejected frontally dominant components with EEG peaked in either the 0–4 Hz delta band (such as the components related to blink/saccade/net-displacement waveforms) or >16 Hz (such as components related to high frequency broadband muscle artefact). The mean of ICA rejected components across subjects was 14,2 (SD=2,9) for EXE and 15,8, (SD=3,4) for OBS. EEG data were then reconstructed in channel space from the remaining set of clean components. More details about EEG processing procedures have been previously described in Thorpe et al. (2016).
For both OBS and EXE trials, EEG data were segmented ± 500 ms around the GRASP STOP event, corresponding to the Stimulus epoch, while the Baseline corresponded to a 1000 ms interval from the initial 3s of each trial, starting 0.5 s after the static cross was presented. This segmentation approach was mainly based on previous studies investigating mu rhythm suppression during the execution and the observation of grasping actions in either humans (Cannon et al., 2014) or monkeys (Bimbi et al., 2018). Artifact-free EEG intervals (EXE trials: M=18.7, SD=3.4; OBS trials: M=23.4, SD=2.7), corresponding to Stimulus and Baseline epochs, were submitted to a fast Fourier transform (FFT) and Spectral power (μV2) was computed for 1-Hz bins from 1 to 30 Hz. Event-related desynchronization (ERD) or synchronization (ERS) was computed in dB units, i.e. 10 log10 Stimulus EEG power/Baseline EEG power. Negative values indicate desynchronization (i.e., decrease in power relative to the baseline) and positive values indicate synchronization (i.e., increase in power relative to the baseline). This computation was performed for each channel of interest. Subsequently, ERDs from EXE and OBS trials separately were averaged over frequency bins (1-Hz bins) for bands of interest and over 4 clusters of electrodes: frontal (F), central (C), parietal (P), and occipital (O) sites.
The two frequency bands of interest were within the alpha (8–12 Hz) and the beta (15–25 Hz) ranges. These two bands were chosen a priori as they have been reported as the two main spectral subcomponents of the sensorimotor mu rhythm by a significant number of previous EEG and MEG investigations (Avanzini et al., 2012; Bimbi et al., 2018; Hari, 2006). ERD spectra confirmed that the actual peak of desynchronization of our EEG data falls within these two frequency bands (see FigureS1 in Supplementary materials). Primary channels of interest for the investigation of mu rhythm reactivity were clusters of electrodes over the central (C3: 42,41,36,30,37; C4: 105,104,103,93,87) and parietal sites (P3: 60,59,53,52,51,47; P4: 98,97,92,91,86,85). In addition, contrast/control frontal and occipital electrodes’ clusters were also analyzed (F3:28,27,24,23,20,19; F4: 124,123,118,117,4,3; O3: 74,71,70,69,66; O4: 89,84,83,82,76), for comparison during analyses. Three-dimensional 128-channel topomaps overlayed on adult head model (University of South Carolina McCausland Brain Imaging Center Neurodevelopmental MRI Database), and showing peak of EEG desynchronization/ synchronization, were generated (see Figure 2A and 4A).
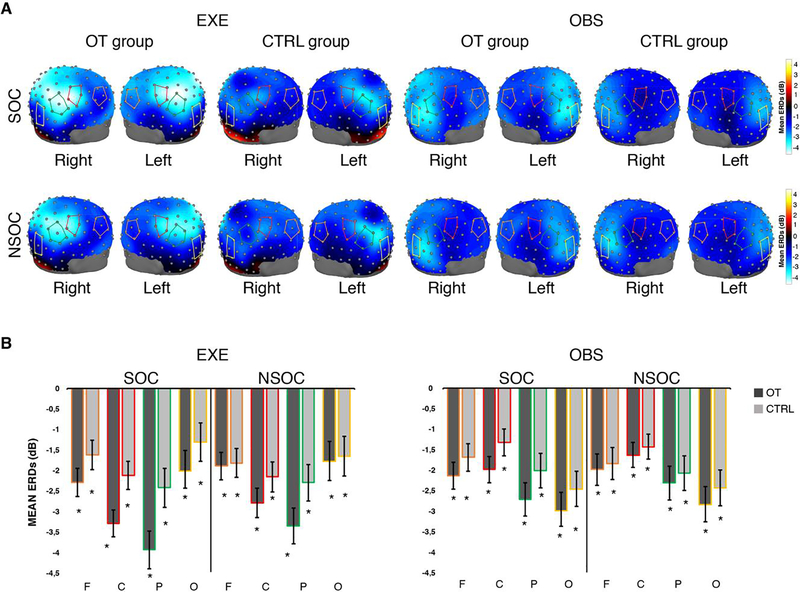
Figure 2: Topographic scalp maps and ERDs for the Alpha band.
A. Three-dimensional topomaps overlaid on adult head model (University of South Carolina McCausland Brain Imaging Center Neurodevelopmental MRI Database) showing peaks of EEG desynchronization across the scalp in the 8 to 12 Hz band, for each group (OT, CTRL), task (EXE, OBS) and condition (SOC, NSOC). The red lines overlaid on the head model indicate the cluster of central electrodes, the green lines indicate the cluster of parietal electrodes, the orange lines indicate the cluster of frontal electrodes, the yellow lines indicate the cluster of occipital electrodes. B. Mean and Standard errors (SE) of alpha ERD across clusters of electrodes (F, C, P, O), conditions and tasks. OT: oxytocin group; CTRL: control group, F: frontal electrodes, C: central electrodes, P: parietal electrodes, O: occipital electrodes; SOC: social condition; NSOC: non-social condition; EXE: execution task; OBS: observation task. * indicates significant desynchronization compared to Baseline.
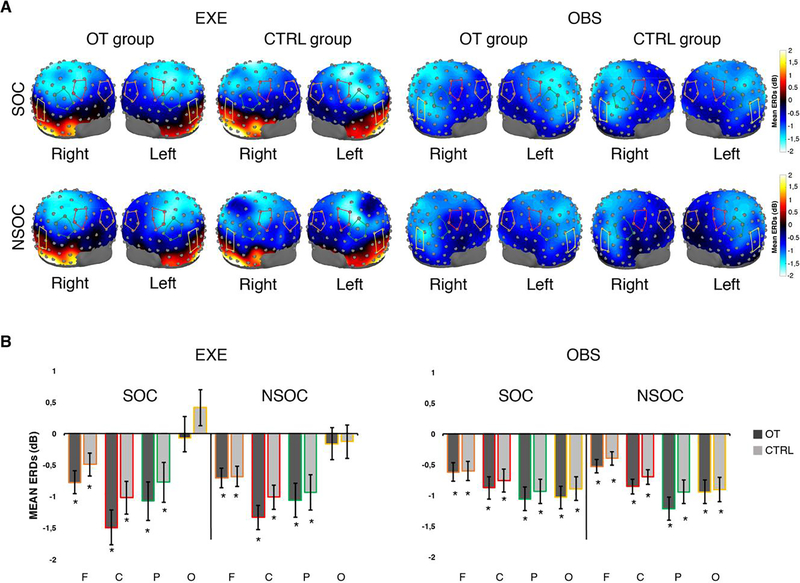
Figure 4: Topographic scalp maps and ERDs for the Beta band.
A. Three-dimensional topomaps overlaid on adult head model (University of South Carolina McCausland Brain Imaging Center Neurodevelopmental MRI Database) showing peaks of EEG desynchronization across the scalp, in the 15 to 25 Hz band, for each group (OT, CTRL), task (EXE, OBS) and condition (SOC, NSOC). The red lines overlaid on the head model indicate the cluster of central electrodes, the green lines indicate the cluster of parietal electrodes, the orange lines indicate the cluster of frontal electrodes, the yellow lines indicate the cluster of occipital electrodes. B. Mean and Standard errors (SE) of alpha ERD across clusters of electrodes (F, C, P, O), conditions and tasks. OT: oxytocin group; CTRL: control group, F: frontal electrodes, C: central electrodes, P: parietal electrodes, O: occipital electrodes; SOC: social condition; NSOC: non-social condition; EXE: execution task; OBS: observation task. * indicates significant desynchronization compared to Baseline.
At this stage subjects whose ERD/ERS values exceeded ± 2.5 SD from the mean sample in at least 3 of the 4 scalp regions analyzed, in one or both experimental tasks, were identified as statistical outliers (as in Festante et al., 2018).
Statistical analyses were run using SPSS software. EEG data were analyzed by means of mixed ANOVAs, scalp mapping analyses and planned comparisons at the electrode-cluster level. Throughout the statistical analysis Greenhouse-Geisser corrected degrees of freedom and p values were used for violations of sphericity. Bonferroni correction was applied for follow up pairwise comparisons and t-tests.
1.3. Results
We examined whether there was a modulatory effect of intranasal OT compared to placebo on EEG alpha and beta rhythms by means of a mixed ANOVA with Task (EXE and OBS), Condition (SOC and NSOC) and Region (F, C, P, O) as within-subject factors and Group (OT and CTRL) as between-subject factor, for each of band of interest (alpha and beta). The assumption of homogeneity of variances was not violated for either the alpha and the beta band as revealed by the Levene’s test (all ps>0.05), see Table S1 in Supplementary materials for detailed results.
1.3.1. Alpha band
The omnibus ANOVA for the alpha band revealed a Condition × Group interaction (p = 0.011), with greater ERD in SOC than NSOC in the OT group (p = 0.006) and no significant differences between conditions in the CTRL group (p=0.378). The omnibus ANOVA also revealed a main effect of Region (p < 0.001), qualified by a Task × Region interaction (p < 0.001). Follow-up analyses showed that ERD was greater in EXE compared to OBS in central (p=0.001) and parietal electrodes (p=0.039). An opposite pattern of desynchronization was found in the occipital electrodes, with greater ERD in OBS than EXE (p < 0.001), while no differences between conditions, in terms of desynchronization, were found in frontal electrodes (p = 0.720). A Condition × Region interaction was also found, which revealed that, across tasks, SOC and NSOC were significantly different only over the parietal region (p= 0.013). A trend toward significance was found for central electrodes (p=0.069), while no differences between conditions were found in frontal (p=0.781) and occipital scalp regions (p= 0.761). Results from this omnibus ANOVA are reported in Table 1.
Table 1:
Omnibus ANOVA results for the alpha band
| Alpha band | ||||||
|---|---|---|---|---|---|---|
| Effect | df | F | p | η2 | ε | |
| Group | 1 | 1.986 | .168 | 0.60 | ||
| Task | 1 | 0.516 | .477 | .017 | ||
| Task × Group | 1 | 0.578 | .452 | .020 | ||
| Condition | 1 | 1.985 | .168 | .057 | ||
| Condition × Group | 1 | 7.245 | .011* | .180 | ||
| Task × Condition | 1 | .097 | .757 | .003 | ||
| Task × Condition × Group | 1 | .273 | .605 | .008 | ||
| Error | 33 | |||||
| Region | 3 | 10.923 | <.001* | .249 | .630 | |
| Region × Group | 3 | 1.495 | .221 | .043 | ||
| Task × Region | 3 | 40.840 | <.001* | .557 | .730 | |
| Task × Region × Group | 3 | 2.159 | .098 | .063 | ||
| Condition × Region | 3 | 3.809 | .012* | .103 | ||
| Condition × Region × Group | 3 | .058 | .098 | .002 | ||
| Task × Condition × Region | 3 | .869 | .460 | .026 | ||
| Task × Condition × Region × Group | 3 | .862 | .463 | .025 | ||
| Error | 99 | |||||
| Pairwise comparisons on significant effects | ||||||
| SOC | NSOC | df | t | p | ||
| OT group (M±SD) | −2.687 (1.3) | −2.237 (1.3) | 17 | −2.95 | .006* | |
| CTRL group | −1.864(1.3) | −1.960 (1.3) | 16 | −1.11 | .378 | |
| SOC | NSOC | df | t | p | ||
| Frontal (M±SD) | −1.865 (1.2) | −1.894 (1.2) | 34 | .279 | .781 | |
| Central | −2.189 (1.3) | −2.041 (1.2) | 34 | −1.886 | .069 | |
| Parietal | −2.772 (1.6) | −2.502 (1.4) | 34 | −2.621 | .013* | |
| Occipital | −2.204 (1.7) | −2.192 (1.6) | 34 | −0.307 | .761 | |
| EXE | OBS | df | t | p | ||
| Frontal (M±SD) | −1.825 (1.3) | −1.905 (1.4) | 34 | .358 | .720 | |
| Central | −2.624 (1.3) | −1.604 (1.2) | 34 | −4.241 | <.001* | |
| Parietal | −2.903 (1.8) | −2.270 (1.6) | 34 | −2.153 | .039* | |
| Occipital | −1.592 (1.9) | −2.643 (1.7) | 34 | 3.835 | .001* | |
SOC: social condition; NSOC: non-social condition; EXE: Execution task; OBS: Observation task; OT Group: oxytocin group; CTRL Group: control group
significant effects after Bonferroni correction.
The 128-channel topomaps in Figure 2A show peaks of desynchronization across the scalp, by group (OT, CTRL), task (EXE, OBS) and conditions (SOC and NSOC), in the alpha band. Finally, t-tests compared to zero confirmed the presence of significant desynchronization in the stimulus epoch compared to baseline in both experimental groups (OT, CTRL), tasks (EXE, OBS), conditions (SOC and NSOC) and at all scalp locations of interest (One samples t-test: all ps< 0.05). Detailed results of this analysis are reported in Supplementary materials (see Table S2), while Figure 2B provides ERD means and standard errors for the alpha band at frontal, central, parietal and occipital electrode clusters.
Taken together these results suggest that sensorimotor EEG alpha frequencies are sensitive to the action of OT during goal-directed action observation and execution, especially in a social context requiring interaction between people. In addition, these results revealed that the desynchronization of the alpha band was greater during the execution than the observation of the goal-directed actions over sensorimotor areas, while an inverse pattern of desynchronization, i.e. greater desynchronization during action observation than action execution, was found in the occipital scalp region.
As shown in Figure 2A, topographically distinct peaks of desynchronization over bilateral central-parietal regions were evident in EXE, while the greatest peaks of desynchronization were evident over the occipital region, during OBS. Therefore, based on our initial hypothesis, we performed further analyses in order to investigate the possible effects of OT on these two scalp regions.
First, by means of a statistical mapping analysis, we contrasted each condition (SOC and NSOC) between groups (OT group vs CTRL group) and the two conditions in each experimental group (OT SOC vs OT NSOC, CTRL SOC vs CTRL NSOC) at the single electrode level (see Figure S2). This analysis, however, did not survive the False Discovery Rate (FDR) correction for multiple comparisons, hence yielding no statistically significant results. See Supplementary materials and Figure S2 for further details on methods and results.
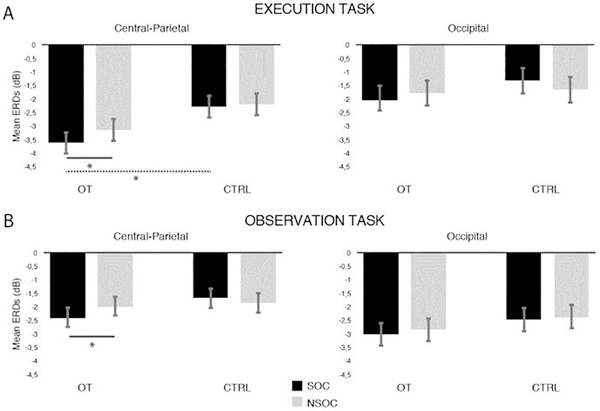
Second, we performed planned comparisons (one-tailed t-tests) on specific clusters of electrodes, namely central-parietal and occipital, to evaluate the possible effects of OT on these scalp regions. entral-parietal regions were considered as a one region/cluster as they resulted to be the most sensitive scalp sites to the action of OT from previous analyses (as evident in both Figure 2A and Figure S2), while the occipital cluster of electrodes was included as control. Bonferroni corrections were applied for multiple comparisons.
For EXE, paired comparisons revealed that, within the OT group, SOC and NSOC significantly differed over the central-parietal region (t(17)= −2.762, p = 0.026), with greater ERD in SOC (M= −3.62, SD= 1.49) than NSOC (M= −3.13, SD= 1.32), while no differences between conditions were found in the CTRL group (t(16)= −0,371, p = 0.715; MSOC= −2.28, SDSOC=1,75; M NSOC=−2.19, SDNSOC=1,98). Over occipital electrodes, SOC and NSOC ERD did not differ in either the OT group (t(17)= −1,355, p = 0.193; MSOC= −2.05 SDSOC=1,31; M NSOC=−1.78, SDNSOC=1,35) and CTRL group (t(16)= −1,333, p = 0.201; MSOC= −1.31 SDSOC=2.42; M NSOC=−1.65, SDNSOC=2.4).
Similar effects to those observed in EXE were found in OBS. Within the OT group, experimental conditions significantly differed over central-parietal electrodes (t(17)= −2.506, p = 0.044), with greater ERD in SOC (M= −2.35, SD= 1.78) than NSOC (M= −1.98, SD= 1.62). No significant ERD differences between experimental conditions were found, instead, within the CTRL group (t(16)= −0.40, p = 0.690; MSOC= −1.67 SDSOC=1.10; M NSOC=−1.76, SDNSOC=1.28). No significant differences were found between SOC and NSOC at the occipital scalp region in both the OT group (t(17)= −0.830, p = 0.417; MSOC= −3.017, SDSOC=1.84; M NSOC=−2.85, SDNSOC=1.96) and the CTRL group (t(16)= −0.107, p = 0.915; MSOC= −2.46, SDSOC=1.68; MNSOC=−2.40, SDNSOC=1.51).
Planned comparisons run to compare each experimental condition between the OT and the CTRL group revealed that during EXE, the OT group showed greater desynchrnization than the CTRL group over the central-parietal region in SOC (t(33)=−2.44 p = 0.038) but not in NSOC (t(33)=−1.64 p = 0.109). No differences between the OT and the CTRL group were found in either SOC (t(33)=−1.13 p = 0.264) and NSOC (t(33)=−0.20 p = 0.842) over occipital scalp locations. As far as OBS is concerned, we did not find any significant difference between the OT and CTRL groups in either SOC (t(33)=−1.33 p = 0.192) and NSOC (t(33)=−0.43 p = 0.666) over the central-parietal region. Also over occipital electrodes no differences between the OT and the CTRL group were found in SOC (t(33)=−0.92 p = 0.362) and NSOC (t(33)=−0.67 p = 0.490). All results relative to this analysis are shown in Figure 3.
Figure 3: Alpha ERD differences over central-parietal and occipital regions.
Mean and Standard errors (SE) of alpha ERD over central-parietal and occipital scalp regions, by tasks (EXE, OBS), conditions (SOC, NSOC) and experimental group (OT, CTRL). Black bars represent the social condition (SOC), grey bars represent the non-social condition (NSOC). *: significant effects; Solid lines indicate significant effects within groups, the dashed line indicates the significant effect between groups.
1.3.2. Beta band
In contrast to the alpha band analysis, the omnibus ANOVA run for the beta band did not reveal any significant effects of Group or Condition. However, a main effect of Region (p < 0.001), qualified by a Task × Region interaction (p < 0.001), was found. Follow-up comparisons revealed that beta desynchronization was grater during EXE than OBS only over central electrodes (p = 0.013), while occipital electrodes showed an inverse pattern of desynchronization, with greater desynchronizion during observation than execution. EXE and OBS did not differ in terms of desynchronizaton over parietal (p = 0.560) and frontal electrodes (p = 0.212). See Table 2 for detailed results of the beta omnibus ANOVA.
Table 2:
Omnibus ANOVA results for the Beta band
| Beta band | |||||||
| Effect | df | F | p | η2 | ε | ||
| Group | 1 | 1.498 | .231 | .043 | |||
| Task | 1 | 1.312 | .260 | .038 | |||
| Task × Group | 1 | 0.702 | .408 | .021 | |||
| Condition | 1 | 0.950 | .337 | .028 | |||
| Condition × Group | 1 | 2.642 | .114 | .074 | |||
| Task × Condition | 1 | 3.242 | .089 | .090 | |||
| Task × Condition × Group | 1 | 3.781 | .068 | .008 | |||
| Error | 33 | ||||||
| Region | 3 | 18.759 | <.001* | .362 | .708 | ||
| Region × Group | 3 | 0.510 | .667 | .017 | |||
| Task × Region | 3 | 35.694 | <.001* | .520 | .765 | ||
| Task × Region × Group | 3 | 0.403 | .751 | .012 | |||
| Condition × Region | 3 | 3.410 | .074 | .094 | .819 | ||
| Condition × Region × Group | 3 | 1.331 | .269 | .039 | |||
| Task × Condition × Region | 3 | 2.116 | .117 | .060 | .812 | ||
| Task × Condition × Region × Group | 3 | .392 | .717 | .012 | |||
| Error | 99 | ||||||
| Pairwise comparisons on significant effects | |||||||
| EXE | OBS | df | t | p | |||
| Frontal (M±SD) | −.668 (0.6) | −.540 (0.4) | 34 | −1,23 | .212 | ||
| Central | −1.130(.0.8) | −.814 (0.5) | 34 | −2.48 | .013* | ||
| Parietal | −.997 (0.8) | −1.066 (0.7) | 34 | −2.15 | .560 | ||
| Occipital | −.085 (1.0) | −.947 (0.7) | 34 | 5.99 | <.001* | ||
EXE: Execution task; OBS: Observation task
significant effects after Bonferroni correction.
The 128-channel topomaps in Figure 4A show peaks of desynchronization across the scalp in the beta band, by group (OT, CTRL), task (EXE, OBS) and condition (SOC and NSOC). One sample t-tests compared to zero confirmed the presence of significant desynchronization, within this band, in both experimental tasks (EXE, OBS), conditions (SOC and NSOC) and groups (OT, CTRL) at all scalp locations of interest (One samples t-test: all ps< 0.05), but the occipital region during grasping execution in both the OT and CTRL group (ps > 0.05) (see Figure 4B). Detailed results of this analysis are reported in Supplementary materials (see Table S3).
To further evaluate the possible effects of OT across the scalp in the beta band, we contrasted each condition (SOC and NSOC) between groups (OT group vs CTRL group) and the two conditions in each experimental group (OT SOC vs OT NSOC, CTRL SOC vs CTRL NSOC), by using the same approach as for the alpha band. Results from the beta band scalp mapping analysis are provided in Supplementary Materials and Figure S3. As for the alpha band, this analysis did not yield any statistically significant results. Since no significant results emerged from previous analyses (see Figure 4 and Figure S3), thus suggesting that beta frequencies were less or not sensitive to the effect of OT or to the context in which the action was embedded, further planned comparisons were not pursued for this band.
1.3.3. Grasping action duration analyses
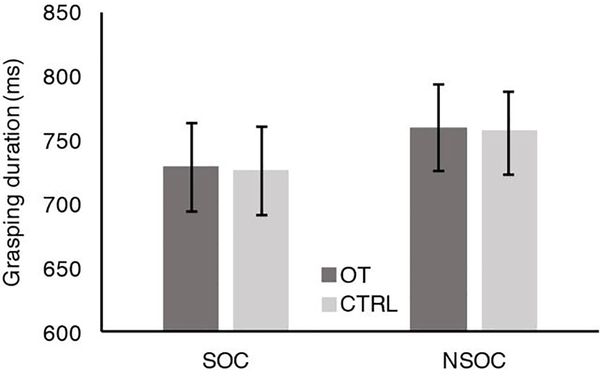
A further analysis was run to verify whether the duration of participants’ grasping actions (DUR) differed between the two experimental conditions (SOC, NSOC) and between the two experimental groups (OT, CTRL). The 2×2 ANOVA, with Condition (SOC, NSOC) as within-subjects factor and Group (OT, CTRL) as between-subjects factor, revealed a main effect of Condition (F(1,33)=8.074, p=0.008), although the grasping duration across groups in the NSOC (M=758.8, SD=23.2) was only approximatively 30 ms longer than the grasping duration in SOC (M=729.9, SD=25.5). Neither main effect of Group (F(1,33)<0.001, p=0.988) nor interaction Condition × Group (F(1,33)=0.005, p=0.946) were found. The duration of the grasping action did not differ between the OT group and the CTRL group in either SOC (DUROT: M= 728,78 ms, SE= 34,59 ms; DURCTRL: M= 727.94 ms, SE= 34.63 ms) and NSOC (DUROT: M= 759.17 ms, SE=33.84 ms; DURCTRL: M= 758.59 ms, SE=31.75 ms). Likewise, the duration of grasping actions did not differ between SOC and NSOC within the OT group and the CTRL group. Mean and standard errors relative to the grasping action duration in each condition (SOC, NSOC) and group (OT, CTRL) are represented in Figure 5. These results suggest that the differences found at neurophysiological level (EEG) between experimental groups and conditions are independent of the duration of the grasping action which was quite similar for both experimental conditions and in either experimental groups.
Figure 5: Grasping Duration.
Mean and Standard errors (SE) of grasping duration in the social condition (SOC) and the non-social condition (NSOC), during the execution task (EXE). In both conditions, the duration of grasping actions corresponded to the time interval that lasts from the frame in which the participant first moved his hand to reach the object to be grasped to the frame in which the participant first made contact with the object. OT: oxytocin group; CTRL: control group; SOC: social condition; NSOC: non-social condition.
1.4. Discussion
The current study investigated the effects of intranasal OT on the modulation of the mu rhythm, a sensorimotor cortical EEG oscillation falling within the alpha and beta frequency ranges, that has been suggested to be a signature of the MNS activity (Bimbi et al., 2018; Fox et al., 2016). We measured EEG activity during the execution and the observation of social and non-social grasping actions, in two groups of young adult males who previously received a single dose (24 IU) of either intranasal OT (OT group) or placebo (CTRL group). We hypothesized that OT would enhance EEG mu desynchronization over sensorimotor areas, especially when grasping actions are aimed at a social goal (i.e. to interact with another person).
In line with our expectations, the Condition (SOC, NSOC) × Group (OT, CTRL) interaction found in the ANOVA analysis relative to the alpha subcomponent of the mu rhythm (8–12 Hz) revealed that intranasal OT, compared to placebo, enhances mu rhythm suppression during the execution and observation of social grasping actions, while it does not affect the alpha band during observed or executed non-social grasps as no differences were found between the OT and CTRL group in the non-social condition. When focusing on specific scalp regions (namely central-parietal and occipital electrode clusters), we found that the OT modulatory effects indeed involved only central-parietal sites, while no significant differences where found at the occipital scalp region. Similar qualitative patterns of cortical activation emerged from the scalp mapping analysis performed to contrast SOC and NSOC within and between experimental groups at each electrode site across the scalp (see Figure S2 panels A–D and I–L). However, this analysis did not yield any statistically significant results after correcting for multiple comparisons, and as such, we caution against strong interpretations of the results.
In contrast, no main effects or interactions of the variable Group or Condition were found when the beta subcomponent of the mu rhythm (15–25 Hz) was investigated, nor clear topographic differences in terms of ERD/ERS emerged when we contrasted SOC and NSOC within and between experimental groups across the scalp (see Figure S3 panels A–D and I–L), thus indicating that this frequency band is overall less sensitive to the action of OT.
These findings confirm that OT may target broad neural networks responding to social relevant stimuli, besides the very delimited areas specifically related to sociality (e.g. amygdala and anterior cingulate cortex). Previously, Perry and colleagues (2010) have described a broad OT-related effect across the scalp of human adults who view spot light displays moving with biological motion. Moreover, Levy and colleagues (2016) in their MEG study have reported a selective activation of the inferior parietal lobule (IPL), the inferior frontal gyrus (IFG) and the posterior superior temporal sulcus (pSTS) while adult subjects (veterans or controls), who received a single dose of intranasal OT, viewed vignettes promoting social synchrony, or, in contrast, showing combatants scenes. The stronger EEG activation found in the current study on electrodes placed over sensorimotor cortical areas in the social condition, during both EXE and OBS, thus further suggests that parietal and motor cortical areas are included in the neural circuitry affected by the action of OT.
Results from the current study also add new insight onto the possible impact that OT has on cortical regions involved in self-other processing. In fact, while the enhanced alpha desynchronization found in the social condition might be generally associated with an increase of perceptual and attentional phenomena, mainly due to the presence of a social context, the fact that we found significant differences only at central-parietal scalp sites is also suggestive of an active role of OT in modulating specific neural fronto-parietal mirror networks involved in the action-perception coupling. Previous neurophysiological studies have supported such speculation (Levy et al., 2016; Perry et al., 2010; Singh et al., 2016). However, the enhanced alpha and beta suppression reported by Perry and colleagues (2010) was not specific to the central-parietal scalp regions, thus leaving open the possibility that the type of stimuli used in their paradigm (i.e., biological motion) was not effective in activating specific central-parietal regions, or that the observed OT-related effect was too unspecific to be interpreted in terms of MN activity. Levy and colleagues (2016), instead, have demonstrated that OT modulates neural activity specifically related to MN cortical regions, namely IFG and IPL, during the observation of socially relevant stimuli. Our results are consistent with findings from this study and, in addition, extend previous research by showing that intranasal OT increases the cortical modulation occurring during social action execution. This finding, indeed, represents the first neurophysiological evidence that, beyond visual perception, OT exerts its modulatory effects also on self-produced actions, by enhancing the suppression of the sensorimotor alpha rhythm over central-parietal scalp regions during the execution of social grasps. We indeed found that, during grasping execution, the OT group significantly differed from the CTRL group in terms of desynchronization but only in the social condition, and that the social and non-social condition significantly differed only within the OT group. No differences between conditions were found in the CTRL group, and the OT and CTRL group did not differ during the production of non social grasps.
Such differences are likely not related to the duration of grasping actions as we found that it was very similar in either experimental conditions and in either groups. It is therefore unlikely that OT targets specific motor programs involved in reach and grasp. More likely, its effects are exerted on neural components involved in coding motor goals at a more general level. Neurophysiological investigations on single neuron recordings in the monkey have shown that MNs are modulated according to the final goals of the action in which a grasp is embedded (i.e. grasp to eat or grasp to place), despite the kinematic parameters of grasping movements do not differ across different actions (Bonini et al., 2010; Fogassi et al., 2005). OT might therefore modulate parietal-premotor networks by facilitating the coding of grasping actions when they are aimed at social goals. This finding is particularly relevant as, in real life-situations, we constantly act toward or jointly with others and, indeed, the adjustment of our own motor behavior, based on the context in which we are required to act (social versus non-social), is extremely important.
It has been well established that MN cortical areas are strictly involved also in imitation tasks (Buccino et al., 2001; Iacoboni et al., 1999; Rizzolatti et al., 2001), in both the observation and the execution/imitation phases. Based on this assumption, a recent behavioral study has investigated the possibility that the MNS is one of the OT targets by exploring the effect of intranasal OT during a motor simulation task (De Coster et al., 2014). Results from this study indicated that OT influences automatic motor simulation by reducing participants’ reaction times during the execution of movements congruent with those observed. Authors argued that such an increase in motor simulation might be due to a direct impact of OT on the MNS. Another recent study, in which transcranial magnetic stimulation (TMS) of the primary motor cortex was used, supports the presence a direct link between the OT action in the brain and the MNS (Prinsen et al., 2018). Prinsen and colleagues showed that intranasal OT enhances individuals’ motor resonance during the observation of hand movements when these are matched with salient social cues, corresponding to the direct gaze of the actor producing the movements. Our results are in line with both these studies, demonstrating that OT facilitates the motor resonance when observing an action and also action production, specifically when the action has a social value and is aimed at interacting with another individual.
In the current study, we found that the cortical OT-modulatory effect during action observation was not as strong as that observed during action execution. Indeed, the OT group showed greater alpha desynchronization during the social condition than the non-social condition, while no statistical significant differences were found between the two groups (OT vs CTRL) in either conditions. There are, however, a number of possible explanations for this weaker OT modulatory effect. Firstly, in contrast to EXE, in which stimuli were live presented, during OBS, stimuli were presented on a computer screen and in a third-person perspective (side view — 90°). Previous studies have provided evidence that motor resonance is stronger during live presentation of actions compared to the presentation of images or video-clips (Fox et al., 2016), as well as during the presentation of actions from a particular perspective, and in particular from the first-person perspective (Angelini et al., 2018; Berntsen et al., 2017; Caggiano et al., 2011; Drew et al., 2015), while weaker responses can be observed for other perspectives. These two factors could have thus made the observation task less effective in eliciting OT-related differences between experimental conditions and groups. Our choice, however, was driven by logistical reasons, as live presentation would have required much more time and effort for the coordination of two experimenters especially in OBS SOC, as well as by our intention to limit the total duration of each visit, which was about 2.5 hours long, in order to perform the entire visuo-motor task within the OT ideal time of actions (within two hours from administration). Therefore, the use of video-presented stimuli and the side view perspective approached in OBS represent a good compromise between the number and type of stimuli that could be used and the quality of stimuli effective in activating sensorimotor regions in relation to others’ actions. Moreover, similar to our methodological approach, previous studies investigating mirror neurons or testing the properties of the MNS using fMRI or EEG have consistently used video or live stimuli presented to subjects in the third person perspective or a later view (see e.g. Arnstein et al., 2011; Bonini et al., 2014; De Klerk et al., 2015). Future investigations should reduce the number of trials per condition in order to assess EEG variations during live-presented stimuli and stimuli presented from the same perspective for both the visual and the motor tasks. Furthermore, as previous EEG studies have reported, the magnitude of mu ERD is greater during execution than observation of goal directed actions (Cannon et al., 2016; Fox et al., 2016). This phenomenon has been long observed also at the single neuron level, with the firing rate of premotor and parietal mirror neurons being typically higher during action execution than action observation (Bonini et al., 2010; Fogassi et al., 2005; Gallese et al., 1996). In the current study, we found that the magnitude of alpha ERD was stronger in EXE than OBS for both central and parietal regions. Such result, besides confirming what has been previously described, might also suggest that the effects exerted by the OT might be related to the strength of ERD and therefore be much more evident when participants are involved first hand in the action.
Importantly, in contrast to Perry and colleagues (2010), who reported both alpha and beta enhanced desynchronization to biological motions following intranasal OT administration, we did not find any effects of OT on the modulation of the beta band. Although, as we expected, beta activity was suppressed, particularly over central-parietal scalp sites during both execution and observation of grasping actions compared to the baseline, our results also revealed that the beta band was not sensitive to the context (social versus non-social) in which the action was performed or observed. It has been shown that the alpha and beta frequency bands originate from different cortical sources, with the beta band originating from the pre-central gyrus and the alpha band having its cortical source in the post-central gyrus (Hari, 2006; Salmelin and Hari, 1994). However, a recent investigation comparing EEG and MN activity in the ventral premotor cortex (area F5) of the monkey has also demonstrated that desynchronization of both EEG alpha and beta frequency bands to grasping action execution and observation correlates with the activity of premotor MNs (Bimbi et al., 2018). It is therefore possible that the beta band better reflects the activation of motor circuits mainly linked to pure motor processing, while the alpha band reflects the activation of networks that integrate sensorimotor information from different domains, such as the social or physical context, and that, in turn, are also more sensitive to the modulatory effect of OT.
In conclusion, results from the current work suggest that OT contributes to the improvement of our social goal-directed actions, probably not only by increasing our attention towards social stimuli but also through the modulation, even indirectly, of sensorimotor networks, which may lead to enhanced automatic processing of action-perception matching and consequently facilitate our understanding of others’ actions. This interpretation is particularly supported by results showing that significant OT-related differences occurred over central-parietal sites which are considered the key hub regions of the MNS. The high concurrence of attentional context-dependent factors should be, however, taken into account as they may have facilitated or directly contributed to the OT-related modulation of the mirror network during both action execution and observation.
Recent studies have argued that mu desynchronization to the observation of goal-directed actions may be contaminated by attentional and visual alpha desynchronization elicited over occipital scalp regions in response to the presentation of visual stimuli (Hobson and Bishop, 2016). However, an alternative explanation relies on the hypothesis that both attentive phenomena, in particular related to social stimuli, and mirroring processes can simultaneously occur during the observation of an action, which in turn would elicit the simultaneous desynchronization of both mu-sensorimotor and visual-alpha rhythms during action observation (Bowman et al., 2017). Confirming this hypothesis, a recent infant EEG study focused on the functional connectivity between central and occipital scalp regions has demonstrated that, during action observation, these two areas are functionally more connected than other brain areas, although they clearly show distinct topographic peaks of desynchronization over the scalp. This would indeed suggest a functional connection between concurrent mirroring and attentional processes during action observation rather than activity volume conduction or mere contamination of visual alpha on sensorimotor scalp regions (Debnath et al., 2019). Results from our study seem to support this hypothesis as well. In fact, despite the high occipital-alpha desynchronization occurring, as expected, during action observation, which might have also dwarfed the desynchronization of the mu rhythm over central and more anterior parietal scalp regions, no differences between groups or conditions were found at the occipital scalp sites, thus suggesting that the effects of OT observed during both action execution and action observation on central-parietal scalp sites are specific to action-related processes. Future investigations should, however, confirm this hypothesis, including further control conditions to disentangle pure attentional phenomena from specific action-related sensorimotor activations.
Importantly, OT might have also affected participants’ emotional state, reducing for instance their social anxiety levels, with a consequent, although indirect, impact on the modulation of the MNS. While this hypothesis remains speculative as the current study did not investigate participants’ emotional state, previous literature showing reduced level of anxiety following the administration of intranasal OT seems to support it (Campbell, 2010; Neumann and Slattery, 2016).
Two limitations of this exploratory study are represented by the between-subjects design approached and by the modest sample included that, although appropriate to detect medium effects at the electrode-cluster level, might have prevented the possibility to detect statistical effects in more comprehensive and powerful analyses involving all electrodes sites over the scalp. Indeed, the statistical mapping analysis performed at the single electrode level did not survived the correction for multiple comparisons thus limiting the interpretation of results. Given the exploratory nature of the study, this is likely related to the fact that data here were not powered enough for such a statistical approach. Future investigation would, therefore, benefit from an increase of the sample size and also from the use of a more powerful within-subject design, in which participants serve as their own control. Finally, as the majority of the behavioral and neurophysiological studies which have investigated the effects of OT so far (De Coster et al., 2014; Levy et al., 2016; Perry et al., 2010; Prinsen et al., 2018) our study targeted a population of young male adults. The rationale for target such population was mainly related to the intention of deliberately avoid the inclusion of confounding experimental variables as age and sex, which may have included more variability in our data and consequently in the interpretation of the results. Future investigation would benefit from the inclusion of female participants and participants of different ages (young adult and adult) in the study sample in order to examine possible sex and age differences, as there is an increasing literature suggesting age and sex effects in the modulation role of intranasal OT (Ebner et al., 2016; Singh et al., 2016). Taken together, all these factors would certainly increase the probability to generalize and expand current results.
The evaluation of the extent to which mu suppression can be altered through pharmacological interventions might in fact allow for the use of these paradigms in people with neuropsychiatric diseases that are known to be, even partially, changeable, which might critically facilitate the development of novel therapeutic interventions for specific social function impairments.
Supplementary Material
Highlights.
We examined the effects of intranasal oxytocin on brain activity during a social and non-social action-perception task.
EEG was used to assess mu rhythm suppression during action execution and observation.
Oxytocin enhanced mu suppression during both execution and observation of social actions.
Oxytocin may affect the mirror neuron network involved in social perception and action understanding.
Acknowledgements
This work was supported by the National Institute of Health, Grant P01HD064653.
The authors would like to thank all participants, the Pharmacist Frank Blatt at UMB for preparing all OT and Placebo solutions, Julie Staple Watson and Jacqueline Kiwanuka for their help during recruitment of participants and for the management of OT and Placebo solutions, and all students at the Child Development Laboratory at UMCP who helped in conducting the study.
Conflict Of Interest
We wish to confirm that the authors have no known conflicts of interest and there has been no significant financial support for this work that could have influenced its outcome. This study was funded by the National Institute of Health, Grant P01HD064653.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andari E, Duhamel J-R, Zalla T, Herbrecht E, Leboyer M, Sirigu A, 2010. Promoting social behavior with oxytocin in high-functioning autism spectrum disorders. Proc. Natl. Acad. Sci. U. S. A 107, 4389–94. 10.1073/pnas.0910249107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angelini M, Fabbri-Destro M, Lopomo NF, Gobbo M, Rizzolatti G, Avanzini P, 2018. Perspective-dependent reactivity of sensorimotor mu rhythm in alpha and beta ranges during action observation: an EEG study. Sci. Rep 10.1038/s41598-018-30912-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnstein D, Cui F, Keysers C, Maurits NM, Gazzola V, 2011. -Suppression during Action Observation and Execution Correlates with BOLD in Dorsal Premotor, Inferior Parietal, and SI Cortices. J. Neurosci 31, 14243–14249. 10.1523/JNEUROSCI.0963-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avanzini P, Fabbri-Destro M, Dalla Volta R, Daprati E, Rizzolatti G, Cantalupo G, 2012. The dynamics of sensorimotor cortical oscillations during the observation of hand movements: An EEG study. PLoS One 7, e37534 10.1371/journal.pone.0037534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakermans-Kranenburg MJ, van I Jzendoorn MH, 2013. Sniffing around oxytocin: review and meta-analyses of trials in healthy and clinical groups with implications for pharmacotherapy. Transl. Psychiatry 3, e258 10.1038/tp.2013.34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartz JA, Zaki J, Bolger N, Ochsner KN, 2011. Social effects of oxytocin in humans: context and person matter. Trends Cogn. Sci 15, 301–9. 10.1016/j.tics.2011.05.002 [DOI] [PubMed] [Google Scholar]
- Berntsen MB, Cooper NR, Romei V, 2017. Transcranial alternating current stimulation to the inferior parietal lobe decreases mu suppression to egocentric, but not allocentric hand movements. Neuroscience. 10.1016/j.neuroscience.2016.12.045 [DOI] [PubMed] [Google Scholar]
- Bethlehem RAI, van Honk J, Auyeung B, Baron-Cohen S, 2013. Oxytocin, brain physiology, and functional connectivity: a review of intranasal oxytocin fMRI studies. Psychoneuroendocrinology 38, 962–74. 10.1016/j.psyneuen.2012.10.011 [DOI] [PubMed] [Google Scholar]
- Bimbi M, Festante F, Coudé G, Vanderwert RE, Fox NA, Ferrari PF, 2018. Simultaneous scalp recorded EEG and local field potentials from monkey ventral premotor cortex during action observation and execution reveals the contribution of mirror and motor neurons to the mu-rhythm. Neuroimage 175, 22–31. 10.1016/j.neuroimage.2018.03.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonini L, Maranesi M, Livi A, Fogassi L, Rizzolatti G, 2014. Space-Dependent Representation of Objects and Other’s Action in Monkey Ventral Premotor Grasping Neurons. J. Neurosci 34, 4108–4119. 10.1523/JNEUROSCI.4187-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonini L, Rozzi S, Serventi FU, Simone L, Ferrari PF, Fogassi L, 2010. Ventral premotor and inferior parietal cortices make distinct contribution to action organization and intention understanding. Cereb. Cortex 20, 1372–1385. 10.1093/cercor/bhp200 [DOI] [PubMed] [Google Scholar]
- Born J, Lange T, Kern W, McGregor GP, Bickel U, Fehm HL, 2002. Sniffing neuropeptides: A transnasal approach to the human brain. Nat. Neurosci 5, 514–516. 10.1038/nn849 [DOI] [PubMed] [Google Scholar]
- Bowman LC, Bakermans-Kranenburg MJ, Yoo KH, Cannon EN, Vanderwert RE, Ferrari PF, van IJzendoorn MH, Fox NA, 2017. The mu-rhythm can mirror: Insights from experimental design, and looking past the controversy. Cortex 96, 121–125. 10.1016/j.cortex.2017.03.025 [DOI] [PubMed] [Google Scholar]
- Buccino G, Binkofski F, Fink GR, Fadiga L, Fogassi L, Gallese V, Seitz RJ, Zilles K, Rizzolatti G, Freund HJ, 2001. Action observation activates premotor and parietal areas in a somatotopic manner: An fMRI study. Eur. J. Neurosci 13, 400–404. 10.1046/j.14609568.2001.01385.x [DOI] [PubMed] [Google Scholar]
- Caggiano V, Fogassi L, Rizzolatti G, Pomper JK, Thier P, Giese MA, Casile A, 2011. View-based encoding of actions in mirror neurons of area F5 in macaque premotor cortex. Curr. Biol 21, 144–148. 10.1016/j.cub.2010.12.022 [DOI] [PubMed] [Google Scholar]
- Campbell A, 2010. Oxytocin and human social behavior. Pers. Soc. Psychol. Rev 14, 281–95. 10.1177/1088868310363594 [DOI] [PubMed] [Google Scholar]
- Cannon EN, Simpson E. a., Fox N. a., Vanderwert RE, Woodward AL, Ferrari PF, 2016. Relations between infants’ emerging reach-grasp competence and event-related desynchronization in EEG. Dev. Sci 19, 50–62. 10.1111/desc.12295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon EN, Yoo KH, Vanderwert RE, Ferrari PF, Woodward AL, Fox NA, 2014. Action experience, more than observation, influences mu rhythm desynchronization. PLoS One 9, e92002 10.1371/journal.pone.0092002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang SWC, Barter JW, Ebitz RB, Watson KK, Platt ML, 2012. Inhaled oxytocin amplifies both vicarious reinforcement and self reinforcement in rhesus macaques (Macaca mulatta). Proc. Natl. Acad. Sci 109, 959–964. 10.1073/pnas.1114621109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang SWC, Platt ML, 2014. Oxytocin and social cognition in rhesus macaques: Implications for understanding and treating human psychopathology. Brain Res. 1580, 57–68. 10.1016/j.brainres.2013.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coudé G, Vanderwert RE, Thorpe S, Festante F, Bimbi M, Fox N. a, Ferrari PF, 2014. Frequency and topography in monkey electroencephalogram during action observation: possible neural correlates of the mirror neuron system. Philos. Trans. R. Soc. Lond. B. Biol. Sci 369, 20130415 10.1098/rstb.2013.0415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dal Monte O, Noble PL, Costa VD, Averbeck BB, 2014a. Oxytocin enhances attention to the eye region in rhesus monkeys. Front. Neurosci 8, 41 10.3389/fnins.2014.00041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dal Monte O, Noble PL, Turchi J, Cummins A, Averbeck BB, 2014b. CSF and blood oxytocin concentration changes following intranasal delivery in macaque. PLoS One 9, e103677 10.1371/journal.pone.0103677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Coster L, Mueller SC, T’Sjoen G, De Saedeleer L, Brass M, 2014. The influence of Oxytocin on automatic motor simulation. Psychoneuroendocrinology 50, 220–226. 10.1016/j.psyneuen.2014.08.021 [DOI] [PubMed] [Google Scholar]
- De Klerk CCJM, Johnson MH, Southgate V, 2015. An EEG study on the somatotopic organisation of sensorimotor cortex activation during action execution and observation in infancy. Dev. Cogn. Neurosci 10.1016/j.dcn.2015.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debnath R, Salo VC, Buzzell GA, Yoo KH, Fox NA, 2019. Mu rhythm desynchronization is specific to action execution and observation: Evidence from time-frequency and connectivity analysis. Neuroimage. 10.1016/j.neuroimage.2018.09.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- di Pellegrino G, Fadiga L, Fogassi L, Gallese V, Rizzolatti G, 1992. Understanding motor events: a neurophysiological study. Exp. brain Res 91, 176–180. 10.1007/BF00230027 [DOI] [PubMed] [Google Scholar]
- Domes G, Heinrichs M, Gläscher J, Büchel C, Braus DF, Herpertz SC, 2007a. Oxytocin Attenuates Amygdala Responses to Emotional Faces Regardless of Valence. Biol. Psychiatry 62, 1187–1190. 10.1016/j.biopsych.2007.03.025 [DOI] [PubMed] [Google Scholar]
- Domes G, Heinrichs M, Kumbier E, Grossmann A, Hauenstein K, Herpertz SC, 2013. Effects of intranasal oxytocin on the neural basis of face processing in autism spectrum disorder. Biol. Psychiatry 74, 164–171. 10.1016/j.biopsych.2013.02.007 [DOI] [PubMed] [Google Scholar]
- Domes G, Heinrichs M, Michel A, Berger C, Herpertz SC, 2007b. Oxytocin improves “mind-reading” in humans. Biol. Psychiatry 61, 731–3. 10.1016/j.biopsych.2006.07.015 [DOI] [PubMed] [Google Scholar]
- Drew AR, Quandt LC, Marshall PJ, 2015. Visual influences on sensorimotor EEG responses during observation of hand actions. Brain Res 10.1016/j.brainres.2014.11.048 [DOI] [PubMed] [Google Scholar]
- Ebner NC, Chen H, Porges E, Lin T, Fischer H, Feifel D, Cohen RA, 2016. Oxytocin’s effect on resting-state functional connectivity varies by age and sex. Psychoneuroendocrinology 69, 50–9. 10.1016/j.psyneuen.2016.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faul F, Erdfelder E, Lang A-G, Buchner A, 2007. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 39, 175–91. 10.3758/BF03193146 [DOI] [PubMed] [Google Scholar]
- Feifel D, Shilling PD, MacDonald K, 2016. A Review of Oxytocin’s Effects on the Positive, Negative, and Cognitive Domains of Schizophrenia. Biol. Psychiatry 79, 222–33. 10.1016/j.biopsych.2015.07.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Festante F, Vanderwert RE, Sclafani V, Paukner A, Simpson EA, Suomi SJ, Fox NA, Ferrari PF, 2018. EEG beta desynchronization during hand goal-directed action observation in newborn monkeys and its relation to the emergence of hand motor skills. Dev. Cogn. Neurosci 30, 142–149. 10.1016/j.dcn.2018.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogassi L, Ferrari P, Gesierich B, Rozzi S, Chersi F, Rizzolatti G, 2005. Parietal Lobe: From Action Organization to Intention Understanding. Science (80-. ). 308, 662–667. 10.1126/science.1106138 [DOI] [PubMed] [Google Scholar]
- Fox NA, Bakermans-Kranenburg MJ, Yoo KH, Bowman LC, Cannon EN, Vanderwert RE, Ferrari PF, Van Ijzendoorn MH, 2016. Assessing Human Mirror Activity With EEG Mu Rhythm: A Meta-Analysis. Psychol. Bull 142, 291–313. 10.1037/bul0000031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallese V, Fadiga L, Fogassi L, Rizzolatti G, 1996. Action recognition in the premotor cortex. Brain 119, 593–609. 10.1093/brain/119.2.593 [DOI] [PubMed] [Google Scholar]
- Gamer M, Zurowski B, Buchel C, 2010. Different amygdala subregions mediate valence-related and attentional effects of oxytocin in humans. Proc. Natl. Acad. Sci 107, 9400–9405. 10.1073/pnas.1000985107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guastella AJ, MacLeod C, 2012. A critical review of the influence of oxytocin nasal spray on social cognition in humans: Evidence and future directions. Horm. Behav 61, 410–418. 10.1016/j.yhbeh.2012.01.002 [DOI] [PubMed] [Google Scholar]
- Guastella AJ, Mitchell PB, Dadds MR, 2008a. Oxytocin Increases Gaze to the Eye Region of Human Faces. Biol. Psychiatry 63, 3–5. 10.1016/j.biopsych.2007.06.026 [DOI] [PubMed] [Google Scholar]
- Guastella AJ, Mitchell PB, Mathews F, 2008b. Oxytocin Enhances the Encoding of Positive Social Memories in Humans. Biol. Psychiatry 64, 256–258. 10.1016/j.biopsych.2008.02.008 [DOI] [PubMed] [Google Scholar]
- Hari R, 2006. Action-perception connection and the cortical mu rhythm. Prog. Brain Res 159, 253–60. 10.1016/S0079-6123(06)59017-X [DOI] [PubMed] [Google Scholar]
- Hobson HM, Bishop DVM, 2016. Mu suppression - A good measure of the human mirror neuron system? Cortex. 82, 290–310. 10.1016/j.cortex.2016.03.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyvärinen A, 1999. Fast and robust fixed-point algorithms for independent component analysis. IEEE Trans. Neural Networks 10, 626–634. 10.1109/72.761722 [DOI] [PubMed] [Google Scholar]
- Iacoboni M, Woods RP, Brass M, Bekkering H, Mazziotta JC, Rizzolatti G, 1999. Cortical mechanisms of human imitation. Science (80-. ). 286, 2526–8. 10.1126/science.286.5449.2526 [DOI] [PubMed] [Google Scholar]
- Illum L, 2000. Transport of drugs from the nasal cavity to the central nervous system. Eur. J. Pharm. Sci. Off. J. Eur. Fed. Pharm. Sci 11, 1–18. 10.1016/S0928-0987(00)00087-7 [DOI] [PubMed] [Google Scholar]
- Kosfeld M, Heinrichs M, Zak PJ, Fischbacher U, Fehr E, 2005. Oxytocin increases trust in humans. Nature 435, 673–676. 10.1038/nature03701 [DOI] [PubMed] [Google Scholar]
- Labuschagne I, Phan KL, Wood A, Angstadt M, Chua P, Heinrichs M, Stout JC, Nathan PJ, 2012. Medial frontal hyperactivity to sad faces in generalized social anxiety disorder and modulation by oxytocin. Int. J. Neuropsychopharmacol 15, 883–896. 10.1017/S1461145711001489 [DOI] [PubMed] [Google Scholar]
- Labuschagne I, Phan KL, Wood A, Angstadt M, Chua P, Heinrichs M, Stout JC, Nathan PJ, 2010. Oxytocin attenuates amygdala reactivity to fear in generalized social anxiety disorder. Neuropsychopharmacology 35, 2403–2413. 10.1038/npp.2010.123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy J, Goldstein A, Zagoory-Sharon O, Weisman O, Schneiderman I, Eidelman-Rothman M, Feldman R, 2016. Oxytocin selectively modulates brain response to stimuli probing social synchrony. Neuroimage 124, 923–930. 10.1016/j.neuroimage.2015.09.066 [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Domes G, Kirsch P, Heinrichs M, 2011. Oxytocin and vasopressin in the human brain: social neuropeptides for translational medicine. Nat. Rev. Neurosci 12, 524–38. 10.1038/nrn3044 [DOI] [PubMed] [Google Scholar]
- Neumann ID, Slattery DA, 2016. Oxytocin in General Anxiety and Social Fear: A Translational Approach. Biol. Psychiatry 79, 213–21. 10.1016/j.biopsych.2015.06.004 [DOI] [PubMed] [Google Scholar]
- Parr LA, Modi M, Siebert E, Young LJ, 2013. Intranasal oxytocin selectively attenuates rhesus monkeys’ attention to negative facial expressions. Psychoneuroendocrinology 38, 1748–56. 10.1016/j.psyneuen.2013.02.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry A, Bentin S, Shalev I, Israel S, Uzefovsky F, Bar-On D, Ebstein RP, 2010. Intranasal oxytocin modulates EEG mu/alpha and beta rhythms during perception of biological motion. Psychoneuroendocrinology 35, 1446–1453. 10.1016/j.psyneuen.2010.04.011 [DOI] [PubMed] [Google Scholar]
- Petrovic P, Kalisch R, Singer T, Dolan RJ, 2008. Oxytocin Attenuates Affective Evaluations of Conditioned Faces and Amygdala Activity. J. Neurosci 28, 6607–6615. 10.1523/JNEUROSCI.4572-07.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pineda JA, 2005. The functional significance of mu rhythms: translating “seeing” and “hearing” into “doing”. Brain Res. Brain Res. Rev 50, 57–68. 10.1016/j.brainresrev.2005.04.005 [DOI] [PubMed] [Google Scholar]
- Prinsen J, Brams S, Alaerts K, 2018. To mirror or not to mirror upon mutual gaze, oxytocin can pave the way: A cross-over randomized placebo-controlled trial. Psychoneuroendocrinology 90, 148–156. 10.1016/j.psyneuen.2018.02.016 [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Fogassi L, Gallese V, 2001. Neurophysiological mechanisms underlying the understanding and imitation of action. Nat. Rev. Neurosci 2, 661–70. 10.1038/35090060 [DOI] [PubMed] [Google Scholar]
- Salmelin R, Hari R, 1994. Spatiotemporal characteristics of sensorimotor neuromagnetic rhythms related to thumb movement. Neuroscience. [DOI] [PubMed] [Google Scholar]
- Simpson EA, Sclafani V, Paukner A, Hamel AF, Novak MA, Meyer JS, Suomi SJ, Ferrari PF, 2014. Inhaled oxytocin increases positive social behaviors in newborn macaques. Proc. Natl. Acad. Sci 111, 6922–6927. 10.1073/pnas.1402471111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh F, Nunag J, Muldoon G, Cadenhead KS, Pineda JA, Feifel D, 2016. Effects of intranasal oxytocin on neural processing within a socially relevant neural circuit. Eur. Neuropsychopharmacol 26, 626–630. 10.1016/j.euroneuro.2015.12.026 [DOI] [PubMed] [Google Scholar]
- Thorpe SG, Cannon EN, Fox NA, 2016. Spectral and source structural development of mu and alpha rhythms from infancy through adulthood. Clin. Neurophysiol 127, 254–269. 10.1016/j.clinph.2015.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zak PJ, Stanton AA, Ahmadi S, 2007. Oxytocin increases generosity in humans. PLoS One 2, e1128 10.1371/journal.pone.0001128 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.