Abstract
Background:
The effects of estradiol on the brain regions involved in the response to stress and emotional processes may be particularly important in women who have alterations in these systems that make them vulnerable to Major Depressive Disorder (MDD). This study examined whether the effect of estradiol administration on the subjective distress and mood response to a laboratory-based psychosocial stress task differs in women with and without a history of MDD.
Methods:
Participants were 65 euthymic postmenopausal women, with and without a history of MDD. They received either 3 months of open-label oral estradiol or did not receive estradiol. After 3 months, participants completed the Montreal Stress Imaging Task (MIST) and subjective distress and mood ratings.
Results:
The effect of estradiol on subjective distress following the MIST differed based on MDD history. In women without a history of MDD, estradiol administration was associated with greater subjective distress than no estradiol. However, in women with a history of MDD, estradiol administration was associated with less subjective distress compared to no estradiol.
Limitations:
This study included open-label administration of estradiol and participants were not blinded to administration. Interpretation of result should include consideration of the relatively small group sizes and that results may not generalize to currently depressed individuals.
Conclusions:
In euthymic women with a history of MDD, estradiol may benefit the affective response to psychosocial stress while having comparatively little benefit in women with no MDD history. Further work should explore whether estradiol administration reduces the risk of depression recurrence in post-menopausal women with past MDD.
Introduction
The incidence and prevalence of major depressive disorder (MDD) is 2-3 times higher in women than in men beginning in puberty and declining after menopause (Kessler, R. C., Berglund, P., Demler, O., Jin, R., Merikangas, K. R., & Walters, 2005; Kessler et al., 2003). Depression risk for women changes across the lifespan, with higher risk during life stages where ovarian hormones fluctuate across the monthly menstrual cycle or during reproductive events such as the perinatal period and the menopause. The concurrence of increased depression risk with the reproductive life phase suggests that ovarian hormone fluctuations may contribute to mood dysregulation in some women. However, many women do not experience depression during reproductive changes, suggesting that ovarian hormone effects may interact with other vulnerability factors to contribute to depression risk (Bloch et al., 2000; Schmidt et al., 2015, 1998). The effect of ovarian hormones on mood in women with differing MDD vulnerability remains to be characterized.
Estrogen fluctuations may increase the risk of MDD through hormonal effects on systems involved in the response to stress (Newhouse and Albert, 2015). The stress exposure model of depression posits that depressive episodes develop from factors increasing vulnerability to depression in the context of stressful life events (Hankin et al., 2007; Liu and Alloy, 2010). Psychosocial stressors are among the top reported antecedents to depressive episodes (Frank et al., 1994; Kendler et al., 2000, 1999), and dysregulation of the hypothalamic-pituitary-adrenal (HPA) axis response is a consistent finding in major depression (Pariante and Lightman, 2008). Psychosocial stress may be especially critical in women as the depressive effects of life stressors are reportedly greater in women than men (Mezulis et al., 2010), even when there is no difference in the number of stressful life events or in the subjective perception of these events (Young and Korszun, 2010). Women are more likely than men to show variations in HPA function in response to stressors (Weiss et al., 1999) and during depressive episodes (Young and Korszun, 2010) .
Ovarian hormone fluctuation may contribute to negative mood by modulating the affective response to stress. Brain activity in the hippocampus and prefrontal cortex during processing of negative emotional information is modulated by changing estradiol levels across the menstrual cycle (Cover et al., 2014; García-García et al., 2016; Goldstein, 2005; Merz et al., 2012). This modulation suggests that estrogen may alter the emotional response to negative information, making this information more or less salient to cognitive processes and thus, mood states. Social-evaluative threat is another element of psychosocial stress that may be especially salient for women (Gordon and Girdler, 2014; Kendler et al., 2000, 1999; Stroud et al., 2002). These differences in affective responses to stress likely contribute to the higher mood disorder risk in women, and may interact with ovarian hormone fluctuations to increase the risk of depression. However, the effect of estrogen on the response to psychosocial stress (including social-evaluative threat) and potential differential effects of estrogen based on depression vulnerability remain unclear.
Estradiol fluctuations and stress exposure have been previously associated with increased negative mood and depressive symptoms in perimenopausal and postmenopausal women (Fang et al., 2014; Gordon et al., 2018, 2016; Schmidt et al., 2015). In randomized clinical trials, estrogen treatment during and after the menopause transition shows mood benefits in women with depression (Cohen et al., 2003; Rasgon et al., 2002; Schmidt et al., 2000; Soares et al., 2001). In contrast to the findings in depressed women, our previous work demonstrates that estradiol administration in post-menopausal women without a history of mood disorders was associated with increased negative mood response to a psychosocial stressor (Newhouse et al., 2008). These findings support that ovarian hormone effects on the affective or emotional response to stress may depend on underlying MDD vulnerability. Periods of low estrogen may represent windows of risk for depressive episodes in women with a previous history of MDD because of differential effects of estrogen on mood regulation, stress, and cognitive systems (Newhouse and Albert, 2015). In women with MDD vulnerability, estrogen may support normal functioning of these systems, while having no benefit in women whose systems are already functioning optimally. Affective benefits of estrogen treatment in postmenopausal women who are currently depressed of who have s history of MDD may result from enhanced function in brain areas that mediate the affective response to stress (e.g. hippocampus, prefrontal cortex). Examining whether estrogen administration differentially effects the mood response to stress in post-menopausal women with and without a history of MDD may help better characterize the importance of stressful life events in effectively 0targeting ovarian hormone treatment.
The purpose of this study was to examine the effects of estradiol administration on subjective distress and mood response following a laboratory-based psychosocial stress task in post-menopausal women with and without a history of MDD. We hypothesized that the effect of estradiol administration on psychosocial stress response would interact with MDD history such that a beneficial effect on mood would be seen only in women with a history of MDD.
Methods
Participants
Postmenopausal women were recruited for a study of estradiol effects cognitive ability. Participants were not informed of the study focus on mood or stress response until debriefing at the end of study participation. This study was approved by the Vanderbilt University Institutional Review Board and informed consent was obtained from all participants and participants repeated consent for data use following debriefing at the end of study participation.
Sixty-five women between the ages of 50 to 75 were enrolled in this study. All participants were postmenopausal, without menses for at least one year, and had an FSH level greater than 27 mIU/mL. All participants had experienced natural menopause; no participants had complete oophorectomy. None of the participants had taken ovarian hormones for at least one year. Both participants with no psychiatric history and a history of unipolar MDD, currently in remission, were enrolled.
Participants were screened for current and past depression, mania, and dysthymia using the partial Structured Clinical Interview for DSM-IV-TR (SCID) (Spitzer et al., 1992). Criteria for study inclusion were: no current or past episodes that met SCID criteria for dysthymia or mania (bipolar depression), a current score of less than 7 on the Beck Depression Inventory (BDI), and a score less than 15 on the Beck Anxiety Inventory (BAI) (Beck et al., 1961). Participants without a history of MDD had no current or past episodes that met SCID criteria for MDD. Participants with a prior history of MDD had at least one episode in the last ten years that met SCID criteria for MDD (Santesso et al., 2008) with no depressive episodes within the last year. Women who reported depressive episodes only during reproductive events (such as perinatal depression) were not included in this study. No participants reported a history of premenstrual dysphoric disorder symptoms using the Composite International Diagnostic Interview for premenstrual dysphoric disorder (CIDI-PMDD). These criteria were designed to enroll currently euthymic women with a past personal history of MDD who have a demonstrated vulnerability to mood disorder (high-risk) and women with no history of MDD who may represent a resilient population (low-risk).
Cognitive/Behavioral Screening
To ensure participants were of at least average intelligence and did not have cognitive impairment, participants were evaluated using the Wechsler Abbreviated Scale of Intelligence (WASI), the Mini Mental State Exam (MMSE), Brief Cognitive Rating Scale, and the Mattis Dementia Rating Scale (DRS) to establish a Global Deterioration Scale (GDS) and exclude participants with evidence of dementia (Folstein et al., 1975; Reisberg et al., 1982; Reisberg and Ferris, 1988; Weschsler, 1997). Participants were required to obtain a GDS score of 1-2 and a MMSE score greater than 26. No participant scored below 123 on the Mattis scale or below 90 on the WASI suggesting that participants were of average or above intelligence with no evidence of dementia or mild cognitive impairment.
Estradiol Administration
Based on their time of recruitment, participants either received open-label oral E2 for 3 months or did not receive E2. This resulted in four participant groups: 1) women who received estradiol treatment with no history of MDD (E2+/MDD−); 2) women who received estradiol treatment with a history of MDD (E2+/MDD+); 3) women who did not receive estradiol treatment with no history of MDD (E2−/MDD−); 4) women who did not receive estradiol treatment with a history of MDD (E2−/MDD+).
Participants received estradiol oral preparation containing 17β-estradiol (Estrace) at a dose of 2.0 mg/day (1 mg for the first month and 2 mg for the following 2 months). This pattern of estradiol administration was used to reduce estradiol-related side effects and includes estradiol doses (1 and 2 mg/ day) that are within the range approved for clinical use by the FDA Three months of estradiol administration at this dose has been found to be sufficient in our prior studies to produce significant behavioral and cognitive treatment differences (Newhouse et al., 2008). After the completion of the Stress Study Day, participants who received estradiol were administered progesterone (Provera) 10 mg per day for 12 days to produce endometrial shedding. To verify compliance, pill counts were performed on a monthly basis.
Subjective Measures
Study procedures occurred over a single day. Before and after the psychosocial stress task, participants completed self-rated measures including the Stress Arousal Checklist (SACL) and the Profile of Mood States (POMS) (King et al., 1983; McNair et al., 1989) . The SACL is a subjective measure that provides separate scores for current distress (SACL-Stress) and current arousal (SACL-Arousal). Change scores (pre to post stress task) were calculated for the SACL-Stress, SACL-Arousal, and POMS subscales (tension/anxiety, depression, anger/hostility, vigor, fatigue, confusion) and total mood disturbance (TMD). The SACL-Stress score was the primary measure of subjective distress response.
Stress Task
For psychosocial stress induction, we employed the Montreal Imaging Stress Task (MIST) (Albert et al., 2015; Dedovic et al., 2005; Pruessner et al., 2008). The MIST produces moderate psychosocial stress through a combination of motivated performance and social-evaluative threat. The MIST was introduced as an arithmetic task and participants were instructed that they should achieve an 80-90% correct performance for their data to be usable in the study. Unknown to participants, the MIST contains an algorithm producing script that automatically adjusts the difficulty of the math task to the participants’ performance; maintaining a low performance rate (between 40-50%) by changing either the problem difficulty or the allotted time. During the MIST, scripted experimenter interaction produces social evaluation threat: the experimenters enter the room between runs and inform the participants that they are not performing well enough and need to improve their performance for the experiment to be successful. This protocol was developed to maintain both performance and social evaluative threat throughout the MIST and to prevent participants from habituating to the performance challenge or giving up on completing the task. Specifically, the interaction of the participants and the experimenters was structured to generate psychosocial stress. Participants were debriefed regarding the purpose of the MIST and task manipulations following the completion of all study procedures.
Data Analysis
Analysis of self-report measures of mood, distress, and arousal included a mixed model repeated measures analysis of variance (ANOVA) utilizing IBM Statistical Package for Social Sciences (SPSS) Version 24. Initial analysis of subjective measures utilized a 2 (E2+, E2−) x 2 (MDD+, MDD−) mixed model ANOVA as an overall test of the effect of E2 treatment and MDD history on subjective distress and mood before (pre-MIST) and after (post-MIST) psychosocial stress on subjective measures: POMS, SACL with p < 0.05. Post-hoc pairwise comparisons using Tukey’s HSD criterion were used to test for differences between the four groups. Change in POMS and SACL scores were compared using the 2X2 ANOVA to examine main and interactive effects of E2 and MDD history on subjective response to the psychosocial stress task including age, study day BDI and BAI as covariates to control for current depression and anxiety symptoms.
Results
Participants included 65 postmenopausal women: 25 women with a history of MDD (E2+:12, E2−:13), and 40 women without a history of MDD (E2+:21, E2−:19). There were no significant group differences in age or screening measures. Participant groups did not differ in reported life-time use of hormonal contraception or ovarian hormone treatment during or following menopause (Table 1). There was a significant group difference in BDI on the study day between E2+/MDD− and E2−/MDD+ groups (Table 1). Mean study day BDI score was significantly lower in the E2+/MDD− group (M=1.24, SD=2.10) than in the E2−/MDD+ group (M=4.46, SD=2.90), F (3, 61) =3.365, p=0.027 (Table 1), although all scores remained in the nonclinical range. All participants similarly scored within the normal range for age on the DRS, WASI, and MMSE.
Table 1.
Demographics and Screening Measures
| MDD+/E2− N=13 | MDD+/E2+ N=12 | MDD−/E2− N=19 | MDD−/E2+ N=21 | F (df) | P value | |
|---|---|---|---|---|---|---|
| Age (years) | 62.54 (7.1) | 62.08 (5.6) | 58.95 (5.9) | 61.57 (7.0) | 1.05 (3, 61) | p= 0.377 |
| DRS | 141.23 (2.0) | 141.08 (2.1) | 140 (4.5) | 141.67 (2.4) | 1.03 (3, 61) | p= 0.384 |
| MMSE | 28.69 (2.2) | 29.58 (.70) | 29.16 (.96) | 29.10 (1.1) | 0.98 (3, 61) | p= 0.410 |
| WASI | 114.83 (15.0) | 116.60 (10.9) | 110.39 (11.8) | 118.11 (10.4) | 1.37 (3, 55) | p= 0.260 |
| Baseline BDI | 4.46 (3.8) | 2.17 (2.6) | 2.63 (2.9) | 2.71 (3.1) | 1.37 (3, 61) | p= 0.262 |
| Baseline BAI | 4.08 (4.4) | 3.08 (3.3) | 3.63 (3.1) | 2.67 (3.0) | 0.54 (3, 61) | p= 0.656 |
| Study BDI | 4.46 (2.9) | 3.75 (4.3) | 3.26 (3.5) | 1.24 (2.1) | 3.37 (3, 61) | p= 0.024 |
| Study BAI | 5.62 (3.9) | 3.92 (4.3) | 4.21 (4.0) | 2.43 (2.8) | 2.07 (3, 61) | p= 0.113 |
| HC Use (%) | 1.00 | 0.83 | 0.90 | 0.89 | x2 = 0.67 | p = 0.88 |
| HT Use (%) | 0.46 | 0.58 | 0.79 | 0.52 | x2 = 4.40 | p = 0.22 |
Demographics and screening measures are reported as the mean (standard deviation) of each group. ANOVA results of group differences are reported as the F value (degrees of freedom) and the p-values. DRS: Mattis Dementia Rating Scale, MMSE: Mini Mental State Exam, WASI: Wechsler Abbreviated Scale of Intelligence, BDI: Beck Depression Index, BAI: Beck Anxiety Index, HC Use: participant reported life-time use of hormonal contraception, HT Use: participant reported use of ovarian hormone treatment during or following menopause
There were main effects of both E2 and MDD history on the pre-MIST POMS TMD, but no significant interaction between E2 and MDD history TMD (Table 2). Women who received E2 had lower pre-MIST TMD scores than women who did not receive E2 (E2+: M = −13.76 SD = 10.63, E2−: M = −6.19, SD = 11.84; t (63) = −2.714, p = 0.009). Women with a history of MDD had higher pre-MIST TMD scores than women without a history of MDD (MDD+: M = −6.20, SD = 10.95, MDD−: M = −12.43, SD = 11.78; t (63) = 2.13, p = 0.037). There as a main effect of E2 on pre-MIST SACL-Arousal, but no significant effect of MDD history or interaction between E2 and MDD history (Table 2). Women who received E2 had higher pre-MIST SACL-Arousal scores than women who did not receive E2 (E2+: M = 10.03, SD = 2.57, E2−: M = 7.88, SD = 3.13; t (63) = 3.04, p = 0.003).
Table 2.
Results of Main Effects & Interaction for SACL & POMS
| MDD+/E2− N=13 | MDD+/E2+ N=12 | MDD−/E2− N=19 | MDD−/E2+ N=21 | E2 F (df) p value Cohen’s d | MDD | Interaction | |
|---|---|---|---|---|---|---|---|
| Pre POMS TMD | −0.85 (10.48) | −12.00 (8.45) | −9.84 (11.55) | −14.76 (11.78) | 7.803 (1,62) p=0.007* d= 0.473 |
4.473 (1,62) p=0.039* d= 0.100 |
1.250 (1,62) p=0.268 d= 0.286 |
| Post POMS TMD | 22.15 (16.45) | 33.67 (52.76) | 20.84 (38.41) | 18.00 (25.51) | 0.073 (1,62) p=0.788 d= 0.222 |
0.911 (1,62) p=0.344 d= 0.274 |
0.657 (1,62) p=0.421 d= 0.208 |
| Δ POMS TMD | 23.00 (17.18) | 45.08 (56.93) | 30.26 (33.02) | 32.76 (24.87) | 1.393 (1,62) p=0.242 d= 0.377 |
0.080 (1,62) p=0.778 d= 0.311 |
1.907 (1,62) p=0.172 d= 0.303 |
| Pre SACL-S | 1.00 (2.04) | 1.17 (2.86) | 0.95 (1.93) | 0.14 (0.48) | 0.966 (1,62) p=0.330 d= 0.181 |
1.27 (1,62) p=0.264 d= 0.340 |
1.046 (1,62) p=0.311 d= 0.262 |
| Post SACL-S | 11.15 (4.34) | 9.58 (6.11) | 8.89 (5.92) | 11.62 (4.64) | 0.663 (1,62) p=0.418 d= 0.331 |
0.008 (1,62) p=0.927 d= 0.380 |
2.542 (1,62) p=0.116 d= 0.408 |
| Δ SACL-S | 10.15 (4.14) | 8.42 (6.34) | 7.95 (5.59) | 11.48 (4.51) | 1.315 (1,62) p=0.256 d= 0.404 |
0.094 (1,62) p=0.760 d= 0.512 |
4.011 (1,61) p=0.050* d= 0.513 |
| Pre SACL-A | 6.92 (2.66) | 9.67 (2.53) | 8.53 (3.32) | 10.24 (2.62) | 9.354 (1,62) p=0.003* d= 0.340 |
2.262 (1,62) p=0.138 d= 0.051 |
0.507 (1,62) p=0.479 d= 0.182 |
| Post SACL-A | 8.85 (2.73) | 8.58 (3.42) | 7.95 (5.59) | 9.24 (3.24) | 0.849 (1,62) p=0.360 d= 0.180 |
0.027 (1,62) p=0.870 d= 0.233 |
1.025 (1,62) p=0.315 d= 0.260 |
| Δ SACL-A | 1.92 (4.59) | −1.08 (3.55) | −0.58 (4.10) | −1.00 (4.23) | 1.870 (1, 62) p=0.176 d= 0.400 |
1.306 (1,62) p=0.257 d= 0.204 |
1.487 (1,61) p=0.227 d= 0.312 |
Results are reported as the mean (standard deviation) change score of each group. Results for the main effect of E2, Depression, and the Interaction are reported as the F value (degrees of freedom)
: p<.05
POMS TMD: Profile of Mood States Total Mood Disturbance (score: 0-200)
SACL-S: Stress and Arousal Checklist Stress Score (score: 0-18)
SACL-A: Stress and Arousal Checklist Arousal Score (core: 0-12)
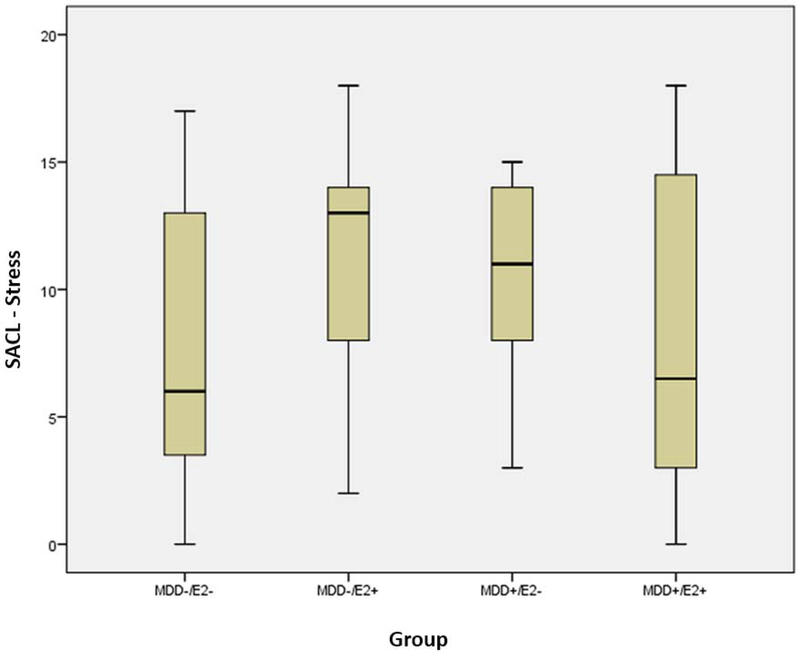
There were no significant main effects of E2 or MDD history for change on SACL measures or POMS subscales or TMD (Table 2). A statistically significant interaction between E2 group and MDD history was observed for change in the SACL-Stress measure following the MIST (F (1, 61) = 4.011, p=0.050). In women with MDD history (MDD+), those who received E2 reported less distress after the MIST (SACL-Stress change, M= 8.42, SD=6.34) than those who did not receive E2 (M=10.15, SD=4.14). However, in women without a history of MDD (MDD−), women who received E2 reported more distress (SACL-Stress change, M=11.48, SD=4.51) after the MIST than women who did not receive E2 (M=7.95, SD=5.59) (Figure 1). Post Hoc pairwise comparisons using Tukey’s HSD showed the average change in SACL-Stress score was greater for the MDD−/E2+ than the MDD−/E2− group (mean difference between groups = 3.53, t (34.64) = −2.18, p=0.036, Cohen’s d = 0.695). Thus in women without a history of MDD, receiving E2 was associated with a significant increase in subjective distress. However, this effect of E2 was not seen in women with a history of MDD and the pattern of means indicates a beneficial effect of E2 in reducing the negative response to psychosocial stress in these women.
Figure 1. Group Differences on SACL (Stress Arousal Checklist) Stress:
Estradiol Effects on subjective distress following the Montreal Imaging Stress Task by Depression History
There were no main or interactive effects of E2 or depression history on POMS subscales or TMD. Neither age nor study day BAI was significantly associated with SACL measures or POMS TMD. Study day BDI was associated with changes in POMS TMD (F=4.87, p=0.031), but not individual POMS subscales or SACL measures.
Discussion
The primary finding of this study was that the effect of estradiol on the subjective distress response to a psychosocial stress task differs between postmenopausal women with and without a history of MDD. In women without a history of MDD, estradiol administration resulted in a greater subjective stress response to the MIST as measured by the SACL-Stress score. This was reversed in women with a history of MDD where estradiol administration was associated with less subjective distress compared to women who did not receive estradiol. Change in measures of arousal and overall mood disturbance did not differ between groups.
The finding that postmenopausal women without a history of MDD who received estradiol had a greater subjective distress response to the MIST than women who did not receive estradiol accords with previous work. Newhouse and colleagues demonstrated that in healthy postmenopausal women with no history of mood disorder, 3 months of estradiol administration was associated with greater negative mood response to the Trier Social Stress Test compared to placebo administration (Newhouse et al., 2008). This finding differs from our previous work in pre-menopausal women demonstrating that high estradiol phases of the menstrual cycle are associated with a less subjective distress response to the MIST (Albert et al., 2015) and reduced activity in brain areas implicated in processing of negative emotional information (Goldstein, 2005; Jacobs et al., 2015). The results from this study taken together with our previous results suggest that the effect of estradiol on affective responses to stress may change following menopause in never depressed women such that estradiol administration increases subjective distress responses to stress rather than protecting against subjective distress as in pre-menopausal women.
This study also supports that the magnitude and directionality of the effects of estradiol on the stress and mood response to psychosocial stress are influenced by MDD history. In women without a history of MDD brain areas important to the regulation of mood and stress response may successfully adapt to the low estradiol state of menopause (perhaps through mechanisms such as changing estrogen receptor density (Milner et al., 2008; Mitterling et al., 2010; Waters et al., 2011; for comprehensive review see McEwen et al., 2012) . In these women fluctuations in estrogen (such as the relatively long-term estradiol administration in this study) may disrupt mood regulation.
In postmenopausal women with a history of MDD, neural systems involved in emotion regulation and the stress response may remain sensitive to the beneficial effects of estradiol that are seen at earlier ages. This hypothesis is supported by previous reports associating estradiol replacement following menopause with reduced depressive symptoms in women with prior MDD (Gordon et al., 2018; Gordon and Girdler, 2014; Kornstein et al., 2013; Schmidt et al., 2015), but not in women without a history of MDD (Almeida et al., 2006; Gordon et al., 2016; Schmidt et al., 2015, 1998). Jacobs and colleagues report that women with and without a history of MDD exhibit a differential effect of high estradiol levels on brain activity in areas important for emotional processing during a mild visual stress task (Jacobs et al., 2015); high estradiol in women without a history of MDD was associated with attenuated activity in response to stress while in women with remitted MDD there was no effect of estradiol level. The response of brain areas important for the regulation of stress and mood responses may be altered in women with MDD such that a shift following menopause may increase the beneficial effects of estradiol in these women as opposed to the negative effect seen in women with no history of MDD. These findings support that for some women (perhaps those with increased risk for MDD), ovarian hormone status is important in regulating stress response and maintaining mood. In women with a history of MDD the low estradiol state of menopause may contribute to greater negative subjective stress responding and thus increased vulnerability to depression recurrence.
Limitations
These results should be interpreted with caution. This study was not a randomized controlled clinical trial. Participants were not blinded to drug administration. Second, the study included a small sample for each group. Although there were no demonstrated effects of age, this study was not designed to examine age or time since menopause which may alter the effect of estrogen (Maki, 2013; Soares, 2017). This study also did not examine severity or duration of MDD history which may be important factors in better characterizing depression vulnerability. Finally, these results may not generalize to women with current or more recently remitted MDD. However, strength of this study include careful characterization of the participant groups to include euthymic women with demonstrated risk for MDD (past personal history of MDD) and with no history of MDD. Additionally, the experimental use of estrogen without concurrent progesterone in this study allows the isolation of estrogen effects which are often not separable from those of progesterone in women who are receiving clinical hormone replacement.
Conclusion
Estradiol administration resulted in less subjective distress following psychosocial stress in postmenopausal women with a history of MDD, but greater subjective distress in women with no depression history. These results accord with previous findings of a negative effect of estradiol administration in postmenopausal women with no mood disorder history and suggests a shift from a beneficial effect of estradiol in pre-menopausal women to a negative effect in post-menopausal women without depression vulnerability. Our finding that women with a history of MDD reported less subjective distress following estradiol administration compared to placebo suggests that in these women brain areas important for the regulation of stress response and mood may remain sensitive to the beneficial effects of estradiol following menopause. Importantly, as all enrolled women were euthymic, this study supports that estradiol administration may benefit mood even in women who are not currently depressed. Future work should examine the potential for estradiol treatment in post-menopausal women with MDD history to reduce depression recurrence risk.
Table 3.
Post-Hoc Pairwise Comparisons for SACL-Stress
| MDD+/E2+ N=13 | MDD+/E2− N=12 | MDD−/E2+ N=19 | MDD−/E2− N=21 | |
|---|---|---|---|---|
| T value (df) p value | ||||
| MDD+/E2 + N=13 | - | |||
| MDD+/E2− N=12 | t=0.80 (18.70) p=0.432 d= 0.323 |
- | ||
| MDD−/E2+ N=19 | t=1.47 (17.47) p=0.159 d= 0.556 |
t=0.87 (27.28) p=0.390 d = 0.079 |
- | |
| MDD−/E2− N=21 | t= −0.21 (21.30) p=0.836 d = 0.224 |
t= −1.28 (29.75) p=0.210 d= 0.556 |
t= −2.18 (34.64) p=0.036 d = 0.695 |
- |
Results for post-hoc pairwise comparisons on SACL (Stress Arousal Checklist) Stress are reported as the t-value (degrees of freedom) p-value, and Cohen’s d.
Highlights.
Estradiol effects on distress to psychosocial stress differs by depression history
Estradiol associated with greater distress in women without depression history
Estradiol associated with less distress in women with depression history
No estradiol effect on arousal or general mood disturbance
Acknowledgments
Funding and Disclosure
This work is supported by NIAR01AG021476, NIHMH110598, and Vanderbilt CTSA grant UL1 TR002243from NCATS/NIH. The authors declare no conflicts of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declarations of interest: none
References
- Albert K, Pruessner J, Newhouse P, 2015. Estradiol levels modulate brain activity and negative responses to psychosocial stress across the menstrual cycle. Psychoneuroendocrinology 59, 14–24. 10.1016/j.psyneuen.2015.04.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida OP, Lautenschlager NT, Vasikaran S, Leedman P, Gelavis A, Flicker L, 2006. A 20-week randomized controlled trial of estradiol replacement therapy for women aged 70 years and older: Effect on mood, cognition and quality of life. Neurobiol. Aging. 10.1016/j.neurobiolaging.2004.12.012 [DOI] [PubMed] [Google Scholar]
- Bloch M, Schmidt PJ, Danaceau M, Murphy J, Nieman L, Rubinow DR, 2000. Effects of Gonadal Steroids in Women With a History of Postpartum Depression. Am. J. Psychiatry 157, 924–930. 10.1176/appi.ajp.157.6.924 [DOI] [PubMed] [Google Scholar]
- Cohen LS, Soares CN, Poitras JR, Prouty J, Alexander AB, Shifren JL, 2003. Short-term use of estradiol for depression in perimenopausal and postmenopausal women: A preliminary report. Am. J. Psychiatry 160, 1519–1522. 10.1176/appi.ajp.160.8.1519 [DOI] [PubMed] [Google Scholar]
- Cover KK, Maeng LY, Lebron-Milad K, Milad MR, 2014. Mechanisms of estradiol in fear circuitry: Implications for sex differences in psychopathology. Transl. Psychiatry. 10.1038/tp.2014.67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dedovic K, Renwick R, Mahani NK, Engert V, Lupien SJ, Pruessner JC, 2005. The Montreal Imaging Stress Task: Using functional imaging to investigate the effects of perceiving and processing psychosocial stress in the human brain, in: Journal of Psychiatry and Neuroscience, 10.1007/s00066-017-1186-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang CY, Egleston BL, Manzur AM, Townsend RR, Stanczyk FZ, Spiegel D, Dorgan JF, 2014. Psychological reactivity to laboratory stress is associated with hormonal responses in postmenopausal women. J. Int. Med. Res 10.1177/0300060513504696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR, 1975. “Mini-mental state.” J. Psychiatr. Res 12, 189–198. 10.1016/0022-3956(75)90026-6 [DOI] [PubMed] [Google Scholar]
- Frank E, Anderson B, Reynolds CF, Ritenour A, Kupfer DJ, 1994. Life Events and the Research Diagnostic Criteria Endogenous Subtype: A Confirmation of the Distinction using the Bedford College Methods. Arch. Gen. Psychiatry. 10.1001/archpsyc.1994.03950070011005 [DOI] [PubMed] [Google Scholar]
- García-García I, Kube J, Gaebler M, Horstmann A, Villringer A, Neumann J, 2016. Neural processing of negative emotional stimuli and the influence of age, sex and task-related characteristics. Neurosci. Biobehav. Rev 10.1016/j.neubiorev.2016.04.020 [DOI] [PubMed] [Google Scholar]
- Goldstein JM, 2005. Hormonal Cycle Modulates Arousal Circuitry in Women Using Functional Magnetic Resonance Imaging. J. Neurosci 25, 9309–9316. 10.1523/JNEUROSCI.2239-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon JL, Girdler SS, 2014. Hormone Replacement Therapy in the Treatment of Perimenopausal Depression. Curr. Psychiatry Rep 10.1007/s11920-014-0517-1 [DOI] [PubMed] [Google Scholar]
- Gordon JL, Rubinow DR, Eisenlohr-Moul TA, Leserman J, Girdler SS, 2016. Estradiol variability, stressful life events, and the emergence of depressive symptomatology during the menopausal transition. Menopause. 10.1097/GME.0000000000000528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon JL, Rubinow DR, Eisenlohr-Moul TA, Xia K, Schmidt PJ, Girdler SS, 2018. Efficacy of transdermal estradiol and micronized progesterone in the prevention of depressive symptoms in the menopause transition: A randomized clinical trial. JAMA Psychiatry, 10.1001/jamapsychiatry.2017.3998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hankin BL, Mermelstein R, Roesch L, 2007. Sex differences in adolescent depression: Stress exposure and reactivity models. Child Dev. 78, 279–295. 10.1111/j.1467-8624.2007.00997.x [DOI] [PubMed] [Google Scholar]
- Jacobs EG, Holsen LM, Lancaster K, Makris N, Whitfield-Gabrieli S, Remington A, Weiss B, Buka S, Klibanski A, Goldstein JM, 2015. 17p-Estradiol differentially regulates stress circuitry activity in healthy and depressed women. Neuropsychopharmacology 40, 566–576. 10.1038/npp.2014.203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Karkowski LM, Prescott CA, 1999. Causal relationship between stressful life events and the onset of major depression. Am. J. Psychiatry. 10.1176/ajp.156.6.837 [DOI] [PubMed] [Google Scholar]
- Kendler KS, Thornton LM, Gardner CO, 2000. Stressful life events and previous episodes in the etiology of major depression in women: An evaluation of the “kindling” hypothesis. Am. J. Psychiatry 157, 1243–1251. 10.1176/appi.ajp.157.8.1243 [DOI] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Dernier O, Jin R, Merikangas KR, & Walters EE, 2005. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch. Gen. Psychiatry 62, 593–602. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Dernier O, Jin R, Koretz D, Merikangas KR, Rush AJ, Walters EE, Wang PS, 2003. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R). JAMA 289, 3095–105. 10.1097/00132578-200310000-00002 [DOI] [PubMed] [Google Scholar]
- King MG, Burrows GD, Stanley GV, 1983. Measurement of stress and arousal: validation of the stress/arousal adjective checklist. Br. J. Psychol 74 (Pt 4), 473–479. 10.1111/j.2044-8295.1983.tb01880.x [DOI] [PubMed] [Google Scholar]
- Kornstein SG, Toups M, Rush AJ, Wisniewski SR, Thase ME, Luther J, Warden D, Fava M, Trivedi MH, 2013. Do Menopausal Status and Use of Hormone Therapy Affect Antidepressant Treatment Response? Findings from the Sequenced Treatment Alternatives to Relieve Depression (STAR*D) Study. J. Women’s Heal 10.1089/jwh.2012.3479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu RT, Alloy LB, 2010. Stress generation in depression: A systematic review of the empirical literature and recommendations for future study. Clin. Psychol. Rev 10.1016/j.cpr.2010.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maki PM, 2013. Critical window hypothesis of hormone therapy and cognition: A scientific update on clinical studies. Menopause, 10.1097/gme.0b013e3182960cf8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNair D, Lorr M, Droppleman LF, 1989. Profile of mood states (POMS). Douglas M. McNair, Maurice Lorr, Leo F. Droppleman. 10.1007/978-1-4419-9893-4_68 [DOI] [Google Scholar]
- Merz CJ, Tabbed K, Schweckendiek J, Klucken T, Vaitl D, Stark R, Wolf OT, 2012. Neuronal correlates of extinction learning are modulated by sex hormones. Soc. Cogn. Affect. Neurosci 10.1093/scan/nsr063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mezulis AH, Funasaki KS, Charbonneau AM, Hyde JS, 2010. Gender differences in the cognitive vulnerability-stress model of depression in the transition to adolescence. Cognit. Ther. Res 10.1007/s10608-009-9281-7 [DOI] [Google Scholar]
- Milner TA, Lubbers LS, Alves SE, McEwen BS, 2008. Nuclear and extranuclear estrogen binding sites in the rat forebrain and autonomic medullary areas. Endocrinology 149, 3306–3312. 10.1210/en.2008-0307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitterling KL, Spencer JL, Dziedzic N, Shenoy S, McCarthy K, Waters EM, McEwen BS, Milner TA, 2010. Cellular and subcellular localization of estrogen and progestin receptor immunoreactivities in the mouse hippocampus. J. Comp. Neurol 518, 2729–2743. 10.1002/cne.22361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newhouse P, Albert K, 2015. Estrogen, stress, and depression: A neurocognitive model. JAMA Psychiatry 72, E1–E3. 10.1001/jamapsychiatry.2015.0487 [DOI] [PubMed] [Google Scholar]
- Newhouse PA, Dumas J, Hancur-Bucci C, Naylor M, Sites CK, Benkelfat C, Young SN, 2008. Estrogen administration negatively alters mood following monoaminergic depletion and psychosocial stress in postmenopausal women. Neuropsychopharmacology. 10.1038/sj.npp.1301530 [DOI] [PubMed] [Google Scholar]
- Pariante CM, Lightman SL, 2008. The HPA axis in major depression: classical theories and new developments. Trends Neurosci. 10.1016/j.tins.2008.06.006 [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Dedovic K, Khalili-Mahani N, Engert V, Pruessner M, Buss C, Renwick R, Dagher A, Meaney MJ, Lupien S, 2008. Deactivation of the Limbic System During Acute Psychosocial Stress: Evidence from Positron Emission Tomography and Functional Magnetic Resonance Imaging Studies. Biol. Psychiatry. 10.1016/j.biopsych.2007.04.041 [DOI] [PubMed] [Google Scholar]
- Rasgon NL, Altshuler LL, Fairbanks LA, Dunkin JJ, Davtyan C, Elman S, Rapkin AJ, 2002. Estrogen replacement therapy in the treatment of major depressive disorder in perimenopausal women. J. Clin. Psychiatry 63 Suppl 7, 45–48. [PubMed] [Google Scholar]
- Reisberg B, Ferris SH, 1988. Brief Cognitive Rating Scale (BCRS). Psychopharmacol. Bull 24,629–36. [PubMed] [Google Scholar]
- Reisberg B, Ferris SH, De Leon MJ, Crook T, 1982. The global deterioration scale for assessment of primary degenerative dementia. Am. J. Psychiatry 139, 1136–1139. 10.1176/ajp.139.9.1136 [DOI] [PubMed] [Google Scholar]
- Santesso DL, Steele KT, Bogdan R, Holmes AJ, Deveney CM, Meites TM, Pizzagalli DA, 2008. Enhanced negative feedback responses in remitted depression. Neuroreport. 10.1097/WNR.0b013e3283036e73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt PJ, Ben Dor R, Martinez PE, Guerrieri GM, Harsh VL, Thompson K, Koziol DE, Nieman LK, Rubinow DR, 2015. Effects of estradiol withdrawal on mood in women with past perimenopausal depression: A randomized clinical trial. JAMA Psychiatry. 10.1001/jamapsychiatry.2015.0111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt PJ, Nieman L, Danaceau MA, Tobin MB, Roca CA, Murphy JH, Rubinow DR, 2000. Estrogen replacement in perimenopause-related depression: A preliminary report. Am. J. Obstet. Gynecol 183, 414–420. 10.1067/mob.2000.106004 [DOI] [PubMed] [Google Scholar]
- Schmidt PJ, Nieman LK, Danaceau M. a, Adams LF, Rubinow DR, 1998. Differential behavioral effects of gonadal steroids in women with and in those without premenstrual syndrome. N. Engl. J. Med 10.1097/00006254-199805000-00019 [DOI] [PubMed] [Google Scholar]
- Soares CN, 2017. Depression and Menopause: Current Knowledge and Clinical Recommendations for a Critical Window. Psychiatr. Clin. North Am 10.1016/j.psc.2017.01.007 [DOI] [PubMed] [Google Scholar]
- Soares CN, Almeida OP, Joffe H, Cohen LSS, de Novaes Soares C, Almedia O, Joff H, Cohen LSS, 2001. Efficacy of estradiol for the treatment of depressive disorders in perimenopausal women: A double-blind, randomized, placebo-controlled trial. Arch. Gen. Psychiatry 58, 529–534. https://doi.org/yoa20238 [pii] [DOI] [PubMed] [Google Scholar]
- Stroud LR, Salovey P, Epel ES, 2002. Sex differences in stress responses: Social rejection versus achievement stress. Biol. Psychiatry, 10.1016/S0006-3223(02)01333-1 [DOI] [PubMed] [Google Scholar]
- Waters EM, Yildirim M, Janssen WGM, Lou WYYW, McEwen BS, Morrison JH, Milner TA, 2011. Estrogen and aging affect the synaptic distribution of estrogen receptor beta-immunoreactivity in the CA1 region of female rat hippocampus. Brain Res. 1379, 86–97. 10.1016/j.brainres.2010.09.069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss EL, Longhurst JG, Mazure CM, 1999. Childhood sexual abuse as a risk factor for depression in women: psychosocial and neurobiological correlates. Am. J. Psychiatry. 10.1176/ajp.156.6.816 [DOI] [PubMed] [Google Scholar]
- Weschsler D, 1997. Wechsler Memory Scale-Third Edition. [Google Scholar]
- Young E, Korszun A, 2010. Sex, trauma, stress hormones and depression. Mol. Psychiatry. 10.1038/mp.2009.94 [DOI] [PubMed] [Google Scholar]