Abstract
Tendon tissue engineering approaches are challenged by a limited understanding of the role mechanical loading plays in normal tendon development. We propose that the increased loading that developing postnatal tendons experience with the onset of locomotor behavior impacts tendon formation. The objective of this study was to assess the onset of spontaneous weight-bearing locomotion in postnatal day (P) 1, 5, and 10 rats, and characterize the relationship between locomotion and the mechanical development of weight-bearing and non-weight-bearing tendons. Movement was video recorded and scored to determine non-weight-bearing, partial weight-bearing, and full weight-bearing locomotor behavior at P1, P5, and P10. Achilles tendons, as weight-bearing tendons, and tail tendons, as non-weight-bearing tendons, were mechanically evaluated. We observed a significant increase in locomotor behavior in P10 rats, compared to P1 and P5. We also found corresponding significant differences in the maximum force, stiffness, displacement at maximum force, and cross-sectional area in Achilles tendons, as a function of postnatal age. However, the maximum stress, strain at maximum stress, and elastic modulus remained constant. Tail tendons of P10 rats had significantly higher maximum force, maximum stress, elastic modulus, and stiffness compared to P5. Our results suggest that the onset of locomotor behavior may be providing the mechanical cues regulating postnatal tendon growth, and their mechanical development may proceed differently in weight-bearing and non-weight-bearing tendons. Further analysis of how this loading affects developing tendons in vivo may inform future engineering approaches aiming to apply such mechanical cues to regulate engineered tendon formation in vitro.
1. Introduction
Tendons transfer mechanical forces from muscle to bone, and are critical for movement and locomotion. Frequent injury and poor healing are key motivators for tendon tissue engineering. To advance tendon tissue engineering, there is a need to better understand the processes that regulate normal tendon development. Tendon development can be characterized by describing the mechanical properties, but there is limited information available on how the mechanical properties of tendon progress during typical development. Structure-function relationships in developing and mature tendon have been reviewed (Benjamin et al., 2008; Connizzo et al., 2013), though few studies have characterized early postnatal tendons. Previous studies demonstrated that the mechanical properties of developing tendon increase throughout embryonic (Marturano et al., 2013; Schiele et al., 2013b) and postnatal growth (Ansorge et al., 2011; Torp et al., 1975). In chick models, significant increases were observed in the ultimate tensile stress of extensor tendons (McBride et al., 1988) and elastic modulus of calcaneal tendons (Marturano et al., 2013) between embryonic developmental stages (e.g., Hamburger and Hamilton (HH) stages) HH 42 and 43, and HH38 and 43, respectively. In developing mice, linear region stiffness and elastic modulus of Achilles tendons (ATs) increased with age from postnatal day (P) 4 to 28 (Ansorge et al., 2011). In humans, children aged 8-10 years and adults aged over 19 years had higher AT stiffness and elastic modulus, compared to children aged 5-7 (Waugh et al., 2012). Collectively, these findings illustrate that the mechanical properties of tendon change throughout embryonic and postnatal development. However, many of the mechanical and biochemical factors that regulate tendon formation during development remain unknown.
Unlike ATs, tail tendons (TTs) are regarded as force-transmitting, but primarily non-weight-bearing tendons, whose main function in rats and most mice is to position the tail (Kondratko-Mittnacht et al., 2015; Screen et al., 2013). In mutant embryonic mice null for scleraxis, a transcription factor and regulator of tenogenesis (Schweitzer et al., 2001), development differed between force-transmitting (forelimb flexors, long trunk, tail) and muscle-anchoring (short-range anchoring, intercostal) tendons, suggesting distinctions in the developmental processes of various tendons (Murchison et al., 2007). Although TTs are considered non-weight-bearing, they were also impacted by scleraxis loss-of-function, much like the force-transmitting limb tendons (Murchison et al., 2007). While mechanical properties of adult mouse (Derwin et al., 2001; Mikic et al., 2008; Reuvers et al., 2011) and rat TTs have been evaluated (Bruneau et al., 2010; Lavagnino et al., 2015), it is unknown how the mechanical development of TTs differs from weight-bearing tendons, such as the ATs.
Tissue engineering studies show that mechanical stimulation regulates tendon formation in vitro (Butler et al., 2008; Chokalingam et al., 2009; Juncosa-Melvin et al., 2007; Kalson et al., 2011; Kuo and Tuan, 2008; Li et al., 2015; Mubyana and Corr, 2018; Nirmalanandhan et al., 2007; Qin et al., 2015; Schiele et al., 2013a; Scott et al., 2011; Shearn et al., 2007; Subramony et al., 2013). Mechanical loading of cells in scaffolds enhanced collagen fibril quantity and diameter, elastic modulus, and ultimate tensile stress (Kalson et al., 2011), and collagen type I and III gene expression (Chokalingam et al., 2009; Juncosa-Melvin et al., 2007). Mechanically stimulated stem cell-seeded scaffolds increased maximum force, linear region stiffness, maximum stress, and elastic modulus of rabbit patellar tendon defects, compared to static controls (Shearn et al., 2007). These results suggest that mechanical loading impacts cell behavior and directs functional tendon tissue formation in vitro, but there is limited information on how mechanical stimulation impacts developing neonatal tendons in vivo.
Embryonic movement is a source of prenatal mechanical stimulation that may contribute to the correct development of musculoskeletal tissues (Hall and Herring, 1990; Hosseini and Hogg, 1991; Huang et al., 2015; Lamb et al., 2003; Schiele et al., 2013b; Schwartz et al., 2013; Scott et al., 1987). Tendon development in chick, mouse, and human is disrupted when embryonic movement is restricted or absent (Beckham et al., 1977; Huang et al., 2015; Mikic et al., 2000; Nemec et al., 2011; Pan et al., 2018). Muscle-less mice develop early-stage condensations of tendon progenitor cells that are subsequently lost by embryonic day 13.5, and this loss may be attributed to a lack of mechanical stimulation from the developing muscles (Huang et al., 2015). While movement in the embryo is important for tissue formation, the effects of mechanical stimulation on postnatal tendon development are not as well understood, partly because changes in postnatal movement patterns and tissue formation have not been characterized.
Throughout postnatal development, the mechanical loads that tendons experience are likely to increase as locomotion and weight-bearing behaviors increase. However, it remains unknown how the development of weight-bearing movement and locomotion in the neonate is associated with the functional development of tendons. Existing research has focused mainly on adult tendon; one study found that daily running uphill significantly increased the elastic modulus and maximum stress of the AT (Heinemeier et al., 2012). Tendons may also adapt to increased mechanical loading in adolescent athletes with increased stiffness (Mersmann et al., 2017). Restriction of motion negatively impacts adult tendon mechanical properties in humans (Kubo et al., 2000), and in normal (De Aro et al., 2012) and healing (Andersson et al., 2012) rats. Based on these studies, we propose that developmental changes in locomotion and weight-bearing increase loading of the musculoskeletal system, and contribute to the increased mechanical properties of developing postnatal tendons. In this study, we explored the relationship between the mechanical properties of neonatal rat tendons and their locomotor behavior. Developing rats are an ideal model system because gradual and significant changes occur in spontaneous posture and locomotion during the first two postnatal weeks. Movement patterns shift from limited loading of the hindlimbs (i.e., crawling) at birth to expression of weight-bearing quadrupedal walking by P10 (Swann and Brumley, 2018). To date, there is limited information on the development of locomotor behavior in rats before P10.
We aimed to characterize the relationship between changes in spontaneous locomotor behavior and mechanical properties of tendon. We hypothesized that developmental changes in locomotion and weight-bearing influence the mechanical properties of tendon in postnatal rats. To test this hypothesis, we measured the structural (maximum force, displacement at maximum force, stiffness, cross-sectional area) and material (maximum stress, strain at maximum stress, elastic modulus) properties of ATs and TTs, and the onset of weight-bearing locomotion as a function of age in neonatal rats at P1, P5, and P10. Evaluating weight-bearing and non-weight-bearing tendons allowed us to compare changes in structural and material properties of tissue subjected to different degrees of in vivo mechanical stimulation at the onset of locomotion.
2. Methods
2.1. Evaluation of weight-bearing locomotion
Subjects were offspring from Sprague-Dawley rats maintained in accordance with NIH guidelines (National Research Council, 2011), and the institutional animal care and use committee. To evaluate locomotion, rat pups were tested at P1, P5, or P10 (n=8 subjects per age). Subjects were removed from the home cage with the dam and individually tested in a clear, 8-in by 8-in Plexiglas box (i.e. open-field) to examine spontaneous locomotion. The open-field box was placed inside a temperature- and humidity-controlled incubator to maintain conditions during testing (35°C at P1, 33°C at P5, 30°C at P10). All behavior was video recorded for 20-minutes from a lateral and dorsal camera view, and stored on DVD. Following behavioral testing, subjects were euthanized via CO2 inhalation, the hindlimbs and tails were removed, packaged in saline-soaked gauze, and stored at −80° C.
Scoring of spontaneous locomotor behavior was conducted during video-playback using Datavyu (Version 1.3.4; Datavyu Team, 2014). Locomotor behaviors scored included non-weight-bearing hindlimb activity (i.e., hindlimb kicking, pivoting, crawling with inactive hindlimbs), partial weight-bearing of the hindlimbs (i.e., partial rearing and hindlimb-active crawling), and full weight-bearing hindlimb activity (i.e., walking, standing, and full rearing). One person scored all of the videos. Intra- and interrater reliability with a standard file was >90%. Differences in durations of each behavior category with developmental age were analyzed with one-way analysis of variance (ANOVA) and Tukey’s post-hoc test. Significance was set at p<0.05.
2.2. Evaluation of mechanical properties
To evaluate tendon mechanical properties, one AT per animal from P1 (n=4), P5 (n=5-7), and P10 (n=6-11) Sprague-Dawley rats was thawed and dissected in saline. Ranges in n-numbers (P5 and P10 ATs) are due to some missing images preventing calculation of cross-sectional area and subsequent determination of material properties, though structural properties were still obtained. After the skin was removed, the muscles and fascia were teased away from the tibia and the fibula, exposing the AT. The tendon was isolated from the limb while maintaining the myotendinous junction at the gastrocnemius muscle and the insertion at the calcaneus. The myotendinous junction and calcaneus bone were mounted facing anteriorly into our custom small-scale tensile load frame with cyanoacrylate (Raveling et al., 2018). A 500 g capacity load cell (Honeywell, Columbus, OH) measured force, and custom LabVIEW software (National Instruments, Austin, TX) recorded force and displacement data. Front and side view images of each tendon were obtained using a digital camera (Thorcam DCC1645C, Thorlabs, Inc, Newton, NJ). Tendon length and width were measured using ImageJ (NIH, Bethesda, MD). ATs are wider mediolaterally than anteroposteriorly, so consistent with previous studies (Lee et al., 2017), ATs were assumed to have an elliptical cross-section. Cross-sectional area was calculated using the narrowest front and side view width of the ATs, whose longitudinal location coincided in both views. ATs were preloaded to 0.05 N, preconditioned with 10 cycles of loading to 1% strain, and pulled to failure at 0.1 mm/s. ATs and TTs were kept hydrated with saline throughout testing. Maximum stress, strain at maximum stress, linear elastic modulus, and linear stiffness were calculated from the force-displacement curves and cross-sectional areas. The linear region was determined from the slope of a line fit to curves that had a R2 > 0.90 (R2= 0.976 ± 0.023, supplementary figure 1). Differences with developmental age were evaluated using a one-way ANOVA and Tukey’s post-hoc test.
TTs from P5 (n=7) and P10 (n=8) rats were dissected in saline using methods previously described (Bruneau et al., 2010). Briefly, whole TTs were grasped at the proximal end of the skinned tail and gently removed. TTs were secured with cyanoacrylate into cardboard c-clamps to hold the tissues during mounting in the load frame. The c-clamps were cut following mounting. TTs were measured using ImageJ as described for ATs, but TTs were assumed to have a circular cross-section based on previous studies (Bruneau et al., 2010; Parent et al., 2010) and using the narrowest diameter (measured three times and averaged) from the side view of the mounted TTs. A preload of 0.01 N for P5 tendons and 0.05 N for P10 TTs was applied to remove the slack. TTs were not preconditioned due to their fragility. TTs were pulled to failure at 0.1 mm/s and force was measured using a 150 g capacity load cell (Honeywell). TTs were evaluated with unpaired, two-tailed t-tests. Significance was set at p<0.05.
3. Results
3.1. Weight-bearing locomotor behavior increases with age
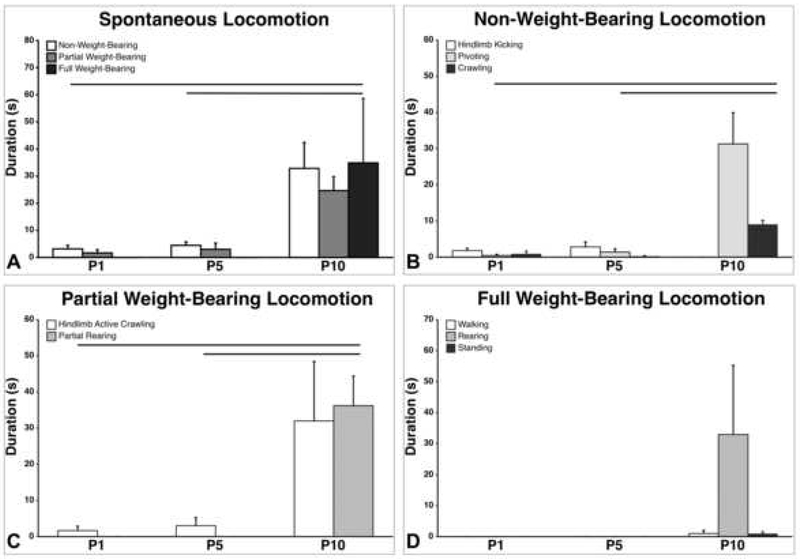
Spontaneous locomotion increased from P1 to P10 (Figure 1A–D). Durations of locomotion significantly increased from P1 to P10 (p=0.002) (Figure 1A). P10 rats showed significantly more non-weight-bearing hindlimb activity compared to P1s (p=0.003) and P5s (p=0.005) (Figure 1B). Specifically, P10 rats showed significantly more pivoting than P1s (p=0.001) and P5s (p=0.001). Differences in hindlimb kicks and crawling (without hindlimbs) across ages were not significant. Partial weight-bearing hindlimb activity increased significantly (p<0.001) during the first 10 postnatal days, with P10s exhibiting significantly more hindlimb-active crawling than P1s (p=0.001) and P5s (p=0.003) (Figure 1C). Only P10 showed partial rearing. For full weight-bearing hindlimb locomotion, the effect of age was not significant (p=0.140); however, only P10 pups showed any full weight-bearing behavior (Figure 1D). There were no significant differences in any category of weight-bearing locomotion between P1 and P5.
Figure 1.
Spontaneous locomotion in postnatal rats, when tested in an open field. A) Durations of non, partial, and full weight-bearing spontaneous locomotion across developmental age. B) Categories of non-weight-bearing locomotion (hindlimb kicking, pivoting, and crawling with inactive hindlimbs). C) Categories of partial weight-bearing locomotion (hindlimb active crawling and partial rearing). D) Categories of full weight-bearing locomotion (walking, full rearing, and standing). Only P10 rats showed any full weight-bearing behavior. Lines denote significant differences between groups. Bars show mean durations; vertical lines depict SEM.
3.2. Achilles tendon mechanical properties
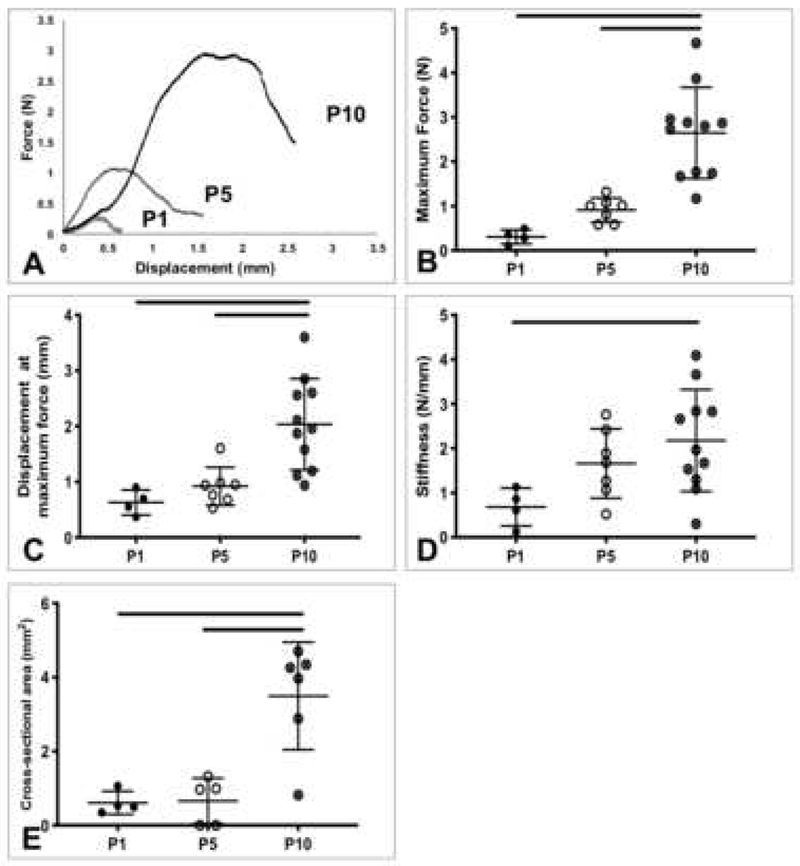
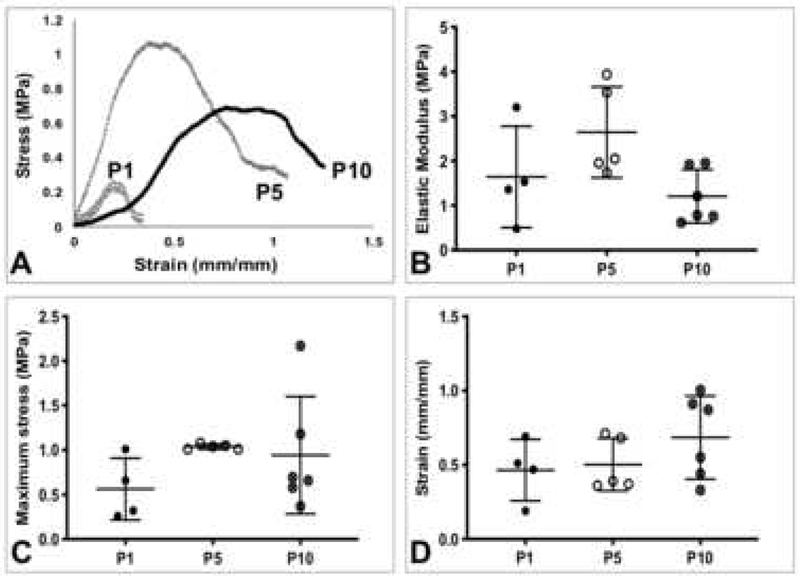
Average mechanical properties of rat tendons and gauge lengths are listed in Table 1. All samples included in the data analysis failed at the midsubstance. Representative force-displacement and stress-strain curves of P1, P5, and P10 ATs showed changes with age (Figure 2A, 3A). The maximum force, displacement at maximum force, stiffness, and cross-sectional area of ATs increased as a function of age (Figure 2B, C, D, E). Maximum force for P10 ATs was significantly higher compared to P1 (p=0.0001) and P5 (p=0.0004) (Figure 2B). Displacement at maximum force was significantly higher for P10 ATs compared to P1 (p=0.0032) and P5 (p=0.0046) (Figure 2C). Stiffness was significantly higher for P10 ATs compared to P1 (p=0.03) (Figure 2D). Cross-sectional area of P10 ATs was significantly larger than P1 (p=0.0023) and P5 (p=0.0016) ATs (Figure 2E). Elastic modulus of P10 ATs trended lower than P5 (p=0.06), while maximum stress and strain at maximum stress remained consistent between age groups (Figure 3B, C, D).
Table 1.
Mechanical properties of P1, P5, and P10 rat ATs, and P5 and P10 rat TTs (mean ± standard deviation)
| Tendon | Max force (N) | Displacement at max force (mm) | Stiffness (N/mm) | Max stress (MPa) | Strain at max stress (mm/mm) | Elastic modulus (MPa) | Cross-sectional area (mm2) | Mechanical testing sample gauge length (mm) |
|---|---|---|---|---|---|---|---|---|
| P1 AT | 0.31±0.16 | 0.63±0.22 | 0.68±0.43 | 0.56±0.34 | 0.47±0.21 | 1.64±1.14 | 0.61±0.31 | 0.89±0.46 |
| P5 AT | 0.91±0.27 | 0.92±0.34 | 1.66±0.78 | 1.04±0.030 | 0.50±0.18 | 2.64±1.02 | 0.66±0.62 | 1.55±0.29 |
| P10 AT | 2.65±1.03 | 2.04±0.82 | 2.18±1.15 | 0.94±0.66 | 0.68±0.28 | 1.21±0.60 | 3.50±1.45 | 3.17±1.40 |
| P5 TT | 0.30±0.18 | 1.59±0.74 | 0.26±0.16 | 1.15±0.55 | 0.61±0.29 | 2.66±1.57 | 0.27±0.12 | 2.89±1.23 |
| P10 TT | 0.66±0.17 | 1.69±0.58 | 0.50±0.21 | 3.23±2.33 | 0.40±0.19 | 10.33±6.48 | 0.31±0.15 | 4.76±1.63 |
Figure 2.
Postnatal rat Achilles tendon structural properties. A) Representative force-displacement curves of P1, P5, and P10 Achilles tendons. B) Maximum force, C) displacement at maximum force, D) stiffness, and E) cross-sectional area. P10 Achilles tendons had significantly increased structural properties, compared to P1 and P5. Lines denote significant differences between groups. Bars represent mean ± standard deviation.
Figure 3.
Postnatal rat Achilles tendon material properties. A) Representative stress-strain curves of P1, P5, and P10 Achilles tendons. B) Elastic modulus, C) maximum stress, and D) strain at maximum stress. P10 Achilles tendon material properties remained relatively constant between groups. Bars represent mean ± standard deviation.
3.3. Tail tendon mechanical properties
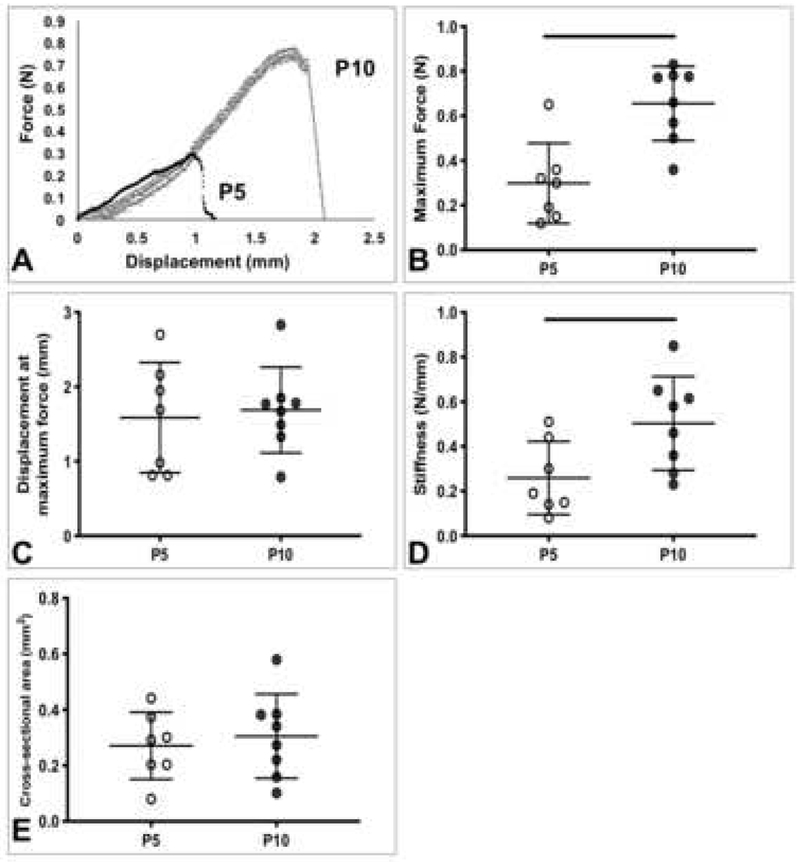
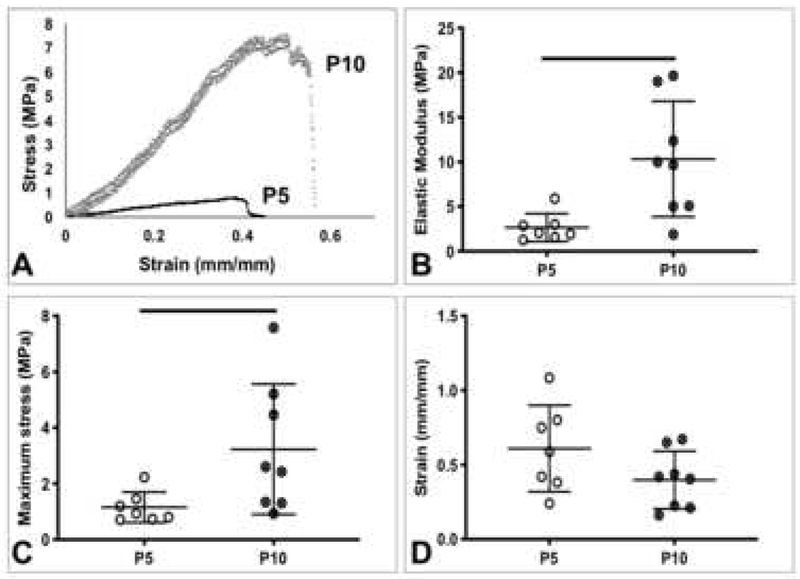
Representative force-displacement and stress-strain curves of TTs showed increases from P5 to P10 (Figure 4A, 5A). The maximum force (p=0.0015), stiffness (p=0.027), elastic modulus (p=0.009), and maximum stress (p=0.039) of P10 TTs were significantly higher than the corresponding properties in P5 TTs (Figure 4B, 4D, Figure 5B, C). No significant differences were observed for displacement at maximum force, cross-sectional area, and strain at maximum stress between age groups (Figure 4C, E, Figure 5D).
Figure 4.
Postnatal rat tail tendon structural properties. A) Representative force-displacement curves of P5 and P10 tail tendons. B) Maximum force, C) displacement at maximum force, D) stiffness, and E) cross-sectional area. P10 tails tendons had significantly increased structural properties, compared to P5 tail tendons. Lines denote significant differences between groups. Bars represent mean ± standard deviation.
Figure 5.
Postnatal rat tail tendon material properties. A) Representative stress-strain curves of P5 and P10 tail tendons. B) Elastic modulus, C) maximum stress, and D) strain at maximum stress. P10 tails tendons had significantly increased material properties, compared to P5 tail tendons. Lines denote significant differences between groups. Bars represent mean ± standard deviation.
4. Discussion
An attractive approach for tendon tissue engineering involves mimicking native tissue development to produce functional constructs. A challenge has been limited information on how the mechanical function of tendon forms during development, and the role mechanical loading plays in tendon formation. Several developmental processes, including the onset of locomotion, may regulate tendon formation. In this study, we examined how the onset of weight-bearing locomotion coincides with changes in the structural and material properties of weight-bearing ATs and non-weight-bearing TTs. We found increases in hindlimb weight-bearing locomotion behavior beginning between P1 and P10. At the same time, structural properties (maximum force, displacement at maximum force, stiffness, and cross-sectional area) of ATs increased significantly at P10, compared to P1 and P5, whereas material properties (maximum stress, strain at maximum stress, and elastic modulus) did not. Structural and material properties of TTs increased between P5 and P10. These findings suggest that increased locomotor behavior coincides with neonatal AT growth and maintained material properties, and that the developmental mechanisms of the non-weight-bearing TTs may be unique from the weight-bearing ATs.
Increased locomotion initiating between P1 and P10 may provide the mechanical loading needed for regulating tendon growth. Narrowing down a specific time point during which important developmental changes might arise is novel information that can aid in the search for potential biomechanical cues to direct tenogenesis in vitro, or to stimulate repair in vivo. Previous characterization of age-related locomotor patterns during postnatal development showed quadrupedal walking is not consistent in rats until P10, and gradually increases in duration throughout the second postnatal week (Swann and Brumley, 2018). The current finding that full weight-bearing locomotion was higher in P10s than at earlier ages supports this, suggesting that the developmental range in which significant mechanical stimulation of hindlimb tendons begins may be quite small, and initiated by mechanical cues from this new movement pattern. Future work should examine the correlation between AT mechanical properties and weight-bearing locomotion in rats during the second and third postnatal weeks (i.e., time points with increased weight-bearing locomotion), to investigate dose effects related to mechanical loading. Experimental manipulation of weight-bearing locomotion during the postnatal period would further elucidate the coordination between locomotor behavior and musculoskeletal development.
Changes in structural and material properties of neonatal tendon during the days immediately following birth are less understood. To our knowledge, this is the first study assessing the mechanical properties of rat ATs and TTs at these early postnatal ages. In rat ATs, we found significant increases in structural properties, specifically maximum force and displacement at maximum force, while no significant differences were found in the material properties (maximum stress, strain at maximum stress, and elastic modulus). Our AT results are consistent with another study that found no significant differences in the material properties of normally developing mouse ATs from P7 to P10 (Ansorge et al., 2011). The significant increase in AT cross-sectional area at P10, compared to both P1 and P5 (Figure 2E), could explain why material properties were unchanged. It is possible that increased mechanical stimuli from initiation of weight-bearing behavior lead to lateral tissue expansion, increasing cross-sectional area. The differences we observed in AT dimensions and structural mechanical properties coincide temporally with changes in the collagen of rat ATs (Chen et al., 2016). At P4, extracellular matrix in the ATs became denser, and long parallel fibers with defined crimping were visible by P7, though at the nanoscale, collagen fibril diameter did not appear to increase until P14 (Chen et al., 2016). Similarly, collagen fibril diameter in postnatal mouse AT was significantly increased at P21, compared to P4, 7, and 10 (Ansorge et al., 2011). Together, these findings indicate that neonatal ATs are still immature and the collagen structure is dynamic and developing. Weight-bearing loading may induce rapid lateral expansion in neonatal ATs and hence increase the dimension-dependent structural properties by P10, but underlying material properties have not yet been significantly impacted. Collagen cross-linking influences elastic modulus (Makris et al., 2014; Marturano et al., 2014), and may be a regulator of material properties in developing ATs. In neonatal ATs, significant increases in dimensions and structural properties may couple with minimal material property changes, to ensure that tendon is responsive to mechanical stimuli before becoming fully cross-linked and less adaptable. Future studies investigating collagen fibril organization, cross-linking, diameter, and density from P1 to P10 and beyond may help to identify impacts of weight-bearing loading on the collagen structure in ATs.
In contrast to AT, both structural and material properties of TTs increased significantly from P5 to P10. Elastic modulus, stiffness, maximum stress, and maximum force were significantly higher in TTs at P10, compared to P5. Since rat TTs are non-weight-bearing, they may experience a smaller increase in mechanical loading as locomotion develops, and hence lateral tissue expansion proceeds more slowly, leading to no significant increase in cross-sectional area at P10. Significant increases in structural and material properties of TTs suggest a more rapid maturation, compared to ATs. Future studies are needed to explore the mechanisms that regulate TT compared to AT development.
Neonatal rat tendons are challenging to mechanically test, as the tissues are small and soft, requiring a small-scale load frame with low force-capacity load cells (e.g., 150 g), leading to some limitations. Tendon isolation and mounting is difficult, and measuring their small cross-sectional areas may be a source of some variability and error. To control for this, all tendons were evaluated in the same way. Cross-sectional areas were measured multiple times and averaged, which reduces error (Parent et al., 2010). Neonatal AT is particularly challenging to evaluate. The AT is anterior to and overlaid by the plantaris tendon, and composed of three different bundles (Lee and Elliott, 2019). To avoid damaging the tissue, the plantaris tendon was not removed and the whole AT complex was used. Neonatal AT is also relatively short, compared to its width, and may be prone to gripping artifacts. The tibialis anterior (TA), another limb tendon, has an increased length to width ratio, and has been evaluated at P2 (Calve et al., 2010). TA tendons may be evaluated in future studies. With regard to the TTs, we could only mechanically evaluate P5 and P10 groups. P1 TTs were too small and fragile to reliably isolate from the tails and mount into our load frame.
Finally, we did not control for sex during this study. At more mature ages, differences have been detected in AT mechanical properties between male and female rodents (Mikic et al., 2010; Pardes et al., 2016). At younger ages (4 weeks), body mass, collagen content, elastic modulus, and maximum stress have been shown to be similar between male and female, and no significant differences were found in TTs (Mikic et al., 2010). Sex differences of the neonates likely did not affect our results, though future studies will control for sex.
Taken together, increased locomotor behavior coincides with AT growth and maintained material properties, and development of non-weight-bearing TTs may be unique from the weight-bearing ATs. Future studies will manipulate the mechanical environment during development to better understand the impacts of locomotion-associated loading on tendon formation. Future investigations will also explore the mechanisms driven by this interaction, and how the developmental process may be different in weight-bearing and non-weight-bearing tendons. Overall, we found a parallel increase in locomotor behavior and functional tendon formation, narrowing down the postnatal time period during which locomotion may affect tendon development. New information on the postnatal developmental timeline of rat tendon and the relationship between locomotion and tendon mechanical properties will provide insights into how these processes occur during normal development of the entire organism. These findings have implications for directing functional tendon formation in engineered tissues.
Supplementary Material
Supplementary Figure. Linear regions of postnatal rat Achilles and tail tendon stress-strain curves. A) Representative stress-strain curve of a P10 Achilles tendon with the linear region selected (dark gray) and the modulus fit showing (dotted line). B) Representative stress-strain curve of a P10 tail tendon with the linear region selected (dark gray) and the modulus fit showing (dotted line). R2 values are shown for the trendlines.
Acknowledgments
This study was supported by the INBRE program, NIH Grant No. P20 GM103408 (National Institutes of General Medical Sciences), a Burroughs Wellcome Fund, Collaborative Research Travel Grant (to MRB and NRS), and the John F. Keegan Fellowship (to SKT). The authors would like to thank Nicholas Pancheri for reading the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement
The authors have no conflicts of interest to disclose.
Contributor Information
Sophia K. Theodossiou, Biological Engineering, University of Idaho, Moscow, ID, Mailing address: 875 Perimeter Dr. MS 0904, Moscow, ID 83844
Aimee L. Bozeman, Psychology, Idaho State University, Pocatello, ID, Mailing address: 921 S 8th Avenue Stop 8021, Pocatello, ID 83209
Nicholas Burgett, Psychology, Idaho State University, Pocatello, ID, Mailing address: 921 S 8th Avenue Stop 8021, Pocatello, ID 83209.
Michele R. Brumley, Psychology, Idaho State University, Pocatello, ID, Mailing address: 921 S 8th Avenue Stop 8021, Pocatello, ID 83209
Hillary E. Swann, Psychology, Idaho State University, Pocatello, ID, Mailing address: 921 S 8th Avenue Stop 8021, Pocatello, ID 83209
Abigail R. Raveling, Biological Engineering, University of Idaho, Moscow, ID, Mailing address: 875 Perimeter Dr. MS 0904, Moscow, ID 83844
Jordan J. Becker, Psychology, University of Idaho, Moscow, ID, Mailing address: 875 Perimeter Dr. MS 3043, Moscow, ID 83844
Nathan R. Schiele, Biological Engineering, University of Idaho, Moscow, ID, Mailing address: 875 Perimeter Dr. MS 0904, Moscow, ID 83844.
References
- Andersson T, Eliasson P, Hammerman M, Sandberg O, Aspenberg P, 2012. Low-level mechanical stimulation is sufficient to improve tendon healing in rats. J Appl Physiol 113, 1398–1402. [DOI] [PubMed] [Google Scholar]
- Ansorge HL, Adams S, Birk DE, Soslowsky LJ, 2011. Mechanical, compositional, and structural properties of the post-natal mouse Achilles tendon. Ann Biomed Eng 39, 1904–1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckham C, Dimond R, Greenlee TK Jr., 1977. The role of movement in the development of a digital flexor tendon. Am J Anat 150, 443–459. [DOI] [PubMed] [Google Scholar]
- Benjamin M, Kaiser E, Milz S, 2008. Structure-function relationships in tendons: a review. J Anat 212, 211–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruneau A, Champagne N, Cousineau-Pelletier P, Parent G, Langelier E, 2010. Preparation of rat tail tendons for biomechanical and mechanobiological studies. Journal of visualized experiments : JoVE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler DL, Juncosa-Melvin N, Boivin GP, Galloway MT, Shearn JT, Gooch C, Awad H, 2008. Functional tissue engineering for tendon repair: A multidisciplinary strategy using mesenchymal stem cells, bioscaffolds, and mechanical stimulation. J Orthop Res 26, 1–9. [DOI] [PubMed] [Google Scholar]
- Calve S, Lytle IF, Grosh K, Brown DL, Arruda EM, 2010. Implantation increases tensile strength and collagen content of self-assembled tendon constructs. J Appl Physiol (1985) 108, 875–881. [DOI] [PubMed] [Google Scholar]
- Chen JL, Zhang W, Liu ZY, Zhu T, Shen WL, Ran JS, Tang QM, Gong XN, Backman LJ, Chen X, Chen XW, Wen FQ, Ouyang HW, 2016. Characterization and comparison of post-natal rat Achilles tendon-derived stem cells at different development stages. Sci Rep 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chokalingam K, Juncosa-Melvin N, Hunter SA, Gooch C, Frede C, Florert J, Bradica G, Wenstrup R, Butler DL, 2009. Tensile stimulation of murine stem cell-collagen sponge constructs increases collagen type I gene expression and linear stiffness. Tissue Eng Part A 15, 2561–2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connizzo BK, Yannascoli SM, Soslowsky LJ, 2013. Structure-function relationships of postnatal tendon development: A parallel to healing. Matrix Biol 32, 106–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Aro AA, De Campos Vidal B, Biancalana A, Tolentino FT, Gomes L, Mattiello SM, Pimentel ER, 2012. Analysis of the Deep Digital Flexor Tendon in Rats Submitted to Stretching after Immobilization. Connect Tissue Res 53, 29–38. [DOI] [PubMed] [Google Scholar]
- Derwin KA, Soslowsky LJ, Kimura JH, Plaas AH, 2001. Proteoglycans and glycosaminoglycan fine structure in the mouse tail tendon fascicle. J Orthop Res 19, 269–277. [DOI] [PubMed] [Google Scholar]
- Hall BK, Herring SW, 1990. Paralysis and growth of the musculoskeletal system in the embryonic chick. Journal of Morphology 206, 45–56. [DOI] [PubMed] [Google Scholar]
- Heinemeier KM, Skovgaard D, Bayer ML, Qvortrup K, Kjaer A, Kjaer M, Magnusson SP, Kongsgaard M, 2012. Uphill running improves rat Achilles tendon tissue mechanical properties and alters gene expression without inducing pathological changes. J Appl Physiol 113, 827–836. [DOI] [PubMed] [Google Scholar]
- Hosseini A, Hogg DA, 1991. The effects of paralysis on skeletal development in the chick embryo. I. General effects. J Anat 177, 159–168. [PMC free article] [PubMed] [Google Scholar]
- Huang AH, Riordan TJ, Pryce BA, Weibel JL, Watson SS, Long F, Lefebvre V, Harfe BD, Stadler HS, Akiyama H, Tufa SF, Keene DR, Schweitzer R, 2015. Musculoskeletal integration at the wrist underlies the modular development of limb tendons. Development 142, 2431–2441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juncosa-Melvin N, Matlin KS, Holdcraft RW, Nirmalanandhan VS, Butler DL, 2007. Mechanical stimulation increases collagen type I and collagen type III gene expression of stem cell-collagen sponge constructs for patellar tendon repair. Tissue Eng 13, 1219–1226. [DOI] [PubMed] [Google Scholar]
- Kalson NS, Holmes DF, Herchenhan A, Lu Y, Starborg T, Kadler KE, 2011. Slow stretching that mimics embryonic growth rate stimulates structural and mechanical development of tendon-like tissue in vitro. Dev Dyn 240, 2520–2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondratko-Mittnacht J, Duenwald-Kuehl S, Lakes R, Vanderby R Jr., 2015. Shear load transfer in high and low stress tendons. J Mech Behav Biomed Mater 45, 109–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubo K, Akima H, Kouzaki M, Ito M, Kawakami Y, Kanehisa H, Fukunaga T, 2000. Changes in the elastic properties of tendon structures following 20 days bed-rest in humans. European Journal of Applied Physiology 83, 463–468. [DOI] [PubMed] [Google Scholar]
- Kuo CK, Tuan RS, 2008. Mechanoactive tenogenic differentiation of human mesenchymal stem cells. Tissue Eng Part A 14, 1615–1627. [DOI] [PubMed] [Google Scholar]
- Lamb KJ, Lewthwaite JC, Lin JP, Simon D, Kavanagh E, Wheeler-Jones CP, Pitsillides AA, 2003. Diverse range of fixed positional deformities and bone growth restraint provoked by flaccid paralysis in embryonic chicks. Int J Exp Pathol 84, 191–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavagnino M, Malek K, Gardner KL, Arnoczky SP, 2015. Thermal energy enhances cell-mediated contraction of lax rat tail tendon fascicles following exercise. Muscles, ligaments and tendons journal 5, 51–55. [PMC free article] [PubMed] [Google Scholar]
- Lee AH, Elliott DM, 2019. Comparative multi-scale hierarchical structure of the tail, plantaris, and Achilles tendons in the rat. J Anat 234, 252–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Chieh H, Lin C, Jou I, Sun Y, Kuo L, Wu P, Su F, 2017. Characteristics of Sonography in a Rat Achilles Tendinopathy Model: Possible Non-invasive Predictors of Biomechanics. Sci Rep 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YH, Ramcharan M, Zhou ZP, Leong DJ, Akinbiyi T, Majeska RJ, Sun HB, 2015. The Role of Scleraxis in Fate Determination of Mesenchymal Stem Cells for Tenocyte Differentiation. Sci Rep 5. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Makris EA, Responte DJ, Paschos NK, Hu JC, Athanasiou KA, 2014. Developing functional musculoskeletal tissues through hypoxia and lysyl oxidase-induced collagen cross-linking. Proc Natl Acad Sci U S A 111, E4832–4841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marturano JE, Arena JD, Schiller ZA, Georgakoudi I, Kuo CK, 2013. Characterization of mechanical and biochemical properties of developing embryonic tendon. Proc Natl Acad Sci U S A 110, 6370–6375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marturano JE, Xylas JF, Sridharan GV, Georgakoudi I, Kuo CK, 2014. Lysyl oxidase-mediated collagen crosslinks may be assessed as markers of functional properties of tendon tissue formation. Acta Biomater 10, 1370–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride DJ, Trelstad RL, Silver FH, 1988. Structural and Mechanical Assessment of Developing Chick Tendon. Int J Biol Macromol 10, 194–200. [Google Scholar]
- Mersmann F, Bohm S, Schroll A, Boeth H, Duda GN, Arampatzis A, 2017. Muscle and tendon adaptation in adolescent athletes: A longitudinal study. Scand J Med Sci Sports 27, 75–82. [DOI] [PubMed] [Google Scholar]
- Mikic B, Amadei E, Rossmeier K, Bierwert L, 2010. Sex matters in the establishment of murine tendon composition and material properties during growth. J Orthop Res 28, 631–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikic B, Entwistle R, Rossmeier K, Bierwert L, 2008. Effect of GDF-7 deficiency on tail tendon phenotype in mice. J Orthop Res 26, 834–839. [DOI] [PubMed] [Google Scholar]
- Mikic B, Johnson TL, Chhabra AB, Schalet BJ, Wong M, Hunziker EB, 2000. Differential effects of embryonic immobilization on the development of fibrocartilaginous skeletal elements. Journal of rehabilitation research and development 37, 127–133. [PubMed] [Google Scholar]
- Mubyana K, Corr DT, 2018. Cyclic Uniaxial Tensile Strain Enhances the Mechanical Properties of Engineered, Scaffold-Free Tendon Fibers. Tissue Eng Part A. [DOI] [PubMed] [Google Scholar]
- Murchison ND, Price BA, Conner DA, Keene DR, Olson EN, Tabin CJ, Schweitzer R, 2007. Regulation of tendon differentiation by scleraxis distinguishes force-transmitting tendons from muscle-anchoring tendons. Development 134, 2697–2708. [DOI] [PubMed] [Google Scholar]
- Nemec SF, Hoftberger R, Nemec U, Bettelheim D, Brugger PC, Kasprian G, Amann G, Rotmensch S, Graham JM Jr., Rimoin DL, Prayer D, 2011. Fetal akinesia and associated abnormalities on prenatal MRI. Prenat Diagn 31,484–490. [DOI] [PubMed] [Google Scholar]
- Nirmalanandhan VS, Dressler MR, Shearn JT, Juncosa-Melvin N, Rao M, Gooch C, Bradica G, Butler DL, 2007. Mechanical stimulation of tissue engineered tendon constructs: effect of scaffold materials. J Biomech Eng 129, 919–923. [DOI] [PubMed] [Google Scholar]
- Pan XS, Li J, Brown EB, Kuo CK, 2018. Embryo movements regulate tendon mechanical property development. Philosophical transactions of the Royal Society of London. Series B, Biological sciences 373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardes AM, Freedman BR, Fryhofer GW, Salka NS, Bhatt PR, Soslowsky LJ, 2016. Males have Inferior Achilles Tendon Material Properties Compared to Females in a Rodent Model. Ann Biomed Eng 44, 2901–2910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parent G, Cyr M, Desbiens-Blais F, Langelier E, 2010. Bias and precision of algorithms in estimating the cross-sectional area of rat tail tendons. Meas Sci Technol 21, 125802. [Google Scholar]
- Qin T-W, Sun Y-L, Thoreson AR, Steinmann SP, Amadio PC, An K-N, Zhao C, 2015. Effect of mechanical stimulation on bone marrow stromal celleseeded tendon slice constructs: A potential engineered tendon patch for rotator cuff repair. Biomaterials 51, 43–50. [DOI] [PubMed] [Google Scholar]
- Raveling AR, Theodossiou SK, Schiele NR, 2018. A 3D printed mechanical bioreactor for investigating mechanobiology and soft tissue mechanics. MethodsX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuvers J, Thoreson AR, Zhao C, Zhang L, Jay GD, An KN, Warman ML, Amadio PC, 2011. The mechanical properties of tail tendon fascicles from lubricin knockout, wild type and heterozygous mice. J Struct Biol 176, 41–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiele NR, Koppes RA, Chrisey DB, Corr DT, 2013a. Engineering cellular fibers for musculoskeletal soft tissues using directed self-assembly. Tissue Eng Part A 19, 1223–1232. [DOI] [PubMed] [Google Scholar]
- Schiele NR, Marturano JE, Kuo CK, 2013b. Mechanical factors in embryonic tendon development: potential cues for stem cell tenogenesis. Curr Opin Biotechnol 24, 834–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz AG, Lipner JH, Pasteris JD, Genin GM, Thomopoulos S, 2013. Muscle loading is necessary for the formation of a functional tendon enthesis. Bone 55, 44–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweitzer R, Chyung JH, Murtaugh LC, Brent AE, Rosen V, Olson EN, Lassar A, Tabin CJ, 2001. Analysis of the tendon cell fate using Scleraxis, a specific marker for tendons and ligaments. Development 128, 3855–3866. [DOI] [PubMed] [Google Scholar]
- Scott A, Danielson P, Abraham T, Fong G, Sampaio AV, Underhill TM, 2011. Mechanical force modulates scleraxis expression in bioartificial tendons. J Musculoskelet Neuronal Interact 11, 124–132. [PubMed] [Google Scholar]
- Scott JE, Haigh M, Neo GE, Gibson S, 1987. The effect of muscle paralysis on the radial growth of collagen fibrils in developing tendon. Clin Sci (Lond) 72, 359–363. [DOI] [PubMed] [Google Scholar]
- Screen HRC, Toorani S, Shelton JC, 2013. Microstructural stress relaxation mechanics in functionally different tendons. Medical Engineering & Physics 35, 96–102. [DOI] [PubMed] [Google Scholar]
- Shearn JT, Juncosa-Melvin N, Boivin GP, Galloway MT, Goodwin W, Gooch C, Dunn MG, Butler DL, 2007. Mechanical stimulation of tendon tissue engineered constructs: Effects on construct stiffness, repair biomechanics, and their correlation. J Biomech Eng-T Asme 129, 848–854. [DOI] [PubMed] [Google Scholar]
- Subramony SD, Dargis BR, Castillo M, Azeloglu EU, Tracey MS, Su A, Lu HH, 2013. The guidance of stem cell differentiation by substrate alignment and mechanical stimulation. Biomaterials 34, 1942–1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swann HE, Brumley MR, 2018. Locomotion and posture development in immature male and female rats (Rattus norvegicus): Comparison of sensory-enriched versus sensory-deprived testing environments. J Comp Psychol Epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torp S, Arridge RGC, Armeniades CD, Baer E, 1975. Structure-property relationships in tendon as a function of age. Structure of Fibrous Biopolymers 26, 197–221. [Google Scholar]
- Waugh CM, Blazevich AJ, Fath F, Korff T, 2012. Age-related changes in mechanical properties of the Achilles tendon. J Anat 220, 144–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure. Linear regions of postnatal rat Achilles and tail tendon stress-strain curves. A) Representative stress-strain curve of a P10 Achilles tendon with the linear region selected (dark gray) and the modulus fit showing (dotted line). B) Representative stress-strain curve of a P10 tail tendon with the linear region selected (dark gray) and the modulus fit showing (dotted line). R2 values are shown for the trendlines.