Abstract
Electronic cigarette (e-cigarette; e-cig) use has grown exponentially in recent years despite their unknown health effects. E-cig aerosols are now known to contain hazardous chemical compounds, including carbonyls and reactive oxygen species (ROS), and these compounds are directly inhaled by consumers during e-cig use. Both carbonyls and ROS are formed when the liquid comes into contact with a heating element that is housed within an e-cig’s atomizer. In the present study, the effect of coil resistance (1.5 Ω and 0.25 Ω coils, to obtain a total wattage of 8±2 W and 40±5 W, respectively) on the generation of carbonyls (formaldehyde, acetaldehyde, acrolein) and ROS was investigated. The effect of the aerosols generated by different coils on the viability of H1299 human lung carcinoma cells was also evaluated. Our results show a significant (p<0.05) correlation between the low resistance coils and the generation of higher concentrations of the selected carbonyls and ROS in e-cig aerosols. Moreover, exposure to e-cig vapor reduced the viability of H1299 cells by up to 45.8%, and this effect was inversely related to coil resistance. Although further studies are needed to better elucidate the potential toxicity of e-cig emissions, our results suggest that these devices may expose users to hazardous compounds which, in turn, may promote chronic respiratory diseases.
Keywords: e-cig, resistance, oxidative stress, carbonyl compounds, lung cancer cells
1. Introduction
Electronic cigarettes (e-cigarettes, e-cigs) have been on the consumer market for almost a decade and are marketed as an alternative to conventional combustion cigarettes. E-cigs have been promoted as a potential approach to aid in smoking cessation (Franks et al., 2018). Of particular concern, e-cig popularity has grown rapidly among young people worldwide. In 2014, e-cig use among US adolescents surpassed the use of conventional cigarette for the first time (Arrazola et al., 2014). While e-cigs do not produce carcinogenic combustion products such as polycyclic aromatic hydrocarbons, and e-cig liquids do not contain tobacco associated carcinogens (e.g., nitrosoamines), there is growing evidence that e-cigs do generate harmful substances during their use. Several authors have reported the presence of reactive carbonyls, including formaldehyde, acetaldehyde, acetone and others, in e-cigarette vapor (Goniewicz et al., 2014; Ogunwale et al., 2017; Bitzer et al., 2019). In addition, both stable, long-lived radicals (Lerner et al., 2015a; Lerner et al., 2015b; Sussan et al., 2015) and short-lived, highly reactive radicals (Goel et al., 2015) have been found in e-cig vapor. Oxidative stress induced by cigarette smoke has been shown to play a key role in the pathogenesis of cancer (Pryor et al., 1997), cardiovascular disease (Messner and Bernhard, 2014), and chronic obstructive pulmonary disease (COPD) (Centers for Disease Control and Prevention, 2010; Domej et al., 2014; Kirkham and Rahman, 2006). Likewise, e-cig vapor can induce oxidative stress that can lead to inflammation (Lerner et al., 2015a; Muthumage et al., 2018; Scott et al., 2018), cytotoxicity in vitro (Scott et al., 2018; Vasanthi Bathrinaraynan et al., 2018; Scheffer et al., 2015; Zhanh et al., 2012) and toxicity in vivo that may increase cancer risk (Canistro et al., 2017).
In contrast to conventional cigarettes, which differ primarily in terms of nicotine content, tobacco variety and filter type, e-cigs are highly customizable with respect to their operating parameters and the chemical composition of their liquids. These liquids are available in a wide-variety of flavors and nicotine levels, and e-cig devices can be programmed to achieve variable power output and variable heating coil resistance levels. Unlike conventional cigarettes, the possibility to choose among such different flavors (e.g., fruit, dessert, tobacco) is particularly appealing to adolescents and young adults (Harrel et al., 2017; Yingst et al., 2017).
In most cases, modern e-cig devices consist of a mouthpiece, a refillable cartridge, a lithium battery and a heating atomizer. Users activate the heating coil in the atomizer by depressing the device’s power button during inhalation; thus, the flavored liquid is rapidly vaporized by passing through the heating element. With new generation devices, users can modulate the e-cig liquid vaporization process by selecting atomizers with different coil resistances, by applying different voltages across the coils, and/or by controlling the operating temperature of the atomizer. According to recent reports, the design and operation settings of e-cigs may have a significant impact on human health (Chausse et al., 2015). Contrary to the initial hypothesis that voltages higher than 3.3 V and up to 5 V are responsible for formaldehyde generation in e-cig aerosols (Jensen et al., 2015), it is now well established that formaldehyde is produced even under lower powered, breath-activated devices (Bitzer et al., 2019). Most of the hazardous carbonyls detected in e-cig vapors are produced during the thermal decomposition of vegetable glycerol (VG) and propylene glycol (PG), the major chemical constituents of most e-cig liquids. In a recent study, significant amounts of formaldehyde and acetaldehyde were detected at temperatures greater or equal to 215 °C when PG and VG were vaporized by an e-cig, while acrolein was observed when VG was subjected to a temperature in excess of 270 °C (Wang et al., 2017). The heating power of the device is a function of the combination of the coil’s resistance value and the voltage applied by the e-cig’s battery (i.e., the Joule effect) (Chausse et al., 2015). As such, consumers who use a low-voltage device are able to obtain the same power of a high-voltage e-cig by selecting an appropriate coil.
The aim of the present study was to determine the effect of coil resistance (1.5 Ω and 0.25 Ω coils, to obtain a total wattage of 8±2 W and 40±5 W, respectively) on carbonyl and ROS generation by e-cigs and to evaluate the biological effects of the resulting e-cig vapors on the viability of H1299 human lung adenocarcinoma cells following exposure using an in vitro air-liquid interface (ALI) exposure system.
2. Material and Methods
2.1. E-cigarette devices and settings
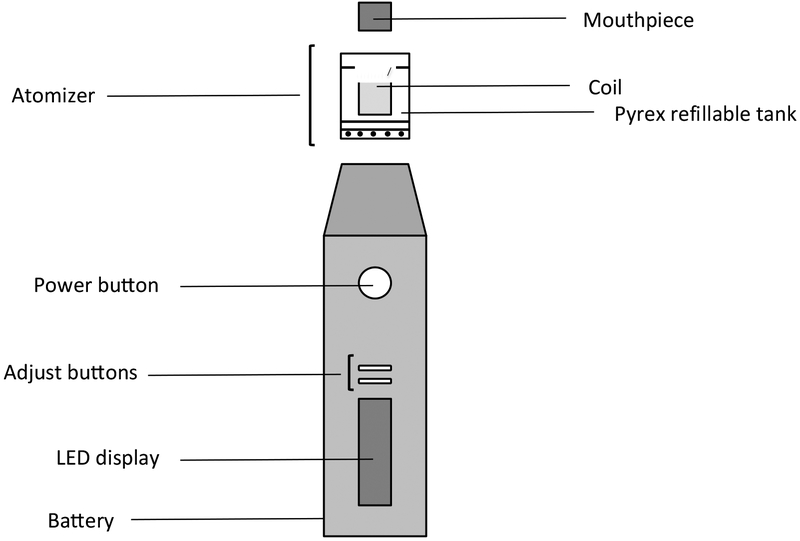
A commercially available Eleaf Pico e-cig consisting of a 2.5 mL liquid tank made of Pyrex glass and a rechargeable lithium battery (MXJO IMR 18650 3000 mAh 35A 3.7 V High Drain Flat Top Rechargeable Battery) was used for all studies (Figure 1). The voltage value was set at 3.5 V and two different coils (Joyetech™, 1.5 Ω and 0.25 Ω) were used to obtain a total wattage of 8±2 W and 40±5 W, respectively. In order to prevent the confounding effect of coil aging, a new element was used for each experiment.
Figure 1.
Schematic representation of the e-cig device used for the study.
2.2. E-liquids
Studies to determine carbonyl compounds in e-cig aerosols used a PG/VG base solution (50/50, v/v) (Fumador S.r.l., Milan, Italy) without nicotine (eL-N) and with nicotine (18%; eL+N). A red fruits flavor concentrate (Chemfont S.r.l., Rome, Italy) was then added to a final concentration of 10% (v/v). For studies examining ROS production and effects cell viability, the e-cig liquid was composed of a PG/VG base (50/50, v/v) (NicVape, USA) without nicotine to which a raspberry flavor concentrate (NicVape, USA) was added at a concentration of 10% (v/v).
2.3. Detection of carbonyl compounds: formaldehyde, acetaldehyde and acrolein
To establish the presence of formaldehyde, acetaldehyde and acrolein, a 30-L propylene box was filled using the following puff profile: puff on 6 s, puff off 5 s; the puffing sequence was repeated twice (9Goel et al., 2015; Canistro et al., 2017; Cardenia et al., 2018). Formaldehyde, acetaldehyde and acrolein were determined by headspace-solid phase microextraction (HS-SPME) coupled to gas chromatography-mass spectrometry (GC/MS), as reported in our previous study (Canistro et al., 2017) with some modifications. A SPME device having a fused-silica fiber (10-mm length) coated with DVB/CAR/PDMS (50/30 mm thickness), was used. After conditioning at 270 °C for 60 min, the SPME fiber was exposed to the box headspace at room temperature. After a 2-min exposure, the fiber was desorbed at 250 °C for 10 min in the injector of the GC/MS system (Q2010 Plus, Shimadzu, Japan). The sample was injected into a RTX-WAX column (30 m, 0.25 mm i.d., 0.25 μm film thickness, Restek, USA) in split mode (1:20 split ratio). Helium was used as carrier gas with a linear velocity of 36.2 cm/sec. The oven temperature was kept at 35 °C for 10 min, then raised to 240 °C at 30 °C/min. Injector and interface temperatures were set at 250 and 230 °C, respectively. Compounds were recognized by comparing their mass spectra and retention time with those of the corresponding chemical standards. The quantification of formaldehyde, acetaldehyde and acrolein signal was carried out by Single Ion Monitoring (SIM), using 29 m/z, 44 m/z and 56 m/z, respectively. The construction of the calibration curves in this case could not provide reproducible results, due to the difference of vapor pressure of carbonyls when used alone or in presence of other compounds (Liu et al., 2016). Therefore, as suggested in literature (Wang et al., 2017; Geiss et al., 2016), a normalized response factor (Rf) was calculated based on the concentration of carbonyls present in the environment as basal level, according to the following expression:
where Ax and Ay represent the peak areas of carbonyls detected after and before (basal) the vaping process in the exposure box, respectively.
2.4. Cell-free ROS
The ROS production was estimated using the dye 2’,7’-dichlorodihydrofluorescein diacetate (DCFH-DA), as previously reported by Lerner et al. (Lerner et al., 2015a). To catalyze the reaction between DCFH and ROS, horseradish peroxidase (HRP) was added. Vapor was pulsed into the bubbler (Ace Glass Inc., Vineland, NJ) at room temperature using the following puffing topography: puff on 4 s, puff off 26 s, flow rate 1.5 L/min; this puffing sequence was repeated 15 times, for a total time of exposure of 7.5 min (total number of puffs: 15). The oxidized dichloro-fluorescein (DCF) fluorescence was measured using a Fluroskan Ascent FL spectrofluorometer (Thermo Fisher Scientific Inc., Waltham, MA) at absorbance/emission maxima of 485 nm/535 nm. H2O2 standards were used to calibrate the fluorescence intensity units (FIU) and DCF fluorescence data are expressed as μM of H2O2 equivalents. The assay was conducted using both phosphate buffer (PBS) and cell medium as reaction mixture.
2.5. Air-liquid interface cell culture and exposure
H1299 human lung adenocarcinoma cells were purchased from ATCC (Manassas, VA). The cells were cultured in RPMI 1640 basal media supplemented with 10% fetal bovine serum, 1% penicillin and 1% streptomycin at 37°C under a 5% CO2 atmosphere. Before aerosol exposure, cells were plated in 60-mm dishes and grown to 50–70% confluence. Cells were then exposed to air or e-cig vapor in a modified vacuum desiccator (500 mL volume, SP Scienceware, Warminster, PA) using the following puffing topography: puff on 4 s, puff off 26 s, flow rate 15 L/min; this puffing sequence was repeated 15 times, for a total time of exposure of 7.5 min (total number of puffs: 15). In addition to the analytical needs, this topography was also more representative of the real human use of e-cig and allowed us to reinforce the correlation between our results and the actual health risks for e-cig consumers (Norton et al., 2014; Robinson et al., 2015). This treatment protocol was repeated once after 2 h. Cells were exposed to a total number of 30 puffs.
2.6. Cell viability measurement
The 3-(4,5-dimethylthiazol-3-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay (Cat#M5655, Sigma Aldrich, St. Louis, MO) was used to assess cell viability 24 h after e-cig vapor exposure. Briefly, 24 h after exposure, H1299 cells were washed twice with PBS and then incubated with MTT (1 mg/mL) in RMPI 1640 medium at 37 °C for 30 min. The medium was then removed and dimethyl sulfoxide (DMSO) was added to solubilize the formazan dye; the absorbance was measured at 550 nm using a Multiskan GO microplate spectrophotometer (Thermo Fisher Scientific Inc., Waltham, MA). The viability of cells exposed to e-cig vapor was normalized to the viability of air-exposed cells.
2.7. Statistical analysis
Data referred to as carbonyls compounds are expressed as mean ± standard deviation (SD) of three independent replicates (n=3) and analyzed by means of one-way ANOVA to evaluate the influence of different conditions tested (p<0.05). Data referred to as DCFH-DA assay in PBS (vapor condensate and air-liquid interface) are expressed as mean ± standard deviation (SD) of six independent replicates. For DCFH-DA assay in cell medium, results are indicated as relative percentage of the ROS content in control samples arbitrarily set at a value of 100% (mean ± SD of six independent replicates). Cell viability determined by MTT assay is expressed as percentage variation of viable cells relative to control group arbitrarily set at 100% (mean ± SD of six independent replicates). One-way ANOVA, followed by Tukey’s multiple comparison test, was carried out at a 95% confidence level (p ≤ 0.05), to separate means of parameters that were statistically different.
3. Results
3.1. Formaldehyde, acetaldehyde and acrolein levels
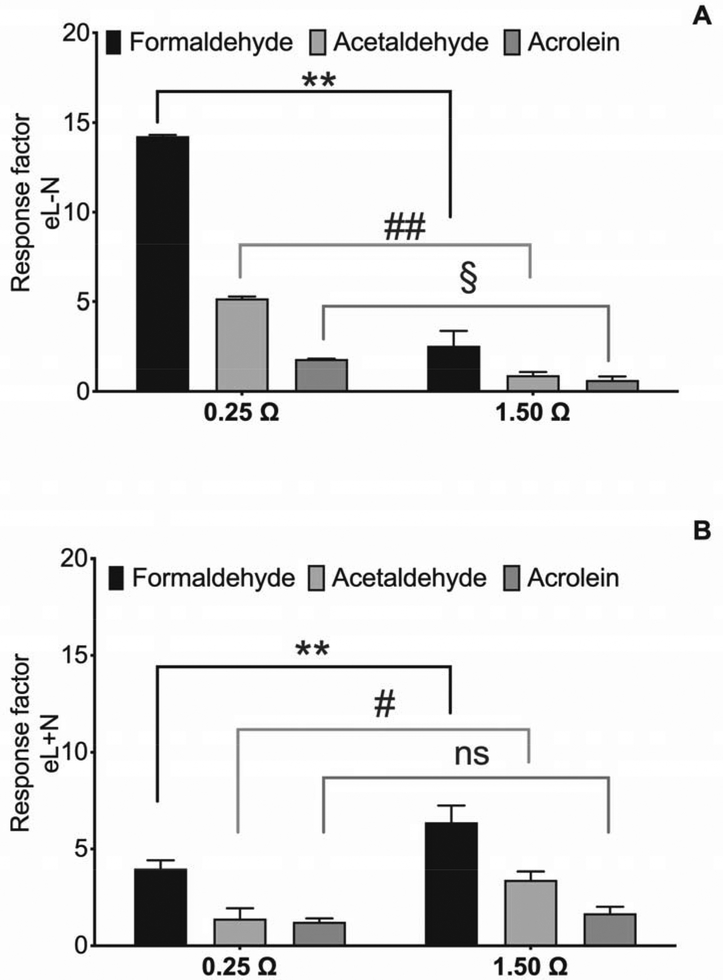
In general, eL-N generated higher levels of carbonyls than eL+N. Formaldehyde was the main aldehyde produced in both liquids, followed by acetaldehyde and acrolein; however, the observed concentrations of carbonyls were strictly related to both the composition of liquids and the resistance value. For eL-N, acrolein, acetaldehyde and formaldehyde levels generated by 0.25 Ω coil were three- to seven-fold higher than those generated by the 1.50 Ω coil (Figure 2A). In contrast, the carbonyl content was higher with the 1.50 Ω coil compared to 0.25 Ω coil when eL+N was used (Figure 2B). Figure 2B shows that when the eL+N was used, the 1.50 Ω coil resulted in the highest generation rate of carbonyls, especially formaldehyde, which was significantly (p<0.05) higher than acetaldehyde and acrolein; however, the latter was not significantly affected by the resistance value. Since the eL-N led to the highest production of hazardous carbonyls, an eL-N was chosen for subsequent ROS generation and cell viability experiments.
Figure 2.
Effects of resistance value (0.25 Ω and 1.5 Ω) on formaldehyde, acetaldehyde and acrolein levels in vapors released by eL-N (without nicotine) (A) and eL+N (with nicotine) (B). Data represent mean ± SD of three independent replicates. Different letters (a-b) for each aldehyde denote statistically different means (Tukey’s test; p<0.05) related to the resistance values; different letters (x-z) for each resistance value denote statistically different means (Tukey’s test; p<0.05) related to carbonyls.
3.2. ROS levels generated by e-cig vapor in PBS
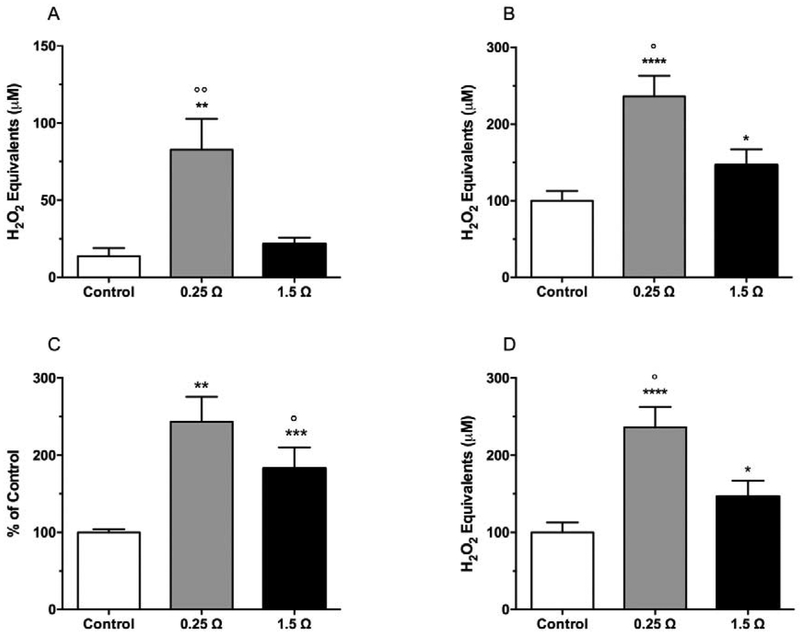
ROS levels generated by e-cigs were determined by collecting vapor in PBS and measured in both the bubbler (vapor condensate) and at the ALI using the exposure chamber described above. Results obtained by analyzing the vapor condensate (Figure 3A) showed more than six-fold higher levels of ROS in samples obtained with the 0.25 Ω coil compared to air control, and about three-fold higher if compared with 1.5 Ω (p<0.01). The same trend was found when ROS levels were measured at the ALI (Figure 3B).
Figure 3.
Effect of resistance values on ROS levels in e-cig vapor. Fresh air control and e-cig vapor were pulsed through DCFH-HRP ROS indicator solution in (A, B) phosphate buffer and (C, D) cell culture media in (A, C) vapor condensate and (B, D) the ALI during a 7.50 min exposure. Data represent mean ± SD of six independent replicates.
**p<0.01; ***p<0.0002 significant results between 1.5 Ω/0.25 Ω groups and control group using one-way ANOVA (Tukey’s multiple comparison test).
°p<0.05; °°p<0.01 significant results between 0.25 Ω and 1.5 Ω group using one-way ANOVA (Tukey’s multiple comparison test).
3.3. ROS levels generated by e-cig vapor in cell medium
To determine if e-cig vapor produces ROS under cell culture conditions, the DCFH-DA assay was conducted using cell media as solvent under the experimental conditions mentioned previously. In both the bubbler and the ALI exposure chamber, the results followed the same trend as those obtained with PBS. The 0.25 Ω coil produced significantly higher levels of ROS when compared to control in both experimental conditions (+82.9%, p<0.001 in vapor condensate; +46.3%, p<0.05 in the air-liquid interface) (Figure 3C and 3D). When compared to the control, the 0.25 Ω coil produced increased ROS levels: 143.9% in condensate (p<0.0002) and 136.2% in air-liquid interface (p<0.0001) (Figure 3C and 3D).
3.4. Cell viability
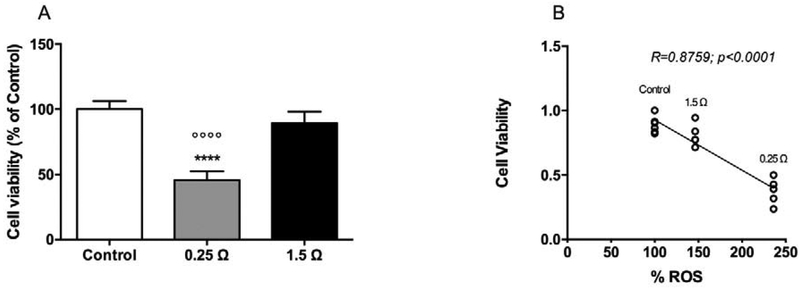
H1299 cell viability was measured 24 h after exposure in the experimental chamber. Results from the MTT assay (Figure 4A) showed that cell survival was inversely related to the total wattage of the devices. The effect of the 1.5 Ω coil group (10% reduction) had borderline statistical significance (p=0.058). Cell viability decreased to 45.8% (p<0.0001) when cells were exposed to the vapor generated by using the 0.25 Ω coil. Cell viability was observed to be inversely correlated to ROS production in cell medium revealed in the exposure chamber (Figure 4B).
Figure 4.
Relationship between cell viability, coil resistance and ROS generation. (A) Effect of vapor generated from e-cigs equipped with 1.5 Ω and 0.25 Ω coils on viability of H1299 cells by MTT assay. Data are expressed as percentage relative to viable cells in control arbitrarily set at a value of 100%; Data represent mean ± SD of six independent replicates. **p<0.01; ***p<0.0002 significant results between 1.5 Ω/0.25 Ω groups and control group using one-way ANOVA (Tukey’s multiple comparison test).
°p<0.05; °°p<0.01 significant results between 0.25 Ω and 1.5 Ω group using one-way ANOVA (Tukey’s multiple comparison test). (B) Cell viability in each group inversely correlates vs ROS generation (R=0.8795; p<0.0001).
4. Discussion
E-cigs are composed of several components which, in many cases, can be modulated or modified according to a consumer’s preferences. Among the modifiable components, the coil that is present in the atomizer is the element that, in combination with the applied voltage, is responsible for heating the liquid, which is generally composed of variable percentages of PG, VG, flavors and nicotine. It is well-established that thermal decomposition of the PG-VG mixture leads to the formation of toxic and carcinogenic carbonyls, such as formaldehyde, acetaldehyde and acrolein (Paschke et al., 2014; Uchiyama et al., 2013). Several studies have also reported that e-cig vapor contains relatively high levels of free radical species (Lerner et al., 2015a; Lerner et al., 2015b; Sussan et al., 2015; Pryor et al., 1997), which are known to be important causal factors in many tobacco related diseases and disorders, such as cardiovascular diseases, COPD and cancer (11Messner et al., 2014; Dekhuijzen, 2004; MacNee and Rahman, 2001).
In the present study, we investigated the role of an e-cig’s heating coil resistance with respect to the generation of carbonyls and ROS. Here, we report for the first time that the levels of selected hazardous carbonyls and ROS increases as coil resistance is reduced. The presence or absence of nicotine was also observed to affect the production of carbonyls with eL-N producing higher levels of carbonyls than eL+N. As reported by Kosmider et al. (2014), the composition of e-cig significantly affects the composition of the released carbonyl compounds. In general, the composition of the volatile fraction is strictly linked to the thermodynamic equilibrium between the vapor and condensed (liquid) phases; for this reason, when the number and/or the concentration of e-cig liquid ingredients decreases, the competition between molecules in the headspace decreases as well, thus leading to an increase and accumulation of some volatile compounds in the headspace, such as the revealed carbonyl compounds that are characterized by a high vapor pressure. Our results are consistent with previous studies demonstrating that the level of carbonyl compounds in e-cig vapors is affected by presence of nicotine and battery voltage (Kosmider et al., 2014). The latter is a relevant issue considering that e-cigs have become popular during the past decade (Korzun et al., 2019) for having the capacity of modulating the nicotine content of the liquid. Human exposure to these low molecular weight carbonyls represents a risk factor for occurrence of neoplastic diseases. In particular, formaldehyde and acetaldehyde are classified as Group 1 and Group 2B carcinogens, respectively, by the International Agency for Research on Cancer (IARC, 2012). Acrolein is listed as a hazardous air pollutant by the United States Environmental Protection Agency (U.S. EPA, 2003). The presence of higher carbonyls levels in eL-N liquid overturns the idea that the absence of nicotine makes the liquid safer. There is no doubt that nicotine is the molecule responsible for addiction and its employment in e-cig is considered a gateway towards the consumption of tobacco cigarette. However, the toxic carbonyls detected in this study represent a risk factor for the onset and development of neoplastic and chronic diseases (Primack et al., 2015; Soneji et al., 2018). This is of particular concern considering that, in many Countries, the nicotine-free e-cig can be sold to adolescent younger than 18 years old.
The generation of carbonyls is related to the formation of radicals (e.g., hydroxyl radicals), which are responsible for the oxidation and fragmentation of glycols (Geiss et al., 2016). For this reason, the ROS levels were measured in both vapor and cell medium using the DCFH-DA assay in a cell-free system. Although the exposure profile was different from the one used for detecting the selected carbonyl compounds, the results exhibited the same trend. The reduction of the coil resistance, and thus the increase of the heating and the total wattage of the device, played an important role in terms of ROS formation. Our results appear to confirm the hypothesis of Lerner et al. (Lerner et al., 2015a) who reported that one of the possible sources of ROS could be the heating element. We examined the biological implications of the ROS and carbonyls by examining the effect of e-cig vapor on the viability of H1299 human lung adenocarcinoma cells. We found that vapor from eL-N significantly reduced cell viability and that these effects were directly related to the levels of ROS produced.
High levels of ROS represent a risk factor for the onset of several diseases and pathological conditions, including cancer and inflammation (Waris and Ahsan, 2006). ROS are able to create DNA strand breaks, cross-links, and can cause modification to the purine, pyrimidine and deoxyribose components of DNA (Halliwell and Gutteridge, 2015). In an in-vivo study, e-cig aerosol caused accumulation of 8-hydroxy-deoxyguanosine (8-OH-dG) which can lead to mutations, with a significant correlation with ROS content, thus probably resulting in inflammatory response and neoplastic development (Canistro et al., 2017).
Others have previously examined the impact of e-cig vapor and e-cig extract on the morphology and viability of lung cells using both air-liquid interface or direct exposure to e-cig extract (Lerner et al., 2015b; Cervellati et al., 2014; Higham et al., 2018). Ex vivo treatment of bronco-epithelial cells from both patients with COPD and healthy subjects with e-cig extracts increased some inflammatory responses including altered cytokine and chemokine production (Higham et al., 2018).
In support of these data, epidemiological studies have indicated an association between e-cig use and respiratory disorders, especially asthma, bronchitis and COPD (Choi and Bernat, 2018; McConnell et al., 2017; Schweitzer et al., 2017; Wills et al., 2019). Altogether, these findings confirm the pivotal role of oxidative stress induced by e-cig use in the onset and development of respiratory diseases in healthy subjects or in the transition of initial symptomatology in chronic disorders.
5. Conclusion
In conclusion, the technology of newer generation e-cigs allow users to easily switch among heating elements in order to generate more or less aerosols and/or to intensify or reduce the flavor intensity of e-liquids. The ability to manipulate these parameters, together with the option that consumers have to select different e-liquid flavors and nicotine content, allows users to use e-cigs without any indication on its potential risks. Since the device and liquids used in this study are commercially-available, we believe that the exposure conditions, and consequently the generated carbonyls and ROS levels, are relevant to public health. Based on the results of this present study, the use of lower resistance coils coupled with an intermediate voltage setting (i.e., 3.5 V) can potentially represent a considerable health concern. Our results indicate how the generation of thermal degradation by-products depends not only on the applied voltage but also on resistance. Our study demonstrates the need for e-cig consumers to be cautious when assuming that low-voltages may be synonymous with “safer” devices (Thomson and Lewis, 2015), and in a broader sense, reiterates that after more than a decade of research, a “safe level” of exposure from e-cig aerosols cannot yet be established.
Highlights.
Nicotine-free liquid led to higher levels of carbonyl compounds in vapor.
Low resistance coil is associated with higher levels of carbonyls and ROS.
0.25 Ω coil vapor was more toxic to lung cancer cells than 1.5Ω coil vapor.
Cell viability in each exposed group inversely correlates vs ROS generation.
Funding
This research was supported by the Basic Research Funding [RFO 2016, Alma Mater Studiorum-Università di Bologna, Italy]; the Marco Polo fellowship program [Alma Mater Studiorum-Università di Bologna, Italy]; and the USDA National Institute of Food and Agriculture and Hatch Appropriations under Project #PEN04691 and Accession #1018545. This work was also supported in part by the National Institute on Drug Abuse of the National Institutes of Health and the Center for Tobacco Products of the U.S. Food and Drug Administration [under Award Number P50-DA-036107]. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the Food and Drug Administration.
Abbreviations
- ROS
reactive oxygen species
- COPD
chronic obstructive pulmonary disease
- PG
propylene glycol
- VG
vegetable glycerol
- DCFH-DA
2’,7’-dichloro-dihydro-fluorescein diacetate
- HRP
horseradish peroxidase
- DCF
dichloro-fluorescein
- H2O2
hydrogen peroxide
- MTT
3-(4,5-Dimethylthiazol-3-yl)-2,5-diphenyl Tetrazolium Bromide
- FIU
fluorescence intensity units
- IARC
International Agency for Research on Cancer
- U.S. EPA
United States Environmental Protection Agency
- HS-SPME
headspace-solid phase microextraction
- GC/MS
gas chromatography/mass spectrometry
- Rf
response factor
- eL-N
liquid without nicotine
- eL+N
liquid with nicotine
- ALI
air-liquid interface
Footnotes
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- (2010) In How Tobacco Smoke Causes Disease: The Biology and Behavioral Basis for Smoking-Attributable Disease: A Report of the Surgeon General, Centers for Disease Control and Prevention, Atlanta, GA. [PubMed] [Google Scholar]
- Arrazola RA, Singh T, Corey CG, Husten CG, Neff LJ, Apelberg BJ, Bunnel RE, Choiniere CJ, King BA, Cox S, McAfee T, Caraballo RS, 2014. Tobacco use among middle and high school students – United States, 2011–2014. MMWR Morb Mortal Wkly Rep. [PMC free article] [PubMed] [Google Scholar]
- Bitzer ZT, Goel R, Reilly SM, Bhangu G, Trushin N, Foulds J, Muscat J, Richie JP, 2019. Emissions of free radicals, carbonyls, and nicotine from the NIDA Standardized Research Electronic Cigarette and comparison to similar commercial devices. Chem Res Toxicol. (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canistro D; Vivarelli F; Cirillo S; Marquillas CB; Buschini A; Lazzaretti M; Marchi L; Cardenia V; Rodriguez-Estrada MTMT; Lodovici M; et al. 2017. E-Cigarettes induce toxicological effects that can raise the cancer risk. Sci. Rep 7 (1), 2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardenia V, Vivarelli F, Cirillo S, Paolini M, Canistro D, Rodriguez-Estrada MT, 2018. The effect of electronic-cigarettes aerosol on rat brain lipid profile. Biochimie. 153; 99–108. [DOI] [PubMed] [Google Scholar]
- Cervellati F, Muresan XM, Sticozzi C, Gambari R, Montagner G, Forman HJ, Torricelli C, Maioli E, Valacchi G, 2014. Comparative effects between electronic and cigarette smoke in human keratinocytes and epithelial lung cells. Toxicol In Vitro. 28(5); 999–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chausse P, Naughton G, Dutheil F, 2015. Electronic cigarettes resistance value of the heating filament could be the key to lung toxicity. Chest. e29–e30. [DOI] [PubMed] [Google Scholar]
- Choi K, Bernat D, 2018. E-cigarette use among Florida youth with and without asthma. Am. J. Prev. Med 51; 446–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dekhuijzen P, 2004. Antioxidant properties of N-acetylcysteine: their relevance in relation to chronic obstructive pulmonary disease. Eur. Respir. J 23(4); 629–636. [DOI] [PubMed] [Google Scholar]
- Domej W, Oettl K, and Renner W, 2014. Oxidative stress and free radicals in COPD–implications and relevance for treatment. Int. J. Chronic Obstruct. Pulm. Dis 9; 1207–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franks AS, Sando K, McBane S, 2018. Do electronic cigarettes have a role in tobacco cessation? Pharmacotherapy. 38(5); 555–i568. [DOI] [PubMed] [Google Scholar]
- Geiss O, Bianchi I, Barrero-Moreno J, 2016. Correlation of volatile carbonyl yields emitted by e-cigarettes with the temperature of the heating coil and the perceived sensorial quality of the generated vapours. Int. J. Hyg. Environ. Health 219 (3); 268–277. [DOI] [PubMed] [Google Scholar]
- Goel R, Durand E, Trushin N, Prokopczyk B, Foulds J, Elias RJ, Richie JP, 2015. Highly reactive free radicals in electronic cigarette aerosols. Chem Res Tox. 28; 1675–1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goniewicz ML Knysak J, Gawron M, Kosmider L, Sobczak A, Kurek J, Prokopowicz A, Jablonska-Czapla M, Rosik-Dulewska C, Havel C, Jacob P 3rd, Benowitz N, 2014. Levels of selected carcinogens and toxicants in vapor from electronic cigarettes. Tob Control 23; 133–139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrell MB, Weaver SR, Loukas A, Creamer M, Marti CN, Jackson CD, Heath JW, Nayak P, Perry CL, Pechacek TF, Eriksen MP, 2017. Flavored e-cigarette use: characterizing youth, young adult, and adult users. Prev. Med. Rep 5; 33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higham A, Bostock D, Booth G, Dungwa JV, Singh D, 2018. The effect of electronic cigarette and tobacco smoke exposure on COPD bronchial epithelial cell inflammatory responses. Int J Chron Obstruct Pulmon Dis 23;13:989–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- International Agency for Research on Cancer (IARC, 2012). Agents classified by IARC (Monographs, Volume 1–112). Geneva, Switzerland: http://monographs.iarc.fr/ENG/Classification/List_of_Classification_Vol1-112.pdf. [Google Scholar]
- Jensen RP, Luo W, Pankow JF, Strongin RM, Peyton DH, 2015. Hidden formaldehyde in e-cigarette aerosols. N Engl J Med. 372(4); 392–394. [DOI] [PubMed] [Google Scholar]
- Kirkham P, and Rahman I, 2006. Oxidative stress in asthma and COPD: Antioxidants as a therapeutic strategy. Pharmacol. Ther 111, 476–494. [DOI] [PubMed] [Google Scholar]
- Korzun T, Munhenzva I, Escobedo JO, Strongin RM, 2019. Synthetic food dyes in electronic cigarettes. Dye. Pigment 160; 509–513. [Google Scholar]
- Kosmider L, Sobczak A, Fik M, Knysak J, Zaciera M, Kurek J, Goniewicz ML, 2014. Carbonyl compounds in electronic cigarette vapors: effects of nicotine solvent and battery output voltage. Nicotine Tob. Res 16 (10); 1319–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner CA, Sundar IK, Watson RM, Elder A, Jones R, Done D, Kurtzman R, Ossip DJ, Robinson R, McIntosh S, and Rahman I, 2015a. Environmental health hazards of e-cigarettes and their components: Oxidants and copper in e-cigarette aerosols. Environ. Pollut 198, 100–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner CA, Sundar IK, Yao H, Gerloff J, Ossip DJ, McIntosh S, Robinson R, and Rahman I, 2015b. Vapors produced by electronic cigarettes and e-juices with flavorings induce toxicity, oxidative stress, and inflammatory response in lung epithelial cells and in mouse lung. PLoS One 10, e0116732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Wang B, Tai C, Wu L, Zhao H, Guan J, Chen L, 2016. An effective method to detect volatile intermediates generated in the bioconversion of coal to methane by gas chromatography-mass spectrometry after in-situ extraction using headspace solid-phase micro-extraction under strict anaerobic conditions. PLoS One 11 (10), e0163949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacNee W, Rahman I, 2001. Is oxidative stress central to the pathogenesis of chronic obstructive pulmonary disease? Trends Mol. Med 7(2); 55–62. [DOI] [PubMed] [Google Scholar]
- McConnell R, Barrington-Trimis JL, Wang K, Urman R, Hong H, Unger J, Samet J, Leventhal A, Berhane K, 2017. Electronic cigarette use and respiratory symptoms in adolescents. Am. J. Respir. Crit. Care Med, 195; 1043–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messner B, and Bernhard D, 2014. Smoking and cardiovascular disease: mechanisms of endothelial dysfunction and early atherogenesis. Arterioscler., Thromb., Vasc. Biol 34, 509–515. [DOI] [PubMed] [Google Scholar]
- Muthumalage T, Prinz M, Ansah KO, Gerloff J, Sundar IK, Rahman I, 2018. Inflammatory and oxidative responses induced by exposure to commonly used e-cigarette flavoring chemicals and flavored e-liquids without nicotine. Front Physiol. 11, 8–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton KJ, June KM, O’Connor RJ, 2014. Initial puffing behaviors and subjective responses differ between an electronic nicotine delivery system and traditional cigarettes. Tob Induc Dis. 12(1):17.http://dx.doi.org.ezproxy.unibo.it/10.1186/1617-9625-12-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogunwale MA, Mingxiao L, Mandapati V, Ramakrishnam R, Chen Y, Nantz H, Conklin DJ, Fu XA,2017. Aldehyde detection in electronic cigarette aerosols. Chem Res Toxicol. 2, 1207–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paschke T, Scherer G, Heller WD, 2014. Effects of ingredients on cigarette smoke composition and biological activity: a literature overview. Beiträge zur Tab 20; 107–247. [Google Scholar]
- Primack BA, Soneji S, Stoolmiller M, Fine MJ, Sargent JD, 2015. Progression to Traditional Cigarette Smoking After Electronic Cigarette Use Among US Adolescents and Young Adults. JAMA Pedr. Nov;169(11):1018–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pryor WA, 1997. Cigarette smoke radicals and the role of free radicals in chemical carcinogenicity. Environ. Health Perspect. 105 (Suppl 4), 875–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson RJ, Hensel EC, Morabito PN, Roundtree KA, 2015. Electronic Cigarette Topography in the Natural Environment. PLoS One. 10(6):e0129296 http://dx.doi.org.ezproxy.unibo.it/10.1371/journal.pone.0129296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheffler S, Dieken H, Krischenowski O, Aufderheide M, 2015. Cytotoxic evaluation of e-liquid aerosol using different lung-derived cell models. Int. J. Environ. Res. Public Health 12, 12466–12474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweitzer RJ, Wills TA, Tam E, Pagano I, Choi K 2017. E-cigarette use and asthma in a multiethnic sample of adolescents. Prev. Med 105; 226–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott A, Lugg ST, Aldridge K, Lewis KE, Bowden A, Mahida RY, Grudzinska FS, Dosanjh D, Parekh D, Foronjy R, Sapey E, Naidu B, Thickett DR, 2018. Pro-inflammatory effects of e-cigarette vapour condensate on human alveolar macrophages. Thorax. August 13 pii: thoraxjnl-2018–211663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soneji S, Barrington-Trimis JL, Wills TA, Leventhal AM, Unger JB, Gibson LA, Yang J, Primack BA, Andrews JA, Miech RA, Spindle TR, Dick DM, Eissenberg T, Hornik RC, Dang R, Sargent JD 2017 2018. JAMA Pediatr 2018 January 1;172(1):98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sussan TE, Gajghate S, Thimmulappa RK, Ma J, Kim JH, Sudini K, Consolini N, Cormier SA, Lomnicki S, Hasan F, Pekosz A, and Biswal S, 2015. Exposure to electronic cigarettes impairs pulmonary anti-bacterial and anti-viral defenses in a mouse model. PLoS One 10, e0116861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson RH, Lewis PM, 2015. More on hidden formaldehyde in e-cigarette aerosols. N Engl J Med. 372(16); 1575–1576. [DOI] [PubMed] [Google Scholar]
- U.S. Environmental Protection Agency, 2003. http://epa.gov/airtoxics/188polls.html. [PubMed]
- Uchiyama S, Ohta K, Inaba Y, Kunugita N, 2013. Determination of carbonyl compounds generated from the e-cigarette using coupled silica cartridges impregnated with hydroquinone and 2,4-dinitrophenylhydrazine, followed by high-performance liquid chromatography. Anal Sci. 29; 1219–1222. [DOI] [PubMed] [Google Scholar]
- Vasanthi Bathrinarayanan P, Brown JEP, Marshall LJ, Leslie LJ, 2018. An investigation into E-cigarette cytotoxicity in-vitro using a novel 3D differentiated co-culture model of human airways. Toxicol In Vitro. 52, 255–264. [DOI] [PubMed] [Google Scholar]
- Wang P, Chen W, Liao J, Matsuo T, Ito K, Fowles J, et al. , 2017. A device-independent evaluation of carbonyl emissions from heated electronic cigarette solvents. PLoS ONE 12(1): e0169811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wills TA, Knight R, Williams R, Pagano I, Sargent JD 2015. Risk factors for exclusive e-cigarette use and dual e-cigarette and tobacco use in adolescents. Pediatrics 135; e43–e51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yingst JM, Veldheer S, Hammett E, Hrabovsky S, Foulds J, 2017. A method for classifying user-reported electronic cigarette liquid flavors. Nicotine Tob. Res, 19 (11); 1381–1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Kleinstreuer C, Feng Y, 2012. Vapor deposition during cigarette smoke inhalation in a subject-specific human airway model. J. Aerosol Sci 53, 40–60. [Google Scholar]