Summary
The 6th International Symposium on Childhood, Adolescent and Young Adult (CAYA) Non-Hodgkin Lymphoma (NHL) was held in Rotterdam, Netherlands, 26–29 September, 2018. This summary manuscript is a perspective on the presentations from the plenary scientific sessions, including wellness and survivorship, B-cell NHL, AYA lymphoma, translational NHL biology, lymphoma immunology, bone marrow transplantation and cell therapy, T/Natural Killer cell lymphoma, anaplastic large cell lymphoma, lymphoblastic lymphoma, novel lymphoma therapeutics and Hodgkin lymphoma. The symposium was attended by over 260 registrants from 42 different countries and included young, middle and senior investigators. Finally, the Angelo Rosolen, MD, Memorial Lecture was delivered by Alfred Reiter, MD.
Keywords: Childhood, adolescent, young adult, non-Hodgkin lymphoma, current perspectives
Wellness and survivorship after diagnosis and treatment of CAYA NHL
The implementation of risk adapted therapy in CAYA with newly diagnosed NHL has catapulted the overall survival (OS) rates to greater than 80–90% during the past 25–30 years (Cairo & Pinkerton, 2016). With these increases in OS in CAYA with NHL these results have facilitated the opportunity to investigate the long-term effects of prior chemoradiotherapy. Several investigators from the Childhood Cancer Survivor Study (CCSS) and St. Jude Lifetime Cohort (SJLIFE) have identified significantly increased risks in CAYA NHL survivors, including chronic health conditions, secondary neoplasms and increased mortality secondary to non-neoplastic aetiologies (Leung et al, 2001; Bluhm et al, 2008; Ehrhardt et al, 2018a,b). It is currently estimated that there will be approximately 35,000 CAYA survivors of NHL by the year 2020 (Ehrhardt et al, 2018a).
The CCSS cohort consists of approximately 36 000 survivors of multiple paediatric and adolescent malignancies including NHL and has been comparing long-term outcomes of this cohort with both sibling cohorts and age- and gender-matched controls from the general population treated between 1970–1999 and sub-comparisons between 1970–1979 vs. 1980–1989 vs. 1990–1999 years of treatment (Armstrong et al, 2016).
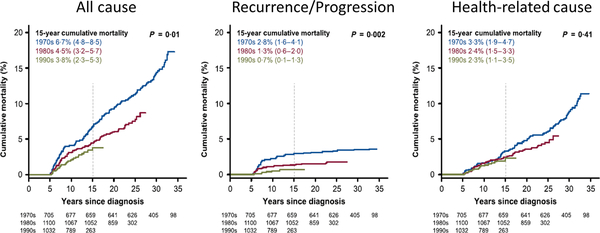
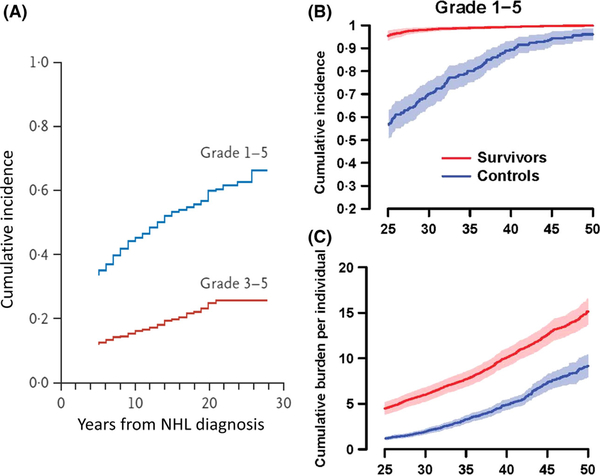
While there has been a decrease in these late effects over each of the above 10-year cohorts, there is still a significant increase in late mortality from all causes, recurrence/progression and health-related causes (Fig 1A–C) compared to control populations, respectively (Armstrong et al, 2016). Among 1085 childhood and adolescent NHL survivors, there was a 3–9-fold (95% confidence interval [CI95] 2–6-5–7) increased risk of developing a second neoplasm with a more significant risk within females (2–2-fold increase) (CI95 1–0-5–2) and those with a mediastinal primary neoplasm (4–6) (CI95 1–6-12–5) (Bluhm et al, 2008). Among 928 childhood and adolescent NHL survivors in the CCSS cohort, there was a significant increase in Grade 1–4 and grade 3 or 4 chronic health conditions versus their sibling comparison group (relative risk [RR] 3–2 and 6–8, respectively) (Fig 2A) (Oeffinger et al, 2006). The SJLIFE childhood and adolescent cancer survivor cohort, consisting of approximately 8300 patients diagnosed between 1962–2012, consists of all cancer diagnoses and includes approximately 440 NHL survivors (Bhakta et al, 2017). Reporting for the STLIFE NHL survivor cohort Bhakta et al (2017) conveyed a significant increase in grade 1–5 chronic health conditions (Fig 2B) and cumulative burden of chronic health conditions for survivors compared to the general population based community controls (Fig 2C).
Fig 1.
All-cause and cause-specific cumulative mortality among 5-year survivors of childhood cancer, according to decade. Shown is the cumulative incidence of death from any cause (A), from disease recurrence or progression (B), and from any health-related cause (C) among 34 033 patients who survived at least 5 years after childhood cancer for which treatment was initiated during the period from 1970 to 1999. The values in parentheses are 95% confidence intervals. The vertical dashed lines indicate 15-year mortality. P values are for the comparisons among the three decades. From New England Journal of Medicine, Armstrong, G.T., Chen, Y., Yasui, Y., Leisenring, W., Gibson, T.M., Mertens, A.C., Stovall, M., Oeffinger, K.C., Bhatia, S., Krull, K.R., Nathan, P.C., Neglia, J.P., Green, D.M., Hudson, M.M. & Robison, L.L., Reduction in Late Mortality among 5-Year Survivors of Childhood Cancer. 374, 833–842. Copyright ©2016 Massachusetts Medical Society. Modified with permission from Massachusetts Medical Society.
Fig 2.
(A) Cumulative incidence of chronic health conditions among 10 397 adult survivors of paediatric cancer, according to the original diagnosis and the severity of the later condition. Among the survivors of various types of childhood cancer, the severity of subsequent health conditions was scored according to the Common Terminology Criteria for Adverse Events (version 3) as either mild (grade 1), moderate (grade 2), severe (grade 3), life-threatening or disabling (grade 4), or fatal (grade 5). For the total survivor cohort, the curves showing the cumulative incidence of the two outcomes by grade are truncated at 28 years, even though the text provides data up to 30 years after the original cancer diagnosis. This was done for consistency with the panels showing data for groups of patients with certain types of cancer, in which smaller samples yielded data that were not as robust at 30 years as they were at 28 years. NHL, non-Hodgkin lymphoma. From New England Journal of Medicine, Oeffinger, K.C., Mertens, A.C., Sklar, C.A., Kawashima, T., Hudson, M.M., Meadows, A.T., Friedman, D.L., Marina, N., Hobbie, W., Kadan-Lottick, N.S., Schwartz, C.L., Leisenring, W., Robison, L.L. & Childhood Cancer Survivor, S. Chronic health conditions in adult survivors of childhood cancer. 355, 1572–1582. Copyright ©2006 Massachusetts Medical Society. Modified with permission from Massachusetts Medical Society. (B) and (C) Cumulative incidence (B) and burden (C) of all (grade 1–5) chronic health conditions in St Jude lifetime cohort study survivors of childhood cancer and in community controls. Reprinted from The Lancet, 390, Bhakta, N., Liu, Q., Ness, K.K., Baassiri, M., Eissa, H., Yeo, F., Chemaitilly, W., Ehrhardt, M.J., Bass, J., Bishop, M.W., Shelton, K., Lu, L., Huang, S., Li, Z., Caron, E., Lanctot, J., Howell, C., Folse, T., Joshi, V., Green, D.M., Mulrooney, D.A., Armstrong, G.T., Krull, K.R., Brinkman, T.M., Khan, R.B., Srivastava, D.K., Hudson, M.M., Yasui, Y. & Robison, L.L., The cumulative burden of surviving childhood cancer: an initial report from the St Jude Lifetime Cohort Study (SJLIFE), 2569–2582. Copyright 2017, with permission from Elsevier.
Significant cause of late mortality in children and adolescent NHL survivors include secondary malignancies, cardiomyopathy and pneumonia. Significant late morbidity also includes defects in neurocognitive function, poor health quality of life and lower social attainment (Armstrong et al, 2016). Similarly, in a smaller cohort of children and adolescent lymphoma survivors, Hochberg et al (2018a) reported significant decrease in working memory, executive function, fine motor skills, emotional quality of life and body image.
In summary, children and adolescent NHL survivors are at significant risk of late mortality from secondary neoplasms, recurrent/progressive disease and chronic health conditions, and late morbidity of multiple organ systems and poor health-related quality of life. These risks are similar to other long-term risks of childhood and adolescent acute lymphoblastic leukaemia (ALL), Wilms tumour and Hodgkin lymphoma (HL) survivors (Oeffinger et al, 2006). Novel approaches are required to reduce the burden of late morbidity and mortality in childhood and adolescent NHL survivors and methods to identify at-risk patients who are at significantly increased risk of these complications.
B-cell NHL in CAYA
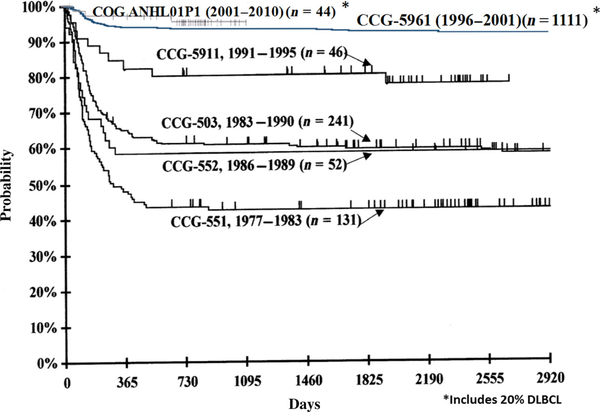
The prognosis has improved dramatically in the last 30 years for CAYA with mature B-cell NHL (Bhakta et al, 2017; Hochberg et al, 2018a), with a 2-year OS of approximately 90% (Cairo & Pinkerton, 2016; Giulino-Roth & Goldman, 2016). The introduction of rituximab in risk-adapted chemotherapy regimens for intermediate and high risk B-cell NHL has been safe and well tolerated in children and adolescents and appears to significantly increase the event-free survival (EFS) in children and adolescents with B-cell NHL (Fig 3) (Goldman et al, 2013, 2014; Cairo & Pinkerton, 2016; Giulino-Roth & Goldman, 2016; Frazer et al, 2018). However, there is a dismal prognosis (≤25% OS) in children and adolescents with B-cell NHL who relapse or progress off of frontline chemotherapy regimens (Cairo et al, 2018).
Fig 3.
Comparison of event-free survival by study among disseminated mature B-NHL patients from CCG/COG studies (551, 503, 552, 5911, CCG-5961, ANHL01P1). CCG, Children’s Cancer Group; COG, Children’s Oncology Group; DLBCL, diffuse large B-cell lymphoma; NHL, non-Hodgkin lymphoma.
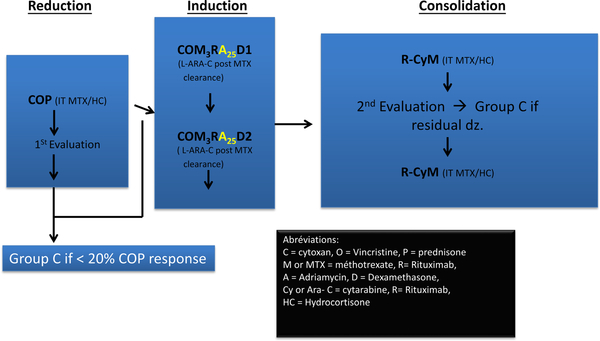
During the symposium, Goldman et al reported 100% EFS/OS in intermediate risk group patients with newly diagnosed B-NHL by the introduction of rituximab and intrathecal liposomal Ara-C concomitant with a significant reduction in the total anthracycline dose utilising a French-American-British (FAB) B4 chemotherapy backbone in the Reduce The Burden of Oncologic Therapy (REBOOT) trial (Fig 4) (Patte et al, 2007; Goldman et al, 2018). Attarbaschi (2018) reported the outcomes of rare subtypes of B-NHL, comprising less than 5% of all B-cell NHL in children and adolescents, including paediatric type follicular lymphoma (FL) nodal and extranodal marginal zone lymphoma (NMZL and EZML) grey zone lymphoma (Attarbaschi, 2018). In children and adolescents with FL and NMZL, a watch and wait approach after biopsy, surgery and diagnosis yielded a 100% OS, together with a 5-year EFS of approximately 7% in a small subgroup of patients with primary central nervous system (CNS) lymphoma (PCNSL) (N = 68), utilizing predominantly Berlin-Frankfurt-Munster (BFM) B-NHL regimens% (Attarbaschi, 2018).
Fig 4.
Reduce the burden of oncological therapy (REBOOT) Group B treatment schema. COM, cyclophosphamide (cytoxan), vincristine, methotrexate; COM3RA25D1, cyclophosphamide, vincristine, methotrexate, rituximab, doxorubicin (adriamycin), dexamethasone; COP, cyclophosphamide, vincristine, prednisone; dz, disease; HC, hydrocortisone; IT, intrathecal; MTX, methotrexate; R-CyM, rituximab, cytarabine, methotrexate.
Shipp (2018) reported on new promising targets in both PCNSL and primary testicular lymphomas (PTL). PCNSL and PTL have a high a high frequency of 9p24.1, programmed cell death protein 1 (PD-1, also termed PDCD1) ligands (PD-L1 [CD274]/PD-L2 [PDCD1LG2]) copy number activators and infrequent translocations (Fig 5) (Monti et al, 2012; Chapuy et al, 2016). Most importantly, Nayak et al (2017) reported on the success of nivolumab, a PD-1 blocker, in 5 patients with relapsed/refractory PCNSL and PTL, resulting in 4 complete responses (CRs) and 1 partial response (PR) (100% overall response [OR]). A current trial is underway investigating the safety and efficacy of nivolumab in relapsed/refractory PCNSL and PTL (CA 209–647) (Nayak et al, 2017). Additionally, targeting the Bruton tyrosine kinase (BTK) in PCNSL has also shown promise as a unique target in this disease (Lionakis et al, 2017).
Fig 5.
PCNSL and PTL harbour frequent 9p24.1 PD-L1/PD-L2 copy number alterations and infrequent translocations. (A) Genomic Identification of Significant Targets in Cancer (GISTIC)-defined copy number alterations (CNAs) in large B-cell lymphoma subtypes. GISTIC-defined recurrent CNAs (amplification in red) in 180 primary DLBCLs14 are compared with those in 11 primary mediastinal B-cell lymphomas (left panel) and 28 PCNSLs/PTLs (right panel) in mirror plots. Chromosome position is on the y-axis, and significance is on the x-axis. The green line denotes q value of 0 25. (B) Genetic alterations of CD274 (PD-L1) and PDCD1LG2 (PD-L2) in PTL and PCNSL. CNs of PD-L1 in 50 PCSNL cases (42 EBV- and 8 EBV1) from the extension cohort. CNs of CD274 (PD-L1) in 43 PTL cases from the extension cohort. Normals include 5 tonsils and 5 reactive lymph nodes. The upper 95% confidence interval of the normals was used as a threshold for CN gain in the PTLs. Indicated cHL cell lines with known CD274 (PD-L1) copy gain were used as controls. Cases with copy gain are highlighted in red. Error bars reflect standard deviation. The scale bar represents 100 mm. CD274/PD-L1: programmed death ligand 1 gene; cHL, classical Hodgkin lymphoma; CN, copy number; DLBCL, diffuse large B-cell lymphoma; EBV, Epstein-Barr virus; PCNSL, primary central nervous system lymphoma; PDCD1LG2/PD-L2, programmed death ligand 2 gene; PTL, primary testicular lymphoma; TL, testicular lymphoma. Republished with permission of the American Society of Hematology, from: Targetable genetic features of primary testicular and primary central nervous system lymphomas., Chapuy, B., Roemer, M.G., Stewart, C., Tan, Y., Abo, R.P., Zhang, L., Dunford, A.J., Meredith, D.M., Thorner, A.R., Jordanova, E.S., Liu, G., Feuerhake, F., Ducar, M.D., Illerhaus, G., Gusenleitner, D., Linden, E.A., Sun, H.H., Homer, H., Aono, M., Pinkus, G.S., Ligon, A.H., Ligon, K.L., Ferry, J.A., Freeman, G.J., van Hummelen, P., Golub, T.R., Getz, G., Rodig, S.J., de Jong, D., Monti, S. & Shipp, M.A., Blood, 127, 869–881, copyright 2016; permission conveyed through Copyright Clearance Center, Inc.
Lastly, Siebert (2018) reported results of differential DNA-methylation of Burkitt lymphoma (BL) versus other B-cell lymphomas, including germinal centre B-cell like diffuse large B-cell lymphoma (GCB DLBCL) and FL (Kreck et al, 2013; Kretzmer et al, 2015; Siebert, 2018). Kretzmer et al (2015) demonstrated significant overexpression of mutant SMARCA4 and differentially methylated regions (DMR) in BL versus other B-cell lymphomas. These abnormalities on the DNA-methylome in BL suggest mechanisms of both lymphomagenesis and also potential future targets for therapy. Finally, Hussain et al (2018) reported that Ubiquitin C-terminal hydrolase L1 bypasses mechanistic target of rapamycin kinase (mTOR) to promote protein synthesis and facilitate MYC-induced lymphomagenesis.
Mature T and natural killer (NK) cell neoplasms excluding anaplastic large cell lymphoma (ALCL) in CAYA
The prognosis in CAYA with advanced (Stage III/IV) mature T and natural killer (NK) cell neoplasms, excluding ALCL, is significantly lower than other NHL in CAYA and is estimated to have a 5-year EFS ≤50% (Cairo & Pinkerton, 2016; Pillai et al, 2016; Suzuki, 2018; Termuhlen, 2018; Yang et al, 2018). Mature T and NK cell neoplasms can be divided into two major subtypes, NK cell, commonly associated with Epstein-Barr virus (EBV), and T cell, either peripheral T-cell lymphomas (PTCL) not otherwise specified or subtypes of PTCL.
Extranodal NK lymphoma (ENKL) is significantly more common in Asian countries compared to Europe and North America (Suzuki, 2015; Cairo & Pinkerton, 2016). The introduction of the SMILE regimen (Steroid [dexamethasone], Methotrexate, Ifosphamide, Etoposide and L-Asparaginase) has induced overall response rates (ORR) of approximately 80% and 2-year OS of approximately 50% in adults with newly diagnosed advanced ENKL (Yamaguchi et al, 2017). More recently, Kwong et al (2017) demonstrated that ENKL express PD-L1 and in relapsed/refractory EBV+/PD-1+ ENKL adult patients, an anti-PD-1 antibody, pembrolizumib, has been associated with an 85% ORR.
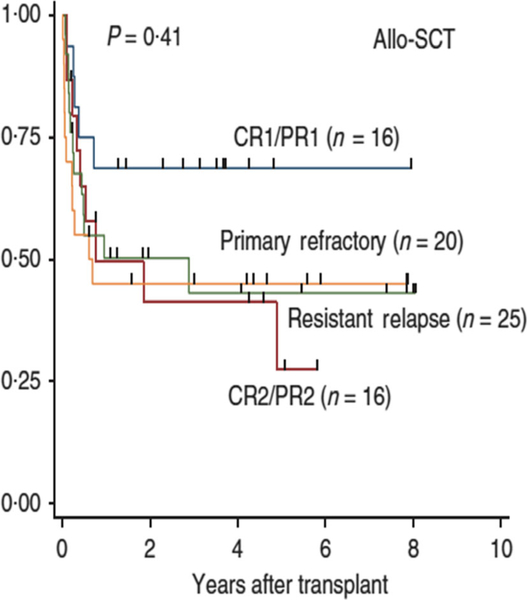
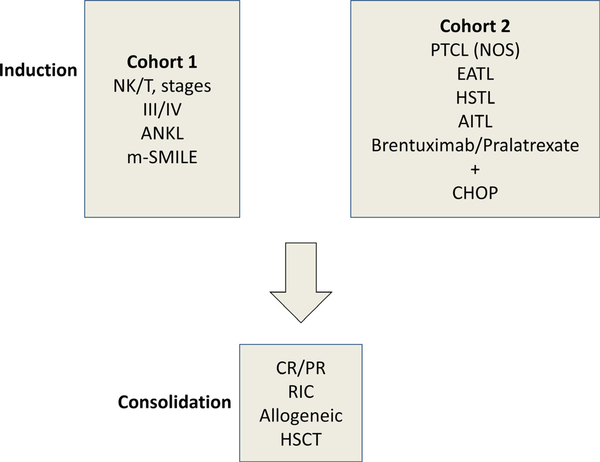
Subtypes of advanced PTCL, including angioimunoblastic T-cell lymphoma (AITL) and hepatosplenic gamma/delta T-cell lymphoma and enteropathy-associated T-cell lymphoma (EATL) have a dismal outcome, especially in patients with advanced and/or refractory disease (Pillai et al, 2016). Novel agents under investigation for PTCL and PTCL subtypes include pralatrexate and brentuximab vedotin. Patients with advanced and/or refractory PTCL and specific subtype who are found to obtain a CR/PR after induction therapy often proceed to an autologous or allogeneic stem cell transplant (Termuhlen, 2018). The results are significantly improved in patients who proceed to bone marrow transplantation (BMT) in first CR or PR (Fig 6) (Kim et al, 2013). An international upfront trial investigating a cohort of CAYA patients with advanced NK/T lymphomas is underway to test the safety of induction chemoimmunotherapy followed by a reduced toxicity allogeneic stem cell transplant (NYMC-575, NCT03719105) (Fig 7) (A. Xavier, MD, University of Alabama, personal communication).
Fig 6.
Probability of overall survival in patients with primary central nervous system lymphoma following allogeneic stem cell transplantation stratified by disease status. Overall survival stratified according to the disease status at transplantation in the allo-SCT group. Allo-SCT, allogeneic stem cell transplantation; CR1/2, first/second complete response; PR1/2, first/second partial response. Reprinted by permission from Springer Nature. Leukemia, 27, 1394–1397, Comparison of outcomes between autologous and allogeneic hematopoietic stem cell transplantation for peripheral T-cell lymphomas with central review of pathology. Kim, S.W., Yoon, S.S., Suzuki, R., Mat-suno, Y., Yi, H.G., Yoshida, T., Imamura, M., Wake, A., Miura, K., Hino, M., Ishikawa, T., Kim, J.S., Maeda, Y., Lee, J.J., Kang, H.J., Lee, H.S., Lee, J.H., Izutsu, K., Fukuda, T., Kim, C.W., Yoshino, T., Ohshima, K., Nakamura, S., Nagafuji, K., Suzumiya, J., Harada, M. & Kim, C.S. Copyright 2013.
Fig 7.
NK/T cell consortium: Experimental design (NYMC-575)NCT03719105). ANKL, aggressive NK cell leukaemia; enteropathy-associated T cell lymphoma; m-SMILE, modified-steroid (dexamethasone), methotrexate, ifosphamide, etoposide, L-asparaginase; NK, natural killer cell; PTCL, peripheral T cell lymphoma; T, T cell.
CAYA translational NHL biology
Over the past two decades there has been a steady increase in translational investigations in CAYA NHL. One of the more novel innovations in the last two decades is the precise measurement of minimal residual disease (MRD) and minimal disseminated disease (MDD) in patients during treatment and at diagnosis, respectively (Shiramizu et al, 2016). Established methods of leukaemia detection have included flow cytometry of aberrant phenotypes, polymerase chain reaction of fusion transcripts and/or antigen receptor genes (Shiramizu et al, 2016). Recently, MDD and MRD analysis has been demonstrated to be associated with risk assessment and prognostication in children and adolescents with NHL (Damm-Welk et al, 2007; Agsalda et al, 2009; Coustan-Smith et al, 2009; Mussolin et al, 2011; Shiramizu et al, 2016). Therefore, MDD and MRD evaluations have been incorporated into the International Paediatric NHL Staging Classification (IPNHLSC) and International Paediatric NHL Response Criteria (IPNHLRC) (Rosolen et al, 2015; Sandlund et al, 2015).
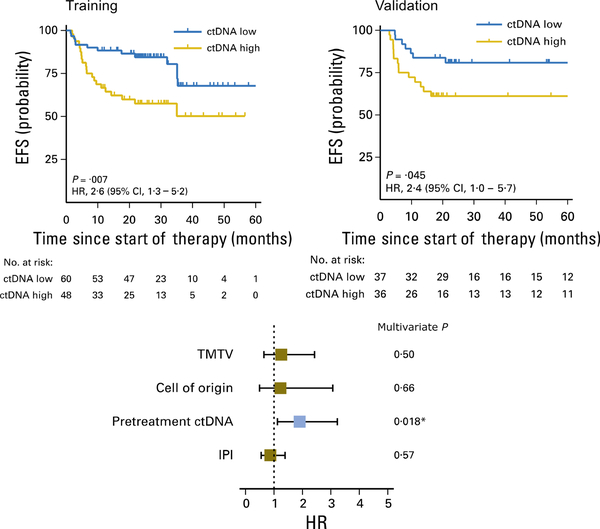
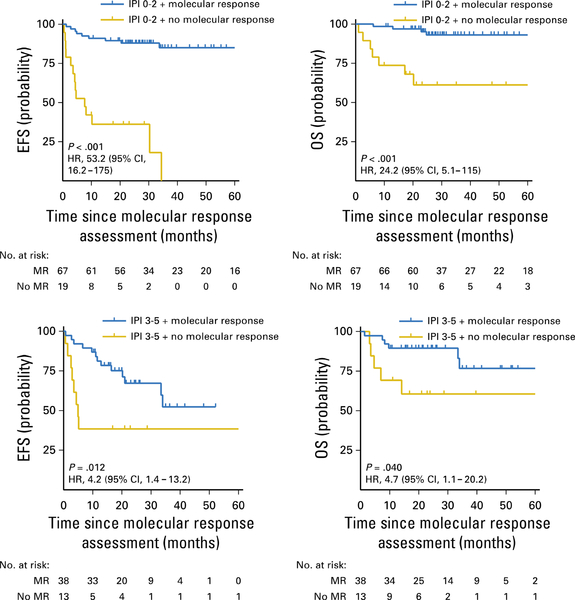
Scherer et al (2018) and Mussolin (2018) reported new methodologies to detect NHL circulating tumour DNA (ctDNA) by high throughput sequencing (HTS), cancer personalizing profiling by deep sequencing (CAPP-Seq), detection of circulating fragments of tumour DNA (cfDNA) and vesicles combining tumour RNA and DNA circulating exosomes. Utilizing ctDNA methodologies, Kurtz et al (2018) demonstrated that adult DLBCL patients with high levels of MDD at diagnosis by ctDNA analysis had a significantly decreased EFS (Fig 8). Furthermore, the same group also demonstrated that early molecular response by measurement of ctDNA levels by CAPP-Seq was significantly associated with 2-year EFS and OS in adult patients with newly diagnosed DLBCL (Fig 9) (Kurtz et al, 2018).
Fig 8.
Non-invasive risk assessment at DLBCL diagnosis by ctDNA. CI, confidence interval; ctDNA, circulating tumour DNA; DLBCL, diffuse large B-cell lymphoma; EFS, event-free survival; HR, hazard ratio; IPI, International Prognostic Index; TMTV, total metabolic tumour volume. From Kurtz, D.M. et al Circulating Tumor DNA Measurements As Early Outcome Predictors in Diffuse Large B-Cell Lymphoma. J Clin Oncol, 36, 2845–2853. Reprinted with permission. © 2018 American Society of Clinical Oncology. All rights reserved.
Fig 9.
Probability of EFS and OS in adult patients with newly diagnosed DLBCL stratified by early molecular response according to circulating tumour DNA analysis by CAncer Personalized Profiling by deep Sequencing. CI, confidence interval; DLBCL, diffuse large B-cell lymphoma; EFS, event-free survival; HR, hazard ratio; IPI, International Prognostic Index; OS, overall survival. From Kurtz, D.M. et al Circulating Tumor DNA Measurements As Early Outcome Predictors in Diffuse Large B-Cell Lymphoma. J Clin Oncol, 36, 2845–2853. Reprinted with permission. © 2018 American Society of Clinical Oncology. All rights reserved.
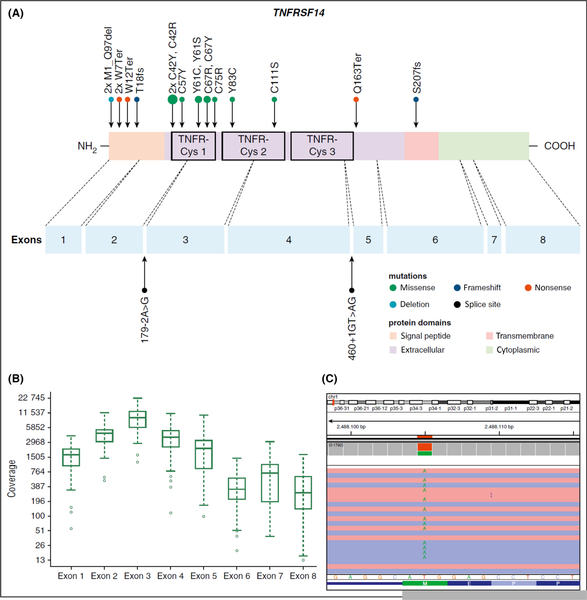
In 2016, the World Health Organization (WHO) designated a subtype of FL as paediatric-type FL (Quintanilla-Fend, 2018). Earlier, Salaverria et al (2011) had identified a subgroup with grade 3 paediatric-type FL with IRF4 translocations presenting predominantly in the head and neck region, including Waldeyer ring, with limited stage disease and a 5-year OS of 100% (Salaverria et al, 2011). IRF4 translocations can also be demonstrated with diffuse large B-cell morphology. More recently, Schmidt et al (2016) demonstrated, by genome-wide analysis, low genetic complexity and recurrent alterations in the TNFRSF14 gene in paediatric-type FL (Fig 10). More recently, this same group also demonstrated MAP2K1 mutation in approximately 49% of paediatric-type FL, such that 81% of children and adolescents with paediatric-type FL carry either a TNFRSF14 and/ or MAP2K1 mutation (Schmidt et al, 2017).
Fig 10.
Distrubtion of TNFRSF14 mutations on protein and exon level in paediatric type follicular lymphoma. (A) TNFRSF14 protein with its different domains and the cysteine repeats (TNFR-Cys 1–3) above. Below, localization of exons is indicated by dashed lines with position of splice site mutations. Domains of the protein are represented according to the Uniprot database (www.uniprot.org). Exact positions of each TNFRSF14 mutation found in 21 cases of paediatric type follicular lymphoma (PTFL) are given. (B) Coverage representation for TNFRSF14 exons 1–8 of all PTFL and reactive hyperplasia (RH) samples included in the study. The spacing of the scale on the y-axis is proportional to the logarithm of the number. (C) Exemplary view of the Integrative Genomics Viewer (IGV) showing the mutation p.M1_97del of PTFL11. Republished with permission of the American Society of Hematology, from: Genome-wide analysis of pediatric-type follicular lymphoma reveals low genetic complexity and recurrent alterations of TNFRSF14 gene., Schmidt, J., Gong, S., Marafioti, T., Mankel, B., Gonzalez-Farre, B., Balague, O., Mozos, A., Cabeca-das, J., van der Walt, J., Hoehn, D., Rosenwald, A., Ott, G., Dojcinov, S., Egan, C., Nadeu, F., Ramis-Zaldivar, J.E., Clot, G., Barcena, C., Perez-Alonso, V., Endris, V., Penzel, R., Lome-Maldonado, C., Bonzheim, I., Fend, F., Campo, E., Jaffe, E.S., Salaverria, I. & Quintanilla-Martinez, L., Blood, 128, 1101–1111, copyright 2016; permission conveyed through Copyright Clearance Center, Inc.
Lymphoma, immunology, BMT and cell therapy in CAYA
The prognosis of CAYA with relapsed/refractory mature B-NHL and T-lymphoblastic lymphoma (LBL) is dismal, with an estimated OS of ≤30% (Burkhardt et al, 2009; Cairo & Pinkerton, 2016; Cairo et al, 2018). The prognosis in CAYA with relapsed/refractory ALCL is significantly better and is influenced by time of relapse and presence or absence of CD3 expression (Woessmann et al, 2011). Woessmann (2018) reviewed the results of blood and stem cell transplantation in children and adolescents with relapsed refractory NHL. The same group reported significant differences in survival with the following variables: histology, time or relapse/ progression from original diagnosis, intensity and efficacy of original therapy, response to reinduction therapy at time of stem cell transplantation and type of stem cell transplant (autologous versus allogeneic) (Woessmann et al, 2011).
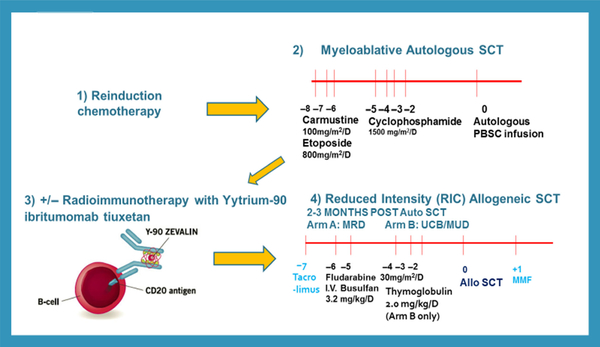
Gardenswartz et al (2018) presented the results of 13 relapsed/refractory CAYA with mature B-NHL (8 BL, 3 DLBCL, 2 primary mediastinal B-cell lymphoma, PMBL) who received reinduction therapy, myeloablative autografts utilizing cyclophosphamide, BCNU (carmustine) and etoposide (CBV) conditioning and reduced intensity allografts (7 of whom received radioimmunotherapy prior to with Zevalin®) who had a 91% 5-year EFS, improving previously reported results by Satwani et al (2015) (Fig 11). With respect to CD19 CAR T cell therapy, two presentations were reported at the symposium (Nakamura, 2018; Rivers et al, 2018). Nakamura (2018) discussed the current limitations of CD19 CAR T cell therapy in adults with DLBCL, including short CAR T cell persistence, excessive T-cell exhaustion following ex vivo expansion, inability to re-stimulate CD19 CAR T cells after relapse and the risk of graft-versus-host disease (GVHD) in patients with a prior allograft. Nakamura et al (2016) previously demonstrated the safety and immunogenicity of a cytomegalovirus (CMV) vaccine (CMVPepVax) in adult allograft recipients. Nakamura (2018) further reported a novel trial of isolating and manufacturing CD19 CAR T cells that were CMV reactive by the Prodigy Cytokine Capture System (CCS) and infusing CMV reactive CD19 CAR T cells in adults with relapsed/refractory CD19+ DLBCL followed by CMV vaccine at approximately 30 and 60 days after both auto and allografts (Nakamura, 2018). This novel approach will potentially circumvent low persistence and CAR T cell exhaustion. Lastly, Rivers et al (2018) presented their early results with CD19 CAR T cells in children and adolescents with relapsed/refractory CD19+ mature B-NHL, demonstrating approximately 50% ORR but few long-term remissions.
Fig 11.
Experimental design of treatment of children, adolescent and young adults with recurrent/refractory mature B-NHL. allo, allogeneic; auto, autologous; MMF, mycophenolate mofetil; MRD, matched related donor; MUD, matched unrelated donor; NHL, non-Hodgkin lymphoma; PBSC, peripheral blood stem cell; SCT, stem cell transplantation; UCB, umbilical cord blood.
Adolescent and young adult NHL and HL
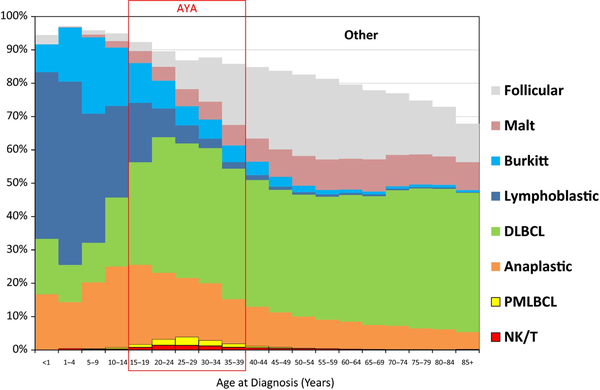
Adolescents and young adults (AYA) comprise an age range of 15–39 years. This age period shows a high change in frequency of different lymphoma types (Fig 12). Differences in epidemiology, disease characteristics, tumour biology and treatment response highlight AYA as a distinct group from younger children and older adults with NHL and HL. The incidence of both NHL and HL is approximately 14% of cancer diagnosis in the AYA group and is found more often in males compared to females (Fig 12) (Brugieres & Brice, 2016; Howlader et al, 2017; Hochberg et al, 2018b). DLBCL, BL, LBL and ALCL are the most common subtypes among AYA, with FL increasing in prominence in patients aged 30–39 years (Jaglowski et al, 2009). Survival in both disease groups have improved during the last three decades. Currently, NHL and HL patients have a 5-year survival of 75 and >90%, respectively, depending on the different entities, see Fig 13 (Gatta et al, 2009; Brugieres & Brice, 2016; Hochberg et al, 2018b; Trama et al, 2018). These excellent results have been obtained with therapies causing acute and long-term toxicities. The challenge now is to develop treatment strategies aimed at reducing these early and late toxicities and maintain these high cure rates. Moreover, patients with high risk of failure requiring new strategies, including targeted therapies, must be identified. Evaluation of treatment outcomes in the AYA population is limited due to variation in staging methodologies, depending on disease subtypes and tumour biology, causing differences in response to therapy. Moreover, no trials, including a representative AYA-population, are currently underway. Historically, they are either included in adult trials or in trials for children.
Fig 12.
Non-Hodgkin lymphoma distribution of histological types by age, United States SEER 2000–2011. AYA, adolescent and young adults; DLBCL, diffuse large B cell lymphoma; NK, Natural killer cell; PMBL, primary mediastinal large B-cell lymphoma; T, T cell.
Fig 13.
Non-Hodgkin lymphoma: 5-year lymphoma specific survival by histological type and age, 2000–2011; United States SEER data. DLBCL, diffuse large B cell lymphoma; NK, Natural killer cell; PMLBCL, primary mediastinal large B-cell lymphoma; T, T cell.
During the symposium, the epidemiology and outcome of AYA with NHL and HL was discussed. Flower (2018) stated that AYA are increasingly recognised as a unique population among patients with NHL and HL. Classical HL has a bimodal age distribution and peaks during the AYA period, making it the most common haematological malignancy among 15- to 29-year olds (Bleyer et al, 2006). High-risk features, including stage III/IV disease, bulk and extra nodal involvement are prominent among AYA with classical HL in comparison to younger children and older adults (Bigenwald et al, 2017). Currently, no established standards of care for AYA with NHL or HL exist. AYA focused programmes dedicated to specific challenges in the diagnosis, treatment and survivorship of malignancies in this age group are developing internationally. Early recognition, development of and enrolment in clinical trials, and consideration of pharmacodynamic variability, fertility, psychosocial barriers and compliance are essential to improving outcomes of lymphoma in the AYA population.
Ansell et al (2015) showed that several immune cells are present in the HL microenvironment. Although these cells have the ability to inhibit the growth of malignant B cells and even eradicate the malignant clone, these intra-tumour immune cells are typically unable to suppress the lymphoma cell, resulting in patients with clinical evidence of tumour progression. Recent data from adult lymphomas have identified some of the mechanisms that account for the suppressed anti-tumour response and have created opportunities for treatment to overcome the deficiencies (Roemer et al, 2016, 2018), particularly in PMBL and grey zone lymphoma, which share overlapping clinical features of DLBCL and HL, PCNSL and PTL, and result in high PD-L1 and PD-L2 expression. The latter two are rare lymphomas in AYA. While the normal immune response can be blocked by stimulating immune checkpoint receptors, such as PD-L1, immune checkpoint inhibitors can overcome this blockade, reversing immune suppression and improving T cell function. Genetic amplification and copy number gain of the chromosomal 9p24.1 gene region in tumour cells results in increased PD-L1/2 expression, causing a downregulation of the T cell response and is associated with response to these immune checkpoint inhibitors (Roemer et al, 2016, 2018). Blockade of inhibitory signals via PD-L1 has resulted in significant clinical activity by allowing intra-tumour T cells to remain activated and target malignant cells. This has resulted in remarkable response rates in HL (Ansell et al, 2015; Ansell, 2017). Other NHL show a more modest response (Chen et al, 2013; Nayak et al, 2017). In the subset of patients with low response rates, combination approaches (such as ipilimumab and nivolumab or rituximab and pembrolizumab in DLBCL and the combination of brentuximab and nivolumab in HL) are being explored, some of which appear promising.
Hochberg (2018) emphasised the significant cost of high cure rates in the form of delayed effects of therapy, such as secondary malignancies, cardiac toxicity and infertility. She stated that various strategies, such as positron emission tomography scan-based treatment responses, have been developed to address the challenge of maintaining current OS while minimizing long-term morbidities. Personalised therapy is needed, based on risk factor assessment, response-based therapy adaptations and incorporation of new agents with improved toxicity profiles to upfront therapy protocols. Brentuximab vedotin could be such a new agent. It is a CD30-targeted antibody conjugated to monomethyl auristatin E, an anti-tubulin agent which selectively induces apoptosis. CD30 is a highly expressed cell surface antigen on HL Reed-Sternberg (RS) cells. Pivotal studies have demonstrated the efficacy of brentuximab vedotin in relapsed/refractory HL and ALCL in adults (Younes et al, 2010) and recently in children (Locatelli et al, 2018). Superior results were also shown in the recent ECHELON-1 randomised trial in patients with previously untreated advanced stage HL (Connors et al, 2018). This has led to US Food and Drug Administration approval for brentuximab vedotin for use in combination with chemotherapy as a frontline treatment for adult patients with stage III or IV HL. A second new agent could be rituximab, an anti-CD20 monoclonal antibody, which showed its value in combination treatment of HL, although most Hodgkin RS cells lack CD20 expression (Younes et al, 2012). The explanation is possibly the circulation of clonotypic B cells in most patients with HL, which can be found in the tumour microenvironment. It remains to be seen if the efficacy of additional rituximab will be maintained long-term.
Xavier and Costa (2018) showed that nonbiological factors, such as lack of insurance or Medicaid insured status and lower income, are associated with increased mortality in AYA lymphomas.
Chen and Yang (2018) showed clinical features and treatment outcome of childhood PMBCL in Taiwan. The treatment outcome seemed as good as in the Western countries, although long-term follow-up is needed (Chen & Yang, 2018).
Anaplastic large cell lymphoma
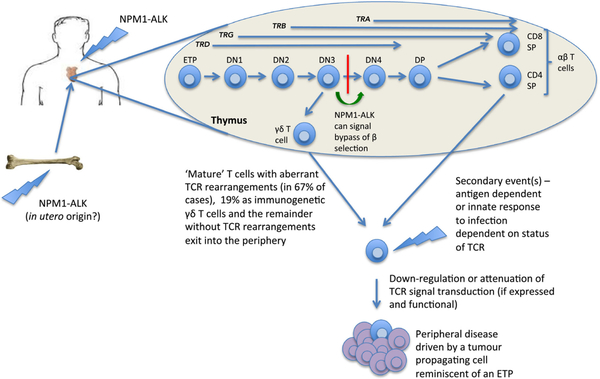
ALCL accounts for approximately 10–15% of all NHL in children and more than 90% of these cases are anaplastic lymphoma kinase (ALK) positive (Brugieres et al, 2009; Turner et al, 2016). ALCL is also characterised by aberrations in the ALK gene, most often occurring as a nucleophosmin (NPM1)-ALK translocation, t(2;5) (p23;q35) (Turner et al, 2016). The primary cutaneous form is characterised by solitary, often ulcerated skin tumours (Willemze et al, 2005). Most patients with systemic ALCL present at advanced stages (stages III-IV) with peripheral intra-abdominal or mediastinal lymph node involvement frequently associated with B symptoms and extra-nodal spread, including skin, liver, lung, soft tissue and bone localization (Brugieres et al, 2009). ALCLs are known as PTCLs based on expression of one or more T-cell antigens (Lamant et al, 2011). Most cases express also the cytotoxic associated-antigens TIA1, granzyme B and perforin. It is therefore considered that peripheral cytotoxic T cells are the cell of origin of ALCL, although recent research calls this into question. Identification of the tumour-propagating cell or cancer stem cell for ALCL identified a gene signature characteristic of an early thymic progenitor within this distinct cellular subset (Moti et al, 2015).
Willemze (2018) showed an extensive overview of the primary cutaneous ALCL (C-ALCL) and other primary cutaneous CD30+ T-cell lymphoproliferative disorders (CTCL) in AYA. He stated that CTCL usually affect adults and are rare in children and adolescents. In the Dutch Cutaneous Lymphoma Registry, C-ALCL, lymphomatoid papulosis and mycosis fungoides variants account for 70%, 20% and 10% of childhood and adult cases, respectively (Willemze, 2018). The first two conditions show overlapping clinical, histological and phenotypical features and form a spectrum of disease. Both have the same favourable prognosis as their adult counterparts. C-ALCL usually does not carry the t(2;5) and is ALK negative. Local radiotherapy and surgical excision are the first choice of treatment. An expectant policy is followed when lymphomatoid papulosis is diagnosed. For cosmetically-disturbing lesions, low dose oral methotrexate should be considered or psoralen and ultraviolet A therapy. Children with mycosis fungoides usually present with early patch/plaque stage disease with nearly no progression to advanced stage disease. Children should be treated with topical steroids or psoralen and ultraviolet A. Recurrences after treatment often occur.
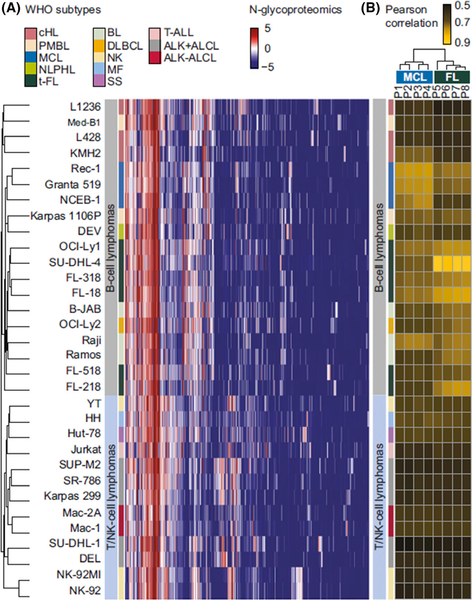
Lim (2018) provided details of novel pathways of oncogenesis in ALCL and showed that novel insights have been gained by leveraging mass spectrometry-based phosphoproteomics, N-glycoproteomics and genomics to elucidate the numerous ALK-driven pathways that contribute to oncogenesis as well as acquired resistance to ALK inhibitor 9 (Fig 14). An example is the identification of IL-31Rβ, a cytokine receptor, exclusively expressed in ALK+ALCL cell lines, by N-glycoproteomics and regulated by ALK through transcription (Rolland et al, 2017). That this protein could be a relevant therapeutic target in ALK-positive ALCL was emphasised in a xenograft model, which showed that tumour growth was in ALK-positive ALCL depleted of IL-31Rβ by RNAi (Rolland et al, 2017). Lim (2018) also showed the elucidation of various ALK-regulated immune surveillance mechanisms.
Fig 14.
N-glycoproteomic profiles classify lymphomas according to lineage, cell of origin and WHO subtype. (A) Thirty-two cell lines included in this study were accurately classified according to their lineage (B or T/natural killer cells), cell of origin (pre-GC- and GC-derived B cells), and World Health Organization subtypes. (B) Clustering of eight blinded primary B-cell lymphoma samples based on their Pearson’s correlations with the 32 cell lines accurately designated the profiles of clinical samples P1 to P4, which are highly correlated to MCL cell lines, whereas clinical samples P5 to P8 are highly correlated to t-FL cell lines. ALCL, anaplastic large cell lymphoma; ALK+, anaplastic lymphoma kinase positive; BL, Burkitt lymphoma; cHL, classical Hodgkin lymphoma; GC, germinal centre; MCL, mantle cell lymphoma; MF, mycosis fungoides; NK, Natural killer cell lymphoma; NLPHL, nodular lymphocyte predominant Hodgkin lymphoma; PMBL, primary mediastinal large B-cell lymphoma; SS, Sezary syndrome; T, T cell; T-ALL, T-lymphoblastic leukaemia/lymphoma; t-FL, transformed follicular lymphoma. From Rolland, D.C.M., Basrur, V., Jeon, Y.K., McNeil-Schwalm, C., Fermin, D., Conlon, K.P., Zhou, Y., Ng, S.Y., Tsou, C.C., Brown, N.A., Thomas, D.G., Bailey, N.G., Omenn, G.S., Nesvizhskii, A.I., Root, D.E., Weinstock, D.M., Faryabi, R.B., Lim, M.S. & Elenitoba-Johnson, K.S.J. (2017) Functional proteogenomics reveals biomarkers and therapeutic targets in lymphomas.
Turner et al (2016) reported a model in which ALK-positive ALCL originates in the thymi of children (Fig 15). This concept was based on several findings in mouse models. NPM-ALK induces a transient arrest in CD4, CD8 double negative cells (DN), a critical point in thymic T-cell development at which successfully rearranged T-cell receptor (TCR) b chains signal cell survival and permit differentiation (Fig 15). Investigations of TCR rearrangements in patient ALCL samples are supportive of aberrant events occurring in the thymus not normally permissive of thymocyte survival and differentiation towards mature T cells, suggestive of NPM-ALK presence in the thymus (Malcolm et al, 2016). These cells escaping from thymus in the mouse model was partly due to the TCR down-regulation during lymphomagenesis, because normally a TCR of some form appeared to be needed for thymic exit. Turner et al (2016) stated that an intricate balance of the overall consequences of signal transduction whether mediated by NPM-ALK or an engaged TCR, is critical for tumour development. Lovisa et al (2018) presented comprehensive characterization of plasma-derived exosomes in childhood ALCL: MIR122–5p, MIR103a-3p and MIR223–3p represent promising biomarkers of ALCL behaviour and might be used to improve diagnosis, treatment and patient prognosis. de los Fayos Alonso et al (2018) examined the function of PDGFRB in ALCL. It was shown that loss of PDGFRB leads to decreased tumour development and dissemination in a CD4-NPM1-ALK mouse model via modulation of the signal transducer and activator of transcription (STAT)-signalling pathway (de los Fayos Alonso et al, 2018). Ducray et al (2018) demonstrated that NPM1-ALK directs expression of the nuclear protein BRG1, which forms a complex that enables cellular survival; inhibitors of BRG1 could represent a novel target for treatment of ALK-positive ALCL (Ducray et al, 2018).
Fig 15.
A thymic origin for ALCL. In this model, the t(2;5) or variant translocation occurs in haemopoietic stem cells or thymic progenitors whereby NPM1-ALK is permissive of cellular survival in the thymus despite aberrant TCR rearrangements. These ‘primed’ cells may go undetected until a secondary event(s) occurs that leads to clonal expansion and tumour development. This event may be induced as a consequence of an inflammatory response as evidenced by ALCL in the context of insect bites but might also be initiated in an innate manner. ALCL, anaplastic large cell lymphoma; DN, double negative thymocyte; DP, double positive thymocyte; ETP, early thymic progenitor; SP, single positive; TCR, T-cell receptor. From Turner, S.D., Lamant, L., Kenner, L. & Brugieres, L. (2016) Anaplastic large cell lymphoma in paediatric and young adult patients. Br J Haematol, 173, 560–572. © 2016 John Wiley and Sons Inc.
Novel therapeutics
The 5-year EFS for the common forms of NHL (BL, DLBCL, LBL and ALCL) that occur in CAYA is excellent (>80%) and has improved dramatically over the last 40 years (Minard-Colin et al, 2015; Cairo & Pinkerton, 2016). Still, the prognosis in refractory/relapsed disease is dismal (EFS <20%). Moreover, the acute and late effects of current upfront treatment protocols are significant (Minard-Colin et al, 2015). Therefore, new targeted therapies in CAYA patients with NHL could potentially reduce toxicity in newly diagnosed patients but, more importantly, significantly increase survival in patients with refractory/relapsed disease. Unfortunately, new agents are difficult to explore in childhood NHL because of the small number of patients with refractory/relapsed disease. Phase I/II studies in newly diagnosed children with NHL are also difficult to undertake due to high survival rates and can only be allowed by diminishing acute and late toxicity through replacing current toxic agents, such as alkylating agents.
New drugs available for the different NHL entities have been summarised and several compounds are currently under investigation pre-clinically (cell-lines and mouse models) (Awasthi et al, 2017) and clinically, especially for B-NHL. Several new strategies employing targeted therapy have emerged, inhibiting different signalling pathways, such as B-cell receptor signalling (the BTK inhibitor, ibrutinib), B-cell lymphoma-2 inhibitors (Venetoclax, Navitoclax), SYK inhibitors (Fostaminib), PI3K (Idelalisib) and Janus kinase-STAT inhibitors (Ruxolitinib, Everolimus and Temsirolimus). Rituximab, an anti-CD20 monoclonal antibody, is the first new agent explored in childhood B-NHL. Its effectiveness was shown in the BFM window study (Meinhardt et al, 2010) and the Children’s Oncology Group (COG) single-arm study (Goldman et al, 2014). Recently, a large randomised trial in high risk B-NHL (group B with high lactate dehydrogenase and stage IV disease), the Inter-Ritux 2010, showed a 10% gain in EFS (83–94%) (Minard-Colin et al, 2016). Currently, ibrutinib is added to standard chemotherapy (R-ICE [rituximab, ifosfamide, carboplatin, etoposide] or R-VICI [rituximab, vincristine, ifosfamide, carboplatin, idarubicin]) for refractory/relapsed B-NHL patients aged 1–30 years in a phase I/II study (NCT02703272). In a preclinical BL model, it was shown that ibrutinib significantly inhibited BTK phosphorylation, in-vitro proliferation and enhanced OS (Nayyar et al, 2018). Levine et al (2018) showed that ruxolitinib significantly enhances in vitro apoptosis in HL and PMBCL and survival in a lymphoma xenograft mouse. These data show that ruxolitinib has the potential to be an alternative targeted therapeutic agent in AYA HL and PMBCL. Ippolito et al (2018) showed promising in vitro and in vivo data concerning a dual pan-PI3K/mTOR inhibitor (Omipalisib; GSK458) in chemo-resistant and -sensitive models of BL. Omipalisib suppresses the PI3K/Akt/mTOR pathway, leading to induction of apoptosis, impaired cell proliferation and exhibits synergistic in vitro activity when combined with cytotoxic chemotherapy (Ippolito et al, 2018). Another category of new drugs are the immunomodulatory drugs that target B-NHL. These include checkpoint blockade inhibitors, such as PD-1 receptor inhibitors, nivolumab and pembrolizumab, bi-specific T-cell engagers, such as Blinatumomab, antibody drug conjugates, such as brentuximab vedotin and inotuzumab ozogamicin and CD19 CAR T cells. Currently, two studies are starting to recruit for refractory/relapsed B-NHL, the first with inotuzumab ozogamicin and the second with CD19 CAR T-cells (A. Younes, MD, Memorial Sloan Kettering Cancer Center, personal communication). In T-LBL fewer new agents are available (nelarabine and bortezomib) and in development (CD5 CAR T-cells).
Lowe (2018) provided details of new agents in ALCL. Brentuximab vedotin and the ALK inhibitors (crizotinib, ceretinib and brigatinib) changed the paradigm for this disease and hopefully lead to less toxicity and improved outcomes. An ORR of 53% (CI95 28–77) was found in a phase I/II study in refractory/relapsed paediatric ALCL patients with single agent brentuximab vedotin (Locatelli et al, 2018). These data are comparable with 57% OS found in 74 patients treated with multi-agent chemotherapy (Woessmann et al, 2011). Crizotinib is an orally bioavailable small molecule inhibitor of receptor tyrosine kinases, including ALK. As discussed earlier, ALK plays a central role in the pathogenesis of ALCL due to a chromosomal translocation that results in expression of an oncogenic kinase fusion protein. Crizotinib inhibits ALK phosphorylation, resulting in anti-tumour activity. ORR for patients with ALCL treated at doses of 165 (ALCL165) and 280 (ALCL280) mg/m2 were 83% and 90%, respectively (Mosse et al, 2017). Currently, we are awaiting the results from the upfront COG study, investigating the safety of brentuximab vedotin and crizotinib in combination with the ALCL99 backbone in stage III-IV ALCL patients under 21 years of age (NCT01979536). The brentuximab vedotin portion of this study is closed, with the crizotinib part still recruiting. The role of checkpoint inhibitors in the treatment of ALCL is promising, as is shown in several case reports in heavily pre-treated patients. A phase I/II study with nivolumab as a single agent in refractory/relapsed ALCL patients is under development and will soon start recruiting.
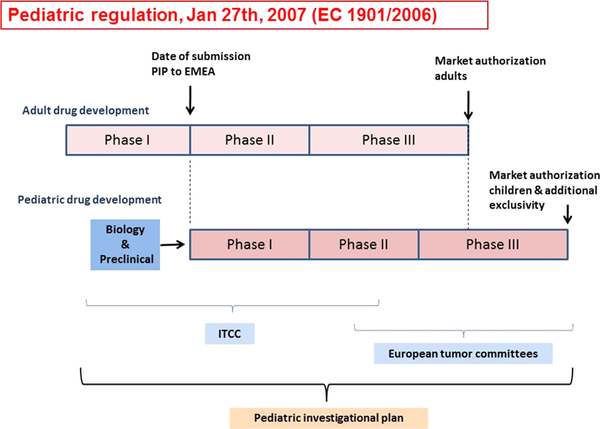
Zwaan et al (2010) reported about the introduction of new legislation (paediatric regulation) in Europe in January, 2007 and what it has meant for the development of new agent studies in paediatric oncology (Fig 16). Companies were obliged to consider paediatric development when developing a drug for which there is a potential paediatric indication. In drug development, prioritization of drugs based on knowledge of tumour biology, molecular ‘drivers’ of disease or drug mechanism of action, and therapeutic unmet needs are key elements and relevant to early phase paediatric trials. The large number of drugs under development in adult NHL caused a high number of approved Paediatric Investigational Plans (PIP). Yet, given the differences in biology between subtypes of NHL in children and adults and the small number of relapses, this appeared to be a challenge for the academic community (Moreno et al, 2017). Against this background, a Paediatric Strategy Forum concerning B-NHL was held in London in November 2017, with all stakeholders involved in paediatric drug development. Collaboration between regulators, pharmaceutical companies, patient representatives and clinical and researcher academics led to the following conclusions:
Fig 16.
A schematic representation of paediatric drug development. The new European Committee legislation (Regulation EC No. 1901/2006; the ‘Paediatric Regulation’) stimulates pharmaceutical companies to consider paediatric indications when they want to authorise a new medicinal product. Plans for the paediatric development of a drug need to be submitted to the European Medicines Agency (EMEA), in a so-called ‘Paediatric Investigation Plan or PIP’, which comprises the entire development process from pre-clinical studies to clinical development. While the PIP should be submitted following the phase I development in adults, the time to start the paediatric development is defined on a case by case approach, as additional safety and efficacy data may be required before launching the first paediatric studies. A PIP is legally binding but can be amended when the development process requires changes. When pharmaceutical companies have performed paediatric studies they will be rewarded by an additional period of market exclusivity. The Innovative Therapies for Children with Cancer consortium has the facilities for both pre-clinical as well as early clinical development, which is performed in close collaboration with the European tumour committees, who are typically responsible for standard of care and late phase II/phase III studies. Reprinted from Cancer Treatment Reviews, 36, Zwaan, C.M., Kearns, P., Caron, H., Verschuur, A., Riccardi, R., Boos, J., Doz, F., Geoerger, B., Morland, B., Vassal, G., The role of the ‘innovative therapies for children with cancer’ (ITCC) European consortium., 328–334. Copyright 2010, with permission from Elsevier.
priority should be directed to relapsed mature B-cell lymphomas;
global strategy, given the small number of relapsed patients;
antibody drug conjugates, CAR T-cells and T-cell engagers have the greatest probability of being beneficial;
joint leukaemia-lymphoma approach is not feasible in view of practical issues
except for PMBCL, specific paediatric studies are needed;
trials for relapsed disease must integrate correlative biological studies;
An international working group should be formed to develop a clinical trial strategy.
An earlier Strategy Forum was held on ALK inhibitors. It was concluded that, in Europe, ALK inhibitors should be accessible for children with ALCL, including the need for long-term safety follow-up data, given the expected prolonged exposure, like with chronic myeloid leukaemia. New ALK-inhibitors should only be developed in children when they have a clear additional utility over the existing compounds in development.
Two investigator studies, initiated by Erasmus MC, Rotterdam, were discussed: the ITCC-059 study with the anti-CD22 directed immunoconjugate inotuzumab ozogamicin (Dutch Trial Registry 5736) and a study with crizotinib in combination with vinblastine in refractory/relapsed ALCL patients (Dutch Trial Registry 5584).
Lymphoblastic lymphoma
LBL includes both precursor T-cell (T-LBL) and precursor B-cell lymphoblastic lymphoma B-LBL). The majority (80%) is of T-cell origin (Minard-Colin et al, 2015). The incidence of LBL in children is high compared to the other NHL entities with a median age of onset at around 9 years. In the AYA group the median age of onset is 25 years, with a much lower incidence (Fig 12) (Burkhardt et al, 2005). T-LBL usually presents with a mediastinal mass and advanced stage disease (stage III or IV in >90% of patients) and may involve bone marrow (25–30%) and, less often, CNS (approximately 5%) at diagnosis. B-LBL is more likely to involve the skin, soft tissue, bone and peripheral lymph nodes and represents most of the localised (stage I or II) stages (Minard-Colin et al, 2015; Cairo & Pinkerton, 2016). The best treatment approaches for childhood advanced LBL are ALL-based therapeutic regimens, with a four-drug induction, CNS prophylaxis with high dose methotrexate courses, re-induction in stage III/IV disease and a maintenance treatment to a total duration of 2 years. Recently reported results of clinical trials show EFS probabilities of 75–80% (Termuhlen et al, 2013; Landmann et al, 2017).
Burkhardt et al stated that three clinical issues remained unsolved: with current protocols, patients face relevant acute and long-term toxicity, the prognosis for patients who suffer disease progression or relapse is still fatal for the majority and treatment intensity is not at all stratified according to patient risk as validated stratification systems are lacking. Clinical factors, such as age, gender, bone marrow or CNS involvement and B-symptoms, were not consistently reported as prognostically relevant. Two recent publications proposed combinations of molecular marker profiles for risk group stratification in adult T-ALL (Trinquand et al, 2013) and paediatric T-LBL (Balbach et al, 2016). Trinquand et al (2013) defined a genetic high-risk group by NOTCH1 mutated and/or FBXW7 mutated, with RAS and PTEN wildtype. Balbach et al (2016) defined a high-risk group by NOTCH1 wildtype, PTEN mutated. Based on these data and analyses of a large T-LBL cohort from the French, Italian and BFM group a new T-LBL study has been developed (B. Burkhardt, MD, PhD, University Children’s Hospital Münster, personal communication).
B-LBL patients are reported to present more commonly with localised disease (64%) than with disseminated disease (Termuhlen et al, 2012, 2013). In patients with localised B-LBL, there was a 5-year EFS of 90% (CI95 74–96%) and a 5-year OS of 94% (CI95 79–99%). In the disseminated B-LBL group, only one of 24 patients relapsed, with a 5-year cumulative incidence of relapse of 5%. The French and BFM groups reported similar results. As such, dose reductions for localised B-LBL are feasible. Current research approaches are focusing on identifying molecular markers and studying response to therapy to develop risk stratification categories (Meyer et al, 2017).
Xu et al (2018) showed with genomic analysis that early T cell precursor (ETP)-LBL does not exhibit a unifying genetic alteration but shows genomic features distinct from both ETP-ALL and non-ETP-LBL. Other data indicates that IL7-induced glucocorticoid (GC) resistance occurs in subsets of healthy thymocytes, suggesting that this is a developmentally retained mechanism of GC resistance in T-ALL/T-LBL that arise from these developmental stages (Meyer et al, 2018). In cells with this phenotype, GCs paradoxically induce steroid resistance by enhancing the activity of a STAT5-mediated survival programme that can be overcome therapeutically with Janus kinase and B-cell lymphoma-2 inhibition. T-LBL were shown to be genetically heterogeneous, with CDKN2A as the most recurrent genetic feature (Martin-Guerrero et al, 2018). Moreover, cases were found displaying a high degree of genomic complexity and chromothripsis-like patterns.
Summary
In summary, the 6th International Childhood, Adolescent and Young Adult NHL symposium increased our knowledge on the pathogenesis, novel therapeutic approaches, new potential therapeutic targets and improved outcomes in low- and middle-income countries. We would like to thank the superb international faculty of speakers who participated in this international symposium (Fig 17).
Fig 17.
Photograph of the majority of the international faculty participating in the 6th International Childhood, Adolescent and Young Adult NHL symposium in Rotterdam, The Netherlands, September 26–29, 2018.
Acknowledgements
M.S.C. and A.B. wrote and reviewed this manuscript and have approved it for submission. The authors would like to thank Erin Morris, RN, for her expert editorial assistance in the preparation of this manuscript. Supported in part by grants from the National Cancer Institute, National Heart, Lung and Blood Institute, Paediatric Cancer Research Foundation, Fondazione Giacomo Ascoli, and the St. Baldrick’s Foundation. All authors have no conflicts of interest to disclose.
References
- Agsalda M, Kusao I, Troelstrup D. & Shiramizu B. (2009) Screening for residual disease in pediatric burkitt lymphoma using consensus primer pools. Advances in Hematology, 2009, 412163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansell SM (2017) Nivolumab in the treatment of Hodgkin lymphoma. Clinical Cancer Research, 23, 1623–1626. [DOI] [PubMed] [Google Scholar]
- Ansell SM, Lesokhin AM, Borrello I, Halwani A, Scott EC, Gutierrez M, Schuster SJ, Millenson MM, Cattry D, Freeman GJ, Rodig SJ, Chapuy B, Ligon AH, Zhu L, Grosso JF, Kim SY, Timmerman JM, Shipp MA & Armand P. (2015) PD-1 blockade with nivolumab in relapsed or refractory Hodgkin’s lymphoma. New England Journal of Medicine, 372, 311–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong GT, Chen Y, Yasui Y, Leisenring W, Gibson TM, Mertens AC, Stovall M, Oeffinger KC, Bhatia S, Krull KR, Nathan PC, Neglia JP, Green DM, Hudson MM & Robison LL (2016) Reduction in late mortality among 5-year survivors of childhood cancer. New England Journal of Medicine, 374, 833–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attarbaschi A. (2018) Diagnosis and treatment of rare B-NHL variants in children and adolescents. British Journal of Haematology, 182, 10. Abstract 19.29676459 [Google Scholar]
- Awasthi A, Rolland DCM, Ayello J, van de Ven C, Basrur V, Conlon K, Fermin D, Barth MJ, Klein C, Elenitoba-Johnson KSJ, Lim MS & Cairo MS (2017) A comparative global phosphoproteomics analysis of obinutuzumab (GA101) versus rituximab (RTX) against RTX sensitive and resistant Burkitt lymphoma (BL) demonstrates differential phosphorylation of signaling pathway proteins after treatment. Oncotarget, 8, 113895–113909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balbach ST, Makarova O, Bonn BR, Zimmermann M, Rohde M, Oschlies I, Klapper W, Rossig C. & Burkhardt B. (2016) Proposal of a genetic classifier for risk group stratification in pediatric T-cell lymphoblastic lymphoma reveals differences from adult T-cell lymphoblastic leukemia. Leukemia, 30, 970–973. [DOI] [PubMed] [Google Scholar]
- Bhakta N, Liu Q, Ness KK, Baassiri M, Eissa H, Yeo F, Chemaitilly W, Ehrhardt MJ, Bass J, Bishop MW, Shelton K, Lu L, Huang S, Li Z, Caron E, Lanctot J, Howell C, Folse T, Joshi V, Green DM, Mulrooney DA, Armstrong GT, Krull KR, Brinkman TM, Khan RB, Srivastava DK, Hudson MM, Yasui Y. & Robison LL (2017) The cumulative burden of surviving childhood cancer: an initial report from the St Jude Lifetime Cohort Study (SJLIFE). Lancet, 390, 2569–2582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigenwald C, Galimard JE, Quero L, Cabannes-Hamy A, Thieblemont C, Boissel N. & Brice P. (2017) Hodgkin lymphoma in adolescent and young adults: insights from an adult tertiary single-center cohort of 349 patients. Oncotarget, 8, 80073–80082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleyer WA, O’Leary M, Barr R. & Ries LAG (eds.) (2006) Cancer Epidemiology in Older Adolescents and Young Adults 15 to 29 Years of Age, Including SEER Incidence and Survival, 1975–2000. National Cancer Institute, Bethesda, MD. [Google Scholar]
- Bluhm EC, Ronckers C, Hayashi RJ, Neglia JP, Mertens AC, Stovall M, Meadows AT, Mitby PA, Whitton JA, Hammond S, Barker JD, Donaldson SS, Robison LL & Inskip PD (2008) Cause-specific mortality and second cancer incidence after non-Hodgkin lymphoma: a report from the Childhood Cancer Survivor Study. Blood, 111, 4014–4021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brugieres L. & Brice P. (2016) Lymphoma in adolescents and young adults. In: Tumors in Adolescents and Young Adults, Progress in Tumor Research (ed. by Stark DP & Vassal G.), Vol. 43, pp. 101–114. Karger, Basel. [DOI] [PubMed] [Google Scholar]
- Brugieres L, Le Deley MC, Rosolen A, Williams D, Horibe K, Wrobel G, Mann G, Zsiros J, Uyttebroeck A, Marky I, Lamant L. & Reiter A. (2009) Impact of the methotrexate administration dose on the need for intrathecal treatment in children and adolescents with anaplastic large-cell lymphoma: results of a randomized trial of the EICNHL Group. Journal of Clinical Oncology, 27, 897–903. [DOI] [PubMed] [Google Scholar]
- Burkhardt B, Zimmermann M, Oschlies I, Niggli F, Mann G, Parwaresch R, Riehm H, Schrappe M. & Reiter A. (2005) The impact of age and gender on biology, clinical features and treatment outcome of non-Hodgkin lymphoma in childhood and adolescence. British Journal of Haematology, 131, 39–49. [DOI] [PubMed] [Google Scholar]
- Burkhardt B, Reiter A, Landmann E, Lang P, Lassay L, Dickerhoff R, Lakomek M, Henze G. & von Stackelberg A. (2009) Poor outcome for children and adolescents with progressive disease or relapse of lymphoblastic lymphoma: a report from the berlin-frankfurt-muenster group. Journal of Clinical Oncology, 27, 3363–3369. [DOI] [PubMed] [Google Scholar]
- Cairo MS & Pinkerton R. (2016) Childhood, adolescent and young adult non-Hodgkin lymphoma: state of the science. British Journal of Haematology, 173, 507–530. [DOI] [PubMed] [Google Scholar]
- Cairo M, Auperin A, Perkins SL, Pinkerton R, Harrison L, Goldman S. & Patte C. (2018) Overall survival of children and adolescents with mature B cell non-Hodgkin lymphoma who had refractory or relapsed disease during or after treatment with FAB/LMB 96: a report from the FAB/LMB 96 study group. British Journal of Haematology, 182, 859–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapuy B, Roemer MG, Stewart C, Tan Y, Abo RP, Zhang L, Dunford AJ, Meredith DM, Thorner AR, Jordanova ES, Liu G, Feuerhake F, Ducar MD, Illerhaus G, Gusenleitner D, Linden EA, Sun HH, Homer H, Aono M, Pinkus GS, Ligon AH, Ligon KL, Ferry JA, Freeman GJ, van Hummelen P, Golub TR, Getz G, Rodig SJ, de Jong D, Monti S. & Shipp MA (2016) Targetable genetic features of primary testicular and primary central nervous system lymphomas. Blood, 127, 869–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S-H & Yang C-P; on behalf of the Taiwan Pediatric Oncology Group. (2018) Clinical features and treatment outcome of children with primary mediastinal large B-cell lymphoma in Taiwan. British Journal of Haematology, 182, 15, Abstract 17. [Google Scholar]
- Chen BJ, Chapuy B, Ouyang J, Sun HH, Roemer MG, Xu ML, Yu H, Fletcher CD, Freeman GJ, Shipp MA & Rodig SJ (2013) PD-L1 expression is characteristic of a subset of aggressive B-cell lymphomas and virus-associated malignancies. Clinical Cancer Research, 19, 3462–3473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connors JM, Jurczak W, Straus DJ, Ansell SM, Kim WS, Gallamini A, Younes A, Alekseev S, Illes A, Picardi M, Lech-Maranda E, Oki Y, Feldman T, Smolewski P, Savage KJ, Bartlett NL, Walewski J, Chen R, Ramchandren R, Zinzani PL, Cunningham D, Rosta A, Josephson NC, Song E, Sachs J, Liu R, Jolin HA, Huebner D. & Radford J.; ECHELON-1 Study Group. (2018) Brentuximab vedotin with chemotherapy for stage III or IV Hodgkin’s lymphoma. New England Journal of Medicine, 378, 331–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coustan-Smith E, Sandlund JT, Perkins SL, Chen H, Chang M, Abromowitch M. & Campana D. (2009) Minimal disseminated disease in childhood T-cell lymphoblastic lymphoma: a report from the children’s oncology group. Journal of Clinical Oncology, 27, 3533–3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damm-Welk C, Busch K, Burkhardt B, Schieferstein J, Viehmann S, Oschlies I, Klapper W, Zimmermann M, Harbott J, Reiter A. & Woessmann W. (2007) Prognostic significance of circulating tumor cells in bone marrow or peripheral blood as detected by qualitative and quantitative PCR in pediatric NPMALK-positive anaplastic large-cell lymphoma. Blood, 110, 670–677. [DOI] [PubMed] [Google Scholar]
- Ducray SP, Garland GD, Truong T, Prokoph N, Elenitoba-Johnson KSJ, Lim MS, Merkel O, Kenner L, Bannister A. & Turner SD (2018) The anaplastic large cell lymphoma (ALCL)-associated nucleophosmin-anaplastic lymphom kinase (NPM-ALK) directs expression of the nuclear protein brahma related gene 1 (BRG1) driving cell survival. British Journal of Haematology, 182, 30, Abstract 41. [Google Scholar]
- Ehrhardt MJ, Hochberg J. & Robison LL (2018a) Childhood and adolescent non-Hodgkin lymphoma survivorship: the childhood cancer survivor study (CCSS) and St. Jude lifetime (SJLIFE) cohorts. British Journal of Haematology, 182, 5, Abstract 1.30230525 [Google Scholar]
- Ehrhardt MJ, Mulrooney DA, Li C, Baassiri MJ, Bjornard K, Sandlund JT, Brinkman TM, Huang IC, Srivastava DK, Ness KK, Robison LL, Hudson MM & Krull KR (2018b) Neurocognitive, psychosocial, and quality-of-life outcomes in adult survivors of childhood non-Hodgkin lymphoma. Cancer, 124, 417–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de los Fayos Alonso IG, Kothmayer M, Schlederer M, Pusch O, Tangermann S, Lagger S. & Kenner L. (2018) Examining the function of PDGFRB in anaplastic large cell lymphoma. British Journal of Haematology, 182, 29, Abstract 40.29741753 [Google Scholar]
- Flower A. (2018) Epidemiology and outcome of adolescents and young adults with NHL and Hodgkin lymphoma. British Journal of Haematology, 182, 13, Abstract 14. [Google Scholar]
- Frazer JK, Li KJ, Galardy PJ, Perkins SL, Auperin A, Anderson JR, Pinkerton R, Buxton A, Gross TG, Michon J, Leverger G, Weinstein HJ, Harrison L, Shiramizu B, Barth MJ, Goldman SC, Patte C. & Cairo MS (2018) Excellent outcomes in children and adolescents with CNS(+) burkitt lymphoma or other mature B-NHL using only intrathecal and systemic chemoimmunotherapy: results from FAB/LMB96 and COG ANHL01P1. British Journal of Haematology. 10.1111/bjh.15520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardenswartz A, Mehta B, El-Mallawany N, Van De Ven C, Hochberg J, Flower A, Morris E, Harrison L, Basso J, Gerard P, Ayello J, Baxter-Lowe LA & Cairo MS (2018) Safety and efficacy of myeloablative conditioning autologous stem cell transplantation, targeted immunotherapy, and reduced intensity conditioning allogeneic stem cell transplantation in children, adolescents, and young adults with relapsed/refractory mature B-cell non Hodgkin lymphoma. British Journal of Haematology, 182, 23, Abstract 28. [DOI] [PubMed] [Google Scholar]
- Gatta G, Zigon G, Capocaccia R, Coebergh JW, Desandes E, Kaatsch P, Pastore G, Peris-Bonet R. & Stiller CA; EUROCARE Working Group. (2009) Survival of European children and young adults with cancer diagnosed 1995–2002. European Journal of Cancer, 45, 992–1005. [DOI] [PubMed] [Google Scholar]
- Giulino-Roth L. & Goldman S. (2016) Recent molecular and therapeutic advances in B-cell non-Hodgkin lymphoma in children. British Journal of Haematology, 173, 531–544. [DOI] [PubMed] [Google Scholar]
- Goldman S, Smith L, Anderson JR, Perkins S, Harrison L, Geyer MB, Gross TG, Weinstein H, Bergeron S, Shiramizu B, Sanger W, Barth M, Zhi J. & Cairo MS (2013) Rituximab and FAB/LMB 96 chemotherapy in children with Stage III/IV B-cell non-Hodgkin lymphoma: a Children’s Oncology Group report. Leukemia, 27, 1174–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman S, Smith L, Galardy P, Perkins SL, Frazer JK, Sanger W, Anderson JR, Gross TG, Weinstein H, Harrison L, Shiramizu B, Barth M. & Cairo MS (2014) Rituximab with chemotherapy in children and adolescents with central nervous system and/or bone marrow-positive Burkitt lymphoma/leukaemia: a Children’s Oncology Group Report. British Journal of Haematology, 167, 394–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman S, Barth M, Hochberg J, Klejmont L, Harrison L, Shi Q, Oesterheld J, Heym K, Perkins S, Miles R, Shiramizu B. & Cairo M. (2018) Reduced burden of oncologic therapy in children, adolescents and young adults with good risk (GR) CD20 + mature B-cell lymphoma. British Journal of Haematology, 182, 11, Abstract 10.29676460 [Google Scholar]
- Hochberg J. (2018) Novel targeted immunotherapy in newly diagnosed adolescent and young adults with Hodgkin lymphoma. British Journal of Haematology, 182, 14, Abstract 15. [Google Scholar]
- Hochberg J, Giblin T, Braniecki S, Batone R, Harrison L, Basso J, Flower A. & Cairo MS (2018a) Long term follow up of pediatric lymphoma survivors upon entry into a wellness and survivorship program. British Journal of Haematology, 182, 8, Abstract 5. [Google Scholar]
- Hochberg J, Flower A, Brugieres L. & Cairo MS (2018b) NHL in adolescents and young adults: a unique population. Pediatric Blood and Cancer, 65, e27073. [DOI] [PubMed] [Google Scholar]
- Howlader N, Mariotto AB, Besson C, Suneja G, Robien K, Younes N. & Engels EA (2017) Cancer-specific mortality, cure fraction, and noncancer causes of death among diffuse large B-cell lymphoma patients in the immunochemotherapy era. Cancer, 123, 3326–3334. [DOI] [PubMed] [Google Scholar]
- Hussain S, Bedekovics T, Liu Q, Hu W, Johnson SH, Vasmatzis G, May DG, Roux KJ & Galardy PJ (2018) UCH-L1 bypasses mTOR to promote protein biosynthesis and is required for MYC driven lymphomagenesis in mice. British Journal of Haematology, 182, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ippolito T, Tang G, Mavis C, Gu J, Hernandez-Illizaliturri F. & Barth MJ (2018) Omipalisib (GSK458), a dual pan-P13K/mTOR inhibitor, exhibits in vitro and in vivo activity in chemotherapy-sensitive and -resistant models of Burkitt lymphoma. British Journal of Haematology, 182, 38, Abstract 53. [Google Scholar]
- Jaglowski SM, Linden E, Termuhlen AM & Flynn JM (2009) Lymphoma in adolescents and young adults. Seminars in Oncology, 36, 381–418. [DOI] [PubMed] [Google Scholar]
- Kim SW, Yoon SS, Suzuki R, Matsuno Y, Yi HG, Yoshida T, Imamura M, Wake A, Miura K, Hino M, Ishikawa T, Kim JS, Maeda Y, Lee JJ, Kang HJ, Lee HS, Lee JH, Izutsu K, Fukuda T, Kim CW, Yoshino T, Ohshima K, Nakamura S, Nagafuji K, Suzumiya J, Harada M. & Kim CS (2013) Comparison of outcomes between autologous and allogeneic hematopoietic stem cell transplantation for peripheral T-cell lymphomas with central review of pathology. Leukemia, 27, 1394–1397. [DOI] [PubMed] [Google Scholar]
- Kreck B, Richter J, Ammerpohl O, Barann M, Esser D, Petersen BS, Vater I, Murga Penas EM, Bormann Chung CA, Seisenberger S, Lee Boyd V, Smallwood S, Drexler HG, Macleod RA, Hummel M, Krueger F, Hasler R, Schreiber S, Rosenstiel P, Franke A. & Siebert R. (2013) Base-pair resolution DNA methylome of the EBV-positive Endemic Burkitt lymphoma cell line DAUDI determined by SOLiD bisulfite-sequencing. Leukemia, 27, 1751–1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kretzmer H, Bernhart SH, Wang W, Haake A, Weniger MA, Bergmann AK, Betts MJ, Carrillo-de-Santa-Pau E, Doose G, Gutwein J, Richter J, Hovestadt V, Huang B, Rico D, Juhling F, Kolarova J, Lu Q, Otto C, Wagener R, Arnolds J, Burkhardt B, Claviez A, Drexler HG, Eberth S, Eils R, Flicek P, Haas S, Humme M, Karsch D, Kerstens HHD, Klapper W, Kreuz M, Lawerenz C, Lenzek D, Loeffler M, Lopez C, MacLeod RAF, Martens JHA, Kulis M, Martin-Subero JI, Moller P, Nage I, Picelli S, Vater I, Rohde M, Rosenstiel P, Rosolowski M, Russell RB, Schilhabel M, Schlesner M, Stadler PF, Szczepanowski M, Trumper L, Stunnenberg HG, Kuppers R, Ammerpohl O, Lichter P, Siebert R, Hoffmann S. & Radlwimmer B. (2015) DNA methylome analysis in Burkitt and follicular lymphomas identifies differentially methylated regions linked to somatic mutation and transcriptional control. Nature Genetics, 47, 1316–1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtz DM, Scherer F, Jin MC, Soo J, Craig AFM, Esfahani MS, Chabon JJ, Stehr H, Liu CL, Tibshirani R, Maeda LS, Gupta NK, Khodadoust MS, Advani RH, Levy R, Newman AM, Duhrsen U, Huttmann A, Meignan M, Casasnovas RO, Westin JR, Roschewski M, Wilson WH, Gaidano G, Rossi D, Diehn M. & Alizadeh AA (2018) Circulating tumor DNA measurements as early outcome predictors in diffuse large B-Cell lymphoma. Journal of Clinical Oncology, 36, 2845–2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwong YL, Chan TSY, Tan D, Kim SJ, Poon LM, Mow B, Khong PL, Loong F, Au-Yeung R, Iqbal J, Phipps C. & Tse E. (2017) PD1 blockade with pembrolizumab is highly effective in relapsed or refractory NK/T-cell lymphoma failing l-asparaginase. Blood, 129, 2437–2442. [DOI] [PubMed] [Google Scholar]
- Lamant L, McCarthy K, d’Amore E, Klapper W, Nakagawa A, Fraga M, Maldyk J, Simonitsch-Klupp I, Oschlies I, Delsol G, Mauguen A, Brugieres L. & Le Deley MC (2011) Prognostic impact of morphologic and phenotypic features of childhood ALK-positive anaplastic large-cell lymphoma: results of the ALCL99 study. Journal of Clinical Oncology, 29, 4669–4676. [DOI] [PubMed] [Google Scholar]
- Landmann E, Burkhardt B, Zimmermann M, Meyer U, Woessmann W, Klapper W, Wrobel G, Rosolen A, Pillon M, Escherich G, Attarbaschi A, Beishuizen A, Mellgren K, Wynn R, Ratei R, Plesa A, Schrappe M, Reiter A, Bergeron C, Patte C. & Bertrand Y. (2017) Results and conclusions of the European Intergroup EURO-LB02 trial in children and adolescents with lymphoblastic lymphoma. Haematologica, 102, 2086–2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung W, Sandlund JT, Hudson MM, Zhou Y, Hancock ML, Zhu Y, Ribeiro RC, Rubnitz JE, Kun LE, Razzouk B, Evans WE & Pui CH (2001) Second malignancy after treatment of childhood non-Hodgkin lymphoma. Cancer, 92, 1959–1966. [DOI] [PubMed] [Google Scholar]
- Levine SR, Shah T, Hochberg J, Ayello J, Morris E, van De Ven C. & Cairo MS (2018) Ruxolitinib significantly enhances in vitro apoptosis in Hodgkin lymphoma (HL) and primary mediastinal B-cell lymphoma (PMBL) and survival in a lymphoma xenograft murine mouse: an AYA therapeutic opportunity. British Journal of Haematology, 182, 37, Abstract 51. [Google Scholar]
- Lim MS (2018) Novel pathways of oncogenesis in anaplastic large cell lymphoma (ALCL). British Journal of Haematology, 182, 27, Abstract 37. [Google Scholar]
- Lionakis MS, Dunleavy K, Roschewski M, Widemann BC, Butman JA, Schmitz R, Yang Y, Cole DE, Melani C, Higham CS, Desai JV, Ceribelli M, Chen L, Thomas CJ, Little RF, Gea-Banacloche J, Bhaumik S, Stetler-Stevenson M, Pittaluga S, Jaffe ES, Heiss J, Lucas N, Steinberg SM, Staudt LM & Wilson WH (2017) Inhibition of B cell receptor signaling by ibrutinib in primary CNS lymphoma. Cancer Cell, 31, e835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locatelli F, Mauz-Koerholz C, Neville K, Llort A, Beishuizen A, Daw S, Pillon M, Aladjidi N, Klingebiel T, Landman-Parker J, MedinaSanson A, August K, Sachs J, Hoffman K, Kinley J, Song S, Song G, Zhang S, Suri A. & Gore L. (2018) Brentuximab vedotin for paediatric relapsed or refractory Hodgkin’s lymphoma and anaplastic large-cell lymphoma: a multicentre, open-label, phase 1/2 study. The Lancet Haematology, 5, e450–e461. [DOI] [PubMed] [Google Scholar]
- Lovisa F, Gaffo E, Bortoluzzi S, Garbin A, Di Battista P, Carraro E, Faruggia P, Sala A, Piglione M, Vinti L, D’Amore E, Basso G, Pillon M. & Mussolin L. (2018) Comprehensive characterization of plasma-derived exosomes in anaplastic large cell lymphoma of childhood. British Journal of Haematology, 182, 29, Abstract 39.29741753 [Google Scholar]
- Lowe E. (2018) Novel targets and outcome in anaplastic large cell lymphoma. British Journal of Haematology, 182, 35, Abstract 49. [Google Scholar]
- Malcolm TIM, Villarese P, Fairbairn CJ, Lamant L, Trinquand A, Hook CE, Burke GAA, Brugieres L, Hughes K, Payet D, Merkel O, Schiefer A-I, Ashankyty I, Mian S, Wasik M, Turner M, Kenner L, Asnafi V, Macintyre E. & Turner SD (2016) Anaplastic large cell lymphoma arises in thymocytes and requires transient TCR expression for thymic egress. Nature Communications, 7, 10087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Guerrero I, Ramis-Zaldivar JE, Celis V, Balague O, Verdu J, Salmeron-Villalobos J, Sabado C, Garrido M, Gonzalez-Farre B, Astigarraga I, Andres M. & Salaverria I. (2018) Global genetic profiling of a pediatric series of T-cell lymphoblastic lymphoma. British Journal of Haematology, 182, 64, Abstract 99. [Google Scholar]
- Meinhardt A, Burkhardt B, Zimmermann M, Borkhardt A, Kontny U, Klingebiel T, Berthold F, Janka-Schaub G, Klein C, Kabickova E, Klapper W, Attarbaschi A, Schrappe M, Reiter A. & Berlin-Frankfurt-Munster G. (2010) Phase II window study on rituximab in newly diagnosed pediatric mature B-cell nonHodgkin’s lymphoma and Burkitt leukemia. Journal of Clinical Oncology, 28, 3115–3121. [DOI] [PubMed] [Google Scholar]
- Meyer JA, Zhou D, Mason CC, Downie JM, Rodic V, Abromowitch M, Wistinghausen B, Termuhlen AM, Angiolillo AL, Perkins SL, Lones MA, Barnette P, Schiffman JD & Miles RR (2017) Genomic characterization of pediatric B-lymphoblastic lymphoma and B-lymphoblastic leukemia using formalin-fixed tissues. Pediatric Blood and Cancer, 64, e26363. [DOI] [PubMed] [Google Scholar]
- Meyer LK, Delgado-Martin C, Huang BJ, Wandler AM, Horton TM, Teachey DT, Shannon KM & Hermiston ML (2018) Glucocorticoids paradoxically induce steroid resistance through a STAT5-mediated survival mechanism in T-cell acute lymphoblastic leukemia/lymphoma. British Journal of Haematology, 182, 63, Abstract 98.29882587 [Google Scholar]
- Minard-Colin V, Brugieres L, Reiter A, Cairo MS, Gross TG, Woessmann W, Burkhardt B, Sandlund JT, Williams D, Pillon M, Horibe K, Auperin A, Le Deley MC, Zimmerman M, Perkins SL, Raphael M, Lamant L, Klapper W, Mussolin L, Poirel HA, Macintyre E, Damm-Welk C, Rosolen A. & Patte C. (2015) non-Hodgkin lymphoma in children and adolescents: progress through effective collaboration, current knowledge, and challenges ahead. Journal of Clinical Oncology, 33, 2963–2974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minard-Colin V, Auperin A, Pillon M, Burke A, Anderson JR, Barkauskas DA, Wheatley K, Delgado R, Alexander S, Uyttebroeck A, Bollard C, Zsiros J, Csoka M, Goma G, Tulard A, Patte C. & Gross TG (2016) Results of the randomized Intergroup trial Inter-B-NHL Ritux 2010 for children and adolescents with high-risk B-cell non-Hodgkin lymphoma (B-NHL) and mature acute leukemia (B-AL): evaluation of rituximab (R) efficacy in addition to standard LMB chemotherapy (CT) regimen. Journal of Clinical Oncology, 34, 10507–10507. [Google Scholar]
- Monti S, Chapuy B, Takeyama K, Rodig SJ, Hao Y, Yeda KT, Inguilizian H, Mermel C, Currie T, Dogan A, Kutok JL, Beroukhim R, Neuberg D, Habermann TM, Getz G, Kung AL, Golub TR & Shipp MA (2012) Integrative analysis reveals an outcome-associated and targetable pattern of p53 and cell cycle deregulation in diffuse large B cell lymphoma. Cancer Cell, 22, 359–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno L, Pearson ADJ, Paoletti X, Jimenez I, Geoerger B, Kearns PR, Zwaan CM, Doz F, Baruchel A, Vormoor J, Casanova M, Pfister SM, Morland B. & Vassal G. (2017) Early phase clinical trials of anticancer agents in children and adolescents - an ITCC perspective. Nature Reviews Clinical Oncology, 14, 497–507. [DOI] [PubMed] [Google Scholar]
- Mosse YP, Voss SD, Lim MS, Rolland D, Minard CG, Fox E, Adamson P, Wilner K, Blaney SM & Weigel BJ (2017) Targeting ALK with crizotinib in pediatric anaplastic large cell lymphoma and inflammatory myofibroblastic tumor: a Children’s Oncology Group Study. Journal of Clinical Oncology, 35, 3215–3221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moti N, Malcolm T, Hamoudi R, Mian S, Garland G, Hook CE, Burke GA, Wasik MA, Merkel O, Kenner L, Laurenti E, Dick JE & Turner SD (2015) Anaplastic large cell lymphoma-propagating cells are detectable by side population analysis and possess an expression profile reflective of a primitive origin. Oncogene, 34, 1843–1852. [DOI] [PubMed] [Google Scholar]
- Mussolin L. (2018) The role of liquid biopsy in non-Hodgkin lymphomas of childhood. British Journal of Haematology, 182, 17, Abstract 20. [Google Scholar]
- Mussolin L, Pillon M, d’Amore ES, Conter V, Piglione M, Lo Nigro L, Garaventa A, Buffardi S, Arico M. & Rosolen A. (2011) Minimal disseminated disease in high-risk Burkitt’s lymphoma identifies patients with different prognosis. Journal of Clinical Oncology, 29, 1779–1784. [DOI] [PubMed] [Google Scholar]
- Nakamura R. (2018) CD19/CMV CAR T cells and post CMV vaccine strategies. British Journal of Haematology, 182, 21, Abstract 27. [Google Scholar]
- Nakamura R, La Rosa C, Longmate J, Drake J, Slape C, Zhou Q, Lampa MG, O’Donnell M, Cai JL, Farol L, Salhotra A, Snyder DS, Aldoss I, Forman SJ, Miller JS, Zaia JA & Diamond DJ (2016) Viraemia, immunogenicity, and survival outcomes of cytomegalovirus chimeric epitope vaccine supplemented with PF03512676 (CMVPepVax) in allogeneic haemopoietic stem-cell transplantation: randomised phase 1b trial. The Lancet Haematology, 3, e87–e98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nayak L, Iwamoto FM, LaCasce A, Mukundan S, Roemer MGM, Chapuy B, Armand P, Rodig SJ & Shipp MA (2017) PD-1 blockade with nivolumab in relapsed/refractory primary central nervous system and testicular lymphoma. Blood, 129, 3071–3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nayyar G, Chu Y, Shah T, Barth M, Miles RR, Ayello J, Morris E, Harrison L, Van De Ven C, Galardy P, Goldman S, Lim MS, Hermiston M, McAllister-Lucas LM, GiulinoRoth L, Perkins SL & Cairo MS (2018) Ibrutinib significantly inhibited bruton’s tyrosine kinase (BTK) phosphorylation, in-vitro proliferation and enhanced overall survival in a pre-clinical burkitt lymphoma (BL) model. British Journal of Haematology, 182, 37, Abstract 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oeffinger KC, Mertens AC, Sklar CA, Kawashima T, Hudson MM, Meadows AT, Friedman DL, Marina N, Hobbie W, Kadan-Lottick NS, Schwartz CL, Leisenring W. & Robison LL; Childhood Cancer Survivor Study. (2006) Chronic health conditions in adult survivors of childhood cancer. New England Journal of Medicine, 355, 1572–1582. [DOI] [PubMed] [Google Scholar]
- Patte C, Auperin A, Gerrard M, Michon J, Pinkerton R, Sposto R, Weston C, Raphael M, Perkins SL, McCarthy K. & Cairo MS (2007) Results of the randomized international FAB/LMB96 trial for intermediate risk B-cell non-Hodgkin lymphoma in children and adolescents: it is possible to reduce treatment for the early responding patients. Blood, 109, 2773–2780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillai V, Tallarico M, Bishop MR & Lim MS (2016) Mature T- and NK-cell non-Hodgkin lymphoma in children and young adolescents. British Journal of Haematology, 173, 573–581. [DOI] [PubMed] [Google Scholar]
- Quintanilla-Fend L. (2018) Mutational landscape of pediatric-type follicular lymphoma (PTFL). British Journal of Haematology, 182, 17, Abstract 21. [Google Scholar]
- Rivers J, Annesley C, Summers C, Finney O, Pulsipher M, Wayne A, Park J, Jensen M. & Gardner R. (2018) Early response data for pediatric patients with non-Hodgkin lymphoma treated with CD19 chimeric antigen receptor (CAR) T-cells. British Journal of Haematology, 182, 23, Abstract 29. [Google Scholar]
- Roemer MG, Advani RH, Ligon AH, Natkunam Y, Redd RA, Homer H, Connelly CF, Sun HH, Daadi SE, Freeman GJ, Armand P, Chapuy B, de Jong D, Hoppe RT, Neuberg DS, Rodig SJ & Shipp MA (2016) PD-L1 and PD-L2 genetic alterations define classical Hodgkin lymphoma and predict outcome. Journal of Clinical Oncology, 34, 2690–2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roemer MGM, Redd RA, Cader FZ, Pak CJ, Abdelrahman S, Ouyang J, Sasse S, Younes A, Fanale M, Santoro A, Zinzani PL, Timmerman J, Collins GP, Ramchandren R, Cohen JB, De Boer JP, Kuruvilla J, Savage KJ, Trneny M, Ansell S, Kato K, Farsaci B, Sumbul A, Armand P, Neuberg DS, Pinkus GS, Ligon AH, Rodig SJ & Shipp MA (2018) Major histocompatibility complex class II and Programmed death ligand 1 expression predict outcome after programmed death 1 blockade in classic Hodgkin lymphoma. Journal of Clinical Oncology, 36, 942–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolland DCM, Basrur V, Jeon YK, McNeilSchwalm C, Fermin D, Conlon KP, Zhou Y, Ng SY, Tsou CC, Brown NA, Thomas DG, Bailey NG, Omenn GS, Nesvizhskii AI, Root DE, Weinstock DM, Faryabi RB, Lim MS & Elenitoba-Johnson KSJ (2017) Functional proteogenomics reveals biomarkers and therapeutic targets in lymphomas. Proceedings of the National Academy of Sciences of United States of America, 114, 6581–6586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosolen A, Perkins SL, Pinkerton CR, Guillerman RP, Sandlund JT, Patte C, Reiter A. & Cairo MS (2015) Revised international pediatric non-hodgkin lymphoma staging system. Journal of Clinical Oncology, 33, 2112–2118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salaverria I, Philipp C, Oschlies I, Kohler CW, Kreuz M, Szczepanowski M, Burkhardt B, Trautmann H, Gesk S, Andrusiewicz M, Berger H, Fey M, Harder L, Hasenclever D, Hummel M, Loeffler M, Mahn F, Martin-Guerrero I, Pellissery S, Pott C, Pfreundschuh M, Reiter A, Richter J, Rosolowski M, Schwaenen C, Stein H, Trumper L, Wessendorf S, Spang R, Kuppers R, Klapper W. & Siebert R. (2011) Translocations activating IRF4 identify a subtype of germinal center-derived B-cell lymphoma affecting predominantly children and young adults. Blood, 118, 139–147. [DOI] [PubMed] [Google Scholar]
- Sandlund JT, Guillerman RP, Perkins SL, Pinkerton CR, Rosolen A, Patte C, Reiter A. & Cairo MS (2015) International pediatric non-Hodgkin lymphoma response criteria. Journal of Clinical Oncology, 33, 2106–2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satwani P, Jin Z, Martin PL, Bhatia M, Garvin JH, George D, Chaudhury S, Talano J, Morris E, Harrison L, Sosna J, Peterson M, Militano O, Foley S, Kurtzberg J. & Cairo MS (2015) Sequential myeloablative autologous stem cell transplantation and reduced intensity allogeneic hematopoietic cell transplantation is safe and feasible in children, adolescents and young adults with poor-risk refractory or recurrent Hodgkin and non-Hodgkin lymphoma. Leukemia, 29, 448–455. [DOI] [PubMed] [Google Scholar]
- Scherer F, Kurtz DM, Newman AM, Stehr H, Craig AFM, Esfahani MS, Lovejoy AF, Chabon JJ, Klass DM, Green MR, Liu CL, Zhou L, Glover C, Visser BC, Poultsides GA, Advani RH, Maeda LS, Gupta NK, Davis RE, Levy R, Ohgami RS, Kunder CA, Rossi D, Westin J, Diehn M. & Alizadeh AA (2018) Circulating tumor DNA (ctDNA) in B-cell lymphoma. British Journal of Haematology, 182, 16, Abstract 19. [Google Scholar]
- Schmidt J, Gong S, Marafioti T, Mankel B, Gonzalez-Farre B, Balague O, Mozos A, Cabecadas J, van der Walt J, Hoehn D, Rosenwald A, Ott G, Dojcinov S, Egan C, Nadeu F, Ramis-Zaldivar JE, Clot G, Barcena C, Perez-Alonso V, Endris V, Penzel R, Lome-Maldonado C, Bonzheim I, Fend F, Campo E, Jaffe ES, Salaverria I. & Quintanilla-Martinez L. (2016) Genome-wide analysis of pediatric-type follicular lymphoma reveals low genetic complexity and recurrent alterations of TNFRSF14 gene. Blood, 128, 1101–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt J, Ramis-Zaldivar JE, Nadeu F, Gonzalez-Farre B, Navarro A, Egan C, MontesMojarro IA, Marafioti T, Cabecadas J, van der Walt J, Dojcinov S, Rosenwald A, Ott G, Bonzheim I, Fend F, Campo E, Jaffe ES, Salaverria I. & Quintanilla-Martinez L. (2017) Mutations of MAP2K1 are frequent in pediatric-type follicular lymphoma and result in ERK pathway activation. Blood, 130, 323–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shipp M. (2018) Promising targets in B cell primary central nervous system lymphoma. British Journal of Haematology, 182, 10, Abstract 17.29676459 [Google Scholar]
- Shiramizu B, Mussolin L, Woessmann W. & Klapper W. (2016) Paediatric non-Hodgkin lymphoma - perspectives in translational biology. British Journal of Haematology, 173, 617–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siebert R. (2018) DNA methylone in Burkitt lymphoma. British Journal of Haematology, 182, 10, Abstract 18.29676459 [Google Scholar]
- Suzuki R. (2015) Extranodal NK/T cell lymphoma, nasal type In: Tropical Hemato-Oncology (ed. by Droz JP, Carme B, Couppie P, Nacher M. & Thieblemont C.), pp. 199–212. Springer Nature, Switzerland. [Google Scholar]
- Suzuki R. (2018) Diagnosis and outcome of extranodal NK/T-cell lymphoma in children and adolescents: the Japan experience. British Journal of Haematology, 182, 31, Abstract 42. [Google Scholar]
- Termuhlen AM (2018) Allogeneic versus autologous bone marrow transplantation for NK/T and non-anaplastic peripheral T cell lymphomas. British Journal of Haematology, 182, 32, Abstract 44. [Google Scholar]
- Termuhlen AM, Smith LM, Perkins SL, Lones M, Finlay JL, Weinstein H, Gross TG & Abromowitch M. (2012) Outcome of newly diagnosed children and adolescents with localized lymphoblastic lymphoma treated on Children’s Oncology Group trial A5971: a report from the Children’s Oncology Group. Pediatric Blood and Cancer, 59, 1229–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Termuhlen AM, Smith LM, Perkins SL, Lones M, Finlay JL, Weinstein H, Gross TG & Abromowitch M. (2013) Disseminated lymphoblastic lymphoma in children and adolescents: results of the COG A5971 trial: a report from the Children’s Oncology Group. British Journal of Haematology, 162, 792–801. [DOI] [PubMed] [Google Scholar]
- Trama A, Bernasconi A, McCabe MG, Guevara M, Gatta G, Botta L, Group RAW, Ries L. & Bleyer A. (2018) Is the cancer survival improvement in European and American adolescent and young adults still lagging behind that in children? Pediatric Blood and Cancer, 66, e27407. [DOI] [PubMed] [Google Scholar]
- Trinquand A, Tanguy-Schmidt A, Ben Abdelali R, Lambert J, Beldjord K, Lengline E, De Gunzburg N, Payet-Bornet D, Lhermitte L, Mossafa H, Lheritier V, Bond J, Huguet F, Buzyn A, Leguay T, Cahn JY, Thomas X, Chalandon Y, Delannoy A, Bonmati C, Maury S, Nadel B, Macintyre E, Ifrah N, Dombret H. & Asnafi V. (2013) Toward a NOTCH1/FBXW7/RAS/PTEN-based oncogenetic risk classification of adult T-cell acute lymphoblastic leukemia: a Group for Research in Adult Acute Lymphoblastic Leukemia study. Journal of Clinical Oncology, 31, 4333–4342. [DOI] [PubMed] [Google Scholar]
- Turner SD, Lamant L, Kenner L. & Brugieres L. (2016) Anaplastic large cell lymphoma in paediatric and young adult patients. British Journal of Haematology, 173, 560–572. [DOI] [PubMed] [Google Scholar]
- Willemze R. (2018) Primary cutaneous anaplastic large cell lymphoma and other cutaneous T-cell lymphomas in children and adolescents. British Journal of Haematology, 182, 27, Abstract 36. [Google Scholar]
- Willemze R, Jaffe ES, Burg G, Cerroni L, Berti E, Swerdlow SH, Ralfkiaer E, Chimenti S, Diaz-Perez JL, Duncan LM, Grange F, Harris NL, Kempf W, Kerl H, Kurrer M, Knobler R, Pimpinelli N, Sander C, Santucci M, Sterry W, Vermeer MH, Wechsler J, Whittaker S. & Meijer CJ (2005) WHO-EORTC classification for cutaneous lymphomas. Blood, 105, 3768–3785. [DOI] [PubMed] [Google Scholar]
- Woessmann W. (2018) Hematopoietic stem cell transplantation in relapsed/refractory NHL. British Journal of Haematology, 182, 21, Abstract 25. [Google Scholar]
- Woessmann W, Zimmermann M, Lenhard M, Burkhardt B, Rossig C, Kremens B, Lang P, Attarbaschi A, Mann G, Oschlies I, Klapper W. & Reiter A. (2011) Relapsed or refractory anaplastic large-cell lymphoma in children and adolescents after Berlin-Frankfurt-Muenster (BFM)-type first-line therapy: a BFM-group study. Journal of Clinical Oncology, 29, 3065–3071. [DOI] [PubMed] [Google Scholar]
- Xavier AC & Costa LJ (2018) Nonbiological factors affecting outcomes in AYA with lymphoma. British Journal of Haematology, 182, 15, Abstract 16. [Google Scholar]
- Xu X, Paxton CN, Hayashi RJ, Perkins SL & Miles RR (2018) Genomic analysis of early Tcell precursor lymphoblastic lymphoma. British Journal of Haematology, 182, 63, Abstract 97.29882587 [Google Scholar]
- Yamaguchi M, Suzuki R, Oguchi M, Asano N, Amaki J, Akiba T, Maeda T, Itasaka S, Kubota N, Saito Y, Kobayashi Y, Itami J, Ueda K, Miyazaki K, Ii N, Tomita N, Sekiguchi N, Takizawa J, Saito B, Murayama T, Ando T, Wada H, Hyo R, Ejima Y, Hasegawa M. & Katayama N. (2017) Treatments and outcomes of patients with extranodal natural killer/ T-cell lymphoma diagnosed between 2000 and 2013: a cooperative study in Japan. Journal of Clinical Oncology, 35, 32–39. [DOI] [PubMed] [Google Scholar]
- Yang J, Jin L, Duan Y-L, Huang S, Zhang MJ, Zhou C-J & Zhang Y-H (2018) Novel treatment and outcome of extranodal NK/T cell lymphoma, the Beijing experience. British Journal of Haematology, 182, 31, Abstract 43. [Google Scholar]
- Younes A, Bartlett NL, Leonard JP, Kennedy DA, Lynch CM, Sievers EL & Forero-Torres A. (2010) Brentuximab vedotin (SGN-35) for relapsed CD30-positive lymphomas. New England Journal of Medicine, 363, 1812–1821. [DOI] [PubMed] [Google Scholar]
- Younes A, Oki Y, McLaughlin P, Copeland AR, Goy A, Pro B, Feng L, Yuan Y, Chuang HH, Macapinlac HA, Hagemeister F, Romaguera J, Samaniego F, Fanale MA, Dabaja BS, Rodriguez MA, Dang N, Kwak LW, Neelapu SS & Fayad LE (2012) Phase 2 study of rituximab plus ABVD in patients with newly diagnosed classical Hodgkin lymphoma. Blood, 119, 4123–4128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwaan CM, Kearns P, Caron H, Verschuur A, Riccardi R, Boos J, Doz F, Geoerger B, Morland B. & Vassal G. (2010) The role of the ‘innovative therapies for children with cancer’ (ITCC) European consortium. Cancer Treatment Reviews, 36, 328–334. [DOI] [PubMed] [Google Scholar]