Abstract
Purpose
Pseudo-progression (PsPD) is a rare phenomenon observed in <5% of cases of non-small cell lung cancer (NSCLC). This event is challenging for both clinicians and patients. Viable biomarkers to distinguish between PsPD and true progressive disease (TPD) are lacking. The aim of our study was to determine the correlation between PsPD and the neutrophil-to-lymphocyte ratio (NLR) in patients with NSCLC treated with immune checkpoint inhibitors (ICIs).
Patients and methods
We retrospectively reviewed the clinical records of NSCLC patients treated with ICI monotherapy from December 2015 to October 2018 at Kobe University Hospital, Japan. Twenty-five patients were determined to have either PsPD (n =4) or TPD (n =21). We focused on longitudinal radiological images and NLRs.
Results
Here, we report four patients with PsPD. The pre- and post-treatment NLRs were significantly lower in patients with PsPD than in patients with TPD (p = 0.019 and p = 0.007, respectively). The receiver operating characteristic curve according to the pre- and post-treatment NLR showed areas under the curve of 0.82 and 0.94, respectively. The optimal cut-off values for pre- and post-treatment NLR were 4.1 and 3.2, respectively. The pre- and post-treatment NLRs were useful in distinguishing between PsPD and TPD. Both a pre-treatment NLR <4.1 and a post-treatment NLR <3.2 were significantly associated with longer overall survival compared to a pre-treatment NLR ≥4.1 (p < 0.001) and post-treatment NLR ≥3.2 (p = 0.004), respectively.
Conclusion
The NLR could be a viable clue for distinguishing between PsPD and TPD. Patients with a high post-treatment NLR in this study all had TPD, suggesting that these subjects should be considered for an early transition to the next drug treatment regimen.
Keywords: pseudo-progression, biomarker, neutrophil-to-lymphocyte ratio, immune checkpoint inhibitor, non-small cell lung cancer
Introduction
Pseudo-progression (PsPD) is when tumour size transiently increases and then shrinks, a phenomenon that has been reported in patients treated with immune checkpoint inhibitors (ICIs). It was first described in patients with malignant melanoma treated with ipilimumab1 and subsequently reported in patients with non-small cell lung cancer (NSCLC) treated with nivolumab.2,3 The frequency was reported as 3% of all cases and 5% of progressive disease cases in a multicentre retrospective study of 542 treated NSCLC patients.4 While PsPD is a rare phenomenon, it is difficult to distinguish between PsPD and true progressive disease (TPD), underscoring the need to identify a viable biomarker.
Physiological inflammation is one of the immune defences against infection and tissue damage. Acute inflammation abates as infection and tissue damage recover, whereas chronic inflammation is associated with many serious conditions including cancer and autoimmune diseases.5 The microenvironment created by chronic inflammation promotes tumour development.6 The tumour microenvironment is mainly composed of various stromal cells such as cancer cells, immune cells, tumour blood vessels, extracellular matrix, and cancer-related fibroblasts, and its properties are defined by cytokines, chemokines, growth factors, and angiogenic factors produced from these cells.7 However, the detailed mechanism of how the microenvironment contributes to tumour development has not yet been elucidated.
Since it is not realistic to repeatedly evaluate changes in the tumour microenvironment, haematological parameters have attracted attention as surrogate markers. Many clinical studies have examined the correlation between blood-based inflammatory markers and prognosis.8 The neutrophil-to-lymphocyte ratio (NLR) reflects systemic inflammation and is widely accepted as a prognostic marker that can be easily calculated for a variety of solid tumours.9 The usefulness of the NLR at various time points after treatment as well as before treatment10 has been reported for immunotherapy of NSCLC.11–15 Recently, baseline-derived NLR (dNLR) and lactate dehydrogenase (LDH) were reported to be useful for determining prognosis and predicting therapeutic effects.16 We hypothesized that the longitudinal behaviour of haematological parameters such as NLR, dNLR, and LDH during treatment might help distinguish PsPD from TPD.
The aim of this study was to assess the correlation between PsPD and the longitudinal behaviour of routine haematological parameters in patients with NSCLC treated with ICIs.
Materials and Methods
Patients
This retrospective monocentric study included 78 patients with NSCLC who were treated with ICI monotherapy from December 2015 to October 2018 at Kobe University Hospital. This retrospective analysis was approved by the Institutional Review Board of Kobe University Hospital (#180169), and all patients signed a comprehensive written informed consent form. The data collected from the patients’ medical records included the following: sex, age, smoking history, Eastern Cooperative Oncology Group Performance Status (ECOG PS) at treatment initiation, histology, the tumour proportion score (TPS) of PD-L1, the targetable driver mutation status with respect to epidermal growth factor receptor (EGFR), anaplastic lymphoma kinase (ALK) value, proto-oncogene tyrosine-protein kinase ROS1 (ROS1) value, prior therapeutics lines (first, second, third, or more), lactate dehydrogenase (LDH) level, and complete blood count (CBC) data. All data were fully anonymized prior to the analysis.
Laboratory Analyses
We longitudinally recorded laboratory data obtained at each visit during therapy. The NLR was defined as the absolute neutrophil count divided by the absolute lymphocyte count. The dNLR defined as the absolute neutrophil count/(white blood cell count – neutrophil count). The pre-treatment NLR and dNLR were determined using the CBC measured within 1 week prior to the first treatment cycle. The post-treatment NLR and dNLR were determined using the CBC measured at the initial treatment evaluation.
Response Assessment
Treatment efficacy was assessed by the treating physician and another physician and was classified according to the Response Evaluation Criteria in Solid Tumours (RECIST) version 1.1. The radiological evaluation of treatment efficacy by computed tomography (CT) scan was performed before treatment and on a schedule determined by each treating physician during treatment.
PsPD was defined as a partial response (PR) following RECIST-defined progressive disease during ICI treatment. The definition of PR in PsPD was assessed according to the changes observed from the time of PD, not from treatment initiation.4,17
Statistical Analysis
All statistical analyses were performed using EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan, version 1.40), which is a graphical user interface for R software (The R Foundation for Statistical Computing, Vienna, Austria, version 3.5.2).18 More precisely, it is a modified version of R Commander (version 2.5-1) designed to add statistical functions frequently used in biostatistics. Receiver operating characteristic (ROC) curve analyses were performed to determine the most appropriate cut-off values for haematological parameters that could identify patients with PsPD. The sensitivity and specificity values were computed to determine the cut-off points that would maximize the sums of the numbers of true positive and true negative predictions. Categorical variables were analysed using Fisher’s exact tests, and continuous variables were analysed using Mann–Whitney U-tests for non-parametric distributions. Overall survival (OS) was estimated using the Kaplan–Meier method and compared using the log-rank test. OS was defined as the time from the date of the initiation of ICI treatment to the date of death due to any cause. Differences were considered significant at p < 0.05.
Results
Patient Characteristics
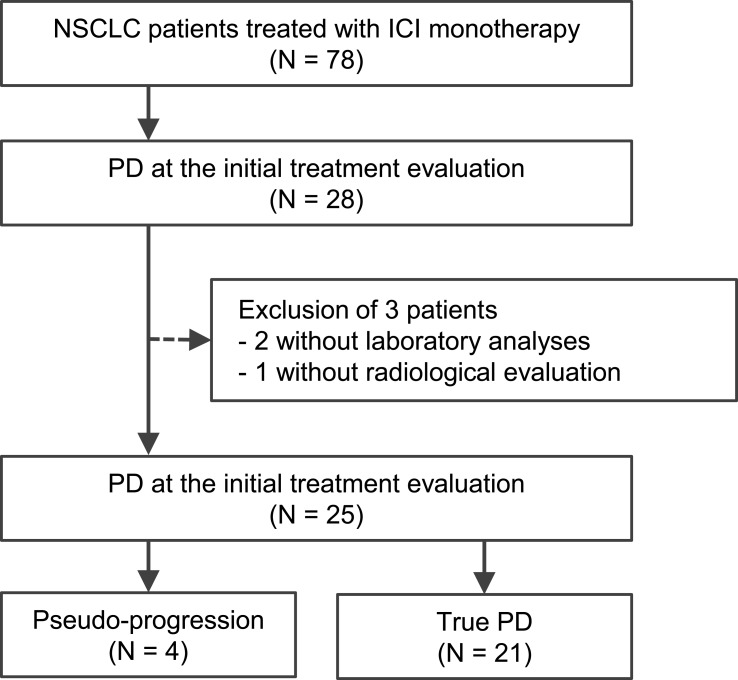
Of the 78 patients, 25 had PD at the initial treatment evaluation and had laboratory data and radiographic records collected during ICI treatment. These 25 patients could be divided into 2 groups that comprised 4 patients with PsPD and 21 patients with TPD (Figure 1). The characteristics of these patient groups are summarized in Table 1.
Figure 1.
Flow diagram of the study selection process.
Table 1.
Patient Characteristics
| PsPD (n = 4) | TPD (n = 21) | p-Value | |
|---|---|---|---|
| Sex | |||
| Male | 4 | 17 | 1 |
| Female | 0 | 4 | |
| Age, years | |||
| Median (range) | 74 (69–75) | 69 (41–78) | 0.22 |
| Performance status | |||
| 0–1 | 4 | 18 | 1 |
| ≥2 | 0 | 3 | |
| Histology | |||
| Adenocarcinoma | 1 | 17 | 0.036 |
| Squamous cell carcinoma | 3 | 2 | |
| Other | 0 | 2 | |
| Tumour stage | |||
| Stage III | 0 | 4 | 1 |
| Stage IV | 3 | 14 | |
| Recurrent | 1 | 3 | |
| Prior lines of therapy | |||
| First | 1 | 4 | 0.80 |
| Second | 2 | 7 | |
| Third or more | 1 | 10 | |
| Targetable driver mutations | |||
| EGFR | 0 | 2 | 1 |
| ALK | 0 | 0 | 1 |
| ROS1 | 0 | 0 | 1 |
| Immune checkpoint inhibitor treatment drug | |||
| Nivolumab | 2 | 13 | 0.48 |
| Pembrolizumab | 1 | 7 | |
| Atezolizumab | 1 | 1 | |
| Use of steroids | |||
| Yes | 0 | 1 | 1 |
| No | 4 | 20 | |
| Programmed death ligand-1 tumour proportion score | |||
| ≥50% | 1 | 5 | 0.67 |
| 1–49% | 0 | 5 | |
| <1% | 2 | 4 | |
| Unknown | 1 | 7 | |
Abbreviations: ALK, anaplastic lymphoma kinase; EGFR, epidermal growth factor receptor; PsPD, pseudo-progression; ROS1, proto-oncogene tyrosine-protein kinase; TPD, true progressive disease.
Comparison of PsPD and TPD
The median time of initial treatment evaluation was 33 days (range, 11–56 days; interquartile range, 20–41 days).
We investigated haematological parameters such as NLR, dNLR, and LDH to identify surrogate markers that distinguish between PsPD and TPD in patients receiving ICI monotherapy. No significant differences were observed between treatment-naïve patients and chemotherapy-resistant patients in NLR (median 3.1 [range, 2.4–92.0] and 4.15 [1.6–14.0], p = 0.658), dNLR (median 2.3 [range, 1.5–11.5] and 2.6 [0.9–6.6], p = 0.946), and LDH (median 224 U/L [range, 208–428] and 273.5 U/L [153–593], p = 0.563).
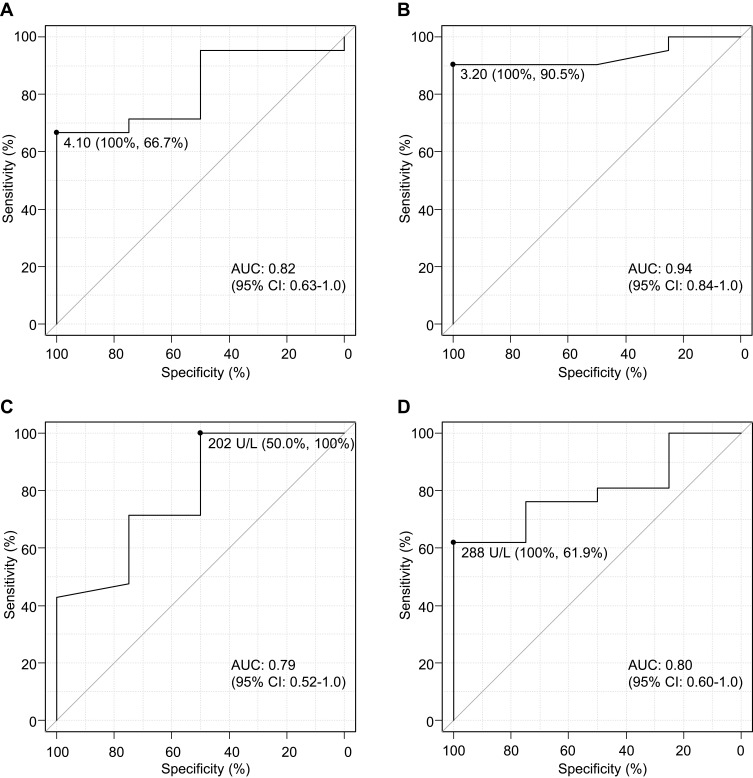
ROC curve analyses were performed to determine the most appropriate cut-off values for the pre- and post-treatment NLR, dNLR, and LDH that could identify patients with PsPD (Figure 2, Supplementary Figure 1). Because NLR showed a larger area under the curve (AUC) than dNLR both pre- and post-treatment, additional analyses were mainly performed using NLR and LDH.
Figure 2.
Receiver operating characteristic (ROC) curve analyses by neutrophil-to-lymphocyte ratio (NLR) and lactate dehydrogenase (LDH). (A) Pre- and (B) post-treatment NLR, (C) pre- and (D) post-treatment LDH. The analyses were performed to determine the most appropriate NLR and LDH cut-off values to identify patients with pseudo-progression. The sensitivity and specificity values were computed to determine the cut-off points that would maximize the sum of the number of true positive and true negative predictions.
The optimal cut-off values for pre-treatment NLR, post-treatment NLR, pre-treatment LDH, and post-treatment LDH were 4.1, 3.2, 202, and 288, respectively (Figure 2). The corresponding AUC values for were 0.82 (95% confidence interval [CI]: 0.63–1.0), with a sensitivity of 66.7% and a specificity of 100%, indicating moderate accuracy (Figure 2A); 0.94 (95% CI: 0.84–1.0), with a sensitivity of 90.5% and a specificity of 100%, indicating high accuracy (Figure 2B); 0.79 (95% CI: 0.52–1.0), with a sensitivity of 100% and a specificity of 50.0%, indicating moderate accuracy (Figure 2C); and 0.80 (95% CI: 0.60–1.0), with a sensitivity of 61.9% and a specificity of 100%, indicating high accuracy (Figure 2D).
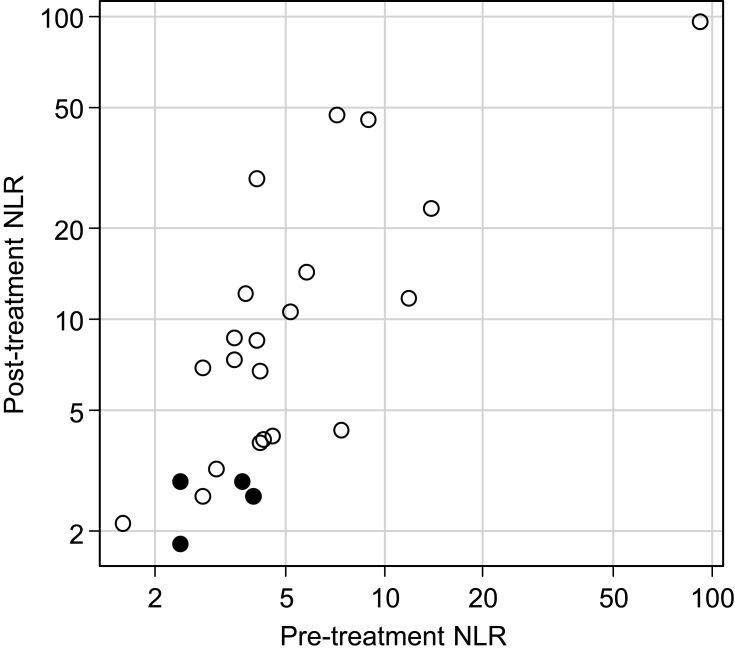
Both median pre- and post-treatment NLRs were significantly lower in patients with PsPD compared to those with TPD (Table 2, p = 0.049 and p = 0.008, respectively). On the other hand, there was no significant difference in the pre- and post-treatment LDH (Table 2, p = 0.075 and p = 0.068, respectively). A scatter plot of the NLRs for all individual patients in this study is shown in Figure 3.
Table 2.
Associations Between PsPD and Haematological Parameters
| PsPD (n = 4) | TPD (n = 21) | p-Value | |
|---|---|---|---|
| Median pre-treatment NLR (range) | 3.05 (2.4–4.0) | 4.2 (1.6–92.0) | 0.049 |
| Median post-treatment NLR (range) | 2.75 (1.8–2.9) | 8.5 (2.1–96.0) | 0.008 |
| Median pre-treatment LDH (range) | 191.5 U/L (153–280) | 271 U/L (202–593) | 0.075 |
| Median post-treatment LDH (range) | 199.5 U/L (133–276) | 313 U/L (153–1560) | 0.068 |
Abbreviations: LDH, lactate dehydrogenase; NLR, neutrophil-to-lymphocyte ratio; PsPD, pseudo-progression; TPD, true progressive disease.
Figure 3.
Scatter plot of the pre- and post-treatment neutrophil-to-lymphocyte ratio. Closed and open circles represent patients with pseudo-progression and true progressive disease, respectively. Note that the coordinates are log scale.
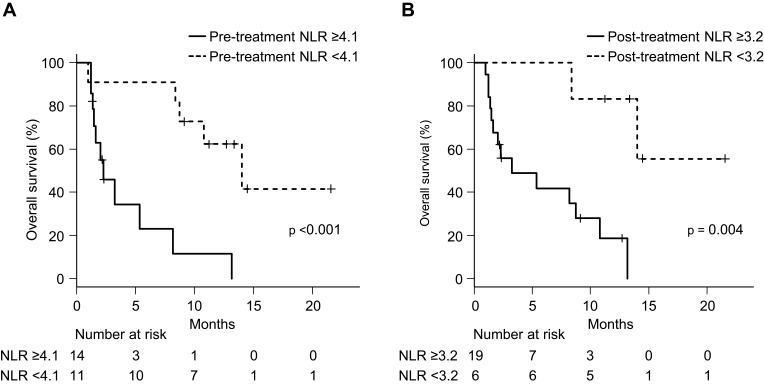
First, we attempted to examine the relationship between PsPD and NLR. We divided the patients with PsPD and TPD into 2 groups according to the pre- and post-treatment NLR (Table 3). Fisher’s exact tests showed that the difference between patients with PsPD and TPD was highly significant (p = 0.026 and p = 0.002, respectively). The median OS values in patients with a pre-treatment NLR <4.1 and ≥4.1 were 14.0 (95% CI: 8.4-not reached) and 2.3 (1.3–8.1) months, respectively (Figure 4A, p < 0.001). The median OS in patients with a post-treatment NLR <3.2 was not reached (95% CI: 8.4-not reached), compared to (1.4–10.8) months in patients with a post-treatment NLR ≥3.2 (Figure 4B, p = 0.004). These results suggest that both a pre-treatment NLR <4.1 and a post-treatment NLR <3.2 were associated with a good prognosis, as reported in the previous studies.11–15 Importantly, these cut-offs were useful for distinguishing between PsPD and TPD.
Table 3.
2×2 Contingency Table for Pseudo-Progression and the NLR
| PsPD (n = 4) | TPD (n = 21) | p-Value | |
|---|---|---|---|
| (A) | |||
| Pre-treatment NLR ≥4.1 | 0 | 14 | 0.026 |
| Pre-treatment NLR <4.1 | 4 | 7 | |
| (B) | |||
| Post-treatment NLR ≥3.2 | 0 | 19 | 0.002 |
| Post-treatment NLR <3.2 | 4 | 2 |
Abbreviations: NLR, neutrophil-to-lymphocyte ratio; PsPD, pseudo-progression; TPD, true progressive disease.
Figure 4.
Survival analysis. Overall survival analysis using the Kaplan–Meier method in 25 patients (A) with a pre-treatment neutrophil-to-lymphocyte ratio (NLR) ≥4.1 and <4.1 and (B) a post-treatment NLR ≥3.2 and <3.2.
Next, we attempted to examine the relationship between PsPD and LDH. We divided the patients with PsPD and TPD into 2 groups according to the pre- and post-treatment LDH values (Supplementary Table 1). Fisher’s exact tests showed that the difference between the patients with PsPD and TPD was highly significant (p = 0.02 and p = 0.039, respectively). The median OS in patients with a pre-treatment LDH <202 U/L was 14.0 (95% CI: 14.0–not reached) compared to 8.1 (2.0–13.2) months in patients with a pre-treatment LDH ≥202 U/L (Supplementary Figure 2A, p = 0.175). The median OS in patients with a post-treatment LDH <288 U/L was 14.0 (95% CI: 8.7–not reached) months, while it was 2.0 (1.2–5.3) months in patients with a post-treatment LDH ≥288 U/L (Supplementary Figure 2B, p <0.001). These results suggest that a post-treatment LDH value <288 U/L was associated with a good prognosis and could be useful for distinguishing between PsPD and TPD.
Based on the above, we propose that the NLR could be useful as a surrogate marker. Further study is needed on the utility of LDH.
Patients with Pseudo-Progression: Case Reports
Patient 1
A 73-year-old male underwent video-assisted thoracic surgery (right lower lobectomy and lymph node dissection) in October 2014 and was diagnosed with stage IIIA (pT1bN2M0) squamous cell carcinoma. Following surgery, he received cisplatin plus vinorelbine as adjuvant chemotherapy. He relapsed with multiple pulmonary metastases 21 months postoperatively. Carboplatin plus nanoparticle albumin-bound paclitaxel was administered as first-line chemotherapy in August 2017. Follow-up after 4 cycles of chemotherapy revealed progression of multiple pulmonary metastases. Subsequently, nivolumab was administered as second-line chemotherapy in August 2018. The TPS of PD-L1 was negative.
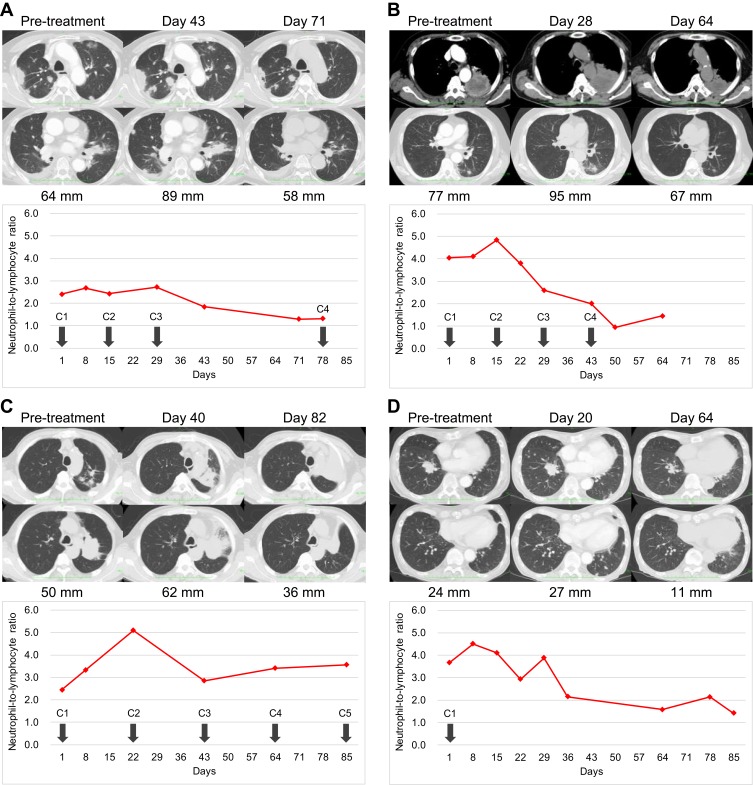
After 6 weeks (3 cycles) of nivolumab administration, multiple intrapulmonary metastases had increased in size (Figure 5A), and his symptoms and general condition worsened. However, CT evaluation at 10 weeks revealed the shrinkage of multiple pulmonary metastases (Figure 5A), and his symptoms and general condition were improved. Nivolumab therapy was resumed and continued over 20 cycles. The best response following treatment initiation was PR. The NLR was not high after nivolumab administration (Figure 5A).
Figure 5.
Longitudinal measurement of representative lesions and the neutrophil-to-lymphocyte ratios (NLRs). (A) Patient 1, (B) Patient 2, (C) Patient 3, (D) Patient 4. Numbers indicate the sum of diameters of target lesions. Only (D) was PD with worsening in non-target lesions and the appearance of new lesions. Solid line, longitudinal NLR measurement; ◆, NLR value at each timepoint; down arrow, immune checkpoint inhibitor administration.
Patient 2
A 75-year-old male was diagnosed with stage IVA (cT3N0M1a) squamous cell carcinoma with malignant pleural effusion in March 2015. He received 2 lines of treatment with chemotherapy (carboplatin plus S-1 [tegafur, 5-chloro-2,4-dihydropyrimidine, and potassium oxonate] as first-line chemotherapy and gemcitabine plus nanoparticle albumin-bound paclitaxel as second-line chemotherapy in a clinical trial19). The primary tumour was enlarged, and mediastinal lymph node metastases were newly noted. Nivolumab was administered as third-line chemotherapy in January 2016. The TPS of PD-L1 was not evaluated.
After 4 weeks (2 cycles) of nivolumab administration, the primary tumour and mediastinal lymph node metastases had increased in size (Figure 5B), but the patient’s symptoms and laboratory data were improved. CT evaluation at 9 weeks (5 cycles) revealed shrinkage of the primary tumour and mediastinal lymph node metastases (Figure 5B). Nivolumab was continued for up to 8 cycles but was discontinued due to the onset of interstitial lung disease. The best response from treatment initiation was stable disease (SD). The NLR peaked 2 weeks after nivolumab administration and remained low thereafter (Figure 5B).
Patient 3
A 75-year-old male was diagnosed with stage IVA (cT4N3M1b) squamous cell carcinoma with liver metastasis in April 2018. Pembrolizumab was administered as first-line chemotherapy in May 2018. The TPS of PD-L1 was 70%.
After 6 weeks (2 cycles) of pembrolizumab administration, the primary tumour had increased in size (Figure 5C), but the patient was asymptomatic. CT evaluation at 12 weeks (4 cycles) revealed shrinkage of the primary tumour (Figure 5C). Pembrolizumab was continued for up to 11 cycles but was discontinued due to the enlargement of multiple mediastinal lymph nodes. The best response from treatment initiation was SD. The NLR peaked 3 weeks after pembrolizumab administration and remained low thereafter (Figure 5C).
Patient 4
A 69-year-old male was diagnosed with stage IVB (cT4N3M1c) adenocarcinoma with brain and cervical lymph node metastases in October 2017. A lung biopsy specimen was negative for EGFR, ALK, ROS1, and the TPS of PD-L1. The patient underwent gamma knife radiosurgery for brain metastasis in November 2017. Cisplatin plus pemetrexed was administered as first-line chemotherapy in December 2017. CT evaluation after 4 cycles of cisplatin plus pemetrexed revealed disease progression. Atezolizumab was administered as second-line chemotherapy in April 2018.
After 10 days of atezolizumab administration, the patient developed a fever. Despite antibiotic administration, the fever and laboratory data worsened. After 3 weeks (1 cycle) of atezolizumab administration, CT evaluation revealed the progression of multiple pulmonary metastases (Figure 5D). Because the patient’s symptoms and general condition had worsened, he was started on 100 mg naproxen 3 times a day on suspicion of tumour-related fever. A week later, he developed acute upper gastrointestinal bleeding and received emergency endoscopic haemostasis. Atezolizumab was discontinued due to fever, upper gastrointestinal bleeding, and the progression of multiple pulmonary metastases. CT evaluation at 9 weeks revealed the shrinkage of the primary tumour (Figure 5D). The patient needed steroid therapy for immune-related hepatic injury supposedly induced by a single dose of atezolizumab. The lesions were stable for more than 3 months. CT imaging in September 2018 revealed lesion worsening, and the patient was switched to docetaxel monotherapy. The best response from treatment initiation was PR. The NLR peaked 1–2 weeks after atezolizumab administration and remained low thereafter (Figure 5D).
Discussion
We have described 4 patients with PsPD treated with ICIs. The results indicate that the NLR is helpful for distinguishing between PsPD and TPD in patients treated with ICIs. To the best of our knowledge, this is the first report to associate PsPD with the NLR in this population.
Chemotherapy after ICI treatment has been reported to improve an objective response in NSCLC.20–23 The synergistic effect of chemotherapy following ICIs is attributable to persistent nivolumab binding to T cells for more than 20 weeks after the last dose.24 On the other hand, patients with early resistance to nivolumab had only a 9% rate of transition to the next drug treatment regimen.25 It is important to promptly shift patients with TPD to the next drug treatment regimen. Notably, patients with a high post-treatment NLR or LDH in this study all had TPD, suggesting that these individuals should be considered for an early transition to the next drug treatment regimen. Identifying differences in immune status between patients with PsPD and TPD with the simple, inexpensive, and repeatable measurements of the NLR and LDH will improve treatment results.
In a retrospective analysis of 3 clinical trials involving 535 pretreated NSCLC patients, 8% of patients with RECIST-defined PD who continued treatment with ICIs reached PR.17 Based on survival analyses, the RECIST 1.1 evaluation underestimated the benefit of ICIs in patients with PD.26,27 Treatment continuation should reportedly be considered in patients with a stable general condition whose laboratory data have not significantly deteriorated.1 While PsPD can be expected to have a delayed anti-tumour effect, there were 2 cases with the temporarily deteriorated general conditions due to fatigue (patient 1) and fever (patient 4). It is therefore desirable to develop non-invasive diagnostic methods that can distinguish between PsPD and TPD.
Lesion enlargement in PsPD is due to T cell lymphocyte infiltration rather than an increase in viable tumour cells.1,28 This suggests that PsPD reflects a treatment response before lesions are visibly smaller on imaging. In metastatic melanoma treated with ICIs, undetectable circulating tumour DNA (ctDNA) at baseline or a marked decrease from the baseline ctDNA value 12 weeks after starting treatment is a powerful biomarker for distinguishing between PsPD and TPD.29 An early decrease in ctDNA using digital-droplet polymerase chain reaction (ddPCR) was observed in two patients with KRAS-mutated lung adenocarcinoma with PsPD.30 It was also reported that a high or increasing ctDNA value on next-generation sequencing (NGS) after treatment is associated with poor clinical outcome.31,32 These results indicate that monitoring changes in ctDNA during ICI treatment is more useful for determining clinical benefit than imaging techniques. In a meta-analysis of EGFR mutation testing using ctDNA in patients with NSCLC, ctDNA was an effective biomarker with high specificity and high association with the primary tumour.33 Although ctDNA measurement using ddPCR or NGS is currently in preliminary stages, it is expected that use will accelerate in the future.
Some limitations should be mentioned. First, our study was a monocentric, small size, retrospective analysis; thus, its generalizability is a possible limitation. However, we included all consecutively enrolled NSCLC patients treated with ICIs, limiting the potential selection bias inherent to this type of analysis. It is hoped that the accumulation of more cases will verify the present results. Second, treatment effects in most cases were determined with radiological evaluations after 3 to 6 weeks, but the schedule was not standardized because this study was retrospective. Third, there were no universal cut-off values of the NLR and LDH. In this study, we used 4.1 and 3.2 for the pre- and post-treatment NLR, and 202 U/L and 288 U/L for the pre- and post-treatment LDH, respectively. The optimal cut-off values should be determined in larger populations. Finally, we could not verify whether changes in the NLR and ctDNA during treatment were correlated. The NLR reflects both systemic inflammation and the degree of local neutrophil and lymphocyte infiltration.34 Although we expect that post-treatment NLR will be normalized by a reduction in ctDNA, which reflects decreased tumour volume and the suppression of systemic and peritumoural inflammation, evidence supporting this hypothesis is needed.
Conclusion
The NLR could be a useful clue for distinguishing between PsPD and TPD. Patients with a high post-treatment NLR in this study all had TPD, suggesting that these individuals should be considered for an early transition to the next drug treatment regimen.
Funding Statement
This research did not receive any specific grants from funding agencies in the public, commercial, or not-for-profit sectors.
Ethical Approval
Written informed consent was obtained from the patients for publication of the study including the patients’ details and accompanying images.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Wolchok JD, Hoos A, O’Day S, et al. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res. 2009;15(23):7412–7420. doi: 10.1158/1078-0432.CCR-09-1624 [DOI] [PubMed] [Google Scholar]
- 2.Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373(2):123–135. doi: 10.1056/NEJMoa1504627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373(17):1627–1639. doi: 10.1056/NEJMoa1507643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fujimoto D, Yoshioka H, Kataoka Y, et al. Pseudoprogression in previously treated patients with non-small cell lung cancer who received nivolumab monotherapy. J Thorac Oncol. 2019;14(3):468–474. doi: 10.1016/j.jtho.2018.10.167 [DOI] [PubMed] [Google Scholar]
- 5.Tamminga M, de Wit S, Hiltermann JT, et al. Circulating tumor cells in advanced non-small cell lung cancer patients are associated with worse tumor response to checkpoint inhibitors. J Immunother Cancer. 2019;7(1):173. doi: 10.1186/s40425-019-0649-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi: 10.1016/j.cell.2011.02.013 [DOI] [PubMed] [Google Scholar]
- 7.Bremnes RM, Dønnem T, Al-Saad S, et al. The role of tumor stroma in cancer progression and prognosis: emphasis on carcinoma-associated fibroblasts and non-small cell lung cancer. J Thorac Oncol. 2011;6(1):209–217. doi: 10.1097/JTO.0b013e3181f8a1bd [DOI] [PubMed] [Google Scholar]
- 8.Dolan RD, McSorley ST, Horgan PG, Laird B, McMillan DC. The role of the systemic inflammatory response in predicting outcomes in patients with advanced inoperable cancer: systematic review and meta-analysis. Crit Rev Oncol Hematol. 2017;116:134–146. doi: 10.1016/j.critrevonc.2017.06.002 [DOI] [PubMed] [Google Scholar]
- 9.Sacdalan D, Lucero J, Sacdalan D. Prognostic utility of baseline neutrophil-to-lymphocyte ratio in patients receiving immune checkpoint inhibitors: a review and meta-analysis. Onco Targets Ther. 2018;11:955–965. doi: 10.2147/OTT.S153290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mei Z, Shi L, Wang B, et al. Prognostic role of pretreatment blood neutrophil-to-lymphocyte ratio in advanced cancer survivors: a systematic review and meta-analysis of 66 cohort studies. Cancer Treat Rev. 2017;58:1–13. doi: 10.1016/j.ctrv.2017.05.005 [DOI] [PubMed] [Google Scholar]
- 11.Khunger M, Patil PD, Khunger A, et al. Post-treatment changes in hematological parameters predict response to nivolumab monotherapy in non-small cell lung cancer patients. PLoS One. 2018;13(10):e0197743. doi: 10.1371/journal.pone.0197743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kiriu T, Yamamoto M, Nagano T, et al. The time-series behavior of neutrophil-to-lymphocyte ratio is useful as a predictive marker in non-small cell lung cancer. PLoS One. 2018;13(2):e0193018. doi: 10.1371/journal.pone.0193018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nakaya A, Kurata T, Yoshioka H, et al. Neutrophil-to-lymphocyte ratio as an early marker of outcomes in patients with advanced non-small-cell lung cancer treated with nivolumab. Int J Clin Oncol. 2018. doi: 10.1007/s10147-018-1250-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Takeda T, Takeuchi M, Saitoh M, Takeda S. Neutrophil-to-lymphocyte ratio after four weeks of nivolumab administration as a predictive marker in patients with pretreated non-small-cell lung cancer. Thorac Cancer. 2018;9(10):1291–1299. doi: 10.1111/1759-7714.12838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Derman B, Macklis J, Azeem M, et al. Relationships between longitudinal neutrophil to lymphocyte ratios, body weight changes, and overall survival in patients with non-small cell lung cancer. BMC Cancer. 2017;17(1):141. doi: 10.1186/s12885-017-3122-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mezquita L, Auclin E, Ferrara R, et al. Association of the lung immune prognostic index with immune checkpoint inhibitor outcomes in patients with advanced non–small cell lung cancer. JAMA Oncol. 2018. doi: 10.1001/jamaoncol.2017.4771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kazandjian D, Keegan P, Suzman DL, Pazdur R, Blumenthal GM. Characterization of outcomes in patients with metastatic non-small cell lung cancer treated with programmed cell death protein 1 inhibitors past RECIST version 1.1-defined disease progression in clinical trials. Semin Oncol. 2017;44(1):3–7. doi: 10.1053/j.seminoncol.2017.01.001 [DOI] [PubMed] [Google Scholar]
- 18.Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2012;48(3):452–458. doi: 10.1038/bmt.2012.244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tachihara M, Kiriu T, Hata A, et al. A multi-center, phase II trial of nab-paclitaxel and gemcitabine in patients with non-small-cell lung cancer previously treated with platinum-based chemotherapy. Cancer Manag Res. 2019;11:7135–7140. doi: 10.2147/CMAR.S208224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shiono A, Kaira K, Mouri A, et al. Improved efficacy of ramucirumab plus docetaxel after nivolumab failure in previously treated non‐small cell lung cancer patients. Thorac Cancer. 2019;10(4):775–781. doi: 10.1111/1759-7714.12998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Costantini A, Corny J, Fallet V, et al. Efficacy of next treatment received after nivolumab progression in patients with advanced nonsmall cell lung cancer. ERJ Open Res. 2018;4:2. doi: 10.1183/23120541.00120-2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Park SE, Lee SH, Ahn JS, Ahn M-J-J, Park K, Sun J-M. Increased response rates to salvage chemotherapy administered after PD-1/PD-L1 inhibitors in patients with non-small cell lung cancer. J Thorac Oncol. 2018;13(1):106–111. doi: 10.1016/j.jtho.2017.10.011 [DOI] [PubMed] [Google Scholar]
- 23.Schvartsman G, Peng S, Bis G, et al. Response rates to single-agent chemotherapy after exposure to immune checkpoint inhibitors in advanced non-small cell lung cancer. Lung Cancer. 2017;112:90–95. doi: 10.1016/j.lungcan.2017.07.034 [DOI] [PubMed] [Google Scholar]
- 24.Osa A, Uenami T, Koyama S, et al. Clinical implications of monitoring nivolumab immunokinetics in non–small cell lung cancer patients. JCI Insight. 2018;3(19):e59125. doi: 10.1172/jci.insight.59125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Costantini A, Fallet V, Corny J, et al. Nivolumab-refractory patients with advanced non-small-cell lung cancer. Lung Cancer. 2019;130:128–134. doi: 10.1016/j.lungcan.2019.01.015 [DOI] [PubMed] [Google Scholar]
- 26.Tazdait M, Mezquita L, Lahmar J, et al. Patterns of responses in metastatic NSCLC during PD-1 or PDL-1 inhibitor therapy: comparison of RECIST 1.1, irRECIST and iRECIST criteria. Eur J Cancer. 2018;88:38–47. doi: 10.1016/j.ejca.2017.10.017 [DOI] [PubMed] [Google Scholar]
- 27.Ricciuti B, Genova C, Bassanelli M, et al. Safety and efficacy of nivolumab in patients with advanced non-small cell lung cancer treated beyond progression. Clin Lung Cancer. 2019;20:208–214. doi: 10.1016/j.cllc.2019.02.001 [DOI] [PubMed] [Google Scholar]
- 28.Tanizaki J, Hayashi H, Kimura M, et al. Report of two cases of pseudoprogression in patients with non–small cell lung cancer treated with nivolumab—including histological analysis of one case after tumor regression. Lung Cancer. 2016;102:44–48. doi: 10.1016/j.lungcan.2016.10.014 [DOI] [PubMed] [Google Scholar]
- 29.Lee JH, Long GV, Menzies AM, et al. Association between circulating tumor DNA and pseudoprogression in patients with metastatic melanoma treated with anti-programmed cell death 1 antibodies. JAMA Oncol. 2018;4(5):717–721. doi: 10.1001/jamaoncol.2017.5332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guibert N, Mazieres J, Delaunay M, et al. Monitoring of KRAS-mutated ctDNA to discriminate pseudo-progression from true progression during anti-PD-1 treatment of lung adenocarcinoma. Oncotarget. 2017;8(23):38056–38060. doi: 10.18632/oncotarget.16935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leprieur E, Herbretau G, Dumenil C, et al. Circulating tumor DNA evaluated by next-generation sequencing is predictive of tumor response and prolonged clinical benefit with nivolumab in advanced non-small cell lung cancer. Oncoimmunology. 2018;7(5):e1424675. doi: 10.1080/2162402X.2018.1424675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Iijima Y, Hirotsu Y, Amemiya K, et al. Very early response of circulating tumour-derived DNA in plasma predicts efficacy of nivolumab treatment in patients with non-small cell lung cancer. Eur J Cancer. 2017;86:349–357. doi: 10.1016/j.ejca.2017.09.004 [DOI] [PubMed] [Google Scholar]
- 33.Qiu M, Wang J, Xu Y, et al. Circulating tumor DNA is effective for the detection of EGFR mutation in non–small cell lung cancer: a meta-analysis. Cancer Epidemiol Prev Biomarkers. 2015;24(1):206–212. doi: 10.1158/1055-9965.EPI-14-0895 [DOI] [PubMed] [Google Scholar]
- 34.Jiang T, Bai Y, Zhou F, et al. Clinical value of neutrophil-to-lymphocyte ratio in patients with non-small-cell lung cancer treated with PD-1/PD-L1 inhibitors. Lung Cancer. 2019;130:76–83. doi: 10.1016/j.lungcan.2019.02.009 [DOI] [PubMed] [Google Scholar]